Introduction
Periodontitis is an inflammatory disease of the
supporting tissues of the teeth (1)
and is considered to be a risk factor for several systemic
conditions (2), such as
cardiovascular disease (3,4), diabetes (5) and chronic obstructive pulmonary
disease (6). Periodontitis is
currently classified as two main forms: chronic periodontitis (CP)
and aggressive periodontitis (AgP). AgP is less prevalent compared
to CP, but is associated with more rapid attachment loss and bone
destruction (7). The
pathophysiology of this disease may be determined by a variety of
microbial, environmental, genetic and behavioural factors and
systemic diseases (8). However, for
patients with a reasonably good oral hygiene who develop this
condition (7), AgP is considered to
be a genetically inherited disease (9). Several family-based studies
demonstrated that AgP is an autosomal recessive disorder (10–13),
indicating a missense mutation of the cathepsin C gene in the 11q14
chromosome as being involved in the development of this disease
(14).
Various candidate genes have been investigated for
association with AgP and polymorphisms in the interleukin-1α
(IL-1α) gene were suggested to affect the transcription of IL-1α,
with −899 (+4845) C→T identified as one of the relevant
polymorphisms. Walker et al (15) first reported that the IL-1α −899
(+4845) C→T polymorphism was not associated with the risk of AgP in
African-American patients. Since then, a number of studies were
conducted to investigate the association between this polymorphism
and the risk of AgP. However, those studies had two limitations: i)
consistent results could not be obtained due to the selection
criteria of patients and controls and their ethnic origin; and ii)
the majority of those studies included a limited number of patients
(≤40). Therefore, the aim of this study was to investigate the
association between IL-1α −899 (+4845) C→T polymorphism and the
risk of AgP by performing a meta-analysis of previously conducted
studies.
Materials and methods
Eligibility criteria
The inclusion criteria were as follows: i) studies
that investigated the association between the IL-1α −899 (+4845)
C→T polymorphism and susceptibility to AgP; ii) the study design
was case-control and the diagnostic criteria of AgP were clearly
reported; iii) the AgP patients were free of any other systemic
disease and the control subjects were healthy or
periodontitis-free; iv) the studies reported the odds ratios (ORs)
and associated 95% confidence intervals (CIs) or the number of
individual genotypes in the case and control groups, or the data
required for their calculation; and v) the publication language was
English or Chinese.
This meta-analysis was conducted in concordance with
the Meta-analysis Of Observational Studies in Epidemiology (MOOSE)
guidelines (16).
Search strategy
The search terms ‘polymorphism’ or ‘mutation’ or
‘variant’, ‘interleukin-1’ or ‘IL-1’ and ‘periodontitis’ or
‘periodontal disease’ were used to conduct a search through PubMed
up to May 1, 2013. The reference lists of the included studies and
recent reviews were manually searched to identify additional
relevant studies.
Data extraction
According to the prespecified selection criteria,
two authors independently selected the studies and extracted the
following data: surname of first author, year of publication,
country of origin and ethnicity, source of controls, number and
genotyping distribution of cases and controls, OR and its 95% CI,
genotyping method and Hardy-Weinberg equilibrium (HWE) for
controls. Any disagreements were resolved by consulting a third
author.
Statistical analysis
We calculated the ORs and corresponding 95% CIs for
the rare and common alleles. Heterogeneity among included studies
was detected using I2 statistics (17). The value of I2≤40% was
considered to indicate no significant heterogeneity and the fixed
effects model was used; otherwise, the random effects model was
used. We also performed subgroup analyses based on ethnicity,
source of controls and the HWE for controls. A sensitivity analysis
was performed by excluding one study at a time. The publication
bias was assessed by funnel plot analysis and the Egger’s linear
regression test (18). All the
analyses were performed using Comprehensive Meta-Analysis software,
version 2 (Biostat, Inc., Englewood, NJ, USA) (19) and the P-values were two-sided.
