1. Introduction
Chaenomeles speciosa (Sweet) Nakai (C.
speciosa, Rosaceae family), also referred to as mugua,
tiegenghaitang, tiejiaoli or zhoupimugua, is distributed in
Central, East and Southwest China and is now cultivated worldwide.
According to the Chinese Pharmacopoeia (2010 edition), the plant
cultivated in Anhui, China, is the genuine medicinal material and
is considered to be of the highest quality. According to
traditional Chinese medicine, the fruit of C. speciosa,
which is warm in nature and sour in flavor, has the ability to calm
the liver, relax the muscles and tendons, harmonize the stomach and
eliminate dampness (1), which may
prevent and cure several clinical conditions, such as rheumatism,
cholera, dysentery, enteritis, beriberi, vitamin C deficiency
syndrome, neuralgia, migraine, stroke and depression (2–6).
Due to the extensive medicinal applications of C.
speciosa, numerous phytochemical and pharmacological studies
have been conducted. The aim of this review was to summarize the
published scientific information that were accumulated over the
last decades regarding this important Chinese medicinal plant for
further investigation.
2. Chemical constituents
Several compounds have been isolated from C.
speciosa (mainly its fruits), including triterpenoid, phenolic
and phenylpropionic acids, flavonoids, saccharides, essential oils
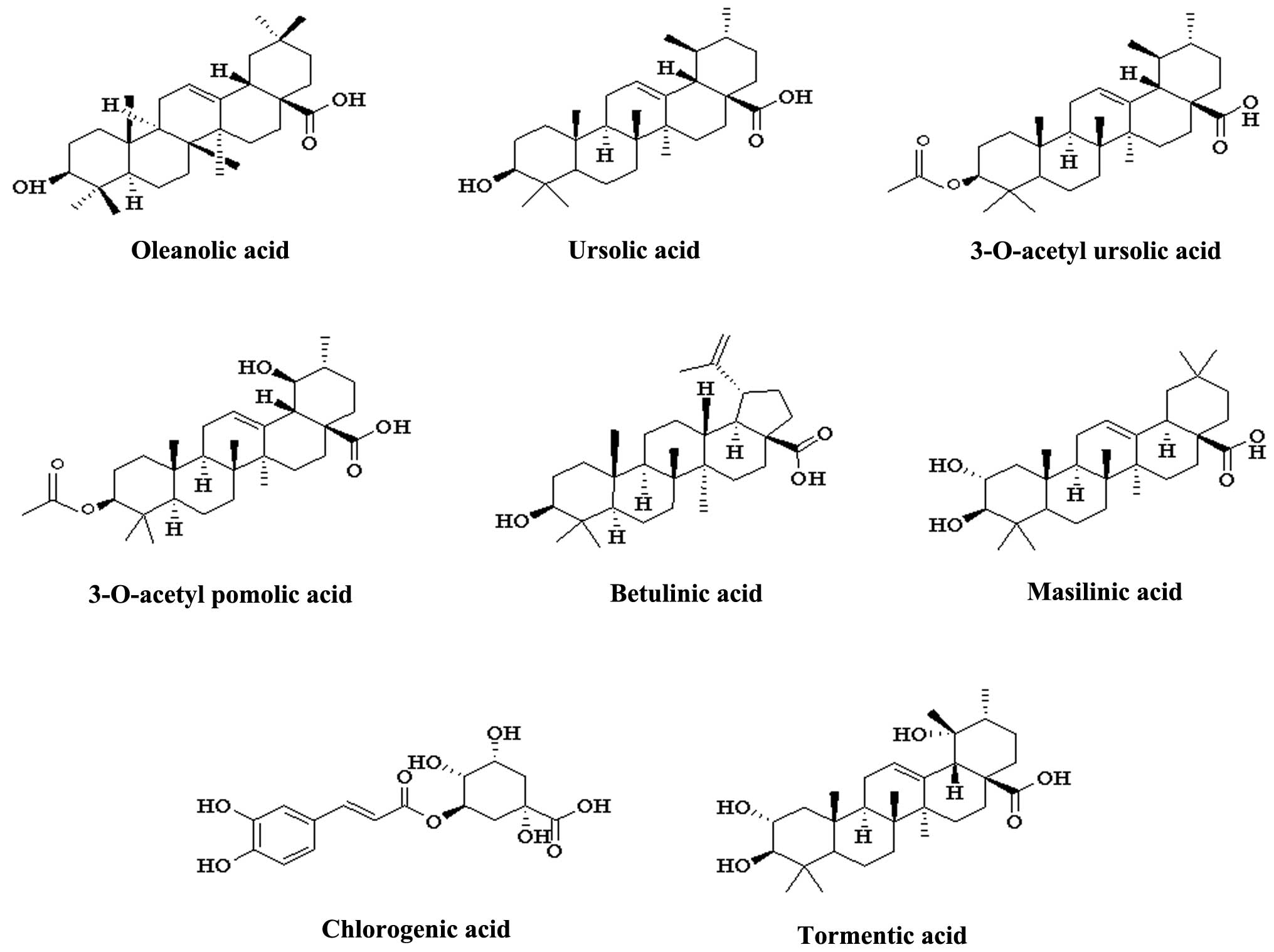
and alkaloids. Oleanolic and ursolic acids, of the triterpenoid
acid family, are the characteristic chemical markers of C.
speciosa and may be used to evaluate the quality of the plant.
