Introduction
DNA double strand breaks (DSBs) generated by
ionizing radiation (IR) and genotoxic agents must be repaired to
protect genetic fidelity. When mammalian cells are exposed to IR,
several physiological processes are invoked, including DSB repair,
cell cycle checkpoints, apoptosis, autophagy and telomere-DSB
fusion. DSBs may: i) be repaired during the G2/M phase and enter
another cell cycle; ii) become apoptotic; or iii) repopulate with
aberrant genome. DSBs are repaired by two main pathways:
non-homologous end-joining (NHEJ) and homologous recombination (HR)
(1,2). The modalities to detect DNA double
strand breaks (DSBs) include immunofluorescent staining of γH2AX
and FACS analysis of G2/M arrest in the cell cycle.
DNA-dependent protein kinase catalytic subunit
(DNA-PKcs) is a crucial factor of NHEJ. Our previous studies and
other research have demonstrated that mouse cells and human cancer
cells deficient in the DNA-PK complex, which is composed of
Ku70/Ku80 heterodimer and DNA-PKcs, are hypersensitive to IR
(3–5). The DNA-PK complex contributes to early
stage damage-induced cell cycle arrest and DNA repair (6). Thus, DNA-PK may be a valid target for
radiotherapeutic intervention in cancer therapy.
The cell cycle is a decisive factor in the choice of
DSB repair pathway. NHEJ is favored in the G1 phase and HR is
active in the S and G2 phases (7).
SCID cells defective in DNA-PKcs are hypersensitive to X-ray during
G1 and early S phase (8). Our
previous research demonstrated that cell cycle arrest in the G2
phase is more common in Ku70−/− and Ku80−/−
mouse embryonic fibroblast (MEF) cells than wild-type (WT) MEF
cells at 6 h post-IR (9).
Inactivation of DNA-PK results in prolonged G2/M phase arrest in
ATM−/− human fibroblasts (10).
DNA damage response (DDR) proteins, including
ataxia-telangiectasia and Rad3-related (ATR), ataxia-telangiectasia
mutated (ATM), checkpoint effector kinase 1 (CHK1), CHK2, and
DNA-PKcs, form a phalanx of kinases in response to DSB. ATM and ATR
trigger the phosphorylation of CHK2 and CHK1, respectively
(11). The ATM-CHK2 and ATR-CHK1
pathways collaboratively modulate cell cycle arrest (12,13).
Akt has been reported to be activated in a DNA-PK-dependent manner
at the site of DSBs and to promote NHEJ via DNA-PK activation
(1,14).
Strategies for improving the efficacy of
conventional radiotherapy with agents inducing DSB repair
deficiency are feasible by better understanding the
interconnectivity between the diverse molecular mechanisms. NU7441,
a highly potent and selective DNA-PK inhibitor, has been reported
to effectively radiosensitize several human cancer cells (15–22).
Nasopharyngeal carcinoma (NPC), an endemic cancer in
southern China, has been cured by radiation therapy in combination
with concurrent chemotherapy. The 5-year survival rate can be
expected to reach 75%; whereas local tumor recurrence remains one
of the main obstacles for successful treatment, especially for
those with locally advanced NPC (23). Novel agents have been found to
enhance radiosensitivity to decrease local recurrence in NPC
patients who are treated with radiation therapy in combination with
targeted therapy. The application of DNA-PK inhibitor to enhance
radiosensitivity may improve treatment outcomes for this cohort of
patients with NPC. However, the cellular specificity and mechanism
of DNA-PK inhibition-mediated antitumor activity is still
unclear.
Comparing DNA-PKcs-deficient and DNA-PKcs-competent
cells, we explored the mechanism of NHEJ repair involving cell
cycle checkpoints and the potential synergistic effect of IR and
DNA-PK inhibitor. We further corroborate the synergistic effect of
DNA-PK inhibition on enhanced radiosensitivity in human NPC
cells.
Materials and methods
Cell lines and treatments
DNA-PKcs−/− and WT MEF cells were kindly
provided by Professor Gloria C. Li from Memorial Sloan-Kettering
Cancer Center, USA (3,24,25).
SUNE-1 cell line, derived from a patient with undifferentiated NPC
(26,27), was a gift from Professor Tiebang
Kang at Sun Yat-sen University Cancer Center. WT,
DNA-PKcs−/− MEF cells and human NPC SUNE-1 cells were
maintained in RPMI-1640 supplemented with 10% fetal bovine serum
(FBS), penicillin, and streptomycin at 37°C in 5%
CO2.
The DNA-PK inhibitor NU7441 (Tocris Bioscience,
Bristol, UK) was dissolved in dimethylsulfoxide (DMSO) as a 5
mmol/l stock solution and stored at −20°C. All drugs were added to
cells with a final DMSO concentration of 0.5%. Cells were exposed
to X-rays generated by a Rad Source RS2000 irradiator (Rad Source
Technologies, Buford, GA, USA) operating at 25 mA with a 0.3 mm Al
filter and effective photon energy of 160 kV. The dose rate at an
irradiation distance of 48.6 cm was 1.31 Gy/min.
Clonogenic survival assays
Clonogenic cell survival was determined by the
colony formation assay as described previously (28). A total of 1.5×105
exponentially growing WT, DNA-PKcs−/− MEF cells and
SUNE-1 cells were supplemented with control or NU7441 (1
µM)-containing medium for 1 h and then exposed to IR at the
indicated dose. After IR, the cells were incubated with or without
NU7441 for a further 24 h. Cells were harvested and plated in
drug-free medium at 20–200 colonies per 100-mm dish in triplicate
and left to develop colonies. Approximately 7–10 days later, the
medium was discarded and the colonies were fixed and stained with
crystal violet. Colonies consisting of >50 cells were counted.
Surviving fractions were normalized by the plating efficiency of
unirradiated controls.
Immunofluorescence microscopy foci
assay
Cells were exposed to IR of 5 Gy in the presence or
absence of 2 µM NU7441 1 h pre-IR and collected at the indicated
time. Cells were fixed with 4% paraformaldehyde, permeabilized with
0.2% Triton X-100, and blocked with 3% bovine serum albumin. The
cover slips were incubated with anti-γH2AX antibody (1:200;
Millipore, Billerica, MA, USA) overnight at 4°C, followed by
incubation with conjugated secondary antibody for 1.5 h in the
dark. Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI;
Roche, Basel, Switzerland). Images were captured using an LSM 710
confocal microscope (Zeiss, Oberkochen, Germany), with foci counted
in 30 cells. Quantitative image analysis was performed by ImageJ
(29).
