Introduction
Neural cell migration is crucial in the formation of
highly organized structures of mammalian brain. Abnormal migration
in prenatal and early postnatal brain causes various types of
psychiatric diseases, including mental retardation, autism, bipolar
disorders and schizophrenia (1,2).
Various molecules have been identified as inducers and modulators
of the migration including growth/neurotrophic factors and certain
types of neurotransmitters (3–5).
Serotonin (5-HT) has a critical role in neural
migration, which is mainly supplied from placental sources and
serotonergic projections from the dorsal raphe nuclei in the fetal
brain (6). Depletion of 5-HT by
injection of DL-P-chlorophenylalanine (PCPA, an inhibitor of 5-HT
synthesis) during the E12-E17 stage inhibited migration and
disorganized the positioning of cortical neurons (7). By contrast, cortical slices exposed to
high doses of 5-HT (100–400 μM) inhibited the migration of GABergic
neurons. The arrested migration was recovered by application of the
5-HT6 antagonist, SB258585 (8,9).
However, the association between 5-HT dose and its effect on
migration remains to be elucidated.
It is also unclear as to which serotonin receptor
mediates 5-HT signal to modulate neural migration. At least 14
classes of 5-HT receptors were identified that are coupled with
various types of G proteins, with the exception of
5-HT3, a ligand-gated ion channel receptor (10,11).
5-HT6 receptor coupled with Gs protein is positively
linked to adenylate cyclase to increase the cAMP level. Recent
studies have demonstrated that 5-HT3 and
5-HT6 are differentially expressed in migrating neurons
in the cerebral cortex (9,12,13).
Vitalis et al(13)
identified 5-HT3 and 5-HT6 as candidates
involved in the mediation of the 5-HT signal for migration of the
pyramidal neurons in the cortex.
To determine the effects of 5-HT on neural cell
migration, we used a PC12 neuron-like cell line that expresses
5-HT3 and 5-HT6 in experiments. 5-HT and
nerve growth factor (NGF) induced PC12 cell migration via
5-HT6 but not 5-HT3, stimulating the cAMP and
extracellular signal-regulated kinase (ERK) signaling pathways.
Materials and methods
Materials
5-HT, NGF, Ondansetron, SB271046, SB258585,
Clozapine and PD98059 were obtained from Sigma-Aldrich (St. Louis,
MO, USA). MDL7222 was obtained from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA).
Cell culture
PC12 cells (RIKEN Tsukuba Institute, Tsukuba, Japan)
were routinely cultured in Dulbecco’s modified Eagle’s medium
(DMEM; Sigma-Aldrich) containing 10% fetal bovine serum (Life
Technologies, Gaithersburg, MD, USA), 10% horse serum (Life
Technologies), 50 U/ml penicillin and 50 μg/ml streptomycin (Life
Technologies). The cells were incubated at 37ºC in a 5%
CO2 atmosphere. For each experiment, 3×105
cells were spread onto a 60 mm dish (Becton Dickinson, Franklin
Lakes, NJ, USA). Following incubation for two days, the cells were
used for RNA extraction and transwell migration assay. To obtain
differentiated cells, the medium was changed to NGF-supplemented
medium (DMEM containing 100 ng/ml NGF, 1% horse serum, 50 U/ml
penicillin and 50 μg/ml streptomycin) and incubated for one
day.
RNA extraction and RT-PCR
Total RNAs were extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA), then cDNAs were synthesized with
ReverTra Ace (Toyobo, Osaka, Japan) according to the manufacturer’s
instructions. PCRs were performed with a denaturation step at 95ºC
for 5 min, followed by 35 (Fig.