In addition, C. speciosa is rich in nutritional constituents
beneficial to the human body (7).
The main compounds are listed in Table
I. The chemical structures of triterpenoid acids are presented
in Fig. 1.
 | Table ICompounds isolated from
Chaenomeles speciosa. |
Table I
Compounds isolated from
Chaenomeles speciosa.
| Type | Compound name
(refs.) | Plant part |
|---|
| Organic acids |
| Triterpenoid
acids | Oleanolic acid
(60,61) | Fruit and leaf |
| Ursolic acid
(62) | Fruit |
| Betulinic acid | Fruit |
| 3-O-acetyl
ursolic acid | Fruit |
| 3-O-acetyl
pomolic acid (63) | Fruit |
|
Speciosaperoxide | Fruit |
| Maslinic acid | Fruit |
| Tormentic acid
(64) | Fruit |
| Phenolic
acids | Protocatechuic
acid | Fruit |
| Gallic acid
(65) | Fruit |
|
2′-Methoxyaucuparin | Fruit |
| p-Hydroxybenzoic
acid (66) | Fruit |
|
3,4-dihydroxybenzoic acid (69) | Fruit |
|
4-Hydroxy-3-methoxy-benzoic acid (67) | Fruit |
| Phenylpropionic
acids | Cinnamic acid | Fruit |
| Chlorogenic
acid | Fruit |
| Caffeic acid
(66) | Fruit |
| Phenyllactic acid
(62) | Fruit |
| Others |
5-(3-Methylphenyl)pentanoic acid | Fruit |
| Butenedioic
acid | Fruit |
| Butanedioic
acid | Fruit |
| Benzoic acid | Fruit |
|
2-Hydroxylbutanedioic acid | Fruit |
| Citramalic
acid | Fruit |
| Benzeneacetic
acid | Fruit |
| Nonanoic acid | Fruit |
| 4-Methoxylbenzoic
acid | Fruit |
|
(Z)-3-Phenyl-2-propenoic acid | Fruit |
| Nonanedioic
acid | Fruit |
|
3-(4-Methoxylphenyl)2-propenoic acid | Fruit |
| Octadecanoic
acid | Fruit |
| Hexadecanoic
acid | Fruit |
|
Methyl-16-heptadecanoic acid | Fruit |
| Octadecatrienoic
acid (68) | Fruit |
| Ethanedioic
acid | Fruit |
| Propandioic
acid | Fruit |
| Furancarboxylic
acid | Fruit |
| 4-Oxo-pentanoic
acid | Fruit |
| 3-Hydroxy-heptanoic
acid | Fruit |
| 3-Hydroxy-hexanoic
acid | Fruit |
| 2-Ketoglutaric
acid | Fruit |
| cis-Aconitic
acid | Fruit |
| Citrate | Fruit |
| 4-Oxo-pimelic
acid | Fruit |
| (E)-2-butenedioic
acid | Fruit |
| Methoxy-butanedioic
acid | Fruit |
|
3-Hydroxy-4-methyl-pentanoic acid | Fruit |
| N-acetyl-L-aspartic
acid | Fruit |
| 15-Octadecenoic
acid | Fruit |
| (Z)-9-octadecenoic
acid (67) | Fruit |
| Flavonoids | Quercetin (69) | Fruit |
| Rutin (70) | Fruit |
| Essential oils | Hexanal | Fruit |
| Ethyl butyrate | Fruit |
| (E)-2-hexenal | Fruit |
| (Z)-3-hexenyl
acetate | Fruit |
| Ethyl
hexanoate | Fruit |
| Linalool | Fruit |
|
trans-Linalool oxide
(furanoid) | Fruit |
| cis-Linalool
oxide (furanoid) | Fruit |
| α-Terpineol | Fruit |
| Ethyl
octanoate | Fruit |
| Edulan I | Fruit |
|
Ethyl(Z)4-decenoate | Fruit |
| Ethyl
p-methoxybenzoate (71) | Fruit |
| Benzaldehyde | Fruit |
| Linaloyl oxide | Fruit |
| n-Octanal | Fruit |
| α-Terpinene | Fruit |
| ϱ-Cymene | Fruit |
| Limonene | Fruit |
| 1,8-Cineole | Fruit |
| (Z)-β-Ocimene | Fruit |
| (E)-β-Ocimene | Fruit |
| γ-Terpinene | Fruit |
| n-Octanol | Fruit |
| (+)-4-Carene | Fruit |
| ϱ-Cymenene | Fruit |
| trans-Limonene
oxide | Fruit |
| n-Nonanal | Fruit |
| Iso-3-thujanol | Fruit |
|
ϱ-Menth-3-3-en-8-ol | Fruit |
| Menthol | Fruit |
| Borneol | Fruit |
| Terpinen-4-ol | Fruit |
| n-Decanal | Fruit |
|
trans-2-Decenal | Fruit |
| Carvenone | Fruit |
| Bornyl acetate | Fruit |
| ϱ-Menth-3-3-en-8-ol
acetate | Fruit |
| α-Longipinene | Fruit |
| β-Elemene | Fruit |
| Longifolene | Fruit |
|
β-Caryophyllene | Fruit |
| Neryl acetone | Fruit |
| E-Ethyl
cinnamate | Fruit |
|
(E,E)-α-Farnesene | Fruit |
| Germacrene A | Fruit |
| δ-Amorphene | Fruit |
| E-Nerolidol | Fruit |
| γ-Eudesmol | Fruit |
| Epi-α-Cadinol | Fruit |
| α-Cadinol (31) | Fruit |
| Others |
3β-acetoxyurs-11-en-13β,28-olide | Fruit |
| Reseoside | Fruit |
| Vomifoliol | Fruit |
|
(6S,7E,9R)-6,9-dihydroxy-4,7-megastigmadien-3-one-9-O-[β-D-xylopyranosyl(1→6)-glucopyranoside]
(64) | Fruit |
| Ethyl
chlorogenate | Fruit |
| Kojic acid
(65) | Fruit |
|
2-Hydroxyl-butanedioicacid-4-methyl
ester | Fruit |
| Esculetin (62) | Fruit |
| Hydroquinone | Fruit |
| Methyl
3-hydroxylbutanedioic ester (69) | Fruit |
3. Anti-inflammatory and antinociceptive
effects
C. speciosa has long been used for the
treatment of rheumatoid arthritis in China and has been shown to
possess anti-inflammatory and antinociceptive properties (8–14).