Results
Study characteristics
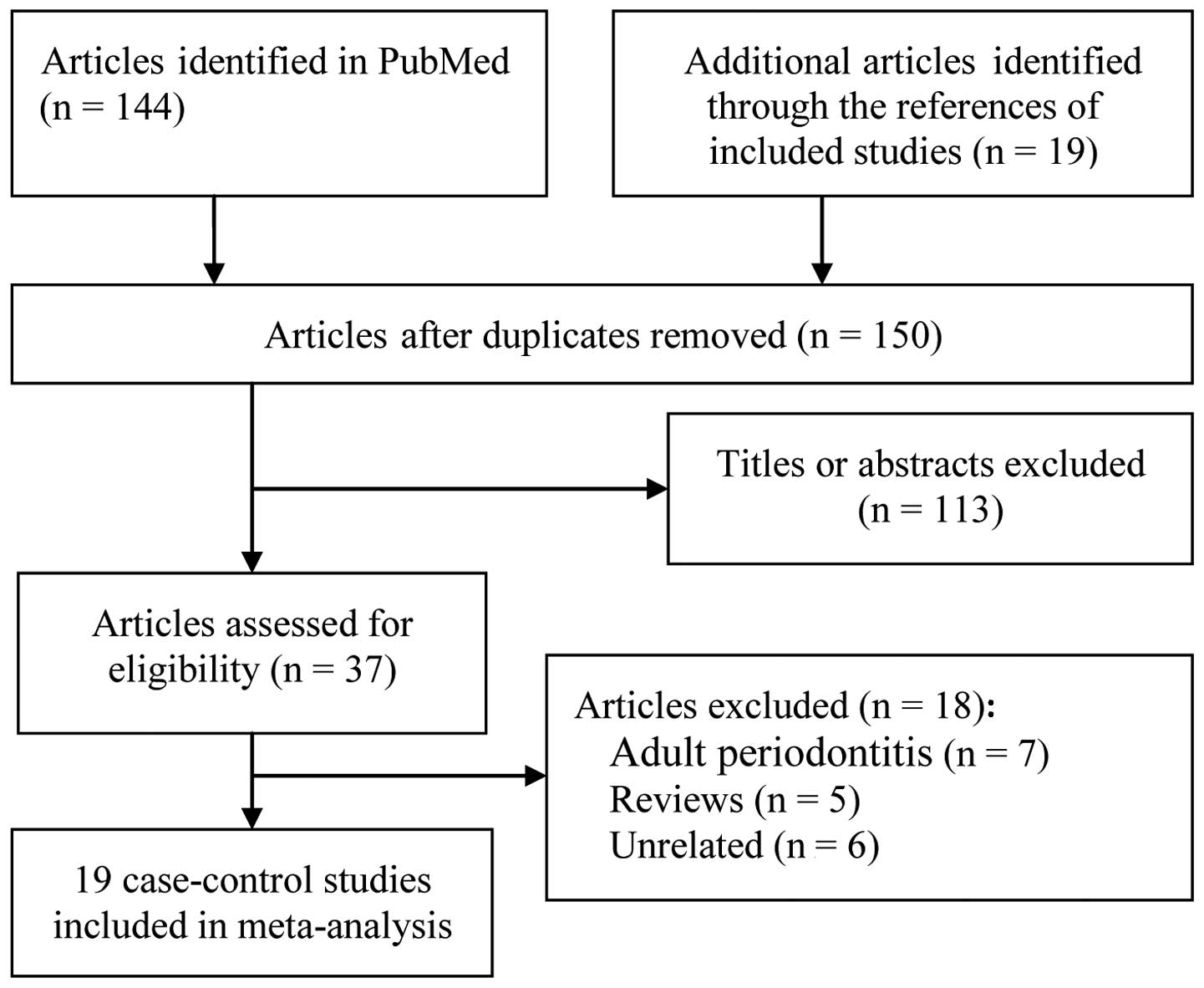
The primary search resulted in the identification of
163 publications. Finally, 19 case-control studies, including a
total of 1,266 AgP patients and 2,134 healthy controls, were deemed
as eligible for inclusion in this meta-analysis (15,20–37).
The study selection process is shown in Fig. 1.
