Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the
fourth leading cause of cancer-related death with a 5-year survival
rate of 2% and a median survival rate of less than 6 months
(1). Despite vast efforts by
researchers, the mortality rate of PDAC patients remains almost
equal to the incidence rate, due to metastasis during the early
asymptomatic stages. Surgery in the early stages, before
metastasis, is the only effective treatment. Thus, diagnosis of
PDAC in early stages is extremely important and finding new
biomarkers seems to be an urgent need (2).
Blood is the ideal biological specimen for detecting
disease biomarkers, due to its availability. Furthermore, blood
biomarkers demonstrate a high degree of accuracy, sensitivity and
specificity, discriminate between harmless and aggressive lesions
and can be detected in early curable stages with convenient and
fast methods (3). Circulating
autoantibodies are a group of serum biomarkers that are produced in
response to tumor microenvironment alterations, such as mutated,
overexpressed and aberrantly glycosylated or localized proteins.
The stability of the autoantibodies compared to proteins and most
importantly, the possibility of being early detected, before
clinical symptoms and signs, are examples of the advantages of
these markers (4). Autoantibodies
can be detectable in the sera of patients, even before detecting
tumor-associated antigens (TAA) and used as a disease-state
reporter to identify the antigenic and physiological changes during
the development and progression of the tumor (5).
Serological proteome analysis (SERPA), also called
two-dimensional (2D) western blot analysis, is a high-throughput
technique for the identification of tumor antigens, in which cell
lysates are firstly separated by 2D gels, and then transferred onto
the membranes and probed with sera. Subsequently, reacting proteins
are identified by mass spectrometry (MS). Using the 2D western
blotting approach or other immunoproteomic approaches, numerous
studies have evaluated and compared the panel of autoantibodies in
healthy individuals and cancer patients against TAAs (6). The presence of autoantibodies against
several proteins, such as p53, MUC1, recombination factor Rad51,
insulin and β-islet cell proteins, calreticulin isoforms,
phosphorylated α-enolase (ENO), as well as DEAD-box protein 48 has
been observed in PDAC (7,8). For example, Tomaino et al
(9) found autoantibodies to ENO as
a hallmark of PDAC. Their data specified that ENO1 was involved in
PDAC cell invasion and that the administration of an anti-ENO1 mAb
can be exploited as a novel therapeutic option to increase the
survival of patients with metastatic PDAC. In this setting, the
combination of elevated CA 19-9 serum levels and anti-ENOA1
autoantibodies improved the diagnostic value of CA 19-9 and
resulted in diagnostic accuracy of 95% (CA 19-9 is the only
FDA-approved blood test for PDAC) (10).
Considering the scarcity of the published studies on
the immunoproteome of PDAC patients, the aim of the present study
was to compare the immunoproteome between PDAC patients and healthy
controls. In order to achieve this goal, we selected two cancer
cell lines, as the antigen source to exploit the autoantibody
repertoire of pancreatic cancer (PC) patients. The screening of
autoantibodies was performed using 2D western blot analysis with
sera from PDAC patients, followed by subsequent identification of
the target proteins by mass spectrometry. This process led to the
identification of a number of new pancreatic immunoreactive
antigens.
Materials and methods
Sera specimens
The present study was approved by the Ethics
Committee of Shiraz University of Medical Sciences. Patients and
controls were informed that their blood samples would be used for
research and their written consent was obtained. The PDAC patients
were recruited from the Surgery Department of Nemazi Hospital
(Shiraz, Iran) during 18 months. Blood samples were collected prior
to surgery or any other treatment. The diagnosis of PDAC was
confirmed by histological analysis. None of the patients had
distant metastasis at the time of diagnosis. The clinical features
of the 20 newly diagnosed PDAC patients (male, 11; female, 9;
median age, 60.2±9.9 years), are described in Table I. The sera from the 20 PDAC
patients, were tested. The control group consisted of 10 healthy
volunteers (male, 7; female, 3; median age, 60.4±8.9 years) who
were recruited at a local Blood Transfusion Center. They had no
history of cancer or autoimmunity. The samples were isolated from
venous blood and stored at −80°C until use.
 | Table I.Clinical features of the 20 PDAC
patients. |
Table I.
Clinical features of the 20 PDAC
patients.
| Patients | Sex | Age (years) | Stage | Tumor site |
|---|
| P1 | Female | 61 | Well
differentiated | Head |
| P2 | Male | 58 | Well
differentiated | Head |
| P3 | Male | 70 | Well
differentiated | Head |
| P4 | Male | 65 | – | Distal |
| P5 | Male | 60 | Well
differentiated | Head |
| P6 | Female | 41 | – | Head |
| P7 | Female | 60 | Moderately
differentiated | Head |
| P8 | Male | 53 | Well
differentiated | Head |
| P9 | Male | 64 | Well
differentiated | Head |
| P10 | Female | 60 | Poorly
differentiated | Head |
| P11 | Male | 83 | Well
differentiated | Head |
| P12 | Male | 58 | Moderately
differentiated | Distal |
| P13 | Male | 52 | – | – |
| P14 | Male | 80 | Well
differentiated | Head |
| P15 | Female | 49 | – | Head |
| P16 | Female | 67 | Well
differentiated | Head |
| P17 | Female | 52 | Well
differentiated | Head |
| P18 | Female | 63 | Well
differentiated | Head |
| P19 | Male | 52 | Well
differentiated | Head |
| P20 | Female | 58 | – | Head |
Cell culture
The human PC cell lines Patu-8902 and Faraz-ICR were
used in this study as antigen sources. Patu-8902, a PC cell line
with full epithelial differentiation and high metastatic potential
(11), was purchased from the
Pasteur Institute of Iran (Tehran, Iran). Faraz-ICR is a PC cell
line that was newly established in the Shiraz Institute for Cancer
Research (Shiraz, Iran). Faraz-ICR has an epithelial-like nature
and is in an undifferentiated state with partial aspects of
epithelial-mesenchymal transition and with significant higher
migration ability than the Patu-8902 cells. Other characteristics
of the Faraz-ICR cell line have been previously described (12). Cell lines were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific) and 1%
penicillin-streptomycin at 37°C in a 5% CO2
atmosphere.