Several triterpenoids, such as oleanolic, ursolic,
betulinic and maslinic acids, possess anti-inflammatory properties
(15–18). Previous studies investigated the
anti-inflammatory effects of oleanolic acid on adjuvant-induced rat
arthritis and carrageenan-induced rat paw edema (19,20).
Oleanolic and ursolic acids display anti-inflammatory activity
through the direct inhibition of secretory phospholipase A2 (sPLA2)
and formation of sPLA2-oleanolic (ursolic) acid complex (21–22).
Oral administration of ursolic acid at doses of 10, 20, 40, 80 and
160 mg/kg was shown to downregulate the production of interleukin
(IL)-2, interferon-γ and tumor necrosis factor α (TNF-α) (23). Oleanolic and ursolic acids were also
shown to suppress the inflammatory cytokine-induced E-selectin
expression in endothelial cells via inhibition of nuclear factor-κB
(NF-κB) activation (24). Betulinic
acid exerts potent inhibitory effects on vascular inflammatory
processes induced by TNF-α in human umbilical vein endothelial
cells, through the direct inhibition of reactive oxygen species
generation and NF-κB activation (25). Maslinic acid was shown to suppress
cyclooxygenase-2 expression in Raji cells, partly via the NF-κB and
activator protein-1 signaling pathways (26).
To evaluate the anti-inflammatory properties of the
glucosides isolated from C. speciosa (GCS), the
collagen-induced arthritis (CIA) rat model was used. The GCS (30,
60, 120 mg/kg, ig × 7 days) significantly suppressed the
inflammatory response, restored body weight and the weight of
immune organs of CIA rats. GCS also reduced lymphocyte
proliferation and IL-1, -2 and TNF-α production in peritoneal
macrophages and synoviocytes in CIA rats. Furthermore, GCS were
shown to inhibit the mRNA expression of G-protein (Gi) and TNF-α of
synoviocytes and increase the mRNA expression of G-protein (Gs) of
synoviocytes in CIA rats. The administration of GCS at
concentrations of 0.5, 2.5, 12.5, 62.5, 125 mg/l were shown to
increase the cAMP levels in the synoviocytes of CIA rats in
vitro. The anti-inflammatory and immunoregulatory activities of
the GCS are mediated through G-protein-adenylate cyclase-cAMP
transmembrane signal transduction in synoviocytes (8). The GCS (60 and 120 mg/kg, ig × 8 days)
were able to dose-dependently inhibit secondary inflammatory paw
edema, pain response and polyarthritis index in rat adjuvant
arthritis (AA) induced by Freund’s complete adjuvant. The
ultrastructural changes of synoviocytes were improved and the
production of IL-1, TNF-α and prostaglandin E2
(PGE2) was suppressed in AA rats (9). The GCS (60 and 120 mg/kg) were also
reported to downregulate the level of serum antibodies in rats with
AA (27). The antinociceptive
bioactivity of the GCS may be evaluated by acetic acid writhing,
mouse formalin and arthritic flexion tests. The GCS (60, 120, 240
mg/kg for mice and 30, 60, 120 mg/kg for rats, ig) were shown to
reverse all the changes in the responses mentioned above, which is
likely associated with their inhibitory effects on peripheral
inflammatory mediators (10).
In addition, the 10% ethanol fraction,
polysaccharides, saponins and total flavonoids isolated from C.
speciosa were also shown to possess anti-inflammatory and
analgesic properties. The 10% ethanol fraction exhibits more potent
anti-inflammatory effects compared to other fractions at the same
dose. Chlorogenic acid, contained in this fraction and identified
by high-performance liquid chromatography, may be responsible for
this anti-inflammatory effect (12). The polysaccharides may inhibit the
development of primary and secondary arthritis in AA mice, which is
possibly associated with the suppression of lymphocyte
proliferation and regulation of inflammatory cytokines (14). The saponins from C. speciosa
may relieve the symptoms in AA rats, inhibit the immunoinflammatory
response, reduce PGE2 synthesis, suppress increased
thymocyte T cells and diminish the CD4+ T lymphocytes in
the peripheral blood of AA rats (13,28).
Total flavonoids were found to exhibit systemic and peripheral
analgesic activity in mouse and rabbit models (11).
