Introduction
The high incidence of cancer leads to high mortality
rates, with one in every four individuals being potential cancer
patients (1). In general, most
cancers are the result of various hereditary and environmental
factors (2). Apoptosis, also known
as programmed cell death, is a necessary genetically controlled
process that is important in sustaining the balance of the
development and maintenance of tissue homeostasis in multi-cellular
organisms. Therefore, inappropriate regulation of apoptosis results
in various human disorders, including cancer (3–6). The
two main apoptotic pathways in humans, the extrinsic or death
receptor-mediated pathway and the intrinsic or mitochondrial
pathway, both utilize the caspase enzyme cascade. Caspases
(cysteine and aspartic proteases, CAPS) are a cascade of enzymes
that regulate apoptosis and are divided into initiator and executor
caspases (7,8). Caspase-2 belongs to initiator caspases
and is primarily involved in the processing and activation of
pro-inflammatory cytokines, and is involved in DNA damage-induced
apoptosis. Caspase-1 and -5 have a similar structure and are
predominantly involved in the maturation of pro-inflammatory
cytokines (1,8,9).
Findings of previous studies demonstrated that caspase genes such
as CASP-3, -7 and -8, are associated with susceptibility to various
types of human cancer (10–12). Investigations into the association
between CASP-1, -2 and -5 and cancer risk are ongoing. Mittal et
al (14) and Dong et al
(13) have found that C allele
carriers of rs507879 in CASP-5 were at higher risk of cancer.
However, a contradictory result was found in another study where no
significant association was identified between CASP-5 and cancer
risk (15). Considering these
inconsistent and inconclusive results, a Human Genome Epidemiology
(HuGE) review and meta-analysis were conducted by including the
most recent and relevant articles in order to identify statistical
evidences to investigate the precise association between CASP-1, -2
and -5 and cancer risk.
Materials and methods
Literary search
Relevant papers published prior to October 1st, 2012
were identified through a search of Pubmed, Embase, Web of Science
and CBM databases using the following terms: (‘Genetic
polymorphism’ or ‘polymorphism’ or ‘SNP’ or ‘gene mutation’ or
‘genetic variants’) and (‘neoplasms’ or ‘neoplasms’ or ‘cancer’ or
‘cancers’ or ‘carcinogenesis’ or ‘carcinoma’) and (‘caspase-1’ or
‘CASP-1’ or ‘caspase-2’ or ‘CASP-2’ or ‘caspase-5’ or ‘CASP-5’).
The references from the eligible studies or textbooks were also
reviewed manually to search for potentially eligible studies.
Inclusion and exclusion criteria
Inclusion criteria for the meta-analysis were: i)
case-control or cohort study focused on the associations between
CASP-1, -2 and -5 gene polymorphisms and cancer risk; ii) patients
diagnosed with malignant tumors were required to be confirmed by
pathological examinations; iii) published data concerning the
frequency of alleles and genotypes was required to be sufficient;
iv) studies were required to have been published in English or
Chinese. Studies were excluded if they were: i) not a case-control
or cohort study; ii) based on incomplete data; iii) duplicates of
previous publications or iv) meta-analyses, letters, reviews or
editorial articles.
Data extraction
Using a standardized form, data from published
studies were extracted independently by two authors to populate the
necessary information. For each study, the following
characteristics were collected: the first author, year of
publication, country, language, ethnicity, study design, number of
subjects, source of cases and controls, pathological type,
detecting sample, genotype method, allele and genotype frequencies
and evidence of Hardy-Weinberg equilibrium (HWE) in controls. In
case of conflicting evaluations, agreement was reached following
discussion between the authors.
Quality assessment of included
studies
Two authors independently assessed the quality of
the studies according to modified STROBE quality score systems
(16,17). Forty assessment items associated
with quality appraisal were used in this meta-analysis, with scores
ranging from 0 to 40. Scores of 0–20, 20–30 and 30–40 were defined
as low, moderate and high quality, respectively. Disagreements were
resolved through discussions between the authors.