2A), 29 (Fig. 2B) or 35 cycles
(Fig. 2C) of denaturation at 95ºC
for 30 sec, primer annealing at 55ºC for 30 sec, and primer
extension at 72ºC for 30 sec. TATA binding protein (TBP) was used
as a control. The primers used were: 5-HT1A (forward)
5′-CTCTGTTGCTGGGTACTCTCATT/(reverse) 5′-AGTCTATAGGGTCGGTGATAGCC-3′,
5-HT1B 5′-3′-, 5-HT2A
5′-TGTACGTGAACCAAGTCAAAGTG-3′/ 5′-GTAGATGATGGGGTTGATGAGAG-3′,
5-HT2A 5′-ATG CTGAAAACAGAACCAACCT-3′/5′-ACATCCAGGTAAAT
CCAGATCG-3′, 5-HT2B 5′-TCGTCAAGATTACGG
TGGTATG-3′/5′-CACCATCTTTTCTGGTGATGAA-3′, 5-HT2C
5′-ATAGGGGGCAACATTCTTGTTAT-3′/5′-ACAGGGATAGGAACTGAAACTCC-3′,
5-HT3, 5′-GGAA GTCTCCAAGCATTCCTTAT-3′/5′-ACGTAGAACTTC
ATTTCCGCATA-3′, 5-HT4 5′-CCAATATTGTGGAC
CCTTTCATA-3′/5′-GACTGGCTTCTTTTCAAGCTACA, 5-HT5A
5′-AAGATTTACAAGGCTGCGAAGTT-3′/5′-ACT GATGAGCTCCGTAACAAAGA-3′,
5-HT5B 5′-CTGG ATCGCTACTGGACTATCAC-3′/5′-GTGA
ATACCGTCTCA GACTCCTG-3′, 5-HT6 5′-CTGGGAATGTTCTTTGT
CACCT-3′/5′-GAAGCGGAGTCTGAATCTGA GTT-3′, 5-HT7
5′-ACTTCTTCTGCAACGTCTTCATC-3′/5′-GCG GCCTTGTAAATCTGATAGTA-3′, TBP
5′-TGCTGGCGG TTTGGCTAGGTTTCTGC-3′/5′-GGTCAGAGTTTGAGAA
TGGAAGAGTT-3′.
Transwell cell migration assay
PC12 cell suspension containing 2×105
cells in DMEM was applied to each upper well of the transwell
chamber (Becton Dickinson), which was previously coated with type I
collagen (50 μg/ml, Becton Dickinson) on both sides. In the bottom
well, DMEM with or without motogen, NGF (100 ng/ml) and/or 5-HT
(0.1–10 μM) were applied to allow the cells to migrate across
filters (8 μm pore size). Ondansetron (1 μM, 5-HT3
antagonist), MDL7222 (1 μM, 5-HT3), SB271046 (1 μM,
5-HT6), SB258585 (1 μM, 5-HT6) and PD98059
(20 μM, ERK inhibitor) were also applied in some of the
experiments. The transwell migration assay was performed at 37ºC
for 5 h. After removal of the remaining cells on the top side of
filters using cotton swabs, the filters were fixed with 4% PFA/PBS
for 15 min. After incubation with Hoechst 33258 (Nacalai Tesque,
Kyoto, Japan) at room temperature for 5 min, the cells on the
bottom side of transwell inserts were washed three times with PBS
and examined by fluorescence microscopy (IX83; Olympus, Tokyo,
Japan). Ten images were captured randomly for one experiment and
the number of nuclei in a 600×600 μm in each image was counted.
Image J was used for counting as previously described (14). Relative percentages of the cell
number to the average of the cell number in the control experiments
(DMEM only in bottom well) were plotted on the graphs.
Statistical analysis
P-values were calculated by one- or two-way ANOVA
followed by Tukey’s HSD. Data are presented as mean ± standard
error.
Results
5-HT induced PC12 cells migration in a
dose-dependent manner
To determine the effect of 5-HT on PC12 cell
migration, the transwell migration assay was used (15,16).
Cells were spread onto the upper side of the transwell insert and
the number of cells migrating across the filter was counted.
