Introduction
Cervical cancer is the most common malignancy of the
female reproductive tract, and 95% of cases are caused by
persistent infections with carcinogenic human papillomaviruses
(HPV) (1). Cervical cancer accounts
for 528,000 new cases and 266,000 deaths worldwide each year, more
than any other gynecologic tumor (2). Effective preventive vaccines are
available for the most important carcinogenic HPV strains, but
their use remains poor (3). At
present, the combination of radiotherapy and platinum-based
chemotherapy is the gold-standard therapy for advanced cervical
cancer (4). Although the addition
of platinum-based chemotherapy to radiotherapy has increased the
5-year survival of advanced-stage cervical cancer patients
(5), systemic toxicity limits the
use of high-dose chemotherapeutic drugs. Therefore, new therapeutic
strategies that are less toxic and that provide higher efficacy
against cervical cancer are urgently needed. Natural components
provide a great deal of opportunity for preventing or changing the
pathogenic course of the disease in a safer and more cost-effective
way.
Apoptosis and autophagy are both cellular
degradative pathways essential for organismal homeostasis.
Dysregulation of apoptosis leads to the accumulation of ‘unwanted’
cells and contributes to the development of cancer (6). Anticancer therapeutics may be used to
promote apoptosis within cancer cells to cause the death of these
cells. Current anticancer therapies, including many
chemotherapeutic agents and ionizing radiation therapy, usually
activate apoptosis and utilize the apoptotic machinery for killing
cancer cells (7,8). Autophagy can remove damaged proteins
and organelles, and it limits their cumulative deleterious effects
intracellularly. Therefore, autophagic defects are found in many
human tumors (9,10). In addition, excessive autophagy has
been involved in autophagic cell death, which is characterized by
morphological changes, such as the accumulation of autophagosomes
in the cell (11). In contrast to
the tumor-suppressor roles for autophagy, stress-activated
autophagy may promote survival of tumor cells, especially when
apoptosis is defective (6).
Previous studies have suggested that fucoidan may exert its
antiproliferative action in cultured HeLa cells by inducing
apoptotic and autophagic cell death (12), and glaucocalyxin B may supress the
proliferation of human cervical cancer cells in vitro
through the induction of apoptosis and autophagy (13). Therefore, the study of the
mechanisms underlying apoptosis and autophagy should provide
therapeutic benefits in human cervical cancer.
Spermidine, a natural polyamine detected in all
eukaryotic organisms, exhibits many biological functions. It is
involved in processes such as cellular proliferation,
differentiation, tissue development, and carcinogenesis (14). Recent studies have revealed that
macrophage ABHD5 suppressed spermidine production and subsequently
promoted colorectal cancer growth (15). In addition, oral spermidine
suppressed liver fibrosis and hepatocellular carcinoma in mice by
activating MAP1S-mediated autophagy (16). It is suggested that spermidine
constitutes a promising agent for the treatment of cancer. However,
the potential effectiveness of spermidine in cervical cancer has
not yet been fully elucidated, and the underlying molecular
mechanism involved remains unknown.
The purpose of this study was to demonstrate the
potential roles and molecular mechanisms underlying the effects of
spermidine in cervical cancer. For this reason, we assessed its
function in HeLa cells and found that spermidine strongly
suppressed proliferation and induced apoptosis in HeLa cells.
Additional mechanistic studies suggested that activation of
autophagy mediated the inhibition of cellular proliferation by
spermidine and spermidine-induced apoptosis in HeLa cells.
Collectively, our results revealed the potent effects of spermidine
in the treatment of cervical cancer and provided experimental
evidence about the details of the underlying mechanisms.
Materials and methods
Reagents and cell culture
Spermidine was dissolved in dimethylsulfoxide (DMSO;
both from Sigma-Aldrich; Merck KGaA, St. Louis, MO, USA).
3-Methyladenine (Sigma-Aldrich; Merck KGaA), an autophagy
inhibitor, was used at a concentration of 4 mM in DMSO.
The human cervical cancer cell lines HeLa
(HPV-18-infected cervical cancer cells), SiHa (HPV-16-infected
cervical cancer cells), and C-33A (HPV-negative cervical cancer
cells) were obtained from the Cell Bank of the Chinese Academy of
Sciences (Shanghai, China). Cells were grown in DMEM (GIBCO; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), supplemented with 10%
fetal bovine serum (Beyotime Institute of Biotechnology, Haimen,
China) and 100 U/ml streptomycin/penicillin at 37°C in a humidified
atmosphere containing 5% CO2 in compressed air. When
80–90% confluence was reached, HeLa cells were digested with 0.25%
trypsin (Beyotime Institute of Biotechnology) for subsequent
experiments.