Three compounds, 3,4-dihydroxybenzoic acid,
quercetin and methyl 3-hydroxybutanedioic ester, were shown to
inhibit the production of TNF-α by 22.73, 33.14 and 37.19%,
respectively. Quercetin was also shown to facilitate the release of
IL-6 in RAW264.7 macrophage cells (29).
4. Antimicrobial activity
C. speciosa has been traditionally used for
the treatment of diarrhea in China. The extract of C.
speciosa was proven to inhibit heat-labile enterotoxin
(LT)-induced diarrhea in mice via blocking the binding of the B
subunit of LT (LTB) to the ganglioside GM1
[Galβ1–3GalNAcβ1–4 (Neu5Acα2–3) Gal-β1–4Glc-ceramide]. The ethyl
acetate (EA) and n-butanol soluble fractions were confirmed to be
the most active, eliminating the interactions between LTB and
GM1. Oleanolic, ursolic and betulinic acids from the EA
fraction are considered as the major therapeutic agents in the
treatment of LT-induced diarrhea. These compounds bind to LTB via
hydrogen bonds and hydrophobic contacts with amino acid residues of
LTB by docking techniques (30).
The essential oil extracted from C. speciosa exhibits a
broad spectrum of antimicrobial activity and is more potent against
gram-positive compared to gram-negative bacteria in the disc
diffusion and broth microdilution tests (31). The avian influenza virus may cause
oxidative stress and severe inflammation; 3,4-dihydroxybenzoic
acid, quercetin and methyl 3-hydroxybutanedioic ester isolated from
C. speciosa may act synergistically in the treatment of
avian influenza and are a potential source of antiviral agents
(29). The ethanol extract of C.
speciosa exhibits potent antibacterial activity, with a minimal
inhibitory concentration of 0.125 mg/ml and a minimal bactericidal
concentration of 0.25mg/ml (32).
5. Antioxidant activity
The 80% methanol extract from C. speciosa
inhibits tyrosinase activity, followed by suppression of
melanogenesis (33). C.
speciosa possesses significant antioxidant properties, partly
due to its abundance in vitamin C and polyphenols. The C.
speciosa powder processed by a specific method exhibits good
scavenging activity against 1,1-diphenyl-2-picrylhydrazyl free
radical (DPPH) and O2−, with a scavenging
index of 945±20 μg DPPH/g and 700±21 U/ml, respectively. and a
ferric reducing antioxidant power of 173±7 μmol Fe2+/g.
C. speciosa may considerably reduce the serum levels of
low-density lipoprotein cholesterol and total cholesterol, increase
glutathione peroxidase activity and decrease the relative
atherosclerotic plaque area of the aortic sinus and aortic arch in
ApoE−/− mice (34). The
total flavonoids from C. speciosa were shown to
significantly reduce the peroxide value in lard, clear DPPH and
deoxidize Fe3+ in a dose-dependent manner, exhibiting a
more potent antioxidant effect compared to that of vitamin C
(35). In addition,
3,4-dihydroxybenzoic acid and quercetin isolated from C.
speciosa exerted a more potent inhibitory effect on DPPH and
neuraminidase (29).
6. Immunoregulatory effect
The GCS were shown to suppress the contact
hypersensitivity (CHS) response. In mice with CHS induced by
2,4-dinitro-I-dinitroflurobenzene, GCS (120 mg/kg) exerted an
inhibitory effect similar to that of the control drug
4-acetylaminophenylacetic acid on the thymus and spleen indices.
The GCS were shown to inhibit splenocyte proliferation induced by
concanavalin A, decrease the CD4+/CD8+ T
lymphocyte ratio and restore the CD4+/CD8−
subset ratio in CHS mice. The GCS were also shown to decrease the
production of IL-2 and transforming growth factor-β1 (TGF-β1) and
increase the IL-4 level in the thymus of CHS mice (36). C. speciosa exerted a
protective effect on mice with immunosuppression induced by
cyclophosphamide (CTX). After the mice were administered C.
speciosa for 15 days, the serum hemolysin and lymphocyte
transformation rates improved significantly and the mRNA expression
of FOXP3, TGF-β, PD1, Fas and Bax was considerably diminished
compared to the CTX-group (37).
7. Dopamine transporter inhibitory and
antiparkinsonian effects
C. speciosa was proven to be effective in
dopamine transporter (DAT) regulation and antiparkinsonism, as
determined by in vitro and in vivo assays. In Chinese
hamster ovary (CHO) cells and two animal models [6-hydroxydopamine
(6-OHDA)-lesioned rats and
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice],
the aqueous extract of C. speciosa was found to markedly
inhibit dopamine uptake by CHO cells and synaptosomes at
concentrations of 1–1,000 μg/ml in a concentration-dependent
manner; however, it had little effect on norepinephrine
transporters at concentrations up to 1,000 μg/ml and no effect on
γ-aminobutyric acid or serotonin transporters. The aqueous extract
of C. speciosa was shown to alleviate
1-methyl-4-phenylpyridinium-induced toxicity in CHO cells stably
expressing DAT. In neurobehavioral studies, the extract
time-dependently mitigated 6-OHDA-induced hemi-parkinsonian
rotations in rats and dose-dependently attenuated MPTP-induced
deficits in mice during endurance performance. The aqueous extract
also significantly reduced the loss of tyrosine
hydroxylase-positive neurons in the substantia nigra of
MPTP-treated mice. The antiparkinsonian-like effects of C.
speciosa may be associated with the suppression of DAT activity
(38).