Fluorescence-activated cell sorting
(FACS)
To detect the percentage of cells with γH2AX, WT MEF
cells were incubated with FBS-free medium to synchronize them into
G1 phase. Twelve hours later, the FBS-free medium was removed and
cells recovered in medium containing 10% FBS for another 24 h. The
cells were irradiated with 5 Gy alone or in combination with 2 µM
NU7441. Following treatment, the cells were trypsinized at the
indicated time, fixed, treated with 0.2% Triton X-100, and blocked
in 3% BSA. The cells were stained with anti-γH2AX antibody (1:100;
Cell Signaling Technology, Inc., Danvers, MA, USA) and DAPI. A
minimum of 10,000 labeled cells were acquired and analyzed by
FlowJo 7.6.1 software (30).
For cell cycle analysis, SUNE-1 cells were exposed
to irradiation of 5-Gy in the presence or absence of 2 µM NU7441.
Cells were collected at indicated time post-IR and fixed with 70%
ethanol. Cellular DNA was labeled with propidium iodide (PI). The
cell cycle distribution was determined with 10,000 cells using FACS
flow cytometry (Gallios; Beckman Coulter, Inc., Brea, CA, USA). The
proportion of cells in different phases was gated and calculated
using Flowjo 7.6.1 software.
Western blot analysis
Total protein and phosphorylated protein levels were
analyzed by western blot analysis. Briefly, following treatment,
cells were lysed and whole-cell lysates resolved by SDS-PAGE (6 or
10%) and immunoblotted with the indicated antibodies. The following
primary antibodies were used for immunoblotting: anti-AKT,
anti-phospho-Akt (S473), anti-CHK1, anti-phospho-CHK1 (S345),
anti-CHK2, anti-ATR, anti-phospho-ATR (S428), anti-ATM,
anti-rabbit/mouse horseradish peroxidase-conjugated secondary
antibodies (Cell Signaling Technology, Inc.), anti-Ku70,
anti-phospho-CHK2 (T68) (Novus Biologicals LLC, Littleton, CO,
USA), anti-phospho-ATM (S1981), anti-RAD51 (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), and anti-β-actin
(Millipore).
Statistical analysis
The data are presented as mean ± SD of at least
three independent experiments. The significance of G2/M phase
arrest between IR and IR and NU7441 was determined by ANOVA and all
other statistical tests were performed using Student's t-test in
Sigma Plot 12.5. Significance was defined as p-values of
<0.05.
Results
Enhancing NHEJ pathway-mediated
radiosensitivity and increasing radiation-induced γH2AX foci by
suppressing DNA-PK activity
To investigate the role of DNA-PK in response to IR,
we utilized WT and DNA-PKcs−/− MEF cells to determine
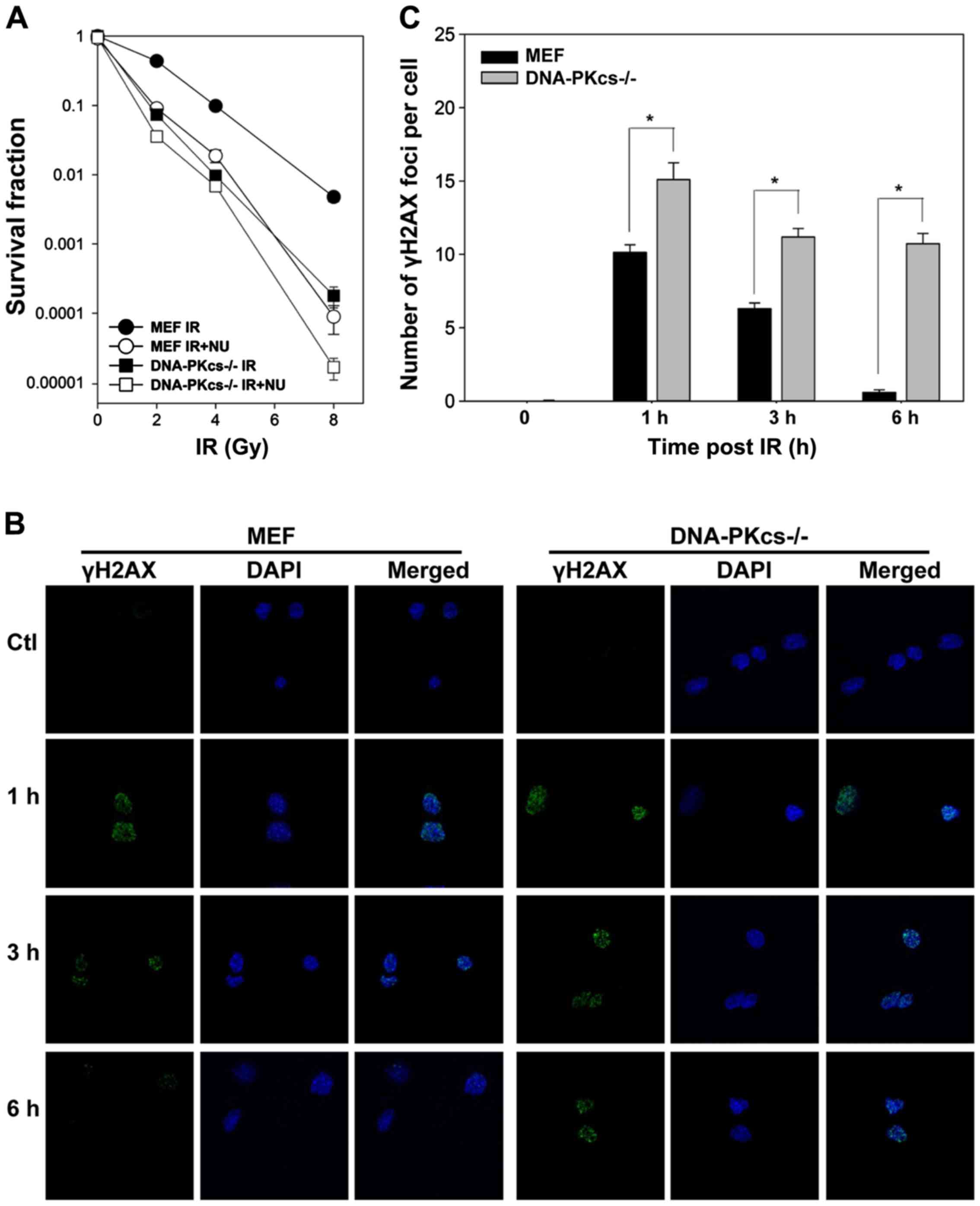
differences in radiosensitivity in colony formation assay.
Irradiation reduced cell survival in DNA-PKcs−/− MEF
cells. NU7441 (1 µmol/l) significantly enhanced radiosensitivity in
WT MEF cells, whereas a modest effect was observed in
DNA-PKcs−/− MEF cells (Fig.
1A), supporting that potentiation was attributable to the
inhibition of the DNA-PK complex involving in DNA-PKcs gene
expression.