Of the 19 studies, 14 investigated probands of
Caucasian (20,22,24,26–33,35–37), 4
of Asian (21,23,25,34),
and 1 of African-American origin (15). Only 1 study was not in HWE (27) and 1 study reported ORs and 95% CIs
for the rare and common alleles (32). The main characteristics of the
identified studies are summarized in Table I.
 | Table ICharacteristics of the studies
included in the meta-analysis. |
Table I
Characteristics of the studies
included in the meta-analysis.
| First author
(year) | Country
(ethnicity) | Cases | Source of
controls | Controls | HWE | Refs. |
|---|
|
|
|---|
| Total | G | T | Total | G | T |
|---|
| Walker (2000) | USA (Negroid) | 37 | 62 | 12 | Population | 104 | 178 | 30 | 0.89 | (15) |
| Hodge (2001) | UK (Caucasian) | 56 | 74 | 38 | Hospital | 56 | 73 | 39 | 0.902 | (20) |
| Duan (2002) | China (Asian) | 20 | 34 | 6 | Hospital | 94 | 170 | 18 | 0.305 | (21) |
| Rogers (2002) | Australia
(Caucasian) | 21 | 31 | 11 | Population | 60 | 90 | 30 | 0.61 | (22) |
| Anusaksathien
(2003) | Thailand
(Asian) | 26 | 48 | 4 | Hospital | 43 | 85 | 1 | 0.94 | (23) |
| Gonzales
(2003) | Germany
(Caucasian) | 43 | 66 | 20 | Population | 47 | 74 | 20 | 0.103 | (24) |
| Li (2004) | China (Asian) | 122 | 226 | 18 | Mixed | 95 | 179 | 11 | 0.55 | (25) |
| Quappe (2004) | Chile
(Caucasian) | 36 | 52 | 20 | Hospital | 75 | 118 | 32 | 0.33 | (26) |
| Brett (2005) | UK (Caucasian) | 50 | 73 | 27 | Population | 103 | 131 | 75 | 0.02 | (27) |
| Scapoli (2005) | Italy