Cell proliferation assay
Cell viability with spermidine treatment was
evaluated using CCK-8 colorimetric assay (Beyotime Institute of
Biotechnology) (17). Briefly,
cells were seeded in 96-well plates at a density of
2×104 cells/well, at 37°C with 5% CO2. After
culturing cells to 80% confluence, the cells were exposed to
varying concentrations of spermidine for 24, 48 or 72 h. CCK-8 was
then added to each well followed by incubation, and the absorbance
was assessed at 450 nm using a microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA). Cell viability was
calculated using the following formula: Cell viability (%) = (OD
treatment - OD blank)/(OD control - OD blank).
Quantitative real-time PCR (qRT-PCR)
assay
HeLa cells were cultured with spermidine at 60, 120
and 180 µM for 24 h, respectively. The control cells were treated
with 0.1% DMSO. Following spermidine incubation, the mRNA from HeLa
cells was extracted (MagnaPure LC RNA Isolation Kit) and underwent
reverse transcription into cDNA according to the Transcription High
Fidelity cDNA Synthesis kit (both from Roche Applied Science,
Penzberg, Germany), following the corresponding manufacturer's
instructions. The expression levels were evaluated using a two-step
qRT-PCR kit with SYBR®-Green (Takara Biotechnology Co.,
Ltd., Dalian, China) with a final volume of 20 µl (10 µl
SYBR®-Green qPCR Mixture, 10 µM forward and reverse
primers) on a 7500 Real-time PCR System (ABI). The threshold cycle
values (Ct values) of the genes and internal control genes for the
different samples were evaluated. The primer pairs are listed in
Table I.
 | Table I.Primer sequences of ODC1, SRM, SMS,
SSAT, SMOX, GAPDH. |
Table I.
Primer sequences of ODC1, SRM, SMS,
SSAT, SMOX, GAPDH.
| Gene | Forward | Reverse |
|---|
| ODC1 |
CAGCTTTCACGCTTGCAGTT |
ATCTTCGTCATCAGAGCCCG |
| SRM |
CCCTCCGTGGAGTCCGTGGTC |
CTGGCAGGAACTTCTTGGAGACTTG |
| SMS |
TGGAAATATTCTCATCCTTAGTGGG |
CGGGTATATGCCAAATCACTCTCT |
| SSAT |
GGCATAGGATCAGAAATTCTGAAGA |
CTGCTACCAAGAAGTGCATGCTG |
| SMOX |
CAATGCTGAAAGTCAAAATAGCGTG | CTCTGGGTCGTCAGGGTC
ATTCC |
| GAPDH |
CATCAATGGAAATCCCATCA |
GACTCCACGACGTACTCAGC |
Cell cycle assay
HeLa cells in each group were trypsinized and fixed
with 70% ethanol at 4°C for 12 h. Subsequent to washing with
phosphate-buffered saline (pH 7.4), the cell cycle analysis was
carried out with a Cell Cycle Detection kit (cat. no. C1052;
Beyotime Institute of Biotechnology), according to the
manufacturer's protocol. The kit contained binding buffer,
propidium iodide (PI) staining buffer (20X), and RNase A (50X). In
brief, the cells were stained in 500 µl of binding buffer
containing 25 µl of PI staining buffer with 10 µl of RNase A for 30
min at 37°C in the dark. Cell cycle kinetics were detected with
flow cytometry (FACSCalibur; BD Biosciences, Franklin Lakes, NJ,
USA). Results are expressed as the percentage of cells at each
phase of the cell cycle.
Cell apoptosis assay
Cellular apoptosis was detected using the Annexin
V-FITC/PI Apoptosis Detection Kit (MultiSciences Biotech Co., Ltd.
Hangzhou, China). Briefly, after treatment with spermidine, HeLa
cells were collected and washed twice with PBS. Cells at
5×105 cells/ml were re-suspended in 400 µl of binding
buffer with 5 µl of Annexin V-FITC and 1 µl of PI in the dark.
After incubation for 15 min, cell apoptosis was assessed by flow
cytometry (FACSCalibur; BD Biosciences, Franklin Lakes, NJ,
USA).
Western blot analysis
HeLa cells were lysed with RIPA lysate to extract
total protein, and concentration was assessed by a bicinchoninic
acid (BCA) protein assay kit (both from Beyotime Institute of
Biotechnology). The same amounts of proteins were separated in 12%
sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE), and transferred to polyvinylidene fluoride (PVDF)
membranes (EMD Millipore, Bedford, MA, USA). Then, 5% skim milk was
used to block proteins for 1 h. Subsequently, the blots were
detected with specific primary antibodies against GAPDH (cat. no.
AF1186), BAX (cat. no. AB026), Bcl-2 (cat. no. AB112),
cleaved-caspase-3 (cat. no. AC033), (all 1:1,000; Beyotime
Institute of Biotechnology), LC3B (cat. no. 3868S), Atg5 (cat. no.