8. Agonist targeting β2-adrenoceptors
β2-adrenoceptor agonists are the most widely used
agents in the treatment of asthma due to their bronchodilator
actions (39). The transfected
human embryonic kidney 293 cell clone was developed for screening
the agonists of human β2-adrenoceptor among Chinese medicinal
herbs. The ethanol extract of C. speciosa exerted
significant activating effects on reporter gene expression at a
half maximal effective concentration of 4.8 μg/ml (40).
9. Inhibitory effect on gastrointestinal
smooth muscle contraction
Total flavonoids from C. speciosa were shown
to relax gastrointestinal smooth muscles, through exerting an
inhibitory effect on the contraction of the isolated rabbit gastric
fundus and ileum induced by acetylcholine and CaCl2 in a
dose-dependent manner and suppressing the contraction of the
isolated rabbit taenia coli elicited by high K+
depolarization. These relaxant effects may be associated with the
voltage-dependent Ca2+ channel blockade by total
flavonoids (40,41).
10. Hepatoprotective effects
The 70% alcohol extract of C. speciosa exerts
a certain protective effect on rats with chronic hepatic
damnification injected with CCl4(42). C. speciosa contained in
high-fat diet may prevent mice from developing non-alcoholic
steatohepatitis by regulating the expression of toll-like and death
receptors and the secretion of inflammatory cytokines (43). Oleanolic acid isolated from C.
speciosa exerts a strong inhibitory effect on hepatitis B virus
replication, with an inhibitory ratio of 29.33% at a concentration
of 20 μg/ml (44). Oleanolic acid
was shown to effectively protect the liver from acute injury
induced by chemicals, as well as from fibrosis and cirrhosis
precipitated by chronic liver diseases (45,46).
Oleanolic acid was shown to increase the expression of hepatic
metallothionein and nuclear factor E2-related factor 2
(Nrf2) against hepatotoxicants (47), but was also found to activate
Nrf2-independent cytoprotective mechanisms in Nrf2-null mice
(46).
11. Antitumor activity
It was reported as early as 1975 that organic acids
from C. speciosa exert antitumor effects in mice with
Ehrlich ascites carcinoma (48);
this antitumor effect is a common property of numerous
triterpenoids (49,50). Among these, oleanolic, ursolic,
betulinic and maslinic acids are the most notable triterpenoid
compounds. When applied to estrogen receptor-negative breast cancer
and osteosarcoma cells, oleanolic acid elicited tumor cell
apoptosis through inhibition of mammalian target of rapamycin
signaling (51,52). Oleanolic and ursolic acids also
caused apoptosis in HuH7 human hepatocellular carcinoma cells via
downregulation of the X-linked inhibitor of apoptotic protein
(53). Oleanolic, ursolic and
maslinic acids were shown to exert potent antiangiogenic effects on
liver and non-small-cell lung cancer cell lines (54,55).
12. Conclusions
C. speciosa is a dual-purpose medicinal and
edible plant. In terms of medicinal application, extensive
pharmacological investigations demonstrated that C. speciosa
is a bioactive species possessing anti-inflammatory,
antinociceptive, antimicrobial, antioxidant and immunoregulatory
properties. These pharmacological activities partly verified the
rationale of the traditional application of C. speciosa in
the treatment of rheumatism, cholera, dysentery, enteritis and
beriberi.
An increasing number of studies are being conducted
to investigate the phytochemistry of C. speciosa and a
number of chemical constituents, including triterpenoid, phenolic
and phenylpropionic acids, flavonoids, saccharides, essential oils
and alkaloids, have been isolated from the fruit and leaves.
Triterpenoid acids, oleanolic and ursolic acid in particular, are
the major active constituents, which possess several
pharmacological properties in vivo and in vitro,
including anti-inflammatory, hepatoprotective and antitumor
properties. The hepatoprotective effects of oleanolic acid allow
its use as an oral medication for the treatment of liver disorders
in China (56,57). Flavonoids, another main bioactive
constituent of C. speciosa, were proven to possess
antioxidant (35), antispasmodic
(41,58), analgesic (11) and anti-influenza (59) properties. However, the specific
ingredients of flavonoids have not been determined. Therefore,
bioassay-guided isolation and identification are required for the
obtained bioactive compounds.
Although various bioactivities of extracts or
compounds obtained from C. speciosa are verified using
laboratory animals or cells, few molecular mechanisms of action
have been determined, which may limit further clinical application
of this plant. In addition, when a drug is used in the clinical
setting, its safety profile is of utmost importance. Of note, there
are few toxicological evaluations reported on other extracts or
compounds.
Apart from the fruit and leaves, other parts of the
C. speciosa plant, including the seed, flower, root, branch
and bark, have been clinically used as medicine. However, the
number of available studies on the chemical components and
pharmacological activities of these parts is limited and further
investigations are required.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundations of China (no. 81173462).
References
|
1
|
Chinese Pharmacopeia Commission, . Chinese
Pharmacopoeia. Chinese Medicine and Technology Publishing House;
Beijing: pp. 572010
|
|
2
|
Zhonghua Bencao Editorial Group, .
