Introduction
Beauveria bassiana is well-known for its
broad spectrum for hosts and has relative resistance to
environmental change (1). For
centuries, adult Bombyx mori infected with Beauveria
bassiana have been used as an oriental medicine for the
treatment of stroke, hives and diabetes (2). To the best of our knowledge,
Beauveria bassiana has a limited virulence in humans.
Notably, only a few cases of invasive disease and keratitis have
been documented, despite the widespread use of the organism
(3). Entomopathogenic fungi,
including Beauveria bassiana, Cordyceps sinensis,
Cordyceps militaris, and Paecilomyces tenuipes, from
a variety of resources have been employed for the treatment of
atopic dermatitis, athlete’s foot and dandruff (4). The use of this agent as a biological
control has received increasing attention as Beauveria
bassiana is used to exterminate a wide variety of pests
(1,5,6). The
anti-bacterial activity of entomopathogenic fungi against
food-borne bacterial growth has also been investigated (7).
Although these entomopathogenic fungi have been
shown to possess valuable properties, including immune-modulation,
anti-diabetic, anti-stress and antitumor activities (8), their application in the cosmetics
industry has not been thoroughly studied. However, investigation
into the whitening effects of fungal fermentation products has been
performed (9), and the results of
those studies indicated that phototoxicity tests are important for
obtaining approval and authorization for the use of test compounds
as functional cosmetic ingredients. Since there are numerous
methods used to measure the toxicity of substances applied to the
skin and skin-related tissues, various trials have been conducted
to assess the biological effects of the cosmetic/cosmeceutical
ingredients that are being approved (10). However, in vitro methods,
such as the 3T3 neutral red uptake (NRU) phototoxicity test
(11) and local lymph node assay
(12), are increasingly being used
instead of animal models due to the ethical aspects involved.
Emerging applications of insect extracts (or fractions) are
employed to broaden the applicability of their biochemicals as
cosmetics/cosmeceuticals. However, whether the agents produced by
entomopathogenic fungi have adverse effects on exposed skin and
eyes has yet to be determined. However, surplus reactions to
cosmetics are frequent in patients with allergic contact
dermatitis. A number of adverse outcomes, such as irritation,
sensitization and acute/chronic toxicity, can be evaluated using
in vitro, in vivo, semi-in vivo, and ex
vivo animal models (13–15).
The individual components or constituents should not exert toxic
effects on the skin and should only be passed and approved in cases
in which no eye lens damage/change is observed in animals or
clinical trials for the development of cosmetics (16).
In the present study, the phototoxicity of
S-(−)-10,11- dihydroxyfarnesic acid methyl ester (DHFAME) was
evaluated using an in vitro phototoxicity test and an in
vivo animal model to determine whether the compound is safe for
development in cosmetic applications.
Materials and methods
Chemicals
8-Methoxypsoralen (8-MOP; M3501), polyethylene
glycol (P3265), chloropromazine (CPZ; C0982), and neutral red
(N4638) were purchased from Sigma-Aldrich Chemical Co., Ltd. (St.
Louis, MO, USA). All media and compositions were commercially
available.
Animal care and use
Seven-week-old Hartley guinea pigs, weighing
319.6–372.9 g, were purchased from Samtako Bio Korea (Osan, Korea)
and used for the skin irritancy and phototoxicity tests,
respectively. The animals were fed a commercial diet (Purina Korea,
Inc., Seoul, Korea) and provided with water ad libitum
throughout all the experiments. The study protocols complied with
the guidelines of the International Association for the Study of
Pain Committee for Research and Ethical Issues (17), and strictly adhered to the internal
guidelines of the Kyungpook National University Animal Ethics
Committee. All animals were acclimatized to the laboratory
environment for ~1 week prior to commencement of the experiments.
Five animals were allocated to each group.