Sample preparation and two-dimensional
gel electrophoresis (2-DE)
Sample preparations and 2-DE were performed
according to methods previously described (6). Briefly, cells at a confluency of
70–80% were harvested using a solution of 0.25% Trypsin-EDTA
(Gibco/Thermo Fisher Scientific Carlsbad, CA, USA). The detached
cells were washed and lysed in urea lysis buffer for 2 h. The
supernatants were collected and stored at −80°C. Protein
concentration was determined using the Bradford assay protocol
(13). The 2-DE analysis was
performed in two steps. For the first dimension, 500 µg protein
lysate was loaded onto immobilized pH gradient strips (pH 3.0–10.0
NL; 18 cm) (GE Healthcare, Uppsala, Sweden). For the second
dimension, the strips were placed on the top of a 12% SDS
polyacrylamide gel and run at a 30 mA constant current for 180 min.
After separation of proteins, the gels were visualized using a
modified Coomassie Brilliant Blue (CBB) (Bio-Rad Laboratories,
Hercules, CA, USA) staining method.
2Dwestern blot analysis
For the 2D western blot analysis. For the 2D western
blotting, proteins from the 2D gels were transferred onto PVDF
membranes by a semi-dry blotter (Bio-Rad Laboratories, Hercules,
CA, USA) at current of 1 mA/cm2 of the membrane for 1 h.
The details of blocking, incubation with primary and secondary
antibodies and washing steps were previously described (6). Finally, immunodetection was
accomplished by incubation of the membranes in diaminobenzedene and
H2O2 for 30 min.
Quantification of protein
immunoreactivity and statistical analysis
The gels were scanned using a densitometer scanner
(Bio-Rad Laboratories) at 300 dpi resolution and recorded in TIFF
format. In order to map the spots with different immunoreactivity,
we analyzed blots using the Prodigy software (version 1.0,
Nonlinear Dynamics, Newcastle, UK). This software aligns and
matches the images by placing 21 manual vectors followed by