(Caucasian) | 40 | 60 | 20 | Population | 96 | 126 | 60 | 0.88 | (28) |
| Sakellari
(2006) | Greece
(Caucasian) | 40 | 51 | 29 | Mixed | 100 | 141 | 59 | 0.53 | (29) |
| Maria de Freitas
(2007) | Brazil
(Caucasian) | 30 | 46 | 14 | Population | 70 | 102 | 38 | 0.61 | (31) |
| Havemose-Poulsen
(2007) | Denmark
(Caucasian) | 45 | 63 | 27 | Hospital | 25 | 38 | 12 | 0.63 | (30) |
| Ren (2008) | China (Asian) | 57 | 106 | 8 | Population | 57 | 99 | 15 | 0.24 | (34) |
| Guzeldemir
(2008) | Turkey
(Caucasian) | 31 | 38 | 24 | Population | 31 | 51 | 11 | 0.23 | (33) |
| Karasneh
(2011) | Jordan
(Caucasian) | 80 | 101 | 59 | Population | 80 | 106 | 54 | 0.15 | (35) |
| Schulz (2011) | Germany
(Caucasian) | 85 | 125 | 45 | Population | 89 | 126 | 52 | 0.76 | (36) |
| Shibani (2011) | Syria
(Caucasian) | 32 | 40 | 24 | Population | 35 | 52 | 18 | 0.54 | (37) |
| Fiebig (2008) | Germany/Netherlands
(Caucasian) | 415 | 0.84a (0.66–1.06)a | Population | 874 | 1.44b (0.93–2.21)b | 0.52 | (32) |
Meta-analysis
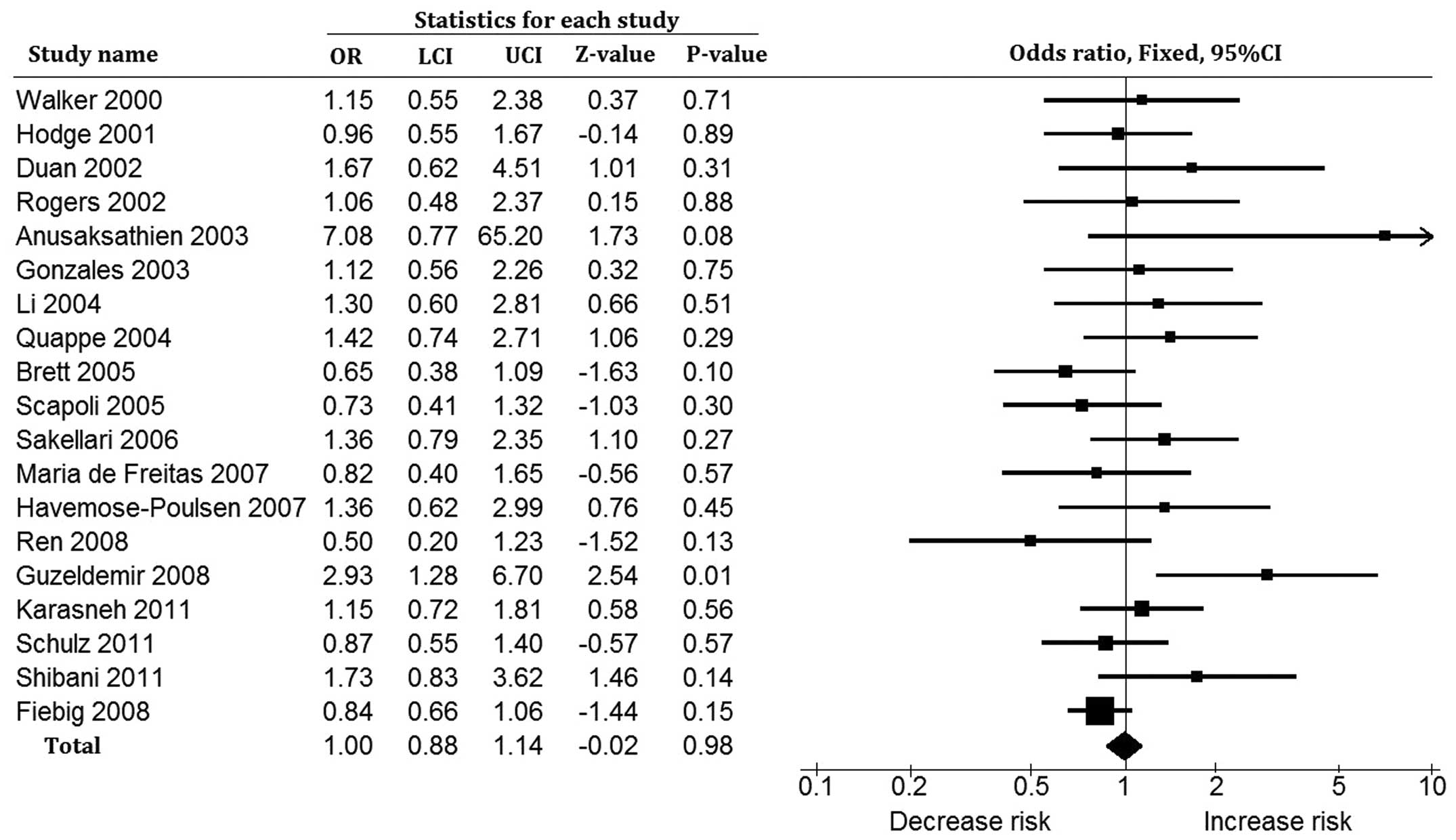
Overall, the rare and common alleles provided no
evidence supporting an association between the IL-1α −899 (+4845)
C→T polymorphism and susceptibility to AgP (OR=1.00, 95% CI:
0.88–1.14, P=0.98 for the T vs. C allele and OR=0.99, 95% CI:
0.85–1.14, P=0.85 for the C vs. T allele; Fig. 2). The subgroup analyses for
ethnicity, source of controls and HWE, also revealed no such
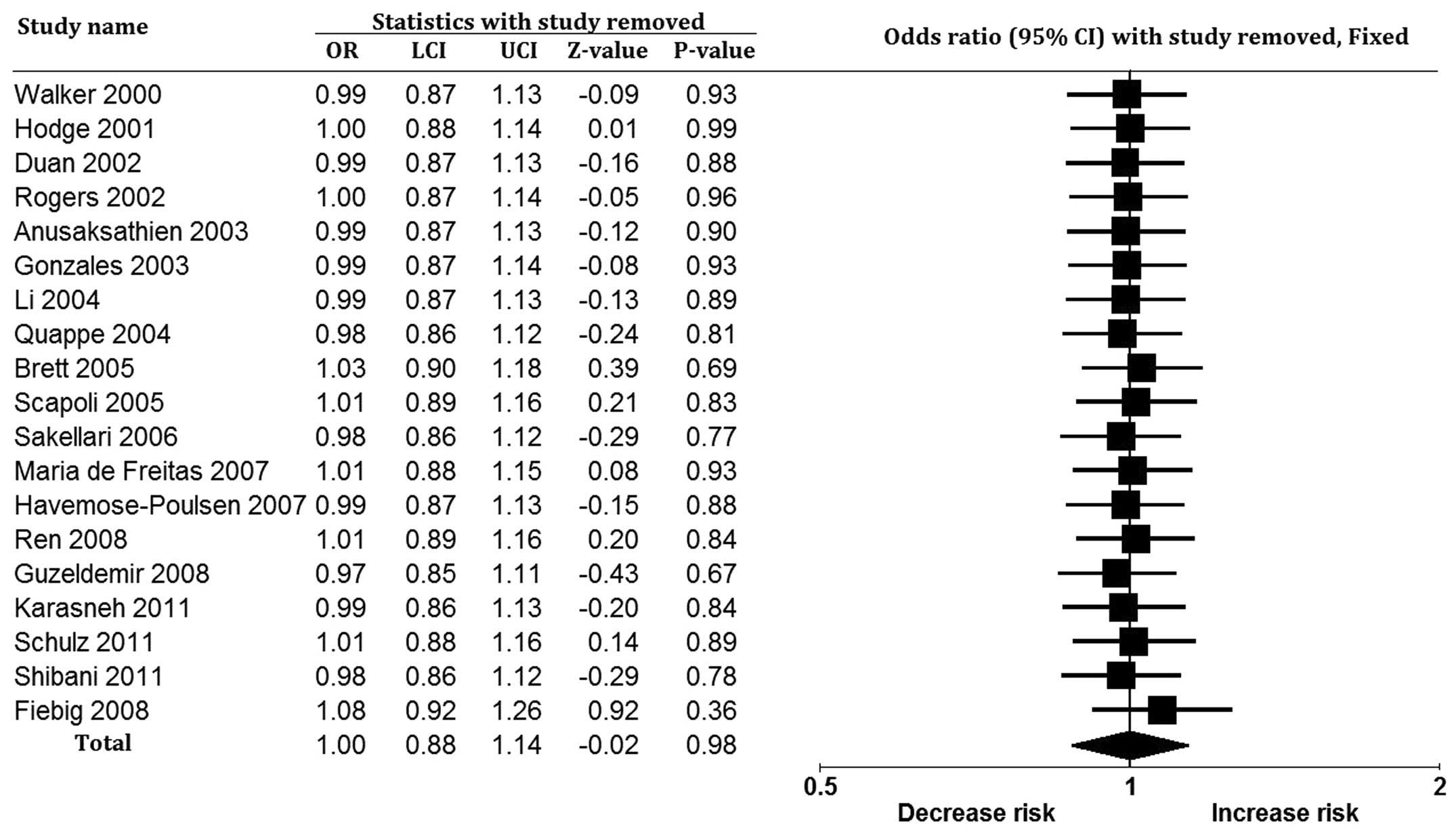
association. The sensitivity analysis, performed by excluding