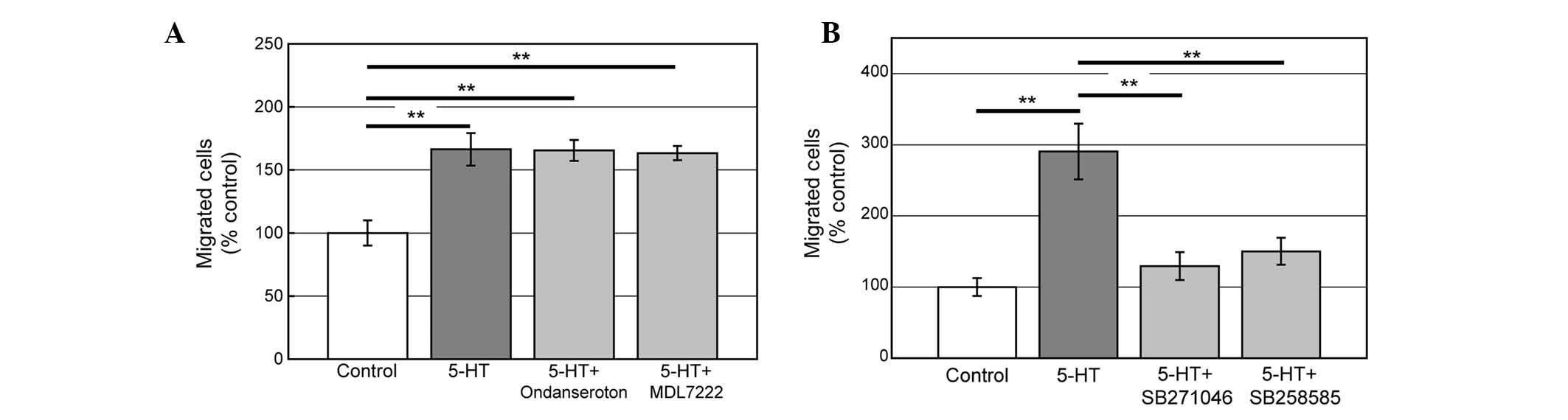
Addition of 5-HT to the bottom well together with DMEM
significantly increased the migrating cells (Fig. 1A). 5-HT-induced migration was
identified in a dose-dependent manner (Fig. 1B). An amount of 1 μM 5-HT
significantly increased cell migration, while 0.1, 0.5, 5.0 and 10
μM 5-HT did not show significant changes compared with the control.
NGF is known to induce PC12 cell migration (15,16),
thus we added 5-HT together with NGF. NGF and 5-HT induce migration
in an additive manner.
5-HT6 receptor mediated
5-HT-induced migration
We examined which serotonin receptor mediates PC12
cell migration induced by 5-HT. Previously, it was reported that
5-HT3 enhanced neurite outgrowth induced by NGF in PC12
cells (17). However, little is
known regarding the expression and molecular function of other 5-HT
receptors in PC12 cells.