Zhonghua Bencao (Chinese Herbal Medicine). 4. Shanghai Science and
Technology Publishing House; Shanghai: pp. 112–120. 1999
|
|
3
|
Nanjing University of Traditional Chinese
Medicine, . Dictionary of Traditional Chinese Medicine. Shanghai
Science and Technology Publishing House; Shanghai: pp. 349–350.
2006
|
|
4
|
He SM and Jiang J: Review on TCM treatment
of migraine and tension-type headache. Chin Arch Tradit Chin Med.
24:1469–1471. 2006.
|
|
5
|
Wang LZ: Therapy of apoplectic sequelae by
six schemes. Henan Tradit Chin Med. 26:31–32. 2006.
|
|
6
|
An CP and Cheng W: Review on mechanisms
and symptoms of depression in TCM (Traditional Chinese Medicine).
Inf Tradit Chin Med. 24:12–14. 2007.
|
|
7
|
Zhang H, Geng YL, Wang DJ, Liu JH, Wang X,
Du JH and Li SB: Research on nutrient components of different
species of Chaenomeles speciosa Nakai. Shandong Sci.
24:24–27. 2011.
|
|
8
|
Chen Q and Wei W: Effects and mechanisms
of glucosides of Chaenomeles speciosa on collagen-induced
arthritis in rats. Int Immunopharmacol. 3:593–608. 2003.PubMed/NCBI
|
|
9
|
Dai M, Wei W, Shen YX and Zheng YQ:
Glucosides of Chaenomeles speciosa remit rat adjuvant
arthritis by inhibiting synoviocyte activities. Acta Pharmacol Sin.
24:1161–1166. 2003.
|
|
10
|
Wang NP, Dai M, Wang H, Zhang LL and Wei
W: Antinociceptive effect of glucosides of Chaenomeles
speciosa. Chin J Pharmacol Toxicol. 19:169–174. 2005.
|
|
11
|
Kong JS and Yang XH: Mechanisms analysis
on the analgesic effect of total flavonoids extracted from
Chaenomeles lagenaria. Lishizhen Med Mater Med Res.
20:549–550. 2009.
|
|
12
|
Li X, Yang YB, Yang Q, Sun LN and Chen WS:
Anti-inflammatory and analgesic activities of Chaenomeles
speciosa fractions in laboratory animals. J Med Food.
12:1016–1022. 2009. View Article : Google Scholar
|
|
13
|
Yang XH, Wu J and Guo LJ:
Anti-inflammatory activity of saponin in Chaenomeles
speciosa in rat adjuvant arthritis. Lishizhen Med Mater Med
Res. 21:2833–2834. 2010.
|
|
14
|
Li SG and Chen Y: Effect of Chaenomeles
speciosa polysaccharide on adjuvant arthritis in mice and its
mechanisms. Chin J Exp Trad Med Form. 17:159–162. 2011.
|
|
15
|
Price KR, Johnson IT and Fenwick GR: The
chemistry and biological significance of saponins in foods and
feedingstuffs. Crit Rev Food Sci Nutr. 26:27–135. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mahato SB, Sarkar SK and Poddar G:
Triterpenoid saponins. Phytochemistry. 27:3037–3067. 1988.
View Article : Google Scholar
|
|
17
|
Hasmeda M, Kweifio-Okai G, Macrides T and
Polya GM: Selective inhibition of eukaryote protein kinases by
anti-inflammatory triterpenoids. Planta Med. 65:14–18. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Salminen A, Lehtonen M, Suuronen T,
Kaarniranta K and Huuskonen J: Terpenoids: natural inhibitors of
NF-kappaB signaling with anti-inflammatory and anticancer
potential. Cell Mol Life Sci. 65:2979–2999. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singh GB, Singh S, Bani S, Gupta BD and
Banerjee SK: Anti-inflammatory activity of oleanolic acid in rats
and mice. J Pharm Pharmacol. 44:456–458. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kapil A and Sharma S: Effect of oleanolic
acid on complement in adjuvant- and carrageenan-induced
inflammation in rats. J Pharm Pharmacol. 47:585–587. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nataraju A, Raghavendra Gowda CD, Rajesh R
and Vishwanath BS: Group IIA secretory PLA2 inhibition by ursolic
acid: a potent anti-inflammatory molecule. Curr Top Med Chem.
7:801–809. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dharmappa KK, Kumar RV, Nataraju A,
Mohamed R, Shivaprasad HV and Vishwanath BS: Anti-inflammatory
activity of oleanolic acid by inhibition of secretory phospholipase
A2. Planta Med. 75:211–215. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ahmad SF, Khan B, Bani S, Suri KA, Satti
NK and Qazi GN: Amelioration of adjuvant-induced arthritis by
ursolic acid through altered Th1/Th2 cytokine production. Pharmacol
Res. 53:233–240. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takada K, Nakane T, Masuda K and Ishii H:
Ursolic acid and oleanolic acid, members of pentacyclic
triterpenoid acids, suppress TNF-alpha-induced E-selectin
expression by cultured umbilical vein endothelial cells.