Statistical analysis
The strength of the association between CASP-1, -2
and -5 gene polymorphisms and cancer susceptibility was measured by
odds ratios (ORs) and 95% confidence intervals (CIs). The
statistical significance of the pooled OR was examined using the Z
test. Between-study variations and heterogeneities were estimated
using Cochran’s Q-statistic test with P<0.05 indicating a
statistically significant heterogeneity (18,19).
The effect of heterogeneity was quantified by using the
I2 test (rang, 0–100%), which represents the proportion
of inter-study variability that can be contributed to heterogeneity
instead of chance. When a significant Q-test (P<0.05) or
I2>50% indicated that heterogeneity among studies
existed, the random-effects model (DerSimonian and Laird method)
was conducted for meta-analysis. Otherwise, the fixed-effects model
(Mantel-Haenszel method) was used.
We also tested whether genotype frequencies of
controls were in HWE using the χ2 test. Begg’s funnel
plots were used to detect publication biases. In addition, Egger’s
linear regression test, which measures funnel plot asymmetry using
a natural logarithm scale of OR, was also used to evaluate the
publication biases (20). To ensure
the reliability and accuracy of the results, two reviewers assessed
the data in the statistical software programs independently and
obtained identical results. P-values were two-sided. Analyses were
calculated using the Stata Version 12.0 software (Stata Corp.,
College Station, TX, USA).
Results
Characteristics of included studies
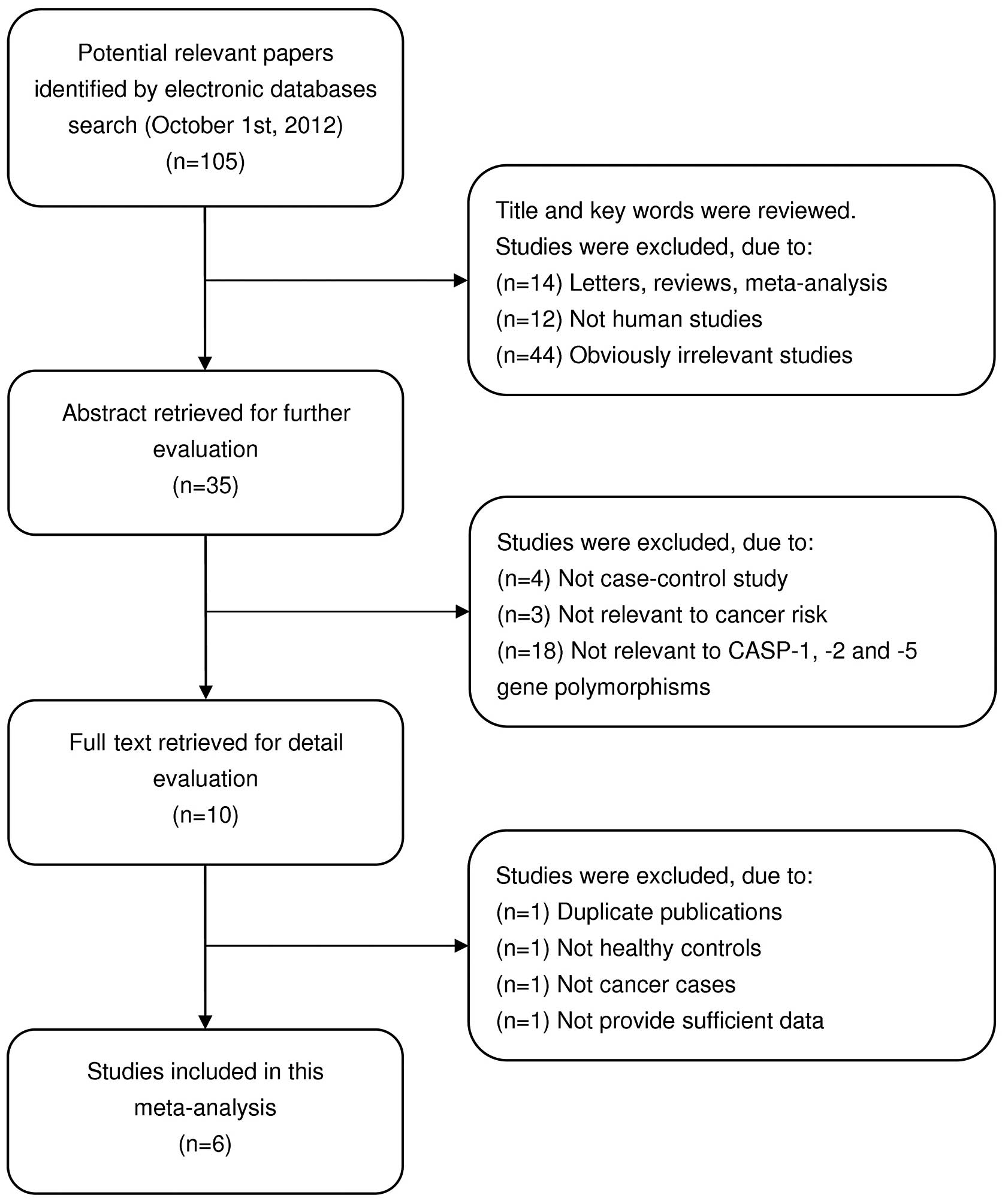
Four studies (14,15,21,22)
were included and 101 articles were excluded in the present
meta-analysis. The flow chart of study selection is shown in
Fig. 1. The publication year of
involved studies ranged from 2009 to 2012. In total, 1,592 cancer