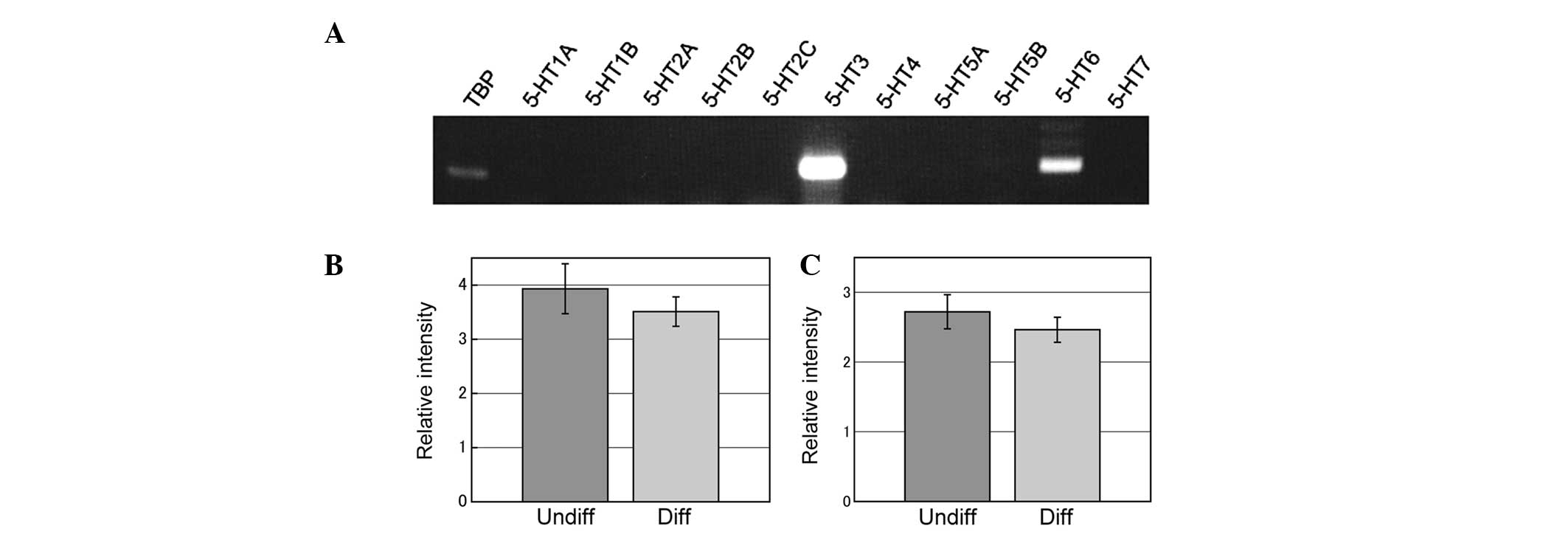
mRNA expression of 5-HT receptors was examined by
RT-PCR and 5-HT6 and 5-HT3 were found to be
expressed (Fig. 2A) in
undifferentiated and differentiated (1 day after application of 100
ng/ml NGF) PC12 cells (Fig. 2B and
C).
Using antagonists against 5-HT3 and
5-HT6 receptors, we investigated which 5-HT receptor
mediates the migration induced by 5-HT. Addition of Ondansetron
(5-HT3 antagonist, 1 μM) or MDL7222 (5-HT3, 1
μM) with 5-HT did not reveal any significant changes (Fig. 3A) while antagonists of
5-HT6, SB258585 (1 μM) or SB271046 (1 μM) inhibited the
migration induced by 5-HT (Fig.
3A). The data indicate that 5-HT6 but not
5-HT3 mediates serotonin-induced PC12 cell
migration.
Involvement of cAMP and ERK in
5-HT6-mediated signaling pathways
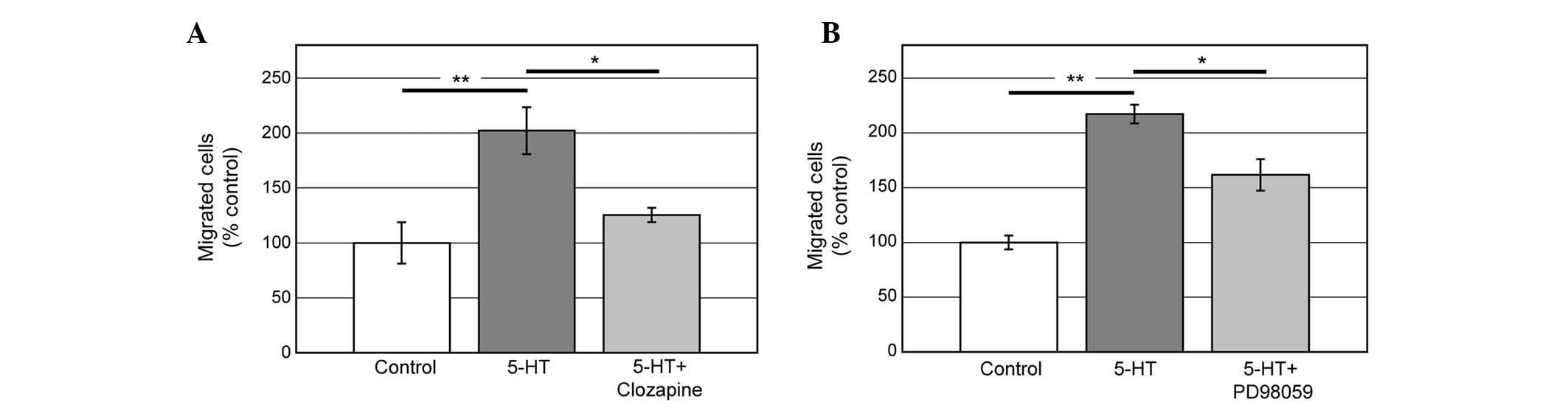
5-HT6 receptor is known to be coupled
with Gs protein which mediates the accumulation of cAMP (18). To examine whether cAMP pathway is
involved in 5-HT6 signaling in order to induce
migration, Clozapine, which was reported to inhibit cAMP
accumulation mediated by 5-HT6, was used (19). Clozapine (1 μM) significantly
reduced the effect of 5-HT on migration (Fig. 4A). Increasing of cAMP activates ERK
through protein kinase A-Rap1 (20). Thus, we examined whether the
inhibition of ERK activation affects 5-HT-induced PC12 cell
migration. Application of ERK inhibitor, PD98059 (20 μM),
significantly suppressed the cell migration induced by 5-HT
(Fig. 4B). These data indicate that
cAMP and ERK are involved in the 5-HT6-mediated
signaling pathways in order to induce PC12 cell migration.
Discussion
5-HT induced PC12 cell migration in a
dose-dependent manner
In the present study, we have demonstrated that 5-HT
induced PC12 cell migration in a transwell migration assay in a
dose-dependent manner (Fig. 1A). A
number of studies have indicated that 5-HT affects proliferation of