2630S), Beclin 1 (cat. no. 3738S) (all 1:1,000; Cell Signaling
Technology, Danvers, MA, USA) at 4°C incubation overnight. Then,
the primary antibodies were washed away, and incubated with
corresponding secondary antibodies [cat. no. A0208 (HRP-labeled
goat anti-rabbit IgG (H+L) and cat. no. A0216 (HRP-labeled goat
anti-mouse IgG (H+L)] (1:5,000; Beyotime Institute of
Biotechnology) for 1 h at room temperature. The enhanced
chemiluminescence (ECL) solution (Qihai Biotec, Shanghai, China)
was used to detect the target bands, and Gel-Pro-Analyzer software
(Media Cybernetics, Inc., Rockville, MD, USA) was employed to
analyze the gray values of the band. GAPDH served as a loading
control.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism v. 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
Data are expressed as the means ± standard deviation (SD). All data
presented represent results from at least 3 independent
experiments. One-way analysis of variance (ANOVA) followed by the
Tukey's multiple comparison test was used to compare differences
between groups. Statistical significance was defined as
P<0.05.
Results
Spermidine reduces the proliferation
of human cervical cancer cell lines
To study the effects of spermidine on the growth of
human cervical cancer cell lines, we first performed CCK-8
analysis. The cells were treated with spermidine at different
concentrations (0 to 200 µM) for 24 h, and then cell viability was
assessed using CCK-8 assays. As shown in Fig. 1A, spermidine significantly inhibited
the viability of HeLa cells in a dose-dependent manner. The
IC50 values of spermidine for HeLa cells was 121.3 µM
(Fig. 1A). Spermidine also
significantly reduced the growth of HeLa cells in a time-dependent
manner (for 24, 48 and 72 h) (Fig.
1B). To further confirm the inhibition of spermidine
specifically in human cervical cancer cells, other human cervical
cancer cell lines, including SiHa (HPV-16-infected cervical cancer
cells) and C-33A (HPV-negative cervical cancer cells) were tested.
Notably, spermidine inhibited the growth of both SiHa (Fig. 1C) and C-33A cell lines in a
dose-dependent manner (Fig. 1D). We
found that HeLa, SiHa and C-33A cell lines were all sensitive to
spermidine-induced cytotoxicity. Collectively, these data revealed
that spermidine reduced the viability of human cervical cancer cell
lines.
 | Figure 1.Spermidine inhibits the proliferation
of HeLa, SiHa, C-33A cell lines. (A) HeLa cells were cultured with
an ascending concentration range of spermidine (from 0 to 200 µM)
for 24 h and cell viability was detected by CCK-8 assay (n=6).
Effects on cell viability were expressed as a function of µM drug
concentration (log scale). Corresponding IC50 values
were calculated with the appropriate software (GraphPad Prism). (B)
HeLa cells were cultured with spermidine at 60, 120 or 180 µM, for
24, 48 and 72 h, respectively (n=6). (C) Spermidine treatment at
60, 120 and 180 µM in SiHa cells after 24 h was performed and cell
viability was assessed by CCK-8 assays, respectively (n=6). (D)
Spermidine treatment at 60, 120, 180 µM in C-33A cells after 24 h
was performed and cell viability was assessed by CCK-8 assays,
respectively (n=6). Data are the means ± SD. *P<0.05,
**P<0.01, compared with the control. The controls were treated
with 0.1% DMSO. |
Spermidine affects the mRNA levels of
the polyamine biosynthesis pathway of HeLa cells
It has been well established that an increase in
intracellular polyamine concentration is correlated with increased
cell proliferation as well as tumorigenesis (18,19).
Depletion of the intracellular polyamine pools using either a
polyamine synthesis inhibitor or a polyamine analog invariably
inhibits cell growth in various cancers (20–22).
To confirm whether exogenous spermidine inhibited the accumulation
of endogenous polyamines, we performed qRT-PCR to detect the
messenger RNA (mRNA) expression levels of principal genes in the
polyamine biosynthesis pathway. The results revealed that the mRNA
level of ornithine decarboxylase (ODC1), spermidine synthase (SRM)
and spermine oxidase (SMOX) were significantly decreased in HeLa
cells after spermidine treatment (Fig.
2A-C), while the mRNA levels of spermidine/spermine
N1-acetyltransferase (SSAT) and spermine synthase (SMS) were
significantly increased in HeLa cells after spermidine treatment
(Fig. 2D and E). Collectively,
these results revealed that exogenous spermidine inhibited the
accumulation of endogenous polyamines.
Spermidine inhibits the viability of
HeLa cells by arresting the cell cycle at the S phase
To further determine whether the viability of HeLa
cells treated with spermidine was decreased, which was due to the
reduction of cellular proliferation, we next investigated the
effects of spermidine on cell cycle distribution. As shown in
Fig. 3A-D, when cells were treated
with 120 or 180 µM spermidine, the percentage of HeLa cells in the
S phase was significantly increased from 23.78±0.75 to 37.42±1.49
and 38.65±1.05%, compared with the control group, respectively. In
addition, the proportion of cells in the G2 phase was not altered
(Fig. 3D). These results revealed
that spermidine inhibited the proliferation of HeLa cells by
arresting the cell cycle at the S phase.