cases and 1,833 health controls were included in this
meta-analysis. The patients diagnosed with cancer were also
confirmed by pathological examinations. Cancer types included lung,
bladder and prostate cancers. Five polymorphisms were evaluated,
including rs501192 (G>A) in the CASP-1 gene, rs4647297 (C>G)
in the CASP-2 gene, as well as rs507879 (T>C), rs3181320
(G>C) and rs523104 (G>C) in the CASP-5 gene. HWE test was
conducted on the genotype distribution of the controls in the
studies. Only the study by Ulybina et al (15) showed significant deviation from HWE
(P<0.05). The quality scores of included studies were >20
(moderate-high quality). The characteristics and methodological
quality of the included studies are shown in Table I. The genotype distributions of
CASP-1, -2 and -5 polymorphisms are presented in Table II.
 | Table I.Characteristics of included studies in
this meta-analysis. |
Table I.
Characteristics of included studies in
this meta-analysis.
| First author
(refs.) | Year | Country | No. of cases | No. of controls | Sample | Detection | Disease | Gene (CASP) | SNP | Quality scores |
|---|
| Ulybina et al
(15) | 2009 | Russia | 111 | 110 | Blood | AS-PCR | Lung cancer | 2 | rs4647297
(C>G) | 24 |
| | | 111 | 110 | | | | 5 | rs507879
(T>C) | |
| | | 647 | 833 | | | | 5 | rs523104
(G>C) | |
| Hart et al
(21) | 2011 | Norway | 442 | 440 | Blood/tissue | TaqMan | NSCLC | 1 | rs501192
(G>A) | 20 |
| Mittal et al
(14) | 2011 | India | 200 | 225 | Blood | AS-PCR/PCR-RFLP | Bladder cancer | 5 | rs3181320
(G>C) | 21 |
| | | | | | | | 5 | rs507879
(T>C) | |
| Mittal et al
(22) | 2012 | India | 192 | 225 | Blood | AS-PCR | Prostate cancer | 5 | rs3181320
(G>C) | 21 |
| | | | | | | | 5 | rs507879
(T>C) | |
 | Table II.Genotype distribution of CASP-1, -2
and -5 gene polymorphisms in case and control groups. |
Table II.