neural cells (7,21–23) as
well as neurite outgrowth (17,24–26),
however, its effect on neural migration remains to be clarified.
Findings of previous studies suggest 5-HT affects cortical neuron
migration during prenatal development (7–9,13).
Depletion of 5-HT by injection of DL-P-chlorophenylalanine (PCPA,
an inhibitor of 5-HT synthesis) during the E12-E17 stage arrested
migration and disorganized the positioning of cortical neurons
(7), suggesting a positive effect
of 5-HT on migration. By contrast, cortical slices exposed to high
doses of 5-HT (100–400 μM) arrested the migration of GABergic
neurons (8,9). Although findings of those studies
reported negative effects of 5-HT, the concentration of 5-HT was
considerably higher than that of another study focusing on the
prenatal cortex (100–200 fmol/mg) (7). The effect of 5-HT on neural migration
therefore remains to be elucidated. A positive effect of 5-HT at
the concentration of 1 μM was observed, which is similar to the
in vivo results obtained in that study.
5-HT induced migration via
5-HT6 receptor-cAMP pathway independently from
NGF-ERK
Blocking of 5-HT stimulation by 5-HT6
antagonists, SB271046 and SB258585 (Fig. 3B) indicate that 5-HT requires
5-HT6 receptor but not 5-HT3 to induce PC12
cell migration (Fig. 3A).
5-HT6 coupled with Gs-protein is known to elevate the
cAMP level that stimulates ERK via protein kinase A (19,26).
Inhibition of cAMP accumulation by Clozapine (19) and ERK by PD98059 (27) reduced the 5-HT effect on the
migration (Fig. 4).
Previous studies suggest that NGF showed a positive
effect on PC12 cell migration by activating ERK signaling (15,16).
EGF and cAMP pathways activate ERK independently in order to
promote PC12 cell differentiation (5,28,29).
Similarly, it is possible that the 5-HT6-cAMP activates
an independent pathway from the NGF-ERK signal to yield an additive
effect on PC12 cell migration (Fig.