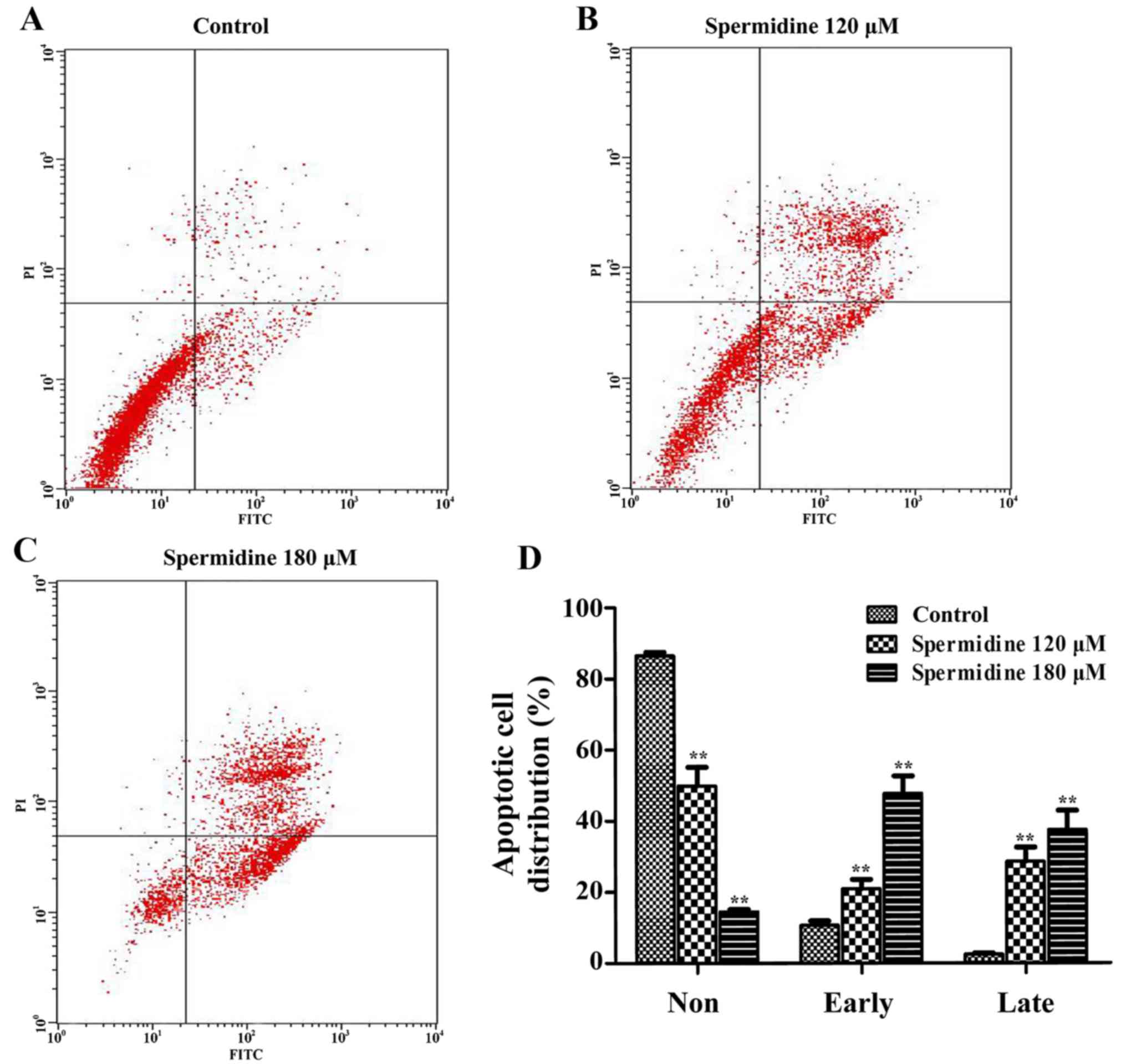
Spermidine induces apoptosis of HeLa
cells through the mitochondrial apoptotic pathway
To confirm whether spermidine inhibition of HeLa
cell proliferation was caused by apoptosis, we used the Annexin
V/PI Cell Apoptosis kit to detect the apoptotic effect of
spermidine on HeLa cells by flow cytometric assays. We found that
120 and 180 µM of spermidine treatment significantly increased the
apoptosis of HeLa cells compared with the control cells (Fig. 4A-D). To further investigate the
promotion of apoptosis of HeLa cells by spermidine, western blot
analysis revealed that the expression of Bcl-2 (an anti-apoptotic
protein) in HeLa cells was significantly decreased after spermidine
treatment (Fig. 5B and E);
conversely, the expression of pro-apoptotic proteins
cleaved-caspase-3 and BAX protein were significantly increased
after spermidine treatment (Fig. 5A, C
and D), compared with the controls. Collectively, these results
strongly demonstrated that spermidine induced apoptosis of HeLa
cells by triggering the mitochondrial apoptotic pathway.