Phytomedicine. 17:1114–1119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoon JJ, Lee YJ, Kim JS, Kang DG and Lee
HS: Protective role of betulinic acid on TNF-alpha-induced cell
adhesion molecules in vascular endothelial cells. Biochem Biophys
Res Commun. 391:96–101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsum YW, Yew WT, Hong PL, et al: Cancer
chemopreventive activity of maslinic acid: suppression of COX-2
expression and inhibition of NF-kappaB and AP-1 activation in Raji
cells. Planta Med. 77:152–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Y, Wei W, Wu H, Tang LQ, Wang XY and
Yang YQ: Down-regulation effect of glucosides of Chaenomeles
speciosa on the levels of serum antibodies in rats with
adjuvant arthritis. Chin Pharmacol Bull. 23:941–944. 2007.(In
Chinese).
|
|
28
|
Yang XH, Wu J and Guo LJ: Optimization on
extraction technology of saponin of Chaenomeles speciosa and
study on its anti-inflammatory immunologic mechanism to rat
adjuvant arthritis. West China J Pharm Sci. 25:49–51. 2010.
|
|
29
|
Zhang L, Cheng YX, Liu AL, Wang HD, Wang
YL and Du GH: Antioxidant, anti-inflammatory and anti-influenza
properties of components from Chaenomeles speciosa.
Molecules. 15:8507–8517. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen JC, Chang YS, Wu SL, et al:
Inhibition of Escherichia coli heat-labile
enterotoxin-induced diarrhea by Chaenomeles speciosa. J
Ethnopharmacol. 113:233–239. 2007.
|
|
31
|
Xie XF, Cai XQ, Zhu SY and Zou GL:
Chemical composition and antimicrobial activity of essential oils
of Chaenomeles speciosa from China. Food Chem.
100:1312–1315. 2007. View Article : Google Scholar
|
|
32
|
Yu Q, Bai ZC, Meng DS, Lu LC, Fu RQ and
Zhang XM: Antibacterial effects of 23 kinds of Chinese herbal
medicine extracts on Streptococcus pneumoniae. China Pharm.
22:2135–2136. 2011.
|
|
33
|
Lee KT, Kim BJ, Kim JH, Heo MY and Kim HP:
Biological screening of 100 plant extracts for cosmetic use (I):
inhibitory activities of tyrosinase and DOPA auto-oxidation. Int J
Cosmet Sci. 19:291–298. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang Y, Yu Xp, Mi MT, Zhao J, Wang J and
Zhang T: Antioxidative property and antiatherosclerotic effects of
the powder processed from Chaenomeles speciosa in
APOE−/−mice. J Food Biochem. 34:535–548. 2010.
|
|
35
|
Liu ZX, Hu SD, Zou K, Pan JR and Liao QB:
Studies on antioxidation in vitro of ethanol extract from
Chaenomeles speciosa(Sweet) Nakai. J China Three Gorges Univ
(Nat Sci). 30:72–75. 2008.(In Chinese).
|
|
36
|
Zheng YQ, Wei W, Dai M and Wang NP:
Glucosides of Chaenomeles speciosa suppressed contact
hypersensitivity response via modulating the thymus T lymphocytes
subsets in mice. Chin Pharmacol Bull. 20:1016–1019. 2004.(In
Chinese).
|
|
37
|
Shi JJ, Liu CQ, Li B, et al: Protecting
effect of Chaenomeles speciosa broth on immunosuppressive
mice induced by cyclophosphamide. J Chin Med Mater. 32:1418–1421.
2009.(In Chinese).
|
|
38
|
Zhao G, Jiang ZH, Zheng XW, Zang SY and
Guo LH: Dopamine transporter inhibitory and antiparkinsonian effect
of common flowering quince extract. Pharmacol Biochem Behav.
90:363–371. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Milic M, Bao X, Rizos D, Liu F and Ziegler
MG: Literature review and pilot studies of the effect of QT
correction formulas on reported β2-agonist-induced QTc
prolongation. Clin Ther. 28:582–590. 2006.PubMed/NCBI
|
|
40
|
Wang H, Li SY, Zhao CK and Zeng X: A
system for screening agonists targeting β2-adrenoceptor
from Chinese medicinal herbs. J Zhejiang Univ Sci B. 10:243–250.
2009.
|
|
41
|
Liu W, Yang XH, Zhou M and Li CD:
Pharmacodynamical mechanisms of total flavonoids from
Chaenomeles lagenaria Koidz in the relaxation of
gastrointestinal smooth muscles. World Chin J Digestol. 15:165–167.
2007.
|
|
42
|
Wang HX: Lab study of effects fructus
Chaenomeles on protecting hepar and decreasing enzyme. World J
Integr Tradit West Med. 2:213–215. 2007.
|
|
43
|
Li B, Liu CQ, Shi JJ, et al: Preventive
effect of Chaenomeles fruits on non-alcoholic
steatohepatitis in mice. Food Sci. 31:258–261. 2010.
|
|
44
|
Liu HJ, Hu JH, Sun LN, Cai Z, Shi J and
Liu T: Inhibition effects of oleanolic acid from Chaenomeles
lagenaria on hepatitis B virus in vitro. Pharm J Chin People’s
Liberation Army. 18:272–274. 2002.
|
|
45
|
Liu J, Liu Y and Klaassen CD: Protective
effect of oleanolic acid against chemical-induced acute necrotic
liver injury in mice. Acta Pharmacologica Sinica. 16:97–102.
1995.PubMed/NCBI
|
|
46
|
Reisman SA, Aleksunes LM and Klaassen CD:
Oleanolic acid activates Nrf2 and protects from acetaminophen
hepatotoxicity via Nrf2-dependent and Nrf2-independent processes.