1A).
Insensitivity of 5-HT3 in
undifferentiated PC12 cells
Antagonists of 5-HT3 did not show any
effects on PC12 cell migration in our experiment (Fig. 3A) although 5-HT was expressed
(Fig. 2A), suggesting independence
of 5-HT3 signaling pathway from cAMP or ERK. Another
possibility is that 5-HT3 is not sensitive to a 5-HT
induction in the experiments of this study. Homma et
al(17) suggest a difference of
5-HT sensitivity between differentiated (pretreated with NGF for 3
days) and undifferentiated PC12 cells to enhance neurite outgrowth
mediated by 5-HT3. In their experiment, 50 μM 5-HT were
required to enhance neurite outgrowth of undifferentiated PC12
cells whereas 5 μM of 5-HT were sufficient for differentiated
cells. In our experiment, undifferentiated PC12 cells were treated
with 1 μM 5-HT together with 5-HT3 antagonists (Fig. 3A). Higher concentrations of 5-HT may
be required to activate the 5-HT3-dependent pathway in
order to modulate PC12 cell migration.
5-HT3 and 5-HT6 are proposed
as candidates to modulate neural migration (30). Although we observed an effect of
5-HT6 but not 5-HT3 on PC12 migration, it is
possible 5-HT3 also modulated the PC12 cell migration in
different conditions, such as in the presence of NGF. Transwell
migration assay with PC12 cells is useful in the study of molecular
mechanisms of the neural migration induced by 5-HT or by NGF in
vitro.
Acknowledgements
This study was supported by JSPS KAKENHI Grant no.
23500427. We thank Kimie Iwasaki for her help.
References
|
1
|
Valiente M and Marín O: Neuronal migration
mechanisms in development and disease. Curr Opin Neurobiol.
20:68–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brennand KJ, Simone A, Tran N and Gage FH:
Modeling psychiatric disorders at the cellular and network levels.
Mol Psychiatry. 17:1239–1253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oliveira SL, Pillat MM, Cheffer A, et al:
Functions of neurotrophins and growth factors in neurogenesis and
brain repair. Cytometry A. 83:76–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang DD and Kriegstein AR: Defining the
role of GABA in cortical development. J Physiol. 587:1873–1879.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hernandez A, Kimball B, Romanchuk G and
Mulholland MW: Pituitary adenylate cyclase-activating peptide
stimulates neurite growth in PC12 cells. Peptides. 16:927–932.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bonnin A, Goeden N, Chen K, et al: A
transient placental source of serotonin for the fetal forebrain.
Nature. 472:347–350. 2011.PubMed/NCBI
|
|
7
|
Vitalis T, Cases O, Passemard S, et al:
Embryonic depletion of serotonin affects cortical development. Eur
J Neurosci. 26:331–344. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Riccio O, Potter G, Walzer C, et al:
Excess of serotonin affects embryonic interneuron migration through
activation of the serotonin receptor 6. Mol Psychiatry. 14:280–290.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Riccio O, Jacobshagen M, Golding B, et al:
Excess of serotonin affects neocortical pyramidal neuron migration.
Transl Psychiatry. 1:e472011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Millan MJ, Marin P, Bockaert J, et al:
Signaling at G-protein-coupled serotonin receptors: recent advances
and future research directions. Trends Pharmacol Sci. 29:454–464.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hannon J and Hoyer D: Molecular biology of
5-HT receptors. Behav Brain Res. 195:198–213. 2008. View Article : Google Scholar
|
|
12
|
Lee S, Hjerling-Leffler J, Zagha E, et al:
The largest group of superficial neocortical GABAergic interneurons
expresses ionotropic serotonin receptors. J Neurosci.