We assessed the capacity of DSB repair by counting
cellular γH2AX foci, which is routinely performed to assess the
amount of DSBs (31). As shown in
Fig. 1B and C, IR led to a rapid
peak of γH2AX foci formation (within 1 h). γH2AX foci in WT MEF
cells gradually resolved to the baseline level at 6 h post-IR but
declined with much slower kinetics in DNA-PKcs−/− MEF
cells. Notably, we observed considerable residual γH2AX foci in
DNA-PKcs−/− MEF cells compared to WT MEF cells at 1, 3,
and 6 h after treatment (Fig. 1C),
indicating that attenuation of the NHEJ pathway impedes DSB repair
with highly significant radiosensitivity.
Effect of NU7441 on radiation-induced
DSB repair
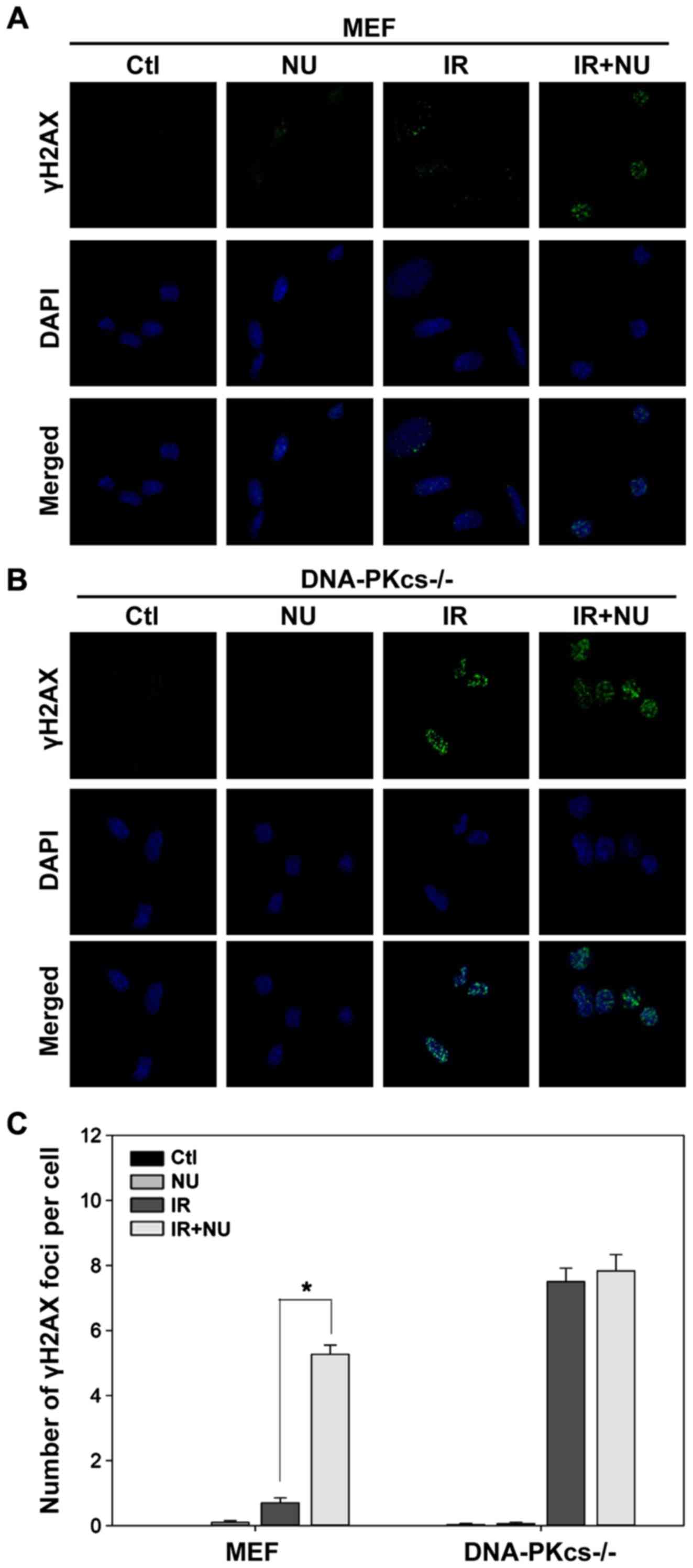
Based on our finding that DSBs are almost completely
repaired in WT MEF cells but persist in DNA-PKcs−/− MEF
cells at 6 h post-IR, we evaluated the effect of NU7441 on DSBs at
6 h after irradiation. We detected a striking accumulation of γH2AX
foci (5.3±0.3 foci per cell) in WT MEF cells treated with both IR
and NU7441 compared to cells treated with IR alone (0.7±0.2 foci
per cell; Fig. 2A and C). NU7441
moderately affected the level of radiation-induced γH2AX foci in
DNA-PKcs−/− MEF cells (Fig.
2B and C). In contrast, treatment with NU7441 alone had a
negligible effect on γH2AX foci formation (Fig. 2). These results were mirrored by a
corresponding decrease in viability (as measured by clonogenic
survival assays).