Spermidine induces autophagy of HeLa
cells
LC3 acts as a marker of the autophagosome membrane,
and is involved in the formation of the autophagic body, and
LC3-I/II conversion reflects the number of autophagosomes (23). Beclin 1 is a specific gene
associated with autophagy in mammals, which is associated with
phospholipid inositol triphosphate-kinase (PI3K), and participates
in the formation of autophagosomes (24). Atg5 is a central regulator necessary
for autophagy in terms of its involvement in autophagosome
elongation (25). Notably, as shown
in Fig. 6, western blotting results
revealed that LC3 II/LC3 I, Atg5 and Beclin 1 protein levels were
significantly increased by spermidine treatment in HeLa cells.
These results revealed that spermidine induced autophagy in HeLa
cells.
Autophagy mediates the
spermidine-induced effects in HeLa cells
To uncover a relationship between apoptosis and
autophagy, we used a specific drug inhibitor (3-MA) to block
autophagy and analyzed the spermidine-induced effect on HeLa cells.
As shown in Fig. 7A, western blots
indeed revealed that 3-MA (a specific inhibitor of autophagy)
blocked the increase induced by treatment with spermidine in LC3
II/LC3 I, Beclin 1, Atg5, and BAX in HeLa cells (Fig. 7A and C-F), and also blocked the
decrease induced by treatment with spermidine in Bcl-2 in HeLa
cells (Fig. 7A and B). Moreover,
3-MA significantly blocked the spermidine-induced apoptosis
(Fig. 8A-E) and
spermidine-inhibited proliferation (Fig. 8F) in HeLa cells. These results
provide additional evidence that autophagy mediates
spermidine-induced inhibition of cellular proliferation and
promotion of apoptosis in HeLa cells.
 | Figure 7.The autophagy inhibitor 3-MA blocks
the effects of spermidine in HeLa cells. (A) Western blotting
detected LC3 II/LC3 I, Beclin 1, Atg5, BAX, and Bcl-2 in HeLa cells
treated with the control, spermidine (120 µM), and/or 3-MA (4 mM).
GAPDH was used as the internal control. (B-F) Quantitative analysis
of the relative protein expression for (B) Bcl-2, (C) BAX, (D) LC3
II/LC3 I, (E) Beclin 1, and (F) Atg5 as displayed in A (n=3). Data
are expressed as the means ± SD. **P<0.01, compared with the
controls. ##P<0.01, compared with spermidine
treatment. The controls were treated with 0.1% DMSO. |
 | Figure 8.The autophagy inhibitor 3-MA blocks
the induction of apoptosis and inhibition of proliferation in HeLa
cells with spermidine treatment. (A-D) Flow cytometric analysis of
Annexin V-FITC/PI-stained HeLa cells treated with (A) the control
medium, (B) 3-MA, (C) spermidine, or (D) spermidine plus 3-MA
(n=3). Cells were characterized as healthy cells (bottom left
quadrant), necrotic (top left quadrant), early apoptotic (bottom
right quadrant), or late apoptotic (top right quadrant). (E)
Quantified data regarding the apoptotic rates of HeLa cells as
displayed in A-D. (F) CCK-8 assays were performed on HeLa cells
after 24 h in control media, or with 3-MA, spermidine, or
spermidine plus 3-MA (n=6). Data are expressed as the means ± SD.
**P<0.01, compared with the controls. ##P<0.01,
compared with spermidine treatment. Controls were treated with 0.1%
DMSO. |
Discussion
Primary polyamines, putrescine, spermidine and
spermine are involved in cellular proliferation, differentiation,
tissue development, and carcinogenesis by maintaining chromatin
structure, membrane stability and regulating ion channels (14,26).
Since 1971, when spermidine was found to be markedly elevated in
the urine of many cancer patients (27), there have been attempts to use the
biosynthetic pathway of polyamines as therapeutic targets or to use
levels of polyamines as prognostic markers for cancer patients.
Polyamine depletion has been suggested for cancer prevention, but
α-difluoromethylornithine (DFMO) was not clinically effective
neither alone nor in combination with other agents (28). Depletion of polyamines by
spermidine/spermine N1-acetyltransferase (SSAT) significantly
inhibited cellular proliferation, migration, and invasion through
the AKT/GSK3β/β-catenin signaling pathway in hepatocellular
carcinoma and colorectal cancer cells (29). However, previous studies reported
that inhibition of spermidine production promoted colorectal cancer
growth (15), and that exogenous
spermidine suppressed hepatocellular carcinomas in mice (16). Also, spermidine can alter protein
acetylation patterns to regulate autophagy and promote longevity in
multiple model systems (30,31).
Therefore, the precise biochemical function of spermidine in cancer
requires clarification.