Isolation and preparation of agent
DHFAME was produced by Beauveria bassiana
KACC46831. Briefly, the fermentation medium consisted of 3%
sucrose, 2% corn steep liquor, 0.05% potassium phosphate dibasic,
0.1% potassium phosphate monobasic and 0.05% MgSO4 •
6H2O. The medium was prepared in a 5l-mini jar fermentor
(Hankook Fermentor, Seoul, Korea) and sterilized at 121°C for 30
min, subsequently it was chilled for inoculation of 5% culture. The
fermentation was then carried out for 3 days, and subsequently the
fermentation broth was centrifuged at 10.000 × g for 10 min and the
supernatant was added as previously described (18). The precipitate was then applied to
an HP column chromatogram and high-performance liquid
chromatography was performed with a reverse column (Waters,
Milford, MA, USA) and a peak was obtained at a retention time of
7.662 min by a detector at 254 nm (2998 PDA; Waters). The peak was
identified as S-(−)-10,11-dihydroxyfarnesic acid methyl ester by
nuclear magnetic resonance and mass spectroscopy (18). A voucher specimen of the methyl
ester produced by Beauveria bassiana KACC46831 has been
deposited in the Laboratory of Food Enzyme Biotechnology, Kyungpook
National University (Daego, Korea).
In vitro 3T3 NRU test
The in vitro 3T3 NRU phototoxicity test was
carried out as described previously (11) and by the OECD guideline 432
(19). Briefly, 96-well plates
(REF353072; BD Falcon, Franklin Lakes, NJ, USA) were seeded with
1.0×104 cells/ml (total 100 μl) 3T3 cells, and
subsequently incubated at 37°C in a humidified 5% CO2
incubator for 24 h. Following the removal of the media and the
washing of cells with Earle’s balanced salt solution (EBSS), the
cells were exposed to various dilutions (three replicate wells per
concentration) of the test materials (100 μl) in EBSS for 60 min.
The cells were treated with an initial range of nine concentrations
ranging from 0 to 100 μM CPZ (as a positive control) or 0 to 250 μM
DHFAME. Following incubation for 24 h in a CO2 incubator
at 37°C, duplicate plates were either exposed to UVA/visible light
at 5 J/cm2 (LF-206.LS; UVitec Strasbourg, France) or
kept in the dark for 50 min. Following irradiation, the media were
discarded from all the plates and the cells were washed with
culture medium. The cells were then reincubated in culture medium
overnight. On day 3, the medium was removed and the cells were
washed with pre-warmed buffer and added to 100 μl of neutral red
medium (50 μg/ml, serum-free). Samples were then incubated for 3 h
in a CO2 incubator at 37°C, and subsequently 150 ml of
neutral red extraction solution (distilled water:ethyl
alcohol:acetone = 49:50:1) was added to the plates. The plates were
then agitated and the optical density was measured at 540 nm using
a spectrophotometer (Perkin Elmer Wallac, Inc., Turku,
Finland).
In vivo phototoxicity test
An in vivo phototoxicity test was conducted
using Hartley guinea pigs. The animals were divided into an
untreated, three experimental (10, 30 and 100 mg/ml of DHFAME) and
a positive control group that was treated with 8-MOP. Each group
contained five guinea pigs (7-week-old males, weighing 319.6–372.9
g). The untreated group was exposed to polyethylene glycol. For the
three experimental groups, 0.5 ml/site of the solution was applied.
The treated skin was then irradiated with UV light at a distance of
10 cm for 10 min using UV irradiation apparatus (UVITEC LF-206.LS)
with a UV lamp (365 nm). The left site was designated as the light
irradiation site, whereas the right site was not irradiated. After
2, 4 and 24 h of irradiation, any skin erythema, eschar and
swelling was scored relative to the control. Transdermal
administration was carried out by removing the fur in a 4×6
cm2 area with an electric hair cutter and then applying
the test sample to two regions (each 2×2 cm2). The test
groups were treated with 0.5 ml of DHFAME at concentrations of 10,
30 and 100 mg/ml, whereas 0.5 ml of a 0.1% 8-MOP solution was
applied to each side of the test site as a positive control
(20). The non-irradiation site was
shielded by aluminum tape.