Genotype distribution of CASP-1, -2
and -5 gene polymorphisms in case and control groups.
| First author
(ref.) | SNP | Case
| Control
| HWE test
|
|---|
| Total | 1 | 2 | 1/1 | 1/2 | 2/2 | Total | 1 | 2 | 1/1 | 1/2 | 2/2 | χ2 | P-value |
|---|
| Ulybina et al
(15) | rs4647297
(C>G) | 111 | 213 | 9 | 102 | 9 | 0 | 110 | 211 | 9 | 101 | 9 | 0 | 0.20 | 0.655 |
| rs507879
(A>G) | 111 | 119 | 103 | 29 | 61 | 21 | 110 | 112 | 108 | 34 | 44 | 32 | 4.39 | 0.036 |
| rs523104
(G>C) | 647 | 772 | 522 | 240 | 292 | 115 | 833 | 1037 | 629 | 328 | 381 | 124 | 0.60 | 0.438 |
| Hart et al
(21) | rs501192
(G>A) | 436 | 706 | 166 | 286 | 134 | 16 | 435 | 705 | 165 | 282 | 141 | 12 | 1.29 | 0.255 |
| Mittal et al
(14) | rs3181320
(G>C) | 200 | 234 | 166 | 75 | 84 | 41 | 225 | 285 | 165 | 96 | 93 | 36 | 2.73 | 0.099 |
| rs507879
(T>C) | 200 | 217 | 183 | 65 | 87 | 48 | 225 | 263 | 187 | 83 | 97 | 45 | 2.85 | 0.092 |
| Mittal et al
(22) | rs3181320
(G>C) | 192 | 219 | 165 | 66 | 87 | 39 | 225 | 285 | 165 | 96 | 93 | 36 | 2.73 | 0.099 |
| rs507879
(T>C) | 192 | 202 | 182 | 62 | 78 | 52 | 225 | 263 | 187 | 83 | 97 | 45 | 2.85 | 0.092 |
Quantitative data synthesis