In the present study, we chose to assess the effects
of spermidine on HeLa cells. Previous studies have revealed that
overexpressed SRM (spermidine synthase) in colorectal cancer cell
lines CT-26 or MC-38 largely increased spermidine production;
consequently, CT-26- or MC-38-inoculated tumors were inhibited
(15). Consistent with these
studies, our results demonstrated that spermidine inhibited the
proliferation of cervical cancer cells in a dose-dependent manner
(Fig. 1). However, we found that
the mRNA level of ODC1, SRM and SMOX were significantly decreased,
while the mRNA levels of SSAT and SMS were significantly increased
in HeLa cells after spermidine treatment (Fig. 2). It suggested that exogenous
spermidine inhibited the synthesis of putrescine and spermidine,
and promoted the spermine and spermidine consumption. Depletion of
the intracellular polyamines inhibits cell growth in HeLa cells. In
addition, we found a significant increase in the number of cells at
the S phase (Fig. 3D), suggesting
that spermidine inhibited the proliferation of HeLa cells by
arresting the cell cycle at the S phase.
Previous studies have shown that excessive polyamine
accumulation induces cellular apoptosis in ornithine
decarboxylase-overproducing L1210 cells (32). Consistent with these studies, we
also found that spermidine significantly induced apoptosis of HeLa
cells based upon flow cytometric analysis of Annexin
V-FITC/PI-stained cells (Fig. 4).
BAX migrates to the mitochondrial membrane and inhibits the action
of Bcl-2, causing damage to the mitochondrial membrane; this in
turn releases cytochrome c and leads to apoptosis via the
activation of caspase-3 and caspase-7 (33). In our study, western blot analysis
indicated that spermidine treatment resulted in attenuated
expression of Bcl-2 and increased expression of BAX and cleaved
caspase-3 levels in HeLa cells (Fig.
5). Thus, these results support the concept that the enhanced
apoptosis of HeLa cells treated with spermidine is due to the
activation of mitochondrial apoptotic pathways.
Autophagy is another cellular degradative pathway
that is essential for organismal homeostasis, and it has been
implicated in protecting organisms from cancer (34,35).
Previous studies have revealed that exogenous spermidine suppresses
hepatocellular carcinoma in mice by activating MAP1S-mediated
autophagy (15). Glaucocalyxin
inhibited the proliferation of human cervical cancer cells in
vitro through the induction of autophagy by the
phosphatidylinositol-4,5-bisphosphate 3-kinase/Akt signaling
pathway (13). The natural agent
HMDB exerted antitumor effects on human cervical cancer cells by
induction of autophagy followed by increased expression of LC3
II/LC3 I and Beclin 1 (36).
Resveratrol-induced cell death in OVCAR-3 human ovarian cancer
cells occured via induced autophagy followed by increased Atg5
expression and promotion of LC3 cleavage (37). Consistent with these studies, we
also found that spermidine treatment resulted in increased LC3
II/LC3 I, Atg 5, and Beclin 1 protein levels in HeLa cells
(Fig. 6). These results revealed
that spermidine inhibited the proliferation of HeLa cells by
activating autophagy.
Previous evidence has suggested the existence of
crosstalk between the apoptotic and autophagic signaling pathways
(38,39). Activation of ROS production
simultaneously induced both autophagy and apoptosis in cancer cells
(40). Autophagy itself can induce
cell death, a process known as autophagic cell death (41). Resveratrol promoted autophagic cell
death in chronic myelogenous leukemia cells via JNK-mediated
p62/SQSTM1 expression and AMPK activation (42). Beclin 1 is an essential autophagic
effector that plays important roles in crosstalk with the apoptotic
pathway, interacting with antiapoptotic Bcl-2 proteins (6). Notably, knockdown of Beclin 1 promoted
cell growth and inhibited apoptosis in the A549 human lung cancer
cell line (43). Atg5 functions as
a switch, shifting the process of cell death from autophagy to
apoptosis. Pre-treatment with 3-methyladenine or Atg5-shRNA
attenuated justicidin A-induced LC3-II expression and LC3 puncta
formation and blocked justicidin A-induced suppression in cell
growth via inhibition of apoptosis (44). The present study indicates that
spermidine inhibits HeLa cells through both apoptosis and
autophagy. We found that the autophagy inhibitor 3-MA attenuated
the levels of LC3 II/LC3 I, Atg 5, Beclin 1 and BAX, and enhanced
the level of Bcl-2 in HeLa cells treated with spermidine (Fig. 7), and blocked spermidine-induced
apoptosis and the spermidine-inhibited proliferation of HeLa cells
(Fig. 8). These results strongly
demonstrated that autophagy mediates the effects of spermidine on
HeLa cells.