Effect of NU7441 on crosstalk between
DSBs and cell cycle
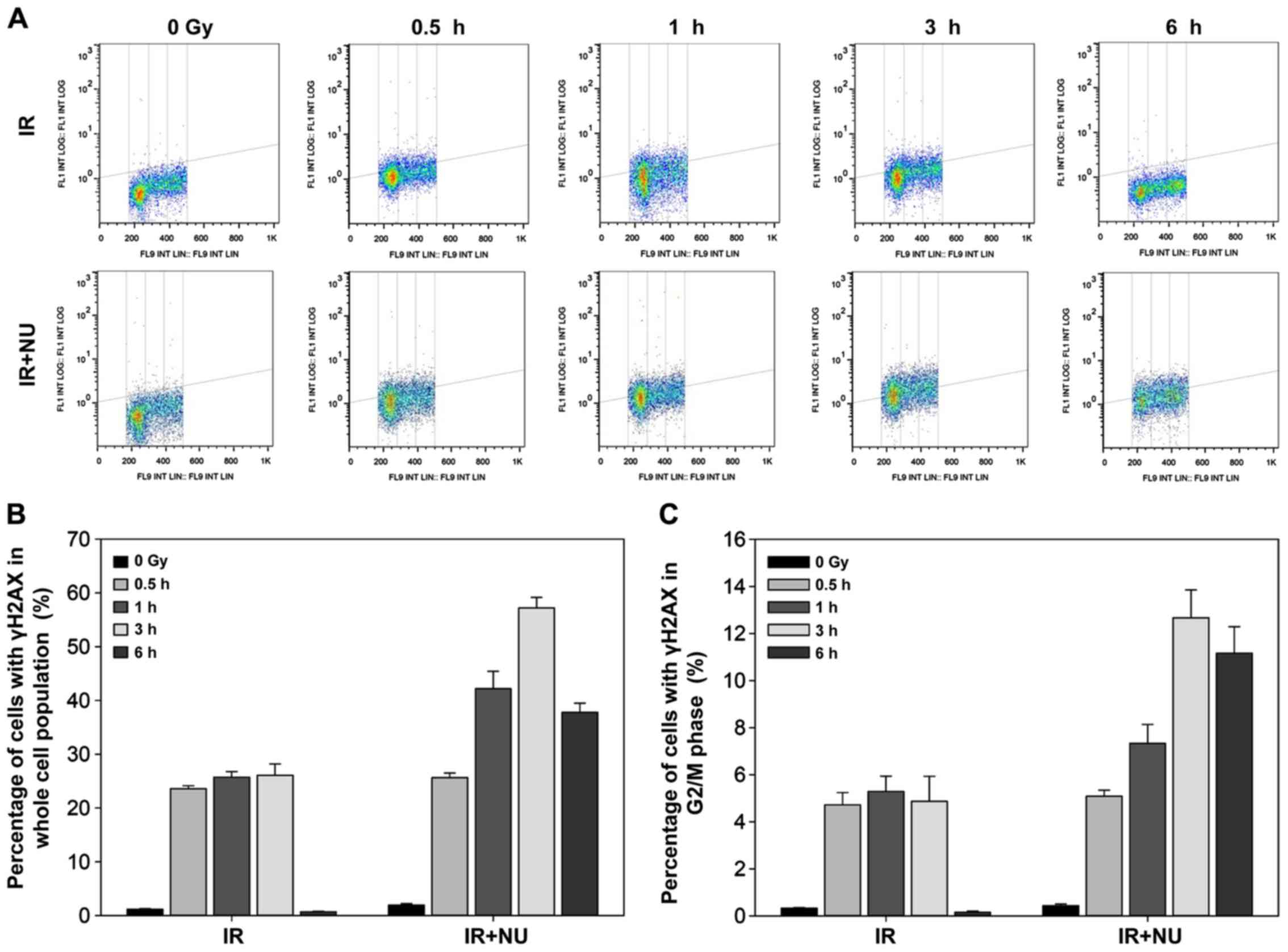
In line with our previous data, deficiency of Ku
heterodimer blocks cell cycle progression (9). NU7441 is designed to target the DNA-PK
complex and may not only impair DSB repair, but also influence the
cell cycle profile. In synchronized WT MEF cells, combined
treatment with NU7441 and IR resulted in more γH2AX-positive cells
than IR alone. The maximum level of γH2AX expression was detected
at 3 h after combined treatment (Fig.
3A and B). Quantification of the percentage of cells with γH2AX
in the G2/M phase showed a slight decrease 6 h after combined
treatment (Fig. 3C).
Mobilization of DDR proteins following
exposure to IR
Because IR-induced DSBs activate the DDR and amplify
the cellular damage signal by phosphorylating cell cycle
checkpoints (32), we assessed the
role of NU7441 in the activation of DDR-related gene expression.
Treatment with NU7441 was associated with a significant increase in
phospho-CHK1 levels in response to radiation (Fig. 4A). Combined treatment with NU7441
and IR resulted in the inhibition of CHK2 phosphorylation compared
to treatment with IR alone (Fig.
4B). IR-induced Akt activity was inhibited by NU7441 only in WT
MEF cells (Fig. 4C). NU7441
potently inhibited the expression of RAD51, a marker of the HR
pathway, suggesting that it may be an efficient inhibitor targeting
both the NHEJ and HR repair pathways (Fig. 4C).
Enhancement of antitumor activity with
the addition of NU7441 to IR in SUNE-1 cells
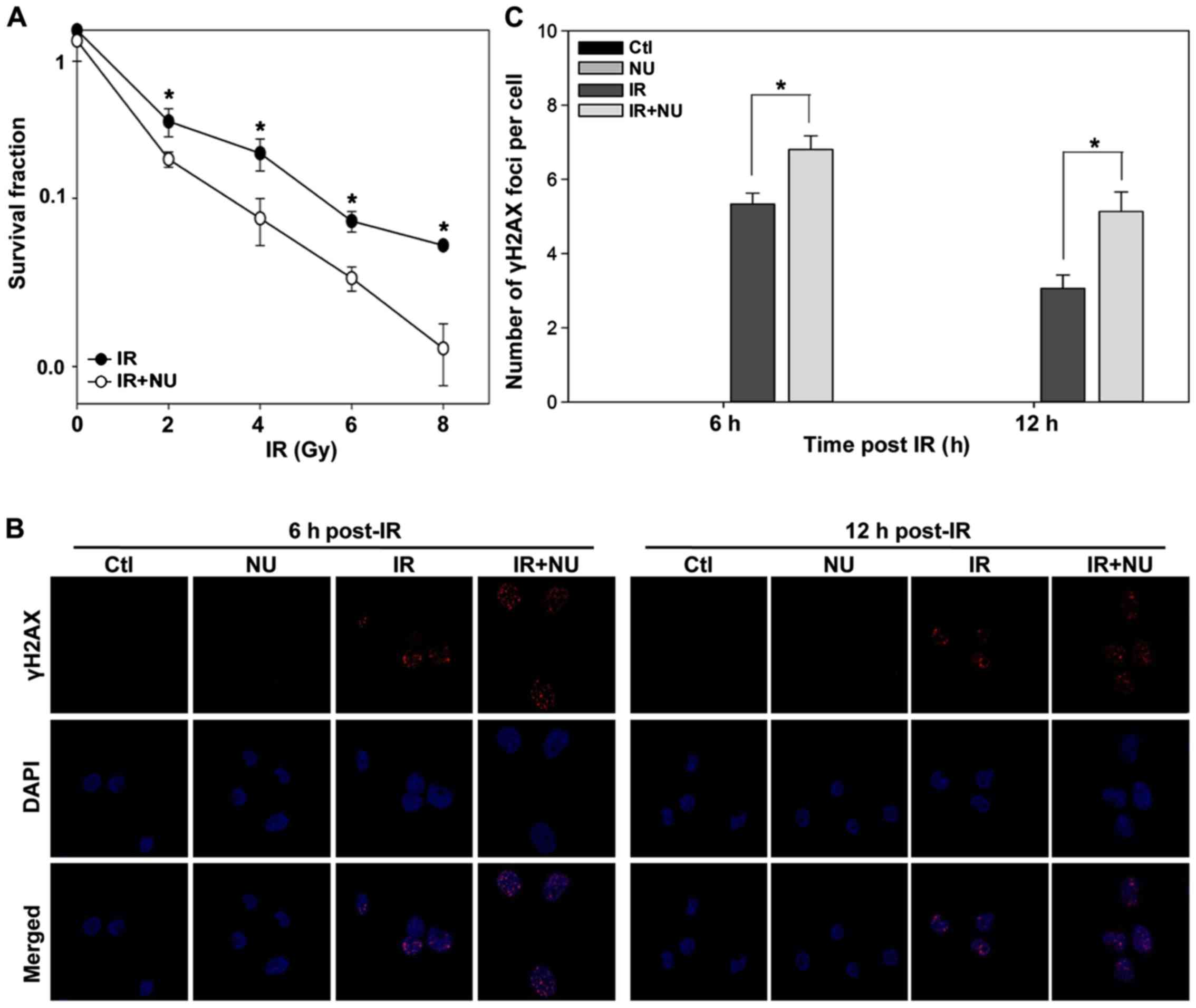
Clonogenic survival assays revealed that NU7441
markedly increased the cytotoxicity of IR (Fig. 5A). Treatment with IR and NU7441
resulted in more pronounced DSBs compared to treatment with IR
alone (Fig. 5B and C) at 6 h or 12
h post-IR, whereas NU7441 alone did not affect γH2AX foci
formation.