Biochem Pharmacol. 77:1273–1282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu J, Wu Q, Lu YF and Pi J: New insights
into generalized hepatoprotective effects of oleanolic acid: key
roles of metallothionein and Nrf2 induction. Biochem Pharmacol.
76:922–928. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jin ZR: The extract of antitumor active
ingredients in Chaenomeles speciosa. Chin Tradit Herb Drug
Commun. 6:181975.
|
|
49
|
Laszczyk MN: Pentacyclic triterpenes of
the lupane, oleanane and ursane group as tools in cancer therapy.
Planta Med. 75:1549–1560. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kuttan G, Pratheeshkumar P, Manu KA and
Kuttan R: Inhibition of tumor progression by naturally occurring
terpenoids. Pharm Biol. 49:995–1007. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chu R, Zhao X, Griffin C, et al: Selective
concomitant inhibition of mTORC1 and mTORC2 activity in estrogen
receptor negative breast cells by BN107 and oleanolic acid. Int J
Cancer. 127:1209–1219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhou R, Zhang Z, Zhao L, et al: Inhibition
of mTOR signaling by oleanolic acid contributes to its anti-tumor
activity in osteosarcoma cells. J Orthop Res. 29:846–852. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shyu MH, Kao TC and Yen GC: Oleanolic acid
and ursolic acid induce apoptosis in HuH7 human hepatocellular
carcinoma cells through a mitochondrial-dependent pathway and
downregulation of XIAP. J Agric Food Chem. 58:6110–6118. 2010.
View Article : Google Scholar
|
|
54
|
Lin CC, Huang CY, Mong MC, Chan CY and Yin
MC: Antiangiogenic potential of three triterpenic acids in human
liver cancer cells. J Agric Food Chem. 59:755–762. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lucio KA, da Rocha GG, Moncao-Ribeiro LC,
Fernandes J, Takiya CM and Gattass CR: Oleanolic acid initiates
apoptosis in non-small cell lung cancer cell lines and reduces
metastasis of a B16F10 melanoma model in vivo. PLoS One.
6:e285962011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu J: Pharmacology of oleanolic acid and
ursolic acid. J Ethnopharmacol. 49:57–68. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu J: Oleanolic acid and ursolic acid:
research perspectives. J Ethnopharmacol. 100:92–94. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kong JS, Yang XH and Liu W: The relaxant
effects and related mechanism of total flavones from Chaenomeles
lagenaria Koidz on gastrointestinal smooth muscles. Lishizhen
Med Mater Med Res. 18:2123–2124. 2007.
|
|
59
|
Wang X, Jia W, Zhao A and Wang X:
Anti-influenza agents from plants and traditional Chinese medicine.
Phytother Res. 20:335–341. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Luo JF: The isolation and identification
of oleanolic acid in Chaenomeles speciosa. Chin Tradit Herb
Drug. 4:481983.
|
|
61
|
Yin B, Yan HG, He B, et al: An orthogonal
experiment to optimize refluxing extraction of oleanolic acid in
leaves of Chaenomeles speciosa s. nakai. Food Sci Technol.
31:101–103. 2006.
|
|
62
|
Chen HC, Ding LS, Peng SL and Liao X:
Study on the chemical constituents in Chaenomeles speciosa.
Chin Tradit Herb Drugs. 36:30–31. 2005.
|
|
63
|
Guo XM, Zhang L, Quan SC, Hong YF, Sun LN
and Liu MZ: The isolation and identification of triterpenoids in
Chaenomeles speciosa. J Chin Med Mater. 23:546–547.
1998.
|
|
64
|
Song YL, Zhang L, Gao JM, Du GH and Cheng
YX: Speciosaperoxide, a new triterpene acid, and other terpenoids
from Chaenomeles speciosa. J Asian Nat Prod Res. 10:214–217.
2008. View Article : Google Scholar
|
|
65
|
Yin K, Gao HY, Li XN and Wu LJ: Chemical
constituents of Chaenomeles speciosa(Sweet) Nakai. J
Shenyang Pharm Univ. 23:760–763. 2006.
|
|
66
|
Yang YB, Yang Y, Li X, et al: Studies on
the chemical constituents of Chaenomeles speciosa. J Chin
Med Mater. 32:1388–1390. 2009.(In Chinese).
|
|
67
|
Gong FJ, Chen L, Lu XC and Wang YW:
Determination of organic acid components from fruits of
Chaenomeles speciosa by GC-MS. J Plant Resour Environ.
14:55–56. 582005.
|
|
68
|
Gao CW, Kan Y, Lei ZM, Duan ZH, et al: The
studies on the acidic constituents in the fresh- fruit of
Chaenomeles speciosa. J Yunnan Univ. 21:319–321. 1999.
|
|
69
|
Song YL, Feng ZB, Cheng YX and Gao JM:
Chemical components of Chaenomeles speciosa(Sweet) Nakai.
Acta Bot Boreali Occident Sin. 27:831–833. 2007.
|
|
70
|
Tang Q, Song SW, Chen HW, Huang B and Song
LH: Determination of the Rutin in Chaenomeles speciosa S.
Nakai by HPLC. J Xuzhou I Technol. 22:65–67. 2007.
|
|
71
|
Horvat RJ, Chapman GW Jr and Payne JA:
Volatiles of ripe flowering quince (Chaenomeles speciosa
Nakai). J Essent Oil Res. 6:81–83. 1994. View Article : Google Scholar
|