In summary, we herein demonstrated that spermidine
suppressed proliferation and promoted apoptosis of HeLa cells, and
that these effects were mediated by autophagic activation. In our
study, the results indicated novel effects of spermidine with
respect to its potential use in cervical cancer therapy and
provided aspects of the details of the underlying mechanisms of
action, focusing on the crosstalk between apoptosis and
autophagy.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by a grant
from the National Natural Science Foundation of China (21503190),
the Education Foundation of Zhejiang province (Y201636954), the
Zhejiang Medical College Youth Dr. start-up funding (2015B07), the
Foundation of Zhejiang province Chinese Medicine, Science and
Technology (2017ZB030), and Medical and Health Science and
Technology Project of Zhejiang Province (2018277310).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YC and XW conceived and designed the study. YC, HZ,
XC and ZS performed the experiments. YC and XW wrote the paper. YC,
XW and HZ reviewed and edited the manuscript. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schiffman M, Wentzensen N, Wacholder S,
Kinney W, Gage JC and Castle PE: Human papillomavirus testing in
the prevention of cervical cancer. J Natl Cancer Ins. 103:368–383.
2011. View Article : Google Scholar
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Uyar D and Rader J: Genomics of cervical
cancer and the role of human papillomavirus pathobiology. Clin
Chem. 60:144–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Malfetano J: Concurrent Cisplatin-based
radiotherapy and chemotherapy for locally advanced cervical
cancer-NEJM. New Eng J Med. 340:1144–1153. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wieringa HW, van der Zee AG, de Vries EG
and van Vugt MA: Breaking the DNA damage response to improve
cervical cancer treatment. Cancer Trea Rev. 42:30–40. 2016.
View Article : Google Scholar
|
|
6
|
Su M, Mei Y and Sinha S: Role of the
Crosstalk between Autophagy and Apoptosis in Cancer. J Oncol.
2013:1027352013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ashkenazi A: Targeting the extrinsic
apoptosis pathway in cancer. Cytokine Growth Factor Rev.
19:325–331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cotter TG: Apoptosis and cancer: The
genesis of a research field. Nat Rev Cancer. 9:501–507. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karantza V and White E: Role of autophagy
in breast cancer. Autophagy. 3:610–613. 1900. View Article : Google Scholar
|
|
10
|
Jin S and White E: Role of autophagy in
cancer: Management of metabolic stress. Autophagy. 3:28–31. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsujimoto Y and Shimizu S: Another way to
die: autophagic programmed cell death. Cell Death Differ. 12 Suppl
2:S1528–S1534. 2005. View Article : Google Scholar
|
|
12
|
Li X, Yang L, Wang YS, LIANG H, SONG SL
and JI AG: Fucoidan induce apoptosis of HeLa cells accompanied by
the induction of autophagy. Lat Am J Pharm. 36:1126–1133. 2017.
|
|
13
|
Pan Y, Bai J, Shen F, Sun L, He Q and Su
B: Glaucocalyxin B induces apoptosis and autophagy in human
cervical cancer cells. Mol Med Rep. 14:1751–1755. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Battaglia V, De Stefano Shields C,
Murray-Stewart T and Casero RA Jr: Polyamine catabolism in
carcinogenesis: Potential targets for chemotherapy and
chemoprevention. Amino Acids. 46:511–519. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miao H, Ou J, Peng Y, Zhang X, Chen Y, Hao
L, Xie G, Wang Z, Pang X, Ruan Z, et al: Macrophage ABHD5 promotes
colorectal cancer growth by suppressing spermidine production by
SRM. Nat Commun. 7:117162016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yue F, Li W, Zou J, Jiang X, Xu G, Huang H
and Liu L: Spermidine prolongs lifespan and prevents liver fibrosis
and hepatocellular carcinoma by activating MAP1S-mediated
autophagy. Cancer Res. 77:2938–2951. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang M, Pan Y, Dorfman RG, Chen Z, Liu F,
Zhou Q, Huang S, Zhang J, Yang D and Liu J: AR-42 induces apoptosis
in human hepatocellular carcinoma cells via HDAC5 inhibition.
Oncotarget. 7:22285–22294. 2016.PubMed/NCBI
|
|
18
|
Durie BG, Salmon SE and Russell DH:
Polyamines as markers of response and disease activity in cancer
chemotherapy. Cancer Res. 37:214–221. 1977.PubMed/NCBI
|
|
19
|
Soda K: The mechanisms by which polyamines
accelerate tumor spread. J Exp Clin Cancer Res. 30:952011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thomas TJ, Thomas T, John S, Hsu HC, Yang
P, Keinänen TA and Hyvönen MT: Tamoxifen metabolite endoxifen
interferes with the polyamine pathway in breast cancer. Amino
Acids. 48:2293–2302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nowotarski SL, Woster PM and Casero RA Jr:
Polyamines and cancer: Implications for chemotherapy and
chemoprevention. Expert Rev Mol Med. 15:e32013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Cameron GA and Wallace HM: Decreased
sensitivity to aspirin is associated with altered polyamine
metabolism in human prostate cancer cells. Amino Acids.
48:1003–1012. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi S and Cao H: Shikonin promotes
autophagy in BXPC-3 human pancreatic cancer cells through the
PI3K/Akt signaling pathway. Oncol Lett. 8:1087–1089. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang IT, Chou SC and Lin YC: Zoledronic
acid induces apoptosis and autophagy in cervical cancer cells.