automatic vectors generated by the software. The statistical
differences in immunoreactive protein spots between PDAC patients
and control groups were also calculated using the Prodigy software.
The spots which exhibited a >2-fold increase in the average
normalized volume between patient and control sera with a P-value
<0.05 were considered as immunoreactive spots. P-values were
calculated using Mann-Whitney U test. The matching process and the
differential immuneoreactivity of these spots were validated by eye
in at least three images.
MS
Immunoreactive protein spots were manually cut from
2D gels derived from Patu-8902 and Faraz-ICR cell lysates and sent
for MALDI-TOF/TOF MS (Ultraflex III; Bruker Daltonics, Bremen,
Germany) analysis to the United Kingdom (Department of Biology,
Proteomics and Analytical Biochemistry Laboratory, University of
York, UK). The peptide mass fingerprinting (PMF) and tandem mass
spectrometry (MS/MS) information were searched against the National
Center for Biotechnology Information non-redundant (NCB Inr)
database, using the Mascot search engine (Matrix Science, London,
UK). One missed cleavage per peptide was permitted. Statistical
confidence limits of 95% were applied for protein. MASCOT protein
scores >67 were considered to indicate statistically significant
difference (P<0.05).
Results
Proteins extracted from Patu-8902 and Faraz-ICR cell
lines were subjected to isoelectric focusing followed by SDS-PAGE.
Comparison of the whole cell proteome between the two cell lines
revealed that, despite some similarities, their patterns shown
certain differences (Figs. 1A and
2A) as we anticipated, because
Patu-8902 is an epithelial cell line (11), however Faraz-ICR seems to be closer
to mesenchymal cells (12).
Therefore, using these two cell lines as a source of antigen, we
expected to get distinct immunoreactive proteins to identify a
wider range of heterogenic biomarkers in PC. There were shared