studies one at a time, reached a similar conclusion (Fig. 3). The results of the meta-analysis
are presented in Table II.
 | Table IIResults of overall and subgroup
analyses of pooled ORs and 95% CIs. |
Table II
Results of overall and subgroup
analyses of pooled ORs and 95% CIs.
| Comparison | Category | Number of
studies | OR (95% CI) | P-value of OR | I2
(%) | Egger’s
P-value |
|---|
| T vs. C | Overall | 19 | 1.00
(0.88–1.14) | 0.98 | 28.86 | 0.02 |
| Caucasian | 14 | 0.98
(0.86–1.13) | 0.82 | 29.28 | |
| Asian | 4 | 1.24
(0.57–2.70) | 0.59 | 53.92 | |
|
African-American | 1 | 1.15
(0.55–2.38) | 0.71 | 0 | |
| PB | 12 | 0.92
(0.80–1.07) | 0.29 | 34.65 | |
| HB | 5 | 1.28
(0.91–1.81) | 0.16 | 0 | |
| Mixed | 2 | 1.34
(0.86–2.09) | 0.2 | 0 | |
| HWE (Yes) | 18 | 1.03
(0.90–1.18) | 0.69 | 24.39 | |
| HWE (No) | 1 | 0.65
(0.38–1.09) | 0.1 | 0 | |
| C vs. T | Overall | 19 | 0.99
(0.85–1.14) | 0.85 | 33.66 | 0.02 |
| Caucasian | 14 | 1.00
(0.86–1.17) | 0.98 | 35.95 | |
| Asian | 4 | 0.81
(0.37–1.77) | 0.59 | 53.92 | |
|
African-American | 1 | 0.87
(0.42–1.81) | 0.71 | 0 | |
| PB | 12 | 1.06
(0.84–1.34) | 0.62 | 41.6 | |
| HB | 5 | 0.78
(0.55–1.10) | 0.16 | 0 | |
| Mixed | 2 | 0.75
(0.48–1.17) | 0.2 | 0 | |
| HWE (Yes) | 18 | 0.95
(0.82–1.11) | 0.5 | 29.34 | |
| HWE (No) | 1 | 1.55
(0.92–2.62) | 0.1 | 0 | |
Publication bias
A weak publication bias was observed in this
meta-analysis (Fig. 4), as
evidenced by Egger’s test (P=0.02), also shown in detail in
Table II.