Statistical analysis
Data are presented as the means ± standard
deviation. Statistical analysis was carried out by Probit analysis
using the SPSS 9.0 program (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
different following analysis using Pearson’s goodness-of-fit
test.
Results and Discussion
Throughout the evaluation of active components that
exhibit whitening activities for application as a cosmetic from
natural resources, Beauveria bassiana KACC46831 was found to
produce a potent compound during liquid culture. The compound was
identified as DHFAME and found to exert anti-tyrosinase activity
in vitro and in vivo [(12) and data not shown].
In a previous study, we examined whether the agent
had the ability to ameliorate skin inflammation, including atopic
dermatitis (18). Initially, insect
biomaterials were obtained and processed into biomaterials using a
variety of methods. Subsequently, microbial fermentation,
biotransformation, supercritical extraction or chemical
modification techniques were employed to convert the raw extracts
into a cosmetic, cosmeceutical, neutraceutical or hit/lead drug.
Therefore, the development of anti-tyrosinase agents from medicinal
insect extracts was tested, which revealed that the methyl ester
had potent whitening activity (18). To determine the toxicity of the
agent, an acute toxicity test was conducted for the application of
cosmetic ingredients.
3T3 NRU phototoxicity was first tested in
vitro according to the OECD 432 guideline. For the assay, CPZ
was selected as a positive control, as the OECD guideline suggests
that this drug exhibits phototoxicity by UV irradiation in 3T3