immunoreactive proteins between these two cell lines, but with
significant reactivity with normal sera that were not picked up
from the gels for MS identification.
The 2D gels were transferred onto PVDF membranes and
sera from 20 patients with PDAC and 10 healthy donors were
individually screened for the presence of autoantibodies. The
immunoblot pattern among patients revealed some differences but was
highly reproducible for each patient. Protein spots that reacted at
least by two-fold ratio (according to Prodigi software estimation)
with at least three patient sera and generally had no visibility or
with minimal reactivity with the control sera were sent for MS
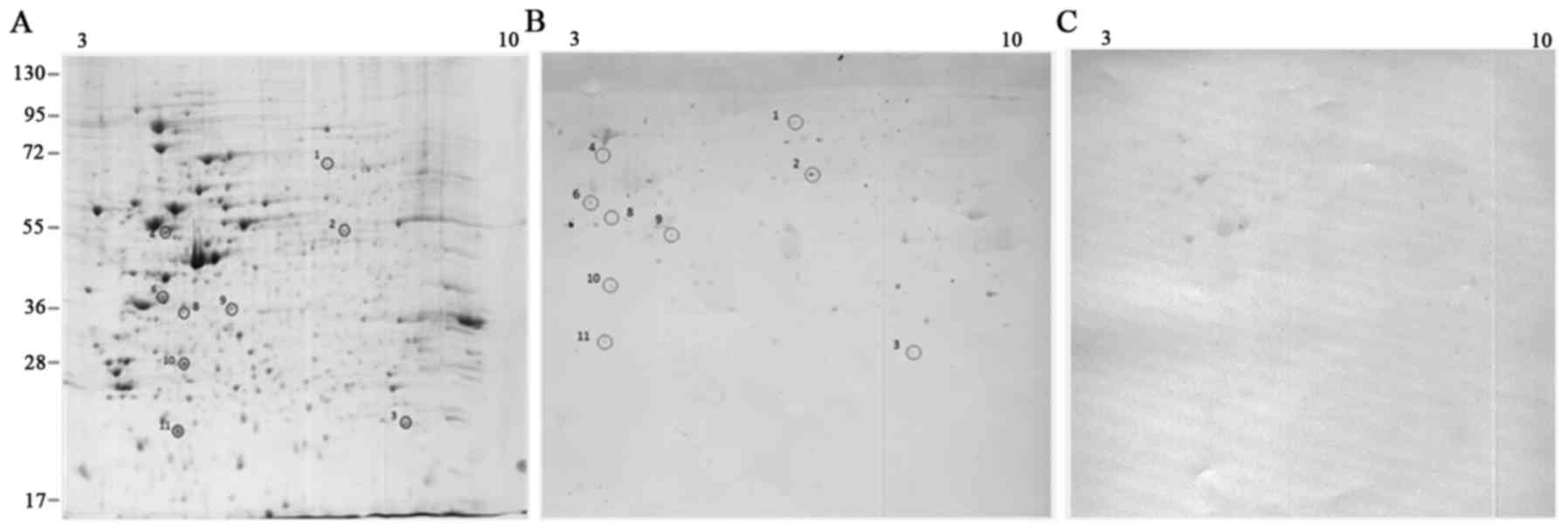
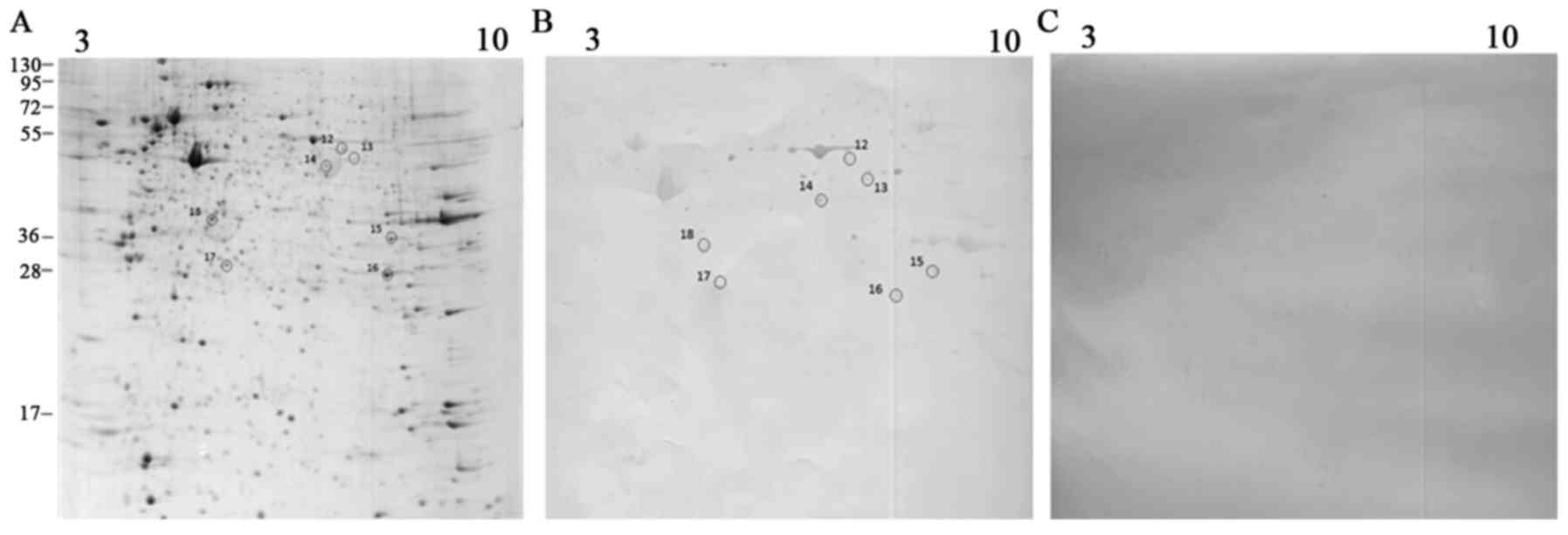
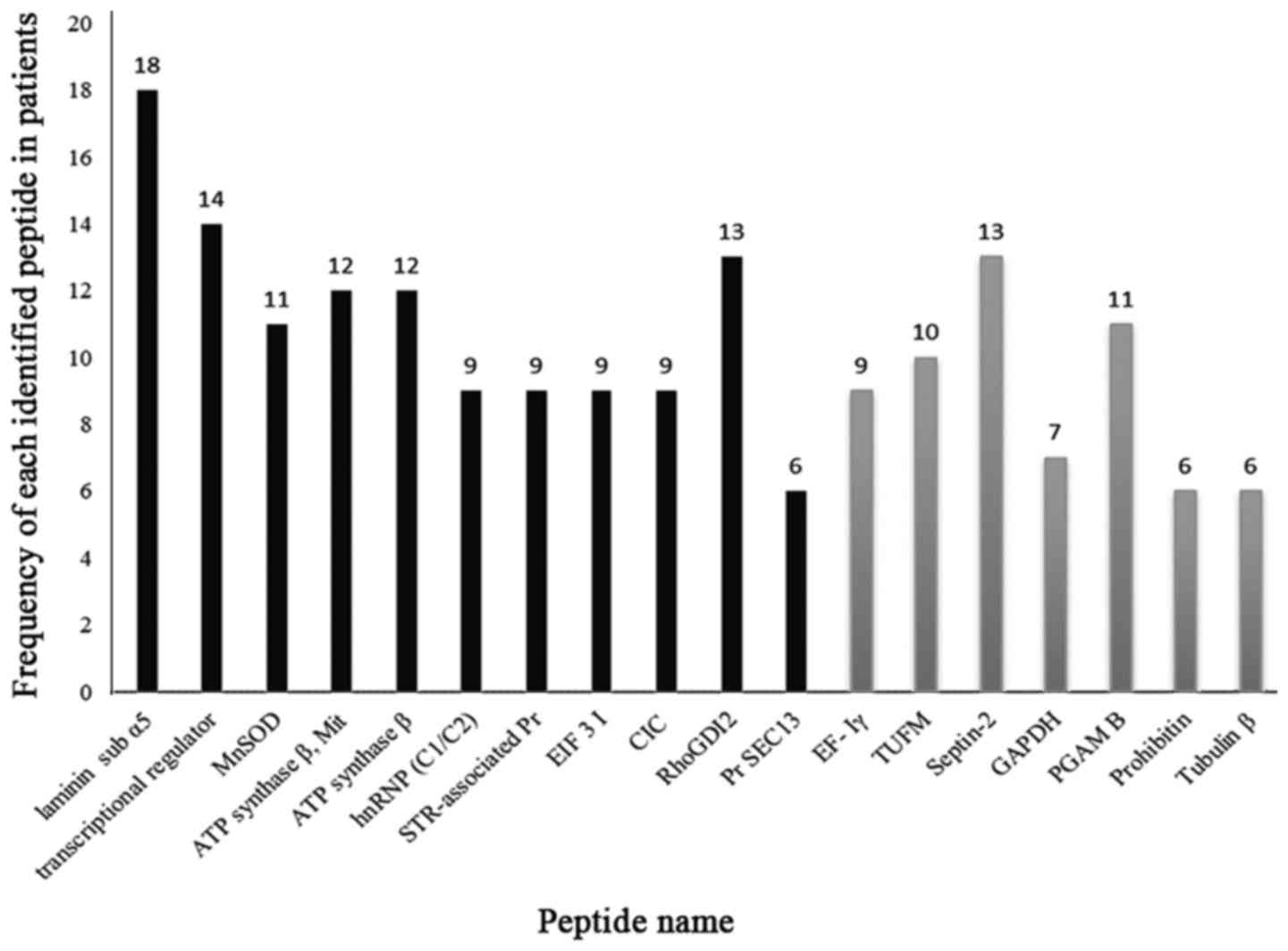
analysis. Fig. 3 displays the
frequency of patients with positive immunoreactivity for the
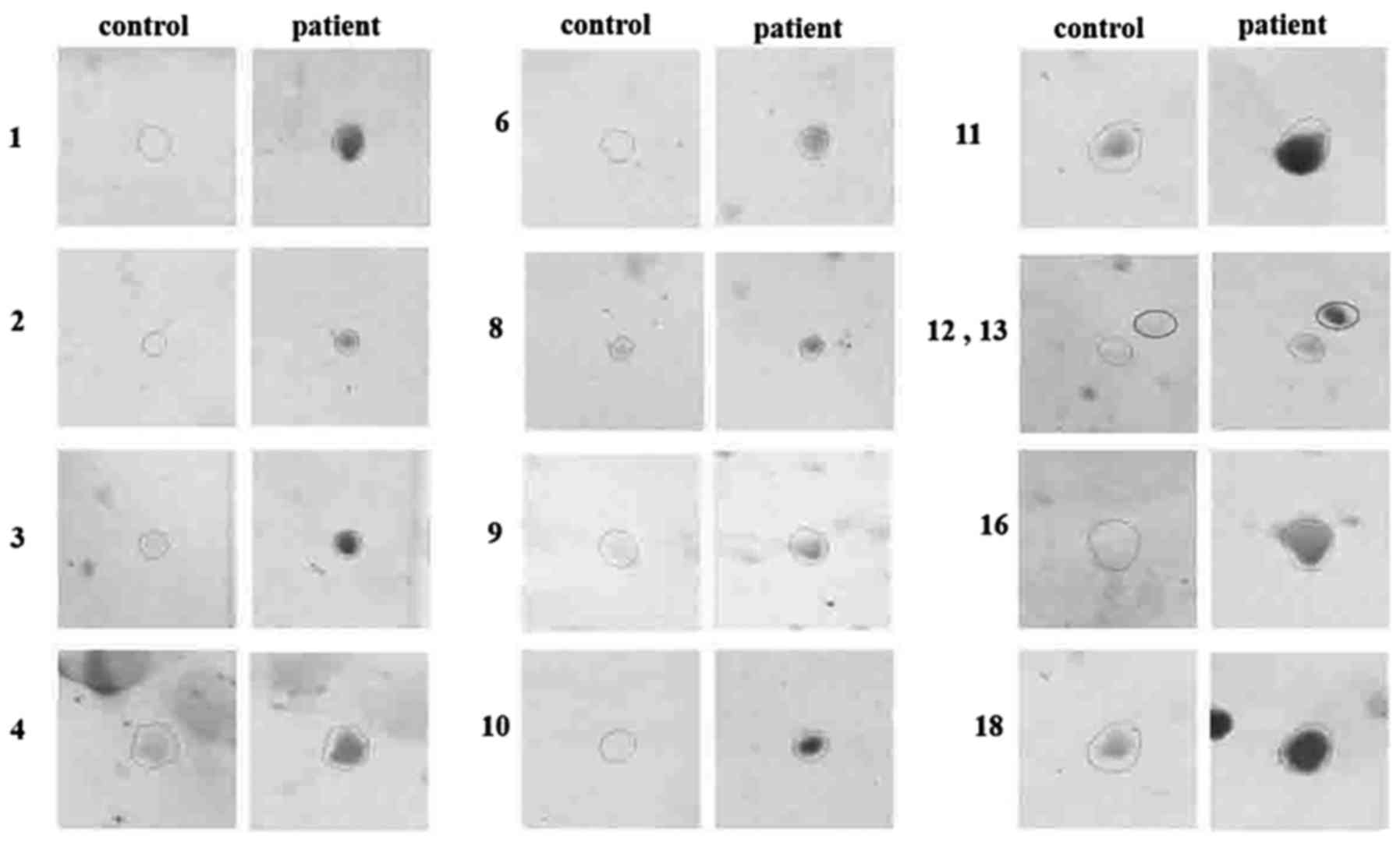
identified spots. The enlarged views of some immunoreactive spots
in patients and controls are displayed in Fig. 4.
The images of 2D gels and blots derived from
Patu-8902 and Faraz-ICR cell lysates are displayed in Figs. 1 and 2. The descriptions of the identified
proteins are shown in Table II. MS
analysis identified two spots as the mixture of proteins. Protein
numbers 4 and 5 were identified in the same spot (mitochondrial ATP
synthase subunit β and ATP synthase subunit β). Protein numbers 6
(heterogeneous nuclear ribonucleoproteins C1/C2) and 7
(serine-threonine kinase receptor-associated protein) were also
identified in the same spot.
 | Table II.Descriptions of the spots that had
differential immunoreactivity with the sera of PDAC patients and
control groups and were identified by mass spectrometry. |
Table II.
Descriptions of the spots that had
differential immunoreactivity with the sera of PDAC patients and
control groups and were identified by mass spectrometry.