30:16796–16808. 2010. View Article : Google Scholar
|
|
13
|
Vitalis T, Ansorge MS and Dayer AG:
Serotonin homeostasis and serotonin receptors as actors of cortical
construction: special attention to the 5-HT3Aand
5-HT6receptor subtypes. Front Cell Neurosci. 7:932013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hassoun AT, Erdélyi F, Szabó G and Davis
MI: A rapid screening method for population-specific neuronal
motogens, substrates and associated signaling pathways. J Neurosci
Methods. 166:178–194. 2007. View Article : Google Scholar
|
|
15
|
Ho W, Uniyal S, Meakin SO, et al: A
differential role of extracellular signal-regulated kinase in
stimulated PC12 pheochromocytoma cell movement. Exp Cell Res.
263:254–264. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshizawa M, Kawauchi T, Sone M, et al:
Involvement of a Rac activator, P-Rex1, in neurotrophin-derived
signaling and neuronal migration. J Neurosci. 25:4406–4419. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Homma K, Kitamura Y, Ogawa H and Oka K:
Serotonin induces the increase in intracellular Ca2+that
enhances neurite outgrowth in PC12 cells via activation of
5-HT3receptors and voltage-gated calcium channels. J
Neurosci Res. 84:316–325. 2006.PubMed/NCBI
|
|
18
|
Choi YH, Kang H, Lee WK, et al: An
inhibitory compound against the interaction between
Gαsand the third intracellular loop region of serotonin
receptor subtype 6 (5-HT6) disrupts the signaling
pathway of 5-HT6. Exp Mol Med. 39:335–342.
2007.PubMed/NCBI
|
|
19
|
Kohen R, Metcalf MA, Khan N, et al:
Cloning, characterization, and chromosomal localization of a human
5-HT6serotonin receptor. J Neurochem. 66:47–56. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vossler MR, Yao H, York RD, et al: cAMP
activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent
pathway. Cell. 89:73–82. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Côté F, Fligny C, Bayard E, et al:
Maternal serotonin is crucial for murine embryonic development.
Proc Natl Acad Sci USA. 104:329–334. 2007.PubMed/NCBI
|
|
22
|
Narboux-Nême N, Angenard G, Mosienko V, et
al: Postnatal growth defects in mice with constitutive depletion of
central serotonin. ACS Chem Neurosci. 4:171–181. 2013.PubMed/NCBI
|
|
23
|
Cheng A, Scott AL, Ladenheim B, et al:
Monoamine oxidases regulate telencephalic neural progenitors in
late embryonic and early postnatal development. J Neurosci.
30:10752–10762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lieske V, Bennett-Clarke CA and Rhoades
RW: Effects of serotonin on neurite outgrowth from thalamic neurons
in vitro. Neuroscience. 90:967–974. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lotto B, Upton L, Price DJ and Gaspar P:
Serotonin receptor activation enhances neurite outgrowth of
thalamic neurones in rodents. Neurosci Lett. 269:87–90. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zachor DA, Moore JF, Brezausek C, et al:
Cocaine inhibits NGF-induced PC12 cells differentiation through
D1-type dopamine receptors. Brain Res. 869:85–97. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Whelchel A, Evans J and Posada J:
Inhibition of ERK activation attenuates endothelin-stimulated
airway smooth muscle cell proliferation. Am J Respir Cell Mol Biol.
16:589–596. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vaudry D, Stork PJ, Lazarovici P and Eiden
LE: Signaling pathways for PC12 cell differentiation: making the
right connections. Science. 296:1648–1649. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lazarovici P, Jiang H and Fink D Jr: The
38-amino-acid form of pituitary adenylate cyclase-activating
polypeptide induces neurite outgrowth in PC12 cells that is
dependent on protein kinase C and extracellular signal-regulated
kinase but not on protein kinase A, nerve growth factor receptor
tyrosine kinase, p21rasG protein, and
pp60c-srccytoplasmic tyrosine kinase. Mol Pharmacol.
54:547–558. 1998.
|
|
30
|
Engel M, Smidt MP and van Hooft JA: The
serotonin 5-HT3receptor: a novel neurodevelopmental
target. Front Cell Neurosci. 7:762013.
|