Our previous study showed that NU7441 alone did not
induce cytotoxicity and DSB (25).
Combination of IR and NU7441 significantly enhances
radiosensitivity and induces more DSB than IR alone. It plays an
important role in radiosensitizing cells but treatment of NU7441
alone might not influence the cell cycle progression. We aimed to
compare the difference of cell cycle distribution between IR alone
and IR + NU7441.
Irradiated cells undergo abnormal cell cycle
progression in the absence of a proficient NHEJ factor (33). Cells are more sensitive to radiation
in the G2/M phase of cell cycle than any other phases (34). We hypothesized that the addition of
NU7441 would augment radiation-induced accumulation of SUNE-1 cells
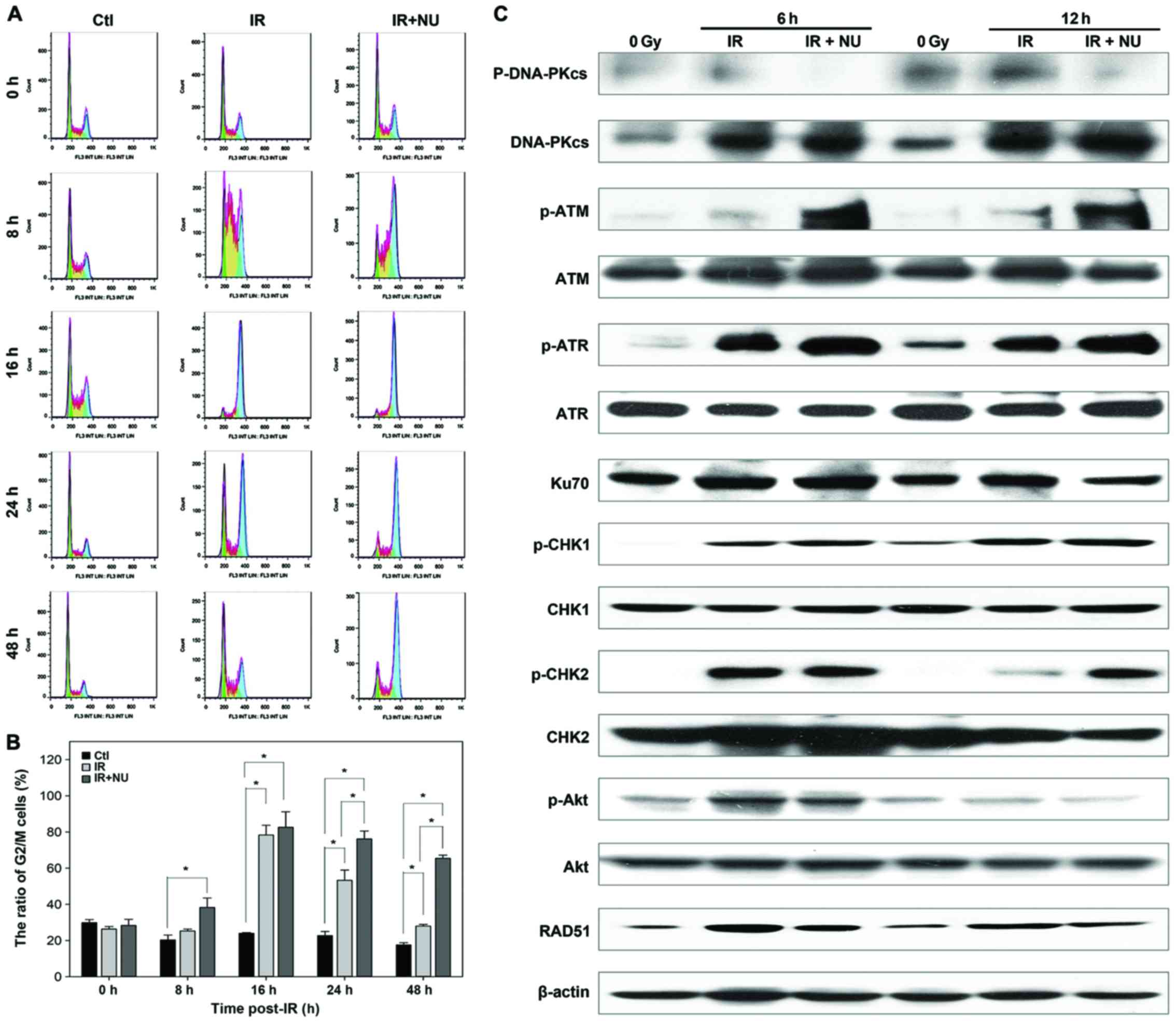
in G2 phase. As expected, the G2/M arrest was observed in SUNE-1
cells when exposed to IR with or without NU7441. The difference
between the groups of IR alone and combination of IR and NU7441 was
statistically significant at 24 and 48 h. The percentage of cells
in the G2/M phase at 24 and 48 h for SUNE1 cells treated with IR
and NU7441 was 76 and 65%, respectively; it was 53 and 27%,
respectively, for the cells treated with IR alone (Fig. 6A and B).
IR induced cell cycle arrest at 16 h
post-irradiation and then the cell cycle arrest gradually released
in IR alone group, but the percentage of SUNE-1 cells in G2/M phase
kept at a high level in IR and NU7441 group. Combined treatment of
IR and NU7441 did not delay the peak of SUNE-1 cells at G2/M arrest
but kept the percentage of cells in G2/M phase at a high level
until 48 h post-irradiation. Comparison of the ratio of G2/M
between IR and IR+NU at the same timing is aimed to examine the
effect of NU7441 on IR-induced cell cycle arrest.