|
| Protein name | Accession no. | Molecular weight
(kDa) | pI | Mascot score | No. of matched
peptides |
|---|
| 1 | Laminin subunit
α-5 | gi|1002609387 | 174.759 | 5.52 | 68 | 1 |
| 2 | transcriptional
regulator | gi|291356655 | 54.090 | 9.54 | 72 | 1 |
| 3 | Superoxide
dismutase (Mn), mitochondrial | gi|30584207 | 25.019 | 8.94 | 102 | 1 |
| 4 | ATP synthase
subunit β, mitochondrial | XP_008323525.1 | 55.109 | 5.26 | 334 | 4 |
| 5 | ATP synthase β
subunit, partial | AAZ30638.1 | 46045 | 5.22 | 251 | 3 |
| 6 | Heterogeneous
nuclear ribonucleoproteins C1/C2 | gi|8393544 | 34.421 | 4.95 | 73 | 1 |
| 7 | Serine-threonine
kinase receptor-associated protein | gi|4063383 | 38.756 | 4.98 | 90 | 2 |
| 8 | Protein SEC13
homolog | gi|12805321 | 36.014 | 5.22 | 104 | 2 |
| 9 | Eukaryotic
translation initiation factor 3 subunit I | gi|4503513 | 36.878 | 5.38 | 232 | 3 |
| 10 | Chloride
intracellular channel protein 1 | gi|4588526 | 27.249 | 5.09 | 270 | 5 |
| 11 | Rho
GDP-dissociation inhibitor 2 | gi|56676393 | 23.031 | 5.10 | 249 | 3 |
| 12 | Elongation factor
I-γ | gi|51948418 | 50.371 | 6.31 | 120 | 3 |
| 13 | Mitochondrial
Ef-Tu, chain A | gi|6137414 | 43.978 | 6.09 | 72 | 2 |
| 14 | Septin 2 | gi|16924010 | 41.737 | 6.15 | 115 | 3 |
| 15 | glyceraldehyde
3-phosphate-dehydrogenase | gi|56188 | 36.098 | 8.43 | 70 | 1 |
| 16 | Phosphoglycerate
mutase B isozyme | gi|206101 | 28.685 | 6.20 | 91 | 1 |
| 17 | Prohibitin isoform
1 | gi|4505773 | 29.843 | 5.57 | 72 | 1 |
| 18 | Tubulin β 8
channel | gi|157383484 | 39.600 | 6.51 | 80 | 1 |
In total, 11 immune reactive proteins with Patu-8902
cell lysates were identified. Laminin subunit α-5, transcriptional
regulator, superoxide dismutase [Mn], mitochondrial, ATP synthase
subunit β and Rho GDP-dissociation inhibitor II were the spots
which reacted with >50% of the patient sera in Patu-8902 blots.
In this regard, laminin had the most frequency of reactivity with
patient sera (18/20). Heterogeneous nuclear ribonucleoproteins
C1/C2, serine-threonine kinase receptor-associated protein,
eukaryotic translation initiation factor 3 subunit I, chloride
intracellular channel protein I and protein SEC13 homolog reacted
with <50% of the patient sera. For each spot, the intensity of
reactivity varied among patients; for example, the intensity of
spot 1 (laminin subunit α-5) was from 1.5- to 4.6-fold compared to
the controls.
To identify possible different antigens, sera from
PDAC patients were reacted against 2D blots derived from Faraz-ICR
cell lysates. Seven proteins were identified, including elongation
factor I-γ, mitochondrial Ef-Tu, septin 2, glyceraldehyde
3-phosphate-dehydrogenase (GAPDH), phosphoglycerate mutase B
isozyme, prohibitin isoform I and tubulin β 8 channel. Among them,
the autoantibody against septin 2 had the most frequency of
reactivity with the patient sera.
Discussion
Circulating autoantibodies against TAAs may be
useful for PC screening and diagnosis and help to detect molecular
changes and cellular processes participating in the tumorigenesis
pathways (14). However, current
research in this area is still at an early stage, but the majority
of examined autoantibodies as biomarkers showed a relative low
sensitivity (85% of autoantibodies demonstrated sensitivity of
<50%) and high specificity (85% of autoantibodies demonstrated
specificity of ≥90%) (15).
Upregulation, isoform replacement, changes in cell
distribution and aberrant or altered glycosylation of proteins can
all stimulate the immune response toward autoantibody formation
(16). We investigated the
autoantibody repertoire in PDAC patients by the high-throughput
technique of SERPA to identify the combination of biomarkers which
is likely more sensitive and specific than a single biomarker
because of the complexity and heterogeneity of the tumor (17). The immunoreactive proteins that we
identified can be classified into extracellular matrix and
cytoskeletal proteins, enzymes, chaperones, signal transduction
proteins and transcriptional regulators (Table III).
 | Table III.Aberrant expression of immunoreactive
spots in different types of cancer. |
Table III.
Aberrant expression of immunoreactive
spots in different types of cancer.
|
| Immunoreactive
peptides | Alteration during
transformation | Cancer type | (Refs.) |
|---|
| ECM and
cytoskeletal abnormal glycosylation | Laminin | Upregulation | Pancreas | (16) |
|
| Septin | Deregulation | Oral/head and neck
Melanoma Renal cell Gastrointestinal Pancreas Hepatocellular | (20) |
|
| Tubulin | Altered expression
of tubulin isotypes Alterations in tubulin post-translational
modifications Changes in the expression of microtubule associated
proteins | Pancreas Breast
cancer Neuroblastoma Melanoma | (21) |
| Enzymes | ATP synthesis α
subunit | Upregulation | Breast | (43) |
|
| ATP synthesis β
subunit | Ectopic
expression | Lung Prostate
Colon | (27) |
|
| MnSOD | Upregulation | Gastric and
esophageal Lung Colorectal | (28) |
|
| GAPDH | Deregulation | Lung Renal Liver
Colorectal Melanoma Pancreas Bladder Thyroid | (25) |
|
| Rho-GDI2 | Overexpression | Ovary | (44) |
|
|
|
| Gastric | (45) |
|
|
| Autoantibody
production | Ovary | (46) |
|
|
|
| Acute leukemia | (47) |
|
|
| Phosphoglycerate
mutase B isozyme | Overexpression or
increased activity | Colorectal Liver
Lung Breast |
|
|
| Lower activity | Brain |
|
|
Transcriptional/translational
proteins | Heterogeneous
nuclear ribonucleoproteins C1/C2 | Upregulation | Hepatocellular | (48) |
|
|
|
| Pancreas | (46) |
|
|
| Autoantibody
production | Ovary |
|
|
| Serine-threonine
kinase receptor-associated protein | Ectopic
expression | Lung | (33) |
|
|
| Upregulation | Colorectal |
|
|
|
| Autoantibody
production | Nasopharyngeal |
|
|
| Elongation
factor | Overexpression of
different subunits | Colorectal Gastric
Hepatocellular Ovarian Pancreas | (39) |
|
| Mitochondrial Ef-Tu
in complex, chain A | Upregulation | Colorectal | (40) |
| Chaperones | Prohibitin | Overexpression | Cervix Esophagus
Lung Bladder Ovary Prostate | (36) |
|
|
| Downregulation G
Somatic mutation (SNP) Trans+location Shedding | Glioma Breast
Prostate Colon |
|
| Membrane
protein | Chloride
intracellular channel Pr 1 | Oncogenic
protein | Pancreas Prostate
Colon Gallblader Gastric | (38) |
The immunoreactive proteins from extracellular
matrix (ECM) and cytoskeletal-associated proteins were laminin-α 5,
septin 2 and tubulin β. Various changes in these proteins have been
identified in a wide variety of cancers (Table III). Immune response to
cytoskeletal proteins in PDAC patients may be the reflection of a
disturbed cytoskeletal structure. Tomaino et al (9) observed an antibody response to the
cytoskeletal proteins cofilin-1 and keratin-type I cytoskeletal 10
in PC. Laminins are basement membrane (BM) proteins belonging to
the glycoprotein family. They are composed of a heterotrimer of α,
β and γ polypeptide chains that through disulfide bonds shape a
cross in the BM (18). Various
combination of α, β and γ chains form over 14 laminin isoforms.