Discussion
The early onset form of periodontitis (juvenile,
post-juvenile, post-adolescent) is less prevalent, but is
associated with more rapid bone destruction at a relatively young
age and is therefore termed ‘AgP’ (32). Unlike CP, AgP is likely a
genetically inherited disease (9).
Numerous studies previously evaluated the association between IL-1α
−899 (+4845) C→T polymorphism and AgP, although the reported
results are inconsistent (Fig. 2).
In addition, the credibility of the results from a single
case-control study is questionable due to the relatively limited
sample size. Meta-analysis has being widely used in genetic
association studies due to its advantage of overcoming this
limitation (38–40). The present meta-analysis, based on
19 case-control studies, aimed to provide a comprehensive analysis
of the association between IL-1α −899 (+4845) C→T polymorphism and
susceptibility to AgP. Our results indicated that there is no such
association, either under T vs. C or C vs. T allele comparison.
These results are consistent with those of the largest-sample and
multicenter study, including a total of 415 AgP patients and 874
healthy controls (32), but not
with the results of the smallest-sample and single-center study,
which included 31 AgP patients and 31 healthy controls (33). This finding may provide evidence
that a meta-analysis has the advantage of overcoming the
limitations of a single case-control study by enlarging the sample
size.
To further investigate the association between IL-1α
−899 (+4845) C→T polymorphism and susceptibility to AgP, subgroup
analyses by ethnicity, source of controls and HWE were performed.
All the results were consistent with those of the overall analysis,
indicating no role for ethnic background or environment. Therefore,
AgP is likely a genetically inherited disease (9) and it was concluded from our results
that the C or T allele did not affect the risk of AgP.
There were some limitations to our meta-analysis.
First, the patient samples of the majority of the included studies
were limited (≤40). Although this may be attributed to the low
prevalence of AgP, a small sample size is associated with the
possibility of bias. Second, despite thorough attempts to collect
relevant studies, publication bias also existed. This may result
from the language limitation to English and Chinese, or from
negative results not being published. Third, due to the lack of
data on origin in the included studies, we were unable to assess
the gene-environment interactions, which may affect the
susceptibility to AgP. Finally, the negative association between
IL-1α −899 (+4845) C→T polymorphism and susceptibility to AgP
should be interpreted with caution.
In conclusion, our meta-analysis, based on a total
of 1,266 AgP patients and 2,134 healthy controls, demonstrated that
the IL-1α −899 (+4845) C→T polymorphism is not associated with the
risk of AgP. However, due to the abovementioned limitations of the
present study, our results may be considered as inconclusive.
Acknowledgements
This study was supported in part by a grant from the
Foundation of Education and Science Planning Project of Hubei
Province (no. 2012A050) and the Intramural Research Program of the
Hubei University of Medicine (no. 2011CZX01), without commercial or
not-for-profit sectors. The funders had no role in study design,
data collection and analysis, decision to publish, or preparation
of the manuscript. No additional external funding was received for
this study.
References
|
1
|
Pihlstrom BL, Michalowicz BS and Johnson
NW: Periodontal diseases. Lancet. 366:1809–1820. 2005. View Article : Google Scholar
|
|
2
|
Cullinan MP, Ford PJ and Seymour GJ:
Periodontal disease and systemic health: current status. Aust Dent
J. 54(Suppl 1): S62–S69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blaizot A, Vergnes JN, Nuwwareh S, Amar J
and Sixou M: Periodontal diseases and cardiovascular events:
meta-analysis of observational studies. Int Dent J. 59:197–209.