A summary of the meta-analysis findings of the
associations between CASP-1, -2 and -5 gene polymorphisms and
cancer risk is provided in Table
III. Heterogeneity was not obvious in the polymorphisms (all
P>0.05), thus the fixed-effects model was used. When all the
eligible studies were pooled into the meta-analysis, the results
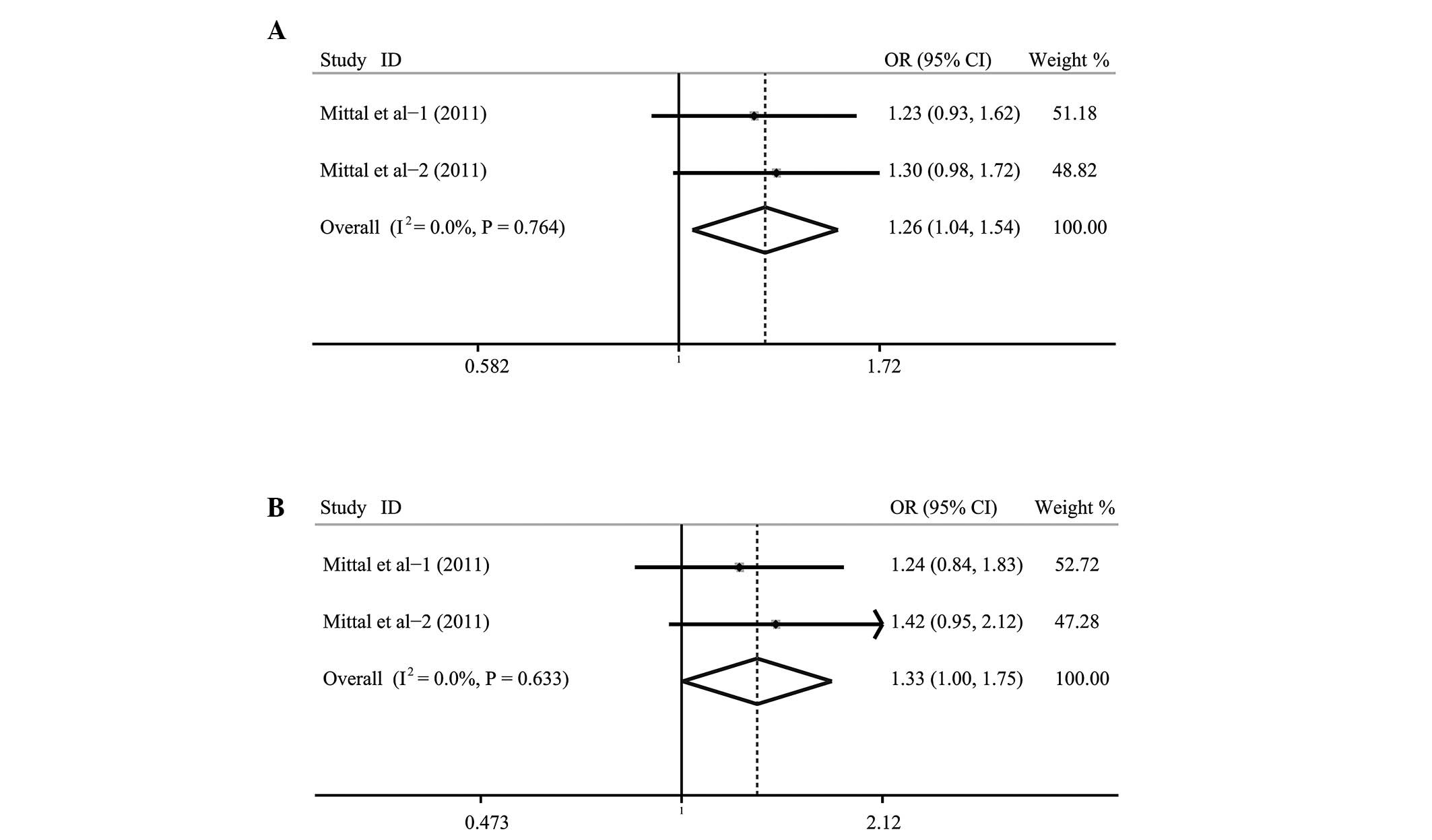
revealed that the rs3181320*C allele/carrier in the CASP-5 gene
were associated with increased risk of various cancers (OR=1.26;
95% CI, 1.04–1.54; P=0.020 and OR=1.33; 95% CI, 1.00–1.75; P=0.047,
respectively) (Fig. 2). Similar
associations were not found in the rs501192, rs4647297, rs507879
and rs523104 polymorphisms (all P>0.05).
 | Table III.Meta-analysis of the association
between polymorphisms of CASP-1, -2 and -5 genes and cancer
susceptibility. |
Table III.
Meta-analysis of the association
between polymorphisms of CASP-1, -2 and -5 genes and cancer
susceptibility.
| Gene name | Gene/SNP | Case (n/N) | Control (n/N) | OR (95% CI) | P-value | Effect model |
|---|
| CASP-1 | | | | | | |
| rs501192
(G>A) | A allele | 166/872 | 165/870 | 1.00
(0.79–1.28) | 0.97 | Fixed |
| A carrier | 150/436 | 153/435 | 0.97