These isoforms have different distributions and functions in normal
and transformed tissues. Isoforms 10, 11 and 15 contain α5 chain
and are the major laminin isoform among other variants involved in
cell proliferation, migration, differentiation, and programmed cell
death (19). Septins belong to a
conserved family of GTP binding proteins that assemble into
filaments and play a role in the process of membrane fusion during
exocytosis (20). Microtubules are
components of the cell cytoskeleton composed of α and β tubulin
heterodimers to form hollow cylindrical structures (21). Lee et al (22) revealed that high tubulin expression
correlated with tumor stages in PDAC.
Metabolic reprogramming has been recognized as a
hallmark of cancer, whereas knockdown or pharmacological
inactivation of some enzymes results in increased cell apoptosis
and retardation of tumor growth (23). ATP synthases,
glyceraldehyde-3-phosphate dehydrogenase (GAPDH), Rho-GDP
dissociation inhibitor 2, superoxide dismutase and serine-threonine
kinase receptor-associated protein, are the immunoreactive enzymes
identified in PDAC patients sera. Despite classification of these
molecules as metabolic enzymes, they take part in other key
processes within cells. For example, GAPDH, in addition to
glycolytic effects, participates in DNA replication and repair,
endocytosis, exocytosis, cytoskeletal organization, iron
metabolism, carcinogenesis and cell death (24). GAPDH was regarded as the main
housekeeping gene for expression quantification in tumors, however
current studies indicated GAPDH deregulation in various tumors.
Remarkably increased GAPDH levels are observed in many human cancer
types and often correlate with reduced survival (25,26).
Among tumor markers, surface and secreted proteins play an
important role. Some TAA through tumor transformation are
dislocated in the cell surface. These molecules could be suitable
targets for tumor therapy as they are absent on the surface of
normal cells. Superoxide dismutase (SOD) and ATP synthases subunit
β are from mitochondria, but translocalization as well as their
ectopic expression in the cell surface membrane of transformed
cells have been proven (27,28).
The altered and elevated expression of mitochondrial form SOD
(MnSOD) in different cancer cells has been observed (Table III) and correlated with increasing
aggressiveness and poor prognosis while in PC the levels of this
protein have been inversely associated with cell growth (29). MnSOD protects the cells against
reactive oxygen species (ROS), ionizing radiation, and inflammatory
cytokines and plays a role as a tumor suppressive protein (30). Its overexpression inhibits many of
the typical properties of cancer such as cell proliferation,
invasiveness and anchorage-independent cell growth (28). ATP synthase is constitutively
expressed in the inner mitochondrial membrane in normal cells.
Overexpression or ectopic appearance on the cell surface of its
subunits (α and β) is reported in breast cancer, colon and prostate
carcinoma cells, as well as lung adenocarcinoma cell line.
Therefore, it may act as a TAA during cancer progression (27).
Another immunoreactive protein identified in the
present study was Rho GDP dissociation inhibitor 2 (RhoGDI2). The
overexpression of RhoA and RhoC induce invasive behavior and
metastatic activity to various tumor types (31). New findings indicate that RhoGDI2 by
regulating the expression of key genes such as E-cadherin, Slug,
Snail and α-Smooth muscle actin both in in vivo and in
vitro models suppressed the metastasis activity of lung cancer
cells through EMT (32).
Serine-threonine kinase receptor-associated protein
(STRAP) is an enzyme that through intervention in TGF-β signaling
promotes the growth and enhances the tumorigenicity. Tumor
progression due to STRAP upregulation and the presence of
autoantibody against STRAP in some tumors has been proven (33). Phosphoglycerate mutase 1 is an
important enzyme in the aerobic glycolysis pathway. Several studies
have revealed that Phosphoglycerate mutase 1 expression and its
activity are increased in a variety of human malignancies (34).
Prohibitin is a conserved chaperone involved in
proteins stabilization that regulates cell cycle progression,
mitochondrial activity and cellular homeostasis. Between two
transcripts, prohibitin1 exhibits more association with human
cancers and has been identified as a potential prognostic biomarker
in human PC (35). Various changes
in prohibitin including overexpression, somatic mutation and
trans-localization to cytoplasm and membrane rafts in different
types of cancer (Table III)
(36) are potential mechanisms that
can stimulate the immune system and autoantibody production.
Protein SEC13 and chloride intracellular channel
(CLIC) are two of the other candidates identified in the present
study. They are both membrane proteins. Protein SEC13 is required
for vesicle biogenesis from the endoplasmic reticulum during the
transport of proteins. CLIC family constitutes a unique class of
mammalian channel proteins that exist as both cytoplasm-soluble
proteins and membrane-bound channels. By regulating the expression
of integrin, CLIC is implicated in diverse biological processes
such as apoptosis, differentiation, cell-cycle regulation and
migration (37). Lu et al
(38) revealed the involvement of
CLIC1 in PC progression and aggressiveness and found that the
classification of PC patients according to the expression of CLIC1
represented a valuable tool to identify PC patients with a poor
prognosis.
Transcriptional regulator, heterogeneous nuclear
ribonucleoproteins C1/C2 (hnRNP), eukaryotic translation initiation
factor 3 subunit I (EIF3I), elongation factor (EF) and
mitochondrial Ef-Tu (TUFM) are nucleus factors involved in
transcription and translation. Overexpression of these compartments
could lead to increased translation rate and overall protein
synthesis. This may enhance cellular proliferation and reduce the
time required for protein production in stimulated cancerous cells.