2009.PubMed/NCBI
|
|
4
|
Leng WD, Zeng XT, Chen YJ, Zhan ZQ and
Yang Y: Periodontal disease is associated with increased coronary
heart disease risk: A meta-analysis based on 38 case-control
studies. World J Meta-Anal. 1:47–56. 2013.
|
|
5
|
Preshaw PM, Alba AL, Herrera D, et al:
Periodontitis and diabetes: a two-way relationship. Diabetologia.
55:21–31. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zeng XT, Tu ML, Liu DY, Zheng D, Zhang J
and Leng W: Periodontal disease and risk of chronic obstructive
pulmonary disease: a meta-analysis of observational studies. PLoS
One. 7:e465082012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sandhu SP, Kakar V, Gogia G and Narula SC:
Unilateral gingival fibromatosis with localized aggressive
periodontitis (involving first molars): An unusual case report. J
Indian Soc Periodontol. 13:109–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meng H, Xu L, Li Q, Han J and Zhao Y:
Determinants of host susceptibility in aggressive periodontitis.
Periodontol 2000. 43:133–159. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hart TC, Pallos D, Bozzo L, et al:
Evidence of genetic heterogeneity for hereditary gingival
fibromatosis. J Dent Res. 79:1758–1764. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saxen L and Nevanlinna HR: Autosomal
recessive inheritance of juvenile periodontitis: test of a
hypothesis. Clin Genet. 25:332–335. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jorgenson RJ, Levin LS, Hutcherson ST and
Salinas CF: Periodontosis in sibs. Oral Surg Oral Med Oral Pathol.
39:396–402. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boughman JA, Astemborski JA and Suzuki JB:
Phenotypic assessment of early onset periodontitis in sibships. J
Clin Periodontol. 19:233–239. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Long JC, Nance WE, Waring P, Burmeister JA
and Ranney RR: Early onset periodontitis: a comparison and
evaluation of two proposed modes of inheritance. Genet Epidemiol.
4:13–24. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hart TC, Hart PS, Michalec MD, et al:
Localisation of a gene for prepubertal periodontitis to chromosome
11q14 and identification of a cathepsin C gene mutation. J Med
Genet. 37:95–101. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Walker SJ, Van Dyke TE, Rich S, Kornman
KS, di Giovine FS and Hart TC: Genetic polymorphisms of the
IL-1alpha and IL-1beta genes in African-American LJP patients and
an African-American control population. J Periodontol. 71:723–728.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stroup DF, Berlin JA, Morton SC, et al:
Meta-analysis of observational studies in epidemiology: a proposal
for reporting. Meta-analysis Of Observational Studies in
Epidemiology (MOOSE) group. JAMA. 283:2008–2012. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huedo-Medina TB, Sanchez-Meca J,
Marin-Martinez F and Botella J: Assessing heterogeneity in
meta-analysis: Q statistic or I2index? Psychol Methods.
11:193–206. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Borenstein M, Hedges L, Higgins J and
Rothstein H: Comprehensive Meta-analysis Version 2. Biostat;
Englewood, NJ, USA: 2005
|
|
20
|
Hodge PJ, Riggio MP and Kinane DF: Failure
to detect an association with IL1 genotypes in European Caucasians
with generalised early onset periodontitis. J Clin Periodontol.
28:430–436. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Duan H, Zhang J and Zhang Y: The
association between IL-1 gene polymorphisms and susceptibility to
severe periodontitis. West Chin J Stomatol. 20:48–51. 2002.(In
Chinese).
|
|
22
|
Rogers MA, Figliomeni L, Baluchova K, et
al: Do interleukin-1 polymorphisms predict the development of
periodontitis or the success of dental implants? J Periodontal Res.