(0.73–1.28) | 0.81 | |
| CASP-2 | | | | | | |
| rs4647297
(C>G) | G allele | 9/222 | 9/220 | 0.99
(0.39–2.54) | 0.98 | Fixed |
| G carrier | 9/111 | 9/110 | 0.99
(0.38–2.60) | 0.98 | |
| CASP-5 | | | | | | |
| rs507879
(T>C) | C allele | 468/1006 | 482/1120 | 1.15
(0.97–1.36) | 0.12 | Fixed |
| C carrier | 347/503 | 360/560 | 1.23
(0.95–1.59) | 0.12 | |
| rs3181320
(G>C) | C allele | 331/784 | 330/900 | 1.26
(1.04–1.54) | 0.02 | Fixed |
| C carrier | 251/392 | 258/450 | 1.33
(1.00–1.75) | 0.05 | |
| rs523104
(G>C) | C allele | 522/1294 | 629/1666 | 1.11
(0.96–1.29) | 0.15 | Fixed |
| C carrier | 407/647 | 505/833 | 1.10
(0.89–1.36) | 0.37 | |
Publication bias
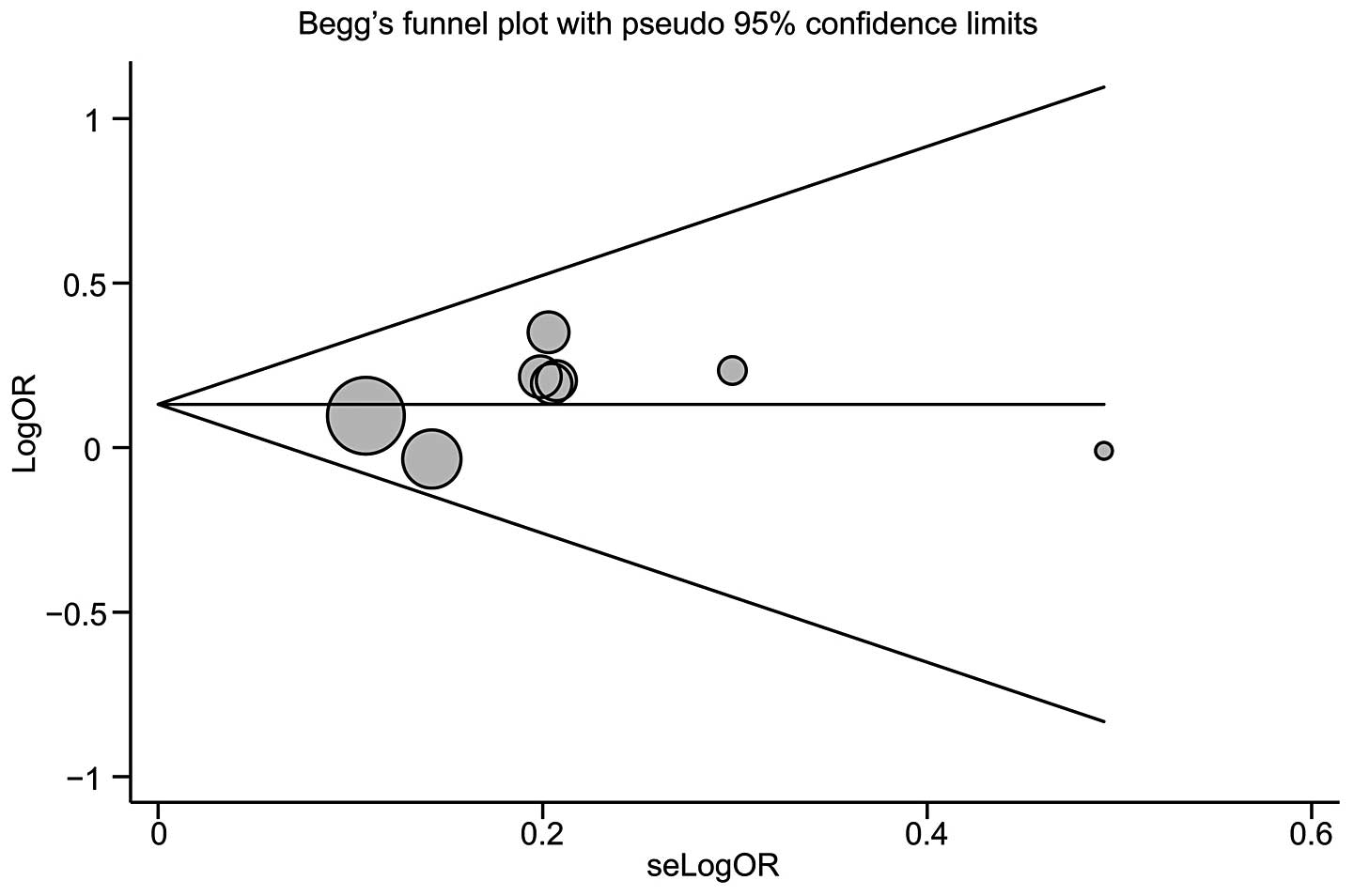
Publication biases within the available research
results may not be representative of the study results. Begg’s
funnel plot and Egger’s linear regression test were performed to
assess the publication biases of included studies. The shapes of
the funnel plots did not reveal any evidence of obvious asymmetry
of the CASP-1, -2 and -5 gene polymorphisms (Fig. 3). Egger’s test also showed that
there was no strong statistical evidence of publication bias
(t=1.03, P=0.343).