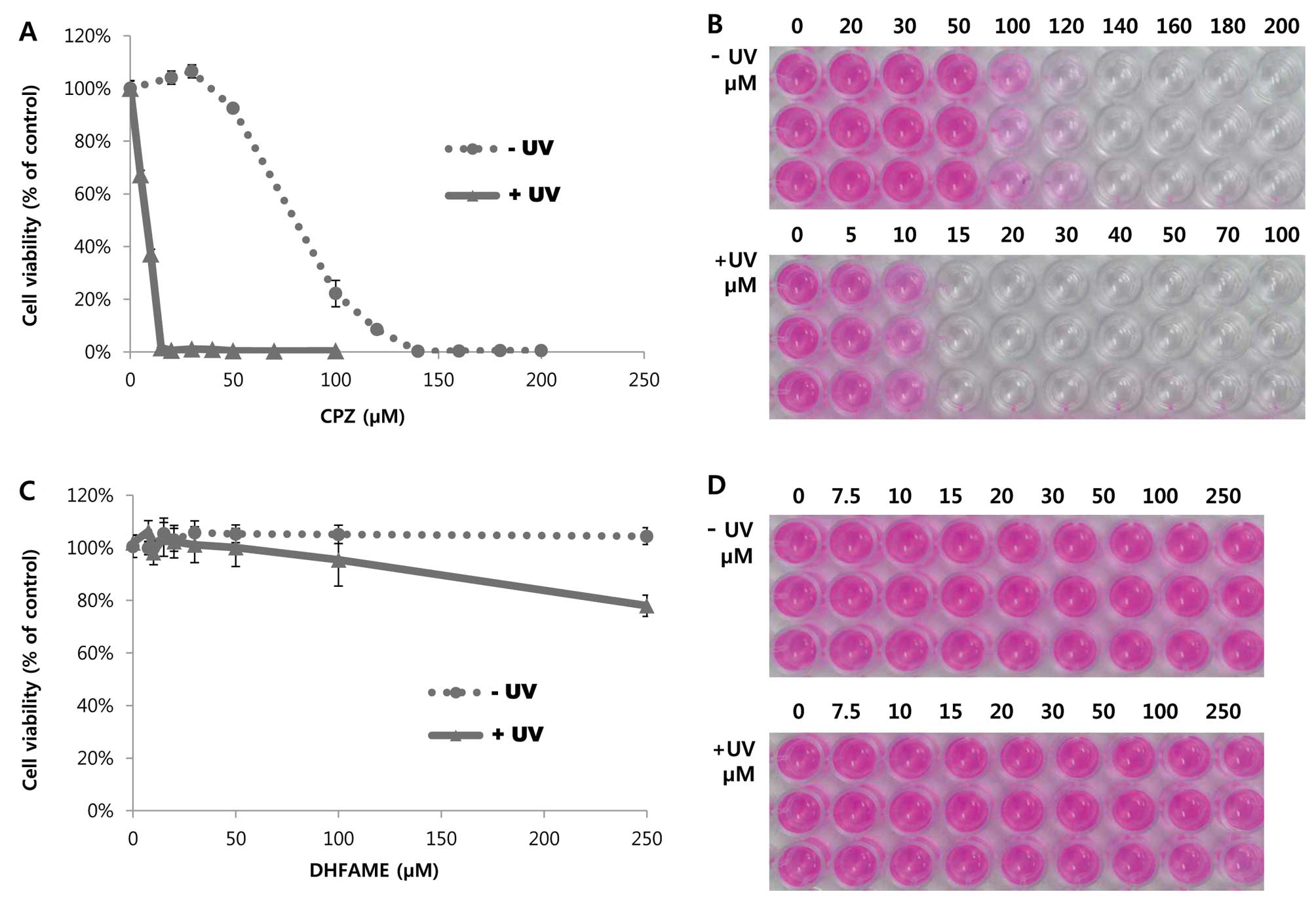
cells. As shown in Fig. 1A, 3T3
cells showed characteristic features of growth in the presence of
various concentrations of CPZ without UV in a
concentration-dependent manner. In particular, 50 and 100 μM CPZ
exhibited 88 and 21.5% cell viability, respectively, when compared
to the control (Fig. 1; dotted and
straight lines). When the cells were treated with UV and 10 μM CPZ,
the growth was decreased significantly by <37.4% (Fig. 1A). Cell viability was 0% in response
to treatment with 15 μM CPZ with UV (Fig. 1B; comparison of upper and lower
panels). The probable toxicity rate of CPZ was 1.000, whereas the
rates of PIF and MPE were 12.016 and 0.781, respectively. This
finding suggested that CPZ treatment results in phototoxicity to UV
irradiation. Under these conditions, various concentrations of
DHFAME were compared to the positive control. As shown in Fig. 1C, a higher concentration of DHFAME
did not cause a notable decrease in cell viability with or without
UV (dotted and straight lines, respectively) at <250 μM.
Moreover, the cell morphology did not change unexpectedly at the
designated concentration (Fig. 1D).
The phototoxicity irritancy factor (PIF) and mean photo effect
(MPE) of CPZ was 12.016 and 0.781, respectively, indicating that
the probable phototoxicity rate was 1.000 and that DHFAME did not
induce phototoxicity in this in vitro 3T3 NRU phototoxicity
test (data not shown). Conversely, the PIF of DHFAME was <1.000
and the MPE was 0.060, indicating that the probable phototoxicity
rate was 0.003 (data not shown).