37:37–41. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Anusaksathien O, Sukboon A, Sitthiphong P
and Teanpaisan R: Distribution of interleukin-1beta(+3954) and
IL-1alpha(−889) genetic variations in a Thai population group. J
Periodontol. 74:1796–1802. 2003.
|
|
24
|
Gonzales JR, Michel J, Rodriguez EL,
Herrmann JM, Bodeker RH and Meyle J: Comparison of interleukin-1
genotypes in two populations with aggressive periodontitis. Eur J
Oral Sci. 111:395–399. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li QY, Zhao HS, Meng HX, et al:
Association analysis between interleukin-1 family polymorphisms and
generalized aggressive periodontitis in a Chinese population. J
Periodontol. 75:1627–1635. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Quappe L, Jara L and Lopez NJ: Association
of interleukin-1 polymorphisms with aggressive periodontitis. J
Periodontol. 75:1509–1515. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brett PM, Zygogianni P, Griffiths GS, et
al: Functional gene polymorphisms in aggressive and chronic
periodontitis. J Dent Res. 84:1149–1153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scapoli C, Trombelli L, Mamolini E and
Collins A: Linkage disequilibrium analysis of case-control data: an
application to generalized aggressive periodontitis. Genes Immun.
6:44–52. 2005.PubMed/NCBI
|
|
29
|
Sakellari D, Katsares V, Georgiadou M,
Kouvatsi A, Arsenakis M and Konstantinidis A: No correlation of
five gene polymorphisms with periodontal conditions in a Greek
population. J Clin Periodontol. 33:765–770. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Havemose-Poulsen A, Sorensen LK, Bendtzen
K and Holmstrup P: Polymorphisms within the IL-1 gene cluster:
effects on cytokine profiles in peripheral blood and whole blood
cell cultures of patients with aggressive periodontitis, juvenile
idiopathic arthritis, and rheumatoid arthritis. J Periodontol.
78:475–492. 2007. View Article : Google Scholar
|
|
31
|
Maria de Freitas N, Imbronito AV, Neves
AC, Nunes FD, Pustiglioni FE and Lotufo RF: Analysis of IL-1A(−889)
and TNFA(−308) gene polymorphism in Brazilian patients with
generalized aggressive periodontitis. Eur Cytokine Netw.
18:142–147. 2007.
|
|
32
|
Fiebig A, Jepsen S, Loos BG, et al:
Polymorphisms in the interleukin-1 (IL1) gene cluster are not
associated with aggressive periodontitis in a large Caucasian
population. Genomics. 92:309–315. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guzeldemir E, Gunhan M, Ozcelik O and
Tastan H: Interleukin-1 and tumor necrosis factor-alpha gene
polymorphisms in Turkish patients with localized aggressive
periodontitis. J Oral Sci. 50:151–159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ren XY, Xu L and Meng HX: Interleukin-1
family polymorphisms in aggressive periodontitis patients and their
relatives. J Peking Univ Health Sci. 40:28–33. 2008.(In
Chinese).
|
|
35
|
Karasneh JA, Ababneh KT, Taha AH,
Al-Abbadi MS and Ollier WE: Investigation of the interleukin-1 gene
cluster polymorphisms in Jordanian patients with chronic and
aggressive periodontitis. Arch Oral Biol. 56:269–276. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schulz S, Stein JM, Altermann W, et al:
Single nucleotide polymorphisms in interleukin-1 gene cluster and
subgingival colonization with Aggregatibacter
actinomycetemcomitans in patients with aggressive
periodontitis. Hum Immunol. 72:940–946. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shibani K, Shhab R and Khattab R: Analysis
of IL-1α(−889) and IL-1B(+3953) gene polymorphism in Syrian
patients with aggressive periodontitis: a pilot study. ISRN Dent.
2011:6825642011.
|
|
38
|
Geng P, Chen Y, Ou J, Yin X, Sa R and
Liang H: The E-cadherin (CDH1) -C160A polymorphism and colorectal
cancer susceptibility: A meta-analysis. DNA Cell Biol.
31:1070–1077. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Leng WD, Zeng XT, Chen YJ, et al:
Cytochrome P450 2E1 Rsa I/Pst I polymorphism and risk
of esophageal cancer: A meta-analysis of 17 case-control studies.
Exp Ther Med. 4:938–948. 2012.
|
|
40
|
Tian GX, Zeng XT, Wang XB, Zhang L, Zhang
W and Wei WL: Association between the endothelial nitric oxide
synthase gene Glu298Asp polymorphism and coronary heart disease: A
metaanalysis of 39 case-control studies. Mol Med Rep. 7:1310–1318.
2013.PubMed/NCBI
|