Discussion
Caspases, which play a key role in biochemical
cell-suicide pathways, have been confirmed by a series of studies
focusing on the association between caspase family members and
cancer risk. Inappropriate regulation of apoptosis contributes to a
number of human disorders (6).
Caspases are also of fundamental importance in mediating apoptosis
(11). Activation of caspases is
essential in cell death commitment. Therefore, understanding of the
mechanisms that underlie their activation is useful in clarifying
the role caspases play in the induction of human disease (23). This is the most comprehensive
meta-analysis examining CASP-1, -2 and -5 gene polymorphisms and
their association with susceptibility to cancer. The strength of
this study was based on the accumulation of published data,
providing additional information to detect significant
differences.
Caspase-1, the first identified caspase, was
previously known as interleukin-1β processing enzyme (1). It may mediate cytokine production
caused by tobacco smoke. The CASP-1 enzyme is essential for the
cleavage of pro-IL1 protein into its active and mature forms and is
involved in the formation and activation of inflammatory processes
(24). Soung et al (25) have detected the probable role of
inflammatory caspase in cancer. However, in the present
meta-analysis, we investigated a polymorphism in CASP-1 gene
rs501192 (G/A) and the results showed that neither the A allele nor
the A allele carrier of rs501192 exhibited any association with
cancer risk. Caspase 2, encoded by the CASP-2 gene, cleaves other
proteins belonging to the caspase family. Ulybina et al
(15) have demonstrated that
rs4647297 was not associated with cancer risk. Similarly, in the
present study, no correlation was found between the rs4647297*G
allele/carrier in the CASP-2 gene and risk for various cancers.
Caspase-5 is also an enzyme that prototypically cleaves other
proteins at an aspartic acid residue. Together with caspase 1,
caspase 4, is important in the immune system. The CASP-5 mutations
include nine mutations in exons, five mutations in introns and one
in the 5′-untranslated region (1).
To date, the biological function of the caspase-5 protein is poorly
understood and is believed to play a role in various aspects of
inflammation. In a recent study, a significant risk was observed in
the rs3181320*C carrier of the CASP-5 gene. In CASP-5 rs507879
T>C, C allele carriers were at a higher risk of breast cancer
(14). By contrast, Ulybina et
al (15) did not find any
significant association with CASP-5 (15). In the present meta-analysis, we
detected the polymorphisms of CASP-5, including rs507879 (T>C),
rs3181320 (G>C) and rs523104 (G>C) and a significant
association was identified with the C allele and C allele carrier
of rs3181320 (G>C). However, no association was detected in the
C allele, C allele carrier of rs507879 (T>C) and rs523104
(G>C).
Similar to other meta-analyses, a number of
limitations of this study should be addressed. Firstly, some
relevant studies could not be included in our analysis due to
incomplete raw data. Secondly, few relevant research articles were
available and the sample size of this meta-analysis was limited.
Thirdly, the source of heterogeneity among the studies could not be
addressed. Fourthly, our meta-analysis was based on unadjusted OR
estimates as adjusted OR was not available in all published studies
and when these studies were available, the OR was not adjusted
using the same potential confounders, such as ethnicity, gender and
geographic distribution. In addition, although the cases and
controls of each study were well defined with similar inclusion
criteria, potential factors that may have affected our results were
not considered. Thus, additional investigation is required and our
conclusions should be interpreted with caution.
In summary, this meta-analysis suggests that the
rs3181320*C allele/carrier in CASP-5 gene might be risk factors for
cancer. However, few studies are currently available concerning
this issue. Therefore, large-scale studies with an adequate
methodological quality, and with appropriate controlling
confounding factors should be conducted to obtain valid
results.
Acknowledgements
We would like to acknowledge the
helpful comments on this paper received from reviewers. We thank
all our colleagues working in the Department of General Surgery,
the Shenjing Hospital of China Medical University.
References
|
1.
|
Ghavami S, Hashemi M, Ande SR, et al:
Apoptosis and cancer: mutations within caspase genes. J Med Genet.