Tumour Biol. 35:11913–11920. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Klionsky DJ: Autophagy: From phenomenology
to molecular understanding in less than a decade. Nat Rev Mol Cell
Biol. 8:931–937. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gerner EW and Meyskens FL Jr: Polyamines
and cancer: Old molecules, new understanding. Nat Rev Cancer.
4:781–792. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Russell DH: Increased polyamine
concentrations in the urine of human cancer patients. Nat New Biol.
233:144–145. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miller-Fleming L, Olin-Sandoval V,
Campbell K and Ralser M: Remaining mysteries of molecular biology:
the role of polyamines in the cell. J Mol Biol. 427:3389–3406.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang C, Ruan P, Zhao Y, Li X, Wang J, Wu
X, Liu T, Wang S, Hou J, Li W, et al: Spermidine/spermine
N1-acetyltransferase regulates cell growth and metastasis via
AKT/β-catenin signaling pathways in hepatocellular and colorectal
carcinoma cells. Oncotarget. 8:1092–1109. 2017.PubMed/NCBI
|
|
30
|
Morselli E, Mariño G, Bennetzen MV,
Eisenberg T, Megalou E, Schroeder S, Cabrera S, Bénit P, Rustin P,
Criollo A, et al: Spermidine and resveratrol induce autophagy by
distinct pathways converging on the acetylproteome. J Cell Biol.
192:615–629. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Eisenberg T, Abdellatif M, Schroeder S,
Primessnig U, Stekovic S, Pendl T, Harger A, Schipke J, Zimmermann
A, Schmidt A, et al: Cardioprotection and lifespan extension by the
natural polyamine spermidine. Nat Med. 22:1428–1438. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Poulin R, Pelletier G and Pegg AE:
Induction of apoptosis by excessive polyamine accumulation in
ornithine decarboxylase-overproducing L1210 cells. Biochem J.
311:723–727. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kumar S, Eroglu E, Stokes JA III,
Scissum-Gunn K, Saldanha SN, Singh UP, Manne U, Ponnazhagan S and
Mishra MK: Resveratrol induces mitochondria-mediated,
caspase-independent apoptosis in murine prostate cancer cells.
Oncotarget. 8:20895–20908. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Levine B: Unraveling the role of autophagy
in cancer. Autophagy. 2:65–66. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu EY and Ryan KM: Autophagy and
cancer-issues we need to digest. J Cell Sci. 125:2349–2358. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsai JH, Hsu LS, Huang HC, Lin CL, Pan MH,
Hong HM and Chen WJ: 1-(2-Hydroxy-5-methylphenyl)-3-phenyl-1,
3-propanedione Induces G1 cell cycle arrest and autophagy in HeLa
cervical cancer cells. Int J Mol Sci. 17:E12742016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lang F, Qin Z, Li F, Zhang H, Fang Z and
Hao E: Apoptotic cell death induced by resveratrol is partially
mediated by the autophagy pathway in human ovarian cancer cells.
PLoS One. 10:e01291962015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hou W, Han J, Lu C, Goldstein LA and
Rabinowich H: Autophagic degradation of active caspase-8: A
crosstalk mechanism between autophagy and apoptosis. Autophagy.
6:891–900. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hui JL, Crowe P and Yang JL: Current
clinical regulation of PI3K/PTEN/Akt/mTOR signalling in treatment
of human cancer. J Cancer Res Clin Oncol. 141:671–89. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wong CH, Iskandar KB, Yadav SK, Hirpara
JL, Loh T and Pervaiz S: Simultaneous induction of non-canonical
autophagy and apoptosis in cancer cells by ROS-dependent ERK and
JNK activation. PLoS One. 5:e99962010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lamy L, Ngo VN, Emre NC, Shaffer AL III,
Yang Y, Tian E, Nair V, Kruhlak MJ, Zingone A, Landgren O and
Staudt LM: Control of autophagic cell death by caspase-10 in
multiple myeloma. Cancer Cell. 23:435–449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Puissant A, Robert G, Fenouille N, Luciano
F, Cassuto JP, Raynaud S and Auberger P: Resveratrol promotes
autophagic cell death in chronic myelogenous leukemia cells via
JNK-mediated p62/SQSTM1 expression and AMPK activation. Cancer Res.
70:1042–1052. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang W, Fan H, Zhou Y, Duan P, Zhao G and
Wu G: Knockdown of autophagy-related gene BECLIN1 promotes cell
growth and inhibits apoptosis in the A549 human lung cancer cell
line. Mol Med Rep. 7:1501–1505. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Won SJ, Yen CH, Liu HS, Wu SY, Lan SH,
Jiang-Shieh YF, Lin CN and Su CL: Justicidin A-induced autophagy
flux enhances apoptosis of human colorectal cancer cells via class
III PI3K and Atg5 pathway. J Cell Physiol. 230:930–946. 2015.
View Article : Google Scholar : PubMed/NCBI
|