Next, we investigated the molecular basis in SUNE-1
cells. Levels of phosphorylated CHK1, ATR, CHK2 and ATM were
significantly increased in cells treated with IR and NU7441
compared to cells treated with IR alone, indicating that the G2
cell cycle checkpoint is necessary to allow time to repair DNA
damage. Treatment with NU7441 attenuated radiation-induced
phosphorylation of Akt. The radiation-induced expression of RAD51
was decreased in the presence of NU7441 (Fig. 6C). Taken together, the results
indicate that radiosensitive effect of NU7441 is mediated by cell
cycle checkpoints by delaying DNA repair, blocking cell cycle
progression, and promoting apoptosis. NPC cells are susceptible to
combined treatment with NU7441 and irradiation due to a resulting
deficiency in DSB repair and activation of the cell cycle
checkpoint. The difference in DSB repair between normal and tumor
cells in the suppression of DNA-PK activity offers promise for the
development of new radiation-related therapeutic approaches.
Discussion
Research on DNA double strand break (DSB) repair by
non-homologous end-joining (NHEJ) remains a hot topic in the field
of radiation oncology, and it is thought to play a very important
role in the therapy of nasopharyngeal carcinoma. DNA-PK is a
crucial member of NHEJ. To suppress NHEJ signaling pathway by the
DNA-PK inhibitor, NU7441, we explored the synergistic effect on
inhibition of DSB repair following exposure to irradiation in
NHEJ-competent or -deficient cells and a cancer cell line from a
nasopharyngeal carcinoma (NPC) patient.
The present study revealed that: i) a deficiency in
DNA-PKcs contributes to the enhancement of radiosensitivity by
delaying DSB repair; ii) mechanistically, the combination of IR and
NU7441 induces a synergistic effect selectively in NHEJ-competent
cells and has a modest effect on DSB repair in cells deficient in
DNA-PK activity; and iii) NU7441 enhances the synergistic antitumor
effect in NPC cells when applied in combination with irradiation by
impairing DSB repair, prolonging cell cycle progression and
activating the cell cycle checkpoints, which suggests that DNA-PK
inhibitor is a potential radiosensitizer in NPC.
Our previous study isolated DNA-PKcs−/−,
Ku70−/−, Ku80−/− MEF cells from relevant gene
knockout mice (3,24,35).
This study first identified Ku70 as an essential factor in DSB
repair and further study demonstrated that decreasing the level of
Ku70 results in radiation hypersensitivity (24,36).
We designed and generated dominant negative fragment Ku70 (DNKu70).
The human cancer cells infected with virus containing DNKu70 were
vulnerable to irradiation (37).
Our previous study showed that the radiosensitivity of
Ku70−/− MEF cells is similar to that of
DNA-PKcs−/− MEF cells, which is mediated by DSB repair,
cell cycle arrest and activation of cell cycle checkpoints
(25). Therefore, we only used
DNA-PKcs-component and DNA-PKcs knockdown cells to explore the
impact of DNA-PK inhibitor on radiation-induced survival. The
results consistently verified that DNA-PKcs−/− MEF cells
are more radiosensitive than WT MEF cells and NU7441 enhanced the
cytotoxicity in WT MEF cells, but not the DNA-PKcs-deficient cells,
implying that it selectively targeted DNA-PK and showed similar
radiobiological effect to DNA-PKcs knockout cells. We then explored
its enhancement in radiosensitivity in human nasopharyngeal
carcinoma cells.
DNA-PK has been shown to be associated with DSB
repair and drive cell cycle progression. Silencing of DNA-PKcs by
small interfering RNA led to increased radiosensitivity and DSBs
(38–40). DNA-PK may coordinate the expression
of cell cycle machinery to damage response in regulating mitotic
entry (41). Treatment with its
small molecular inhibitor significantly increased DSBs in WT MEF
cells but did not alter the level of γH2AX foci in
DNA-PK−/− MEF cells after exposure to IR (Fig. 2). In addition, NU7441 affected the
interaction between IR-induced DSBs and G2/M phase (Fig. 3). These data confirmed the crucial
role of DNA-PK in response to radiation and demonstrated that cells
with individual component of DNA-PK knockdown may not be affected
by NU7441 after IR treatment.
Cell cycle checkpoints are biological pathways that
provide cells with a mechanism by which to respond to DNA damage by
arresting the cell cycle to allow DNA repair. Robust
phosphorylation of CHK1 has been reported in cells deficient in
DNA-PK (9). We also observed that
phosphorylated CHK1 is markedly increased in WT MEF cells treated
with IR and NU7441 when compared to cells irradiated alone.
Radiation therapy is one of the important treatment
modalities in addition to surgical operation, and chemotherapy for
all of those solid cancers. Whereas radiation therapy is the main
treatment modality in the management of NPC and has achieved
excellent outcomes. The 5-year survival rate is >95% for
patients with early stage disease treated with radiation alone and
60% for patients with locally advanced disease in combination with
chemotherapy. The addition of chemotherapy produced 5–7%
improvement in overall survival when compare to radiation alone. A
recent phase 3 clinical trial demonstrated statistically
significant survival advantage of the addition of induction
chemotherapy (i.e., cisplatin, fluorouracil, and docetaxel; TPF) to
concurrent chemo-radiotherapy in patients with locoregionally
advanced NPC (42). Whereas
surgical operation is not accessible to its proximity of
nasopharynx by it complexity with extensive involvement to cranical
nerves and skull base. Local cancer recurrence and distant
metastasis remains the obstacle for the successful treatment of
NPC. Previously we showed that precise radiotherapy is an effective
treatment (43), and comprehensive
treatment had a favorable prognosis (44). However, local tumor recurrence
remains the main obstacle to the successful treatment for patients
with locally advanced NPC. Further study will explore the
application and mechanism of combined treatment with DNA-PK
inhibitor and conventional chemotherapeutic agents in NPC.
Overexpression of Ku70 or DNA-PKcs in NPC patients
correlates with a worse clinical outcome, probably because of the
capacity to repair DNA damage and to be resistant to radiotherapy
(45). For the first time, we have
shown that NU7441 can effectively radiosensitize SUNE-1 cells by
interfering with DNA repair and delaying the progress through the
cell cycle. Combined treatment of IR and NU7441 aggravated G2/M
accumulation in SUNE-1 cells. The limitation of the study is that
the effect of NU7441 was not examined in normal human cells. Our
data provide novel evidence for the application of DNA-PK inhibitor
in NPC which needs further investigation. It may be a promising
paradigm in the management of NPC.
In conclusion, our study elucidated the functional
ramification of DNA-PK in the modification of DSB repair and the
cell cycle checkpoints. NU7441 has been shown to significantly
enhance radiosensitivity in human NPC cells by antagonizing
radiation-induced cellular defense mechanisms, suggesting that
DNA-PK inhibitor may enhance the efficacy of radiotherapy in NPC
which merits further investigation.