46:497–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Dang TP: Notch, apoptosis and cancer. Adv
Exp Med Biol. 727:199–209. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Raff M: Cell suicide for beginners.
Nature. 396:119–122. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Shivapurkar N, Reddy J, Chaudhary PM and
Gazdar AF: Apoptosis and lung cancer: a review. J Cell Biochem.
88:885–898. 2003. View Article : Google Scholar
|
|
5.
|
Hajra KM and Liu JR: Apoptosome
dysfunction in human cancer. Apoptosis. 9:691–704. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Son JW, Kang HK, Chae MH, et al:
Polymorphisms in the caspase-8 gene and the risk of lung cancer.
Cancer Genet Cytogenet. 169:121–127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Degterev A, Boyce M and Yuan J: A decade
of caspases. Oncogene. 22:8543–8567. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kumar S and Dorstyn L: Analysing caspase
activation and caspase activity in apoptotic cells. Methods Mol
Biol. 559:3–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Siegel RM: Caspases at the crossroads of
immune-cell life and death. Nat Rev Immunol. 6:308–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
MacPherson G, Healey CS, Teare MD, et al:
Association of a common variant of the CASP8 gene with reduced risk
of breast cancer. J Natl Cancer Inst. 96:1866–1869. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Zhang X, Miao X, Sun T, et al: Functional
polymorphisms in cell death pathway genes FAS and FASL contribute
to risk of lung cancer. J Med Genet. 42:479–484. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Yang M, Sun T, Wang L, et al: Functional
variants in cell death pathway genes and risk of pancreatic cancer.
Clin Cancer Res. 14:3230–3236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Dong LM, Brennan P, Karami S, et al: An
analysis of growth, differentiation and apoptosis genes with risk
of renal cancer. PloS One. 4:e48952009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Mittal RD, Srivastava P, Mittal T, et al:
Association of death receptor 4, caspase 3 and 5 gene polymorphism
with increased risk to bladder cancer in North Indians. Eur J Surg
Oncol. 37:727–733. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Ulybina YM, Kuligina ESh and Mitiushkina
NV: et al Coding polymorphisms in Casp5, Casp8 and DR4 genes may
play a role in predisposition to lung cancer. Cancer Lett.
278:183–191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
von Elm E, Altman DG, Egger M, et al: The
Strengthening the Reporting of Observational Studies in
Epidemiology (STROBE) statement: guidelines for reporting
observational studies. Epidemiology. 18:800–804. 2007.
|
|
17.
|
Zhang L, Liu JL, Zhang YJ and Wang H:
Association between HLA-B*27 polymorphisms and ankylosing
spondylitis in Han populations: a meta-analysis. Clin Exp
Rheumatol. 29:285–292. 2011.
|
|
18.
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Zintzaras E and Ioannidis JP:
Heterogeneity testing in meta-analysis of genome searches. Genet
Epidemiol. 28:123–137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Peters JL, Sutton AJ, Jones DR, et al:
Comparison of two methods to detect publication bias in
meta-analysis. JAMA. 295:676–680. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Hart K, Landvik NE, Lind H, et al: A
combination of functional polymorphisms in the CASP8, MMP1, IL10
and SEPS1 genes affects risk of non-small cell lung cancer. Lung
Cancer. 71:123–129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Mittal RD, Mittal T, Singh AK and Mandal
RK: Association of caspases with an increased prostate cancer risk
in north Indian population. DNA Cell Biol. 31:67–73. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kumar S: Measurement of caspase activity
in cells undergoing apoptosis. Methods Mol Biol. 282:19–30.
2004.PubMed/NCBI
|
|
24.
|
Belogubova EV, Ulibina YM, Suvorova IK, et
al: Combined CYP1A1/GSTM1 at-risk genotypes are overrepresented in
squamous cell lung carcinoma patients but underrepresented in
elderly tumor-free subjects. J Cancer Res Clin Oncol. 132:327–331.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Soung YH, Jeong EG, Ahn CH, et al:
Mutational analysis of caspase 1, 4, and 5 genes in common human
cancers. Hum Pathol. 39:895–900. 2008. View Article : Google Scholar : PubMed/NCBI
|