Due to their important functions in cancer cell growth, these
molecules can be targeted by chemotherapeutic agents (act as
translation inhibitors) in rapidly growing tumor tissues (39) and their selective inhibition may
present a new avenue for the targeted therapy of cancer (40). The expression level of EF-Tu in
several types of cancers has been investigated and changes in its
expression level were specified. Upregulation of both EF-Tu and the
cytoplasmic elongation factor EF-1α in PDAC patients has been
reported (41).
Data obtained using high-throughput techniques are
generally required to be validated by other methods. The generation
of an autoantibody is usually the reflection of an aberrant
expression of an autoantigen. Identified immunogenic proteins in 2D
western blotting can be validated in terms of their aberrant
expression, such as overexpression using immunohistochemistry.
Furthermore, autoantibodies against identified autoantigens can be
investigated in a larger number of patients using ELISA. Although
further investigation is warranted, the present study identified
eighteen potential PC biomarkers. Among the identified proteins, a
combination of those with the most reactivity with patient sera,
such as laminin and septin, are considered appropriate candidate
biomarkers for future studies.
In conclusion, with the aim of identifying new
biomarkers in PC, we investigated the autoantibody repertoire
against TAAs in PC using a high-throughput method. Eighteen immune
reactive proteins were identified. Some of them have been
identified as PC biomarkers in prior studies, while others need to
be further investigated in order to explore their applicability as
widespread biomarkers in PC.
Acknowledgements
The present study was part of the PhD thesis of
Marzieh Rezaei and was financially supported by Shiraz University
of Medical Sciences, Shiraz, Iran (grant no. 93-7197). This study
was also financially supported by the Shiraz Institute for Cancer
Research (grant no. ICR-100-508).
References
|
1
|
Urayama S: Pancreatic cancer early
detection: Expanding higher-risk group with clinical and
metabolomics parameters. World J Gastroenterol. 21:17072015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gräntzdörffer I, Carl-McGrath S, Ebert MP
and Röcken C: Proteomics of pancreatic cancer. Pancreas.
36:329–336. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Desmetz C, Mange A, Maudelonde T and
Solassol J: Autoantibody signatures: Progress and perspectives for
early cancer detection. J Cell Mol Med. 15:2013–2024. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Caron M, Choquet-Kastylevsky G and
Joubert-Caron R: Cancer immunomics using autoantibody signatures
for biomarker discovery. Mol Cell Proteomics. 6:1115–1122. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dudas SP, Chatterjee M and Tainsky MA:
Usage of cancer associated autoantibodies in the detection of
disease. Cancer Biomark. 6:257–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mojtahedi Z, Safaei A, Yousefi Z and
Ghaderi A: Immunoproteomics of HER2-positive and HER2-negative
breast cancer patients with positive lymph nodes. OMICS.
15:409–418. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xia Q, Kong XT, Zhang GA, Hou XJ, Qiang H
and Zhong RQ: Proteomics-based identification of DEAD-box protein
48 as a novel autoantigen, a prospective serum marker for
pancreatic cancer. Biochem Biophys Res Commun. 330:526–532. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hong SH, Misek DE, Wang H, Puravs E,
Giordano TJ, Greenson JK, Brenner DE, Simeone DM, Logsdon CD and
Hanash SM: An autoantibody-mediated immune response to calreticulin
isoforms in pancreatic cancer. Cancer Res. 64:5504–5510. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tomaino B, Cappello P, Capello M,
Fredolini C, Ponzetto A, Novarino A, Ciuffreda L, Bertetto O, De
Angelis C, Gaia E, et al: Autoantibody signature in human ductal
pancreatic adenocarcinoma. J Proteome Res. 6:4025–4031. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Principe M, Ceruti P, Shih NY,
Chattaragada MS, Rolla S, Conti L, Bestagno M, Zentilin L, Yang SH,
Migliorini P, et al: Targeting of surface alpha-enolase inhibits
the invasiveness of pancreatic cancer cells. Oncotarget.
6:110982015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Elsässer HP, Lehr U, Agricola B and Kern
HF: Structural analysis of a new highly metastatic cell line PaTu
8902 from a primary human pancreatic adenocarcinoma. Virchows Arch
B Cell Pathol Incl Mol Pathol. 64:201–207. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rezaei M, Hosseini A, Nikeghbalian S and
Ghaderi A: Establishment and characterization of a new human acinar
cell carcinoma cell line, faraz-ICR, from pancreas. Pancreatology.
17:303–309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okutucu B, Dınçer A, Habib Ö and Zıhnıoglu
F: Comparison of five methods for determination of total plasma
protein concentration. J Biochem Biophys Methods. 70:709–711. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Casiano CA, Mediavilla-Varela M and Tan
EM: Tumor-associated antigen arrays for the serological diagnosis
of cancer. Mol Cell Proteomics. 5:1745–1759. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dumstrei K, Chen H and Brenner H: A
systematic review of serum autoantibodies as biomarkers for
pancreatic cancer detection. Oncotarget. 7:111512016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pan S, Brentnall TA and Chen R:
Glycoproteins and glycoproteomics in pancreatic cancer. World J
Gastroenterol. 22:9288–9299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J, Wang LJ, Ying X, Han SX, Bai E,
Zhang Y and Zhu Q: Immunodiagnostic value of combined detection of
autoantibodies to tumor-associated antigens as biomarkers in
pancreatic cancer. Scand J Immunol. 75:342–349. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Durbeej M: Laminins. Cell Tissue Res.
339:259–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kikkawa Y, Sanzen N and Sekiguchi K:
Isolation and characterization of laminin-10/11 secreted by human
lung carcinoma cells laminin-10/11 mediates cell adhesion through
integrin alpha3 beta1. J Biol Chem. 273:15854–15859. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Connolly D, Abdesselam I, Verdier-Pinard P
and Montagna C: Septin roles in tumorigenesis. Biol Chem.
392:725–738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Parker AL, Kavallaris M and McCarroll JA:
Microtubules and their role in cellular stress in cancer. Front
Oncol. 4:1532014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee KM, Cao D, Itami A, Pour PM, Hruban
RH, Maitra A and Ouellette MM: Class III beta-tubulin, a marker of
resistance to paclitaxel, is overexpressed in pancreatic ductal
adenocarcinoma and intraepithelial neoplasia. Histopathology.
51:539–546. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang D, Jin N, Sun W, Li X, Liu B, Xie Z,
Qu J, Xu J, Yang X, Su Y, et al: Phosphoglycerate mutase 1 promotes
cancer cell migration independent of its metabolic activity.