Acknowledgements
The study was supported in part by National
Institutes of Health (grant nos. PO1 CA115675); National Institutes
of Health/National Cancer Institute (grant nos. R33 CA109772);
National Natural Science Foundation of China (grant nos. 81172209
and 81673088). We thank San Francisco Edit Service for help in
English language editing.
Glossary
Abbreviations
Abbreviations:
|
DSB
|
DNA double strand breaks
|
|
IR
|
ionizing radiation
|
|
NHEJ
|
non-homologous end-joining
|
|
HR
|
homologous recombination
|
|
DNA-PKcs
|
DNA-dependent protein kinase catalytic
subunit
|
|
MEF
|
mouse embryonic fibroblast
|
|
WT
|
wild-type
|
|
DDR
|
DNA damage response
|
|
ATR
|
ataxia-telangiectasia and
Rad3-related
|
|
ATM
|
ataxia-telangiectasia mutated
|
|
CHK
|
checkpoint effector kinase
|
|
NPC
|
nasopharyngeal carcinoma
|
|
FBS
|
fetal bovine serum
|
|
DMSO
|
dissolved in dimethylsulfoxide
|
|
DAPI
|
4,6-diamidino-2-phenylindole
|
|
FACS
|
fluorescence-activated cell
sorting
|
|
PI
|
propidium iodide
|
|
DNKu70
|
dominant negative fragment Ku70
|
References
|
1
|
Liu P, Gan W, Guo C, Xie A, Gao D, Guo J,
Zhang J, Willis N, Su A, Asara JM, et al: Akt-mediated
phosphorylation of XLF impairs non-homologous end-joining DNA
repair. Mol Cell. 57:648–661. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang S, Matsunaga S, Lin YF, Sishc B,
Shang Z, Sui J, Shih HY, Zhao Y, Foreman O, Story MD, et al:
Spontaneous tumor development in bone marrow-rescued DNA-PKcs
(3A/3A) mice due to dysfunction of telomere leading strand
deprotection. Oncogene. 35:3909–3918. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kurimasa A, Ouyang H, Dong LJ, Wang S, Li
X, Cordon-Cardo C, Chen DJ and Li GC: Catalytic subunit of
DNA-dependent protein kinase: Impact on lymphocyte development and
tumorigenesis. Proc Natl Acad Sci USA. 96:pp. 1403–1408. 1999;
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang S, Guo M, Ouyang H, Li X,
Cordon-Cardo C, Kurimasa A, Chen DJ, Fuks Z, Ling CC and Li GC: The
catalytic subunit of DNA-dependent protein kinase selectively
regulates p53-dependent apoptosis but not cell-cycle arrest. Proc
Natl Acad Sci USA. 97:pp. 1584–1588. 2000; View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chitnis MM, Lodhia KA, Aleksic T, Gao S,
Protheroe AS and Macaulay VM: IGF-1R inhibition enhances
radiosensitivity and delays double-strand break repair by both
non-homologous end-joining and homologous recombination. Oncogene.
33:5262–5273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee SH and Kim CH: DNA-dependent protein
kinase complex: A multifunctional protein in DNA repair and damage
checkpoint. Mol Cells. 13:159–166. 2002.PubMed/NCBI
|
|
7
|
Bunting SF and Nussenzweig A: End-joining,
translocations and cancer. Nat Rev Cancer. 13:443–454. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Branzei D and Foiani M: Regulation of DNA
repair throughout the cell cycle. Nat Rev Mol Cell Biol. 9:297–308.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Li GC, Iliakis G and Wang Y: Ku
affects the CHK1-dependent G (2) checkpoint after ionizing
radiation. Cancer Res. 62:6031–6034. 2002.PubMed/NCBI
|
|
10
|
Shang ZF, Huang B, Xu QZ, Zhang SM, Fan R,
Liu XD, Wang Y and Zhou PK: Inactivation of DNA-dependent protein
kinase leads to spindle disruption and mitotic catastrophe with
attenuated checkpoint protein 2 Phosphorylation in response to DNA
damage. Cancer Res. 70:3657–3666. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weber AM and Ryan AJ: ATM and ATR as
therapeutic targets in cancer. Pharmacol Ther. 149:124–138. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Curtin NJ: DNA repair dysregulation from
cancer driver to therapeutic target. Nat Rev Cancer. 12:801–817.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bouwman P and Jonkers J: The effects of
deregulated DNA damage signalling on cancer chemotherapy response
and resistance. Nat Rev Cancer. 12:587–598. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bozulic L, Surucu B, Hynx D and Hemmings
BA: PKBalpha/Akt1 acts downstream of DNA-PK in the DNA
double-strand break response and promotes survival. Mol Cell.
30:203–213. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao Y, Thomas HD, Batey MA, Cowell IG,
Richardson CJ, Griffin RJ, Calvert AH, Newell DR, Smith GC and
Curtin NJ: Preclinical evaluation of a potent novel DNA-dependent
protein kinase inhibitor NU7441. Cancer Res. 66:5354–5362. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu L, Tumati V, Tseng SF, Hsu FM, Kim DN,
Hong D, Hsieh JT, Jacobs C, Kapur P and Saha D: DAB2IP regulates
autophagy in prostate cancer in response to combined treatment of
radiation and a DNA-PKcs inhibitor. Neoplasia. 14:1203–1212. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tavecchio M, Munck JM, Cano C, Newell DR
and Curtin NJ: Further characterisation of the cellular activity of
the DNA-PK inhibitor, NU7441, reveals potential cross-talk with
homologous recombination. Cancer Chemother Pharmacol. 69:155–164.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Javvadi P, Makino H, Das AK, Lin YF, Chen
DJ, Chen BP and Nirodi CS: Threonine 2609 phosphorylation of the
DNA-dependent protein kinase is a critical prerequisite for
epidermal growth factor receptor-mediated radiation resistance. Mol
Cancer Res. 10:1359–1368. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ciszewski WM, Tavecchio M, Dastych J and
Curtin NJ: DNA-PK inhibition by NU7441 sensitizes breast cancer
cells to ionizing radiation and doxorubicin. Breast Cancer Res
Treat. 143:47–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tichy A, Durisova K, Salovska B, Pejchal
J, Zarybnicka L, Vavrova J, Dye NA and Sinkorova Z:
Radio-sensitization of human leukaemic MOLT-4 cells by
DNA-dependent protein kinase inhibitor, NU7441. Radiat Environ
Biophys. 53:83–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cornell L, Munck JM, Alsinet C, Villanueva
A, Ogle L, Willoughby CE, Televantou D, Thomas HD, Jackson J, Burt
AD, et al: DNA-PK-A candidate driver of hepatocarcinogenesis and
tissue biomarker that predicts response to treatment and survival.
Clin Cancer Res. 21:925–933. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu L, Shang ZF, Hsu FM, Zhang Z, Tumati V,
Lin YF, Chen BP and Saha D: NSCLC cells demonstrate differential
mode of cell death in response to the combined treatment of
radiation and a DNA-PKcs inhibitor. Oncotarget. 6:3848–3860. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chua MLK, Wee JTS, Hui EP and Chan ATC:
Nasopharyngeal carcinoma. Lancet. 387:1012–1024. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ouyang H, Nussenzweig A, Kurimasa A,
Soares VC, Li X, Cordon-Cardo C, Li W, Cheong N, Nussenzweig M,
Iliakis G, et al: Ku70 is required for DNA repair but not for T
cell antigen receptor gene recombination in vivo. J Exp Med.
186:921–929. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dong J, Zhang T, Ren Y, Wang Z, Ling CC,
He F, Li GC, Wang C and Wen B: Inhibiting DNA-PKcs in a
non-homologous end-joining pathway in response to DNA double-strand
breaks. Oncotarget. 8:22662–22673. 2017.PubMed/NCBI
|
|
26
|
Lu QP, Chen WD, Peng JR, Xu YD, Cai Q,
Feng GK, Ding K, Zhu XF and Guan Z: Antitumor activity of 7RH, a
discoidin domain receptor 1 inhibitor, alone or in combination with
dasatinib exhibits antitumor effects in nasopharyngeal carcinoma
cells. Oncol Lett. 12:3598–3608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao M, Luo R, Liu Y, Gao L, Fu Z, Fu Q,
Luo X, Chen Y, Deng X, Liang Z, et al: miR-3188 regulates
nasopharyngeal carcinoma proliferation and chemosensitivity through
a FOXO1-modulated positive feedback loop with
mTOR-p-PI3K/AKT-c-JUN. Nat Commun. 7:113092016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wen B, Deutsch E, Marangoni E, Frascona V,
Maggiorella L, Abdulkarim B, Chavaudra N and Bourhis J: Tyrphostin
AG 1024 modulates radiosensitivity in human breast cancer cells. Br
J Cancer. 85:2017–2021. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kataoka Y, Bindokas VP, Duggan RC, Murley
JS and Grdina DJ: Flow cytometric analysis of phosphorylated
histone H2AX following exposure to ionizing radiation in human
microvascular endothelial cells. J Radiat Res (Tokyo). 47:245–257.
2006. View Article : Google Scholar
|
|
30
|
Cuneo KC, Morgan MA, Davis MA, Parcels LA,
Parcels J, Karnak D, Ryan C, Liu N, Maybaum J and Lawrence TS: Wee1
kinase inhibitor AZD1775 radiosensitizes hepatocellular carcinoma
regardless of TP53 mutational status through induction of
replication stress. Int J Radiat Oncol Biol Phys. 95:782–790. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bonner WM, Redon CE, Dickey JS, Nakamura
AJ, Sedelnikova OA, Solier S and Pommier Y: GammaH2AX and cancer.
Nat Rev Cancer. 8:957–967. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sulli G, Di Micco R and d'Adda di Fagagna
F: Crosstalk between chromatin state and DNA damage response in
cellular senescence and cancer. Nat Rev Cancer. 12:709–720. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou XY, Wang X, Wang H, Chen DJ, Li GC,
Iliakis G and Wang Y: Ku affects the ATM-dependent S phase
checkpoint following ionizing radiation. Oncogene. 21:6377–6381.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Morgan MA and Lawrence TS: Molecular
pathways: Overcoming radiation resistance by targeting DNA damage
response pathways. Clin Cancer Res. 21:2898–2904. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nussenzweig A, Chen C, da Costa Soares V,
Sanchez M, Sokol K, Nussenzweig MC and Li GC: Requirement for Ku80
in growth and immunoglobulin V (D)J recombination. Nature.
382:551–555. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li GC, He F, Shao X, Urano M, Shen L, Kim
D, Borrelli M, Leibel SA, Gutin PH and Ling CC: Adenovirus-mediated
heat-activated antisense Ku70 expression radiosensitizes tumor
cells in vitro and in vivo. Cancer Res. 63:3268–3274.
2003.PubMed/NCBI
|
|
37
|
He F, Li L, Kim D, Wen B, Deng X, Gutin
PH, Ling CC and Li GC: Adenovirus-mediated expression of a dominant
negative Ku70 fragment radiosensitizes human tumor cells under
aerobic and hypoxic conditions. Cancer Res. 67:634–642. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Peng Y, Zhang Q, Nagasawa H, Okayasu R,
Liber HL and Bedford JS: Silencing expression of the catalytic
subunit of DNA-dependent protein kinase by small interfering RNA
sensitizes human cells for radiation-induced chromosome damage,
cell killing, and mutation. Cancer Res. 62:6400–6404.
2002.PubMed/NCBI
|
|
39
|
An J, Xu QZ, Sui JL, Bai B and Zhou PK:
Downregulation of c-myc protein by siRNA-mediated silencing of
DNA-PKcs in HeLa cells. Int J Cancer. 117:531–537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Toulany M, Kehlbach R, Florczak U, Sak A,
Wang S, Chen J, Lobrich M and Rodemann HP: Targeting of AKT1
enhances radiation toxicity of human tumor cells by inhibiting
DNA-PKcs-dependent DNA double-strand break repair. Mol Cancer Ther.
7:1772–1781. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Goodwin JF and Knudsen KE: Beyond DNA
repair: DNA-PK function in cancer. Cancer Discov. 4:1126–1139.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie
FY, Sun Y, Chen XZ, Li JG, Zhu XD, et al: Induction chemotherapy
plus concurrent chemoradiotherapy versus concurrent
chemoradiotherapy alone in locoregionally advanced nasopharyngeal
carcinoma: A phase 3, multicentre, randomised controlled trial.
Lancet Oncol. 17:1509–1520. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ren Y, Zhao Q, Liu H, Huang Y, Wang Z, Cao
X, Teh BS and Wen B: 3D-image-guided HDR-brachytherapy versus 2D
HDR - brachytherapy after external beam radiotherapy for early
T-stage nasopharyngeal carcinoma. BMC Cancer. 14:8942014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang C, Huang P, Guo L, Wang Z, Zhang T,
Dong J, Shi J, Li Y, Guo X, Cao K, et al: Prognostic analysis in
patients with metastatic nasopharyngeal carcinoma at initial
diagnosis. Jacobs J Radiat Oncol. 1:102014.
|
|
45
|
Lee SW, Cho KJ, Park JH, Kim SY, Nam SY,
Lee BJ, Kim SB, Choi SH, Kim JH, Ahn SD, et al: Expressions of Ku70
and DNA-PKcs as prognostic indicators of local control in
nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys.
62:1451–1457. 2005. View Article : Google Scholar : PubMed/NCBI
|