Oncogene. 36:2900–2909. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Colell A, Green DR and Ricci JE: Novel
roles for GAPDH in cell death and carcinogenesis. Cell Death
Differ. 16:1573–1581. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo C, Liu S and Sun MZ: Novel insight
into the role of GAPDH playing in tumor. Clin Transl Oncol.
15:167–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Giusti L, Iacconi P, Ciregia F,
Giannaccini G, Donatini GL, Basolo F, Miccoli P, Pinchera A and
Lucacchini A: Fine-needle aspiration of thyroid nodules: Proteomic
analysis to identify cancer biomarkers. J Proteome Res.
7:4079–4088. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu ZJ, Song QF, Jiang SS, Song Q, Wang W,
Zhang GH, Kan B, Chen LJ, Yang JL, Luo F, et al: Identification of
ATP synthase beta subunit (ATPB) on the cell surface as a non-small
cell lung cancer (NSCLC) associated antigen. BMC Cancer. 9:162009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Borrelli A, Schiattarella A, Bonelli P,
Tuccillo FM, Buonaguro FM and Mancini A: The functional role of
MnSOD as a biomarker of human diseases and therapeutic potential of
a new isoform of a human recombinant MnSOD. Biomed Res Int.
2014:4767892014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weydert C, Roling B, Liu J, Hinkhouse MM,
Ritchie JM, Oberley LW and Cullen JJ: Suppression of the malignant
phenotype in human pancreatic cancer cells by the overexpression of
manganese superoxide dismutase. Mol Cancer Ther. 2:361–369.
2003.PubMed/NCBI
|
|
30
|
Liou GY and Storz P: Reactive oxygen
species in cancer. Free Radic Res. 44:479–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fujita A, Shida A, Fujioka S, Kurihara H,
Okamoto T and Yanaga K: Clinical significance of Rho GDP
dissociation inhibitor 2 in colorectal carcinoma. Int J Clin Oncol.
17:137–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Niu H, Wu B, Jiang H, Li H, Zhang Y, Peng
Y and He P: Mechanisms of RhoGDI2 mediated lung cancer
epithelial-mesenchymal transition suppression. Cell Physiol
Biochem. 34:2007–2016. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Halder SK, Anumanthan G, Maddula R, Mann
J, Chytil A, Gonzalez AL, Washington MK, Moses HL, Beauchamp RD and
Datta PK: Oncogenic function of a novel WD-domain protein, STRAP,
in human carcinogenesis. Cancer Res. 66:6156–6166. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang X, Sun Q, Li H, Li K and Ren X: The
role of phosphoglycerate mutase 1 in tumor aerobic glycolysis and
its potential therapeutic implications. Int J Cancer.
135:1991–1996. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhong N, Cui Y, Zhou X, Li T and Han J:
Identification of prohibitin 1 as a potential prognostic biomarker
in human pancreatic carcinoma using modified aqueous two-phase
partition system combined with 2D-MALDI-TOF-TOF-MS/MS. Tumor Biol.
36:1221–1231. 2015. View Article : Google Scholar
|
|
36
|
Leal MF, Cirilo PD, Mazzotti TK, Calcagno
DQ, Wisnieski F, Demachki S, Martinez MC, Assumpção PP, Chammas R,
Burbano RR and Smith MC: Prohibitin expression deregulation in
gastric cancer is associated with the 3′ untranslated region 1630
C> T polymorphism and copy number variation. PloS One.
9:e985832014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tung JJ and Kitajewski J: Chloride
intracellular channel 1 functions in endothelial cell growth and
migration. J Angiogenes Res. 2:232010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lu J, Dong Q, Zhang B, Wang X, Ye B, Zhang
F, Song X, Gao G, Mu J, Wang Z, et al: Chloride intracellular
channel 1 (CLIC1) is activated and functions as an oncogene in
pancreatic cancer. Med Oncol. 32:1–9. 2015. View Article : Google Scholar
|
|
39
|
Al-Maghrebi M, Anim JT and Olalu AA:
Up-regulation of eukaryotic elongation factor-1 subunits in breast
carcinoma. Anticancer Res. 25:2573–2577. 2005.PubMed/NCBI
|
|
40
|
Shi H, Hayes M, Kirana C, Miller R,
Keating J, Macartney-Coxson D and Stubbs R: TUFM is a potential new
prognostic indicator for colorectal carcinoma. Pathology.
44:506–512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xu C, Wang J, Li J and Fang R: Expression
of elongation factor (EF)-Tu is correlated with prognosis of
gastric adenocarcinomas. Int J Mol Sci. 12:6645–6655. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pan J, Sun LC, Tao YF, Zhou Z, Du XL, Peng
L, Feng X, Wang J, Li YP, Liu L, et al: ATP synthase
ecto-α-subunit: A novel therapeutic target for breast cancer. J
Transl Med. 9:2112011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tapper J, Kettunen E, Seppälä M, Andersson
LC and Knuutila S: Changes in gene expression during progression of
ovarian carcinoma. Cancer Genet Cytogenet. 128:1–6. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cho HJ, Baek KE, Park SM, Kim IK, Choi YL,
Cho HJ, Nam IK, Hwang EM, Park JY, Han JY, et al: RhoGDI2
expression is associated with tumor growth and malignant
progression of gastric cancer. Clin Cancer Res. 15:2612–2619. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yoneyama K, Kojima S, Kodani Y, Yamaguchi
N, Igarashi A, Kurose K, Kawase R, Takeshita T, Hattori S and
Nagata K: Proteomic identification of autoantibodies in sera from
patients with ovarian cancer as possible diagnostic biomarkers.
Anticancer Res. 35:881–889. 2015.PubMed/NCBI
|
|
46
|
Cui JW, Li WH, Wang J, Li AL, Li HY, Wang
HX, He K, Li W, Kang LH, Yu M, et al: Proteomics-based
identification of human acute leukemia antigens that induce humoral
immune response. Mol Cell Proteomics. 4:1718–1724. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yan-Sanders Y, Hammons GJ and Lyn-Cook BD:
Increased expression of heterogeneous nuclear ribonucleoprotein
A2/B1 (hnRNP) in pancreatic tissue from smokers and pancreatic
tumor cells. Cancer Lett. 183:215–220. 2002. View Article : Google Scholar : PubMed/NCBI
|