To determine whether DHFAME exhibited phototoxicity
in vivo, DHFAME produced by Beauveria bassiana
KACC46831 was soaked on the skin of guinea pigs and the toxicity
was determined compared to guinea pigs treated with 8-MOP. The
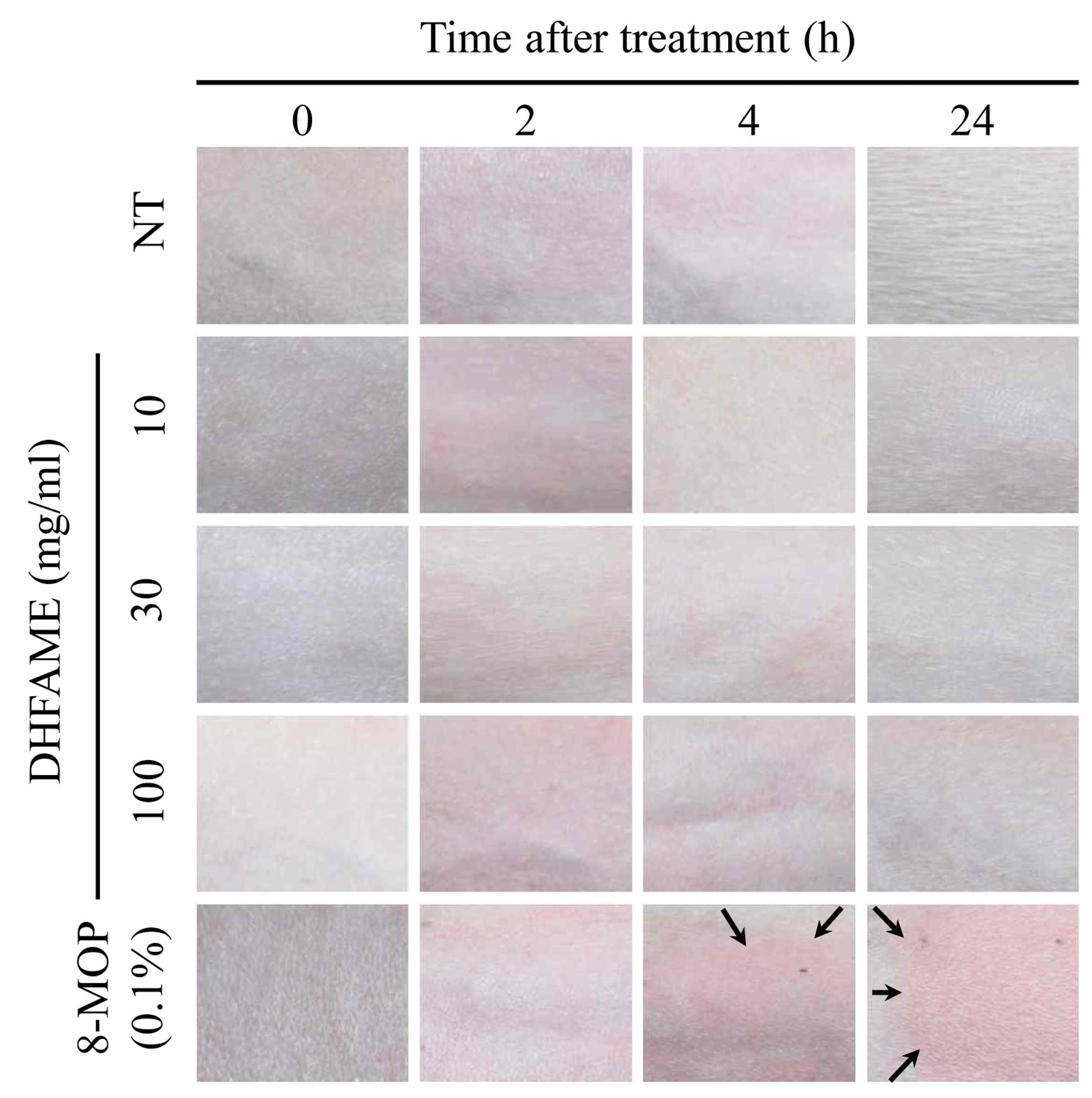
lesions were examined at 2, 4 and 24 h after application of DHFAME
to evaluate phototoxicity. In particular, erythema and eschar were
determined by observation with the naked eye using the following
scale: 0, no erythema; 1, extremely slight; 2, well-defined; 3,
moderate to severe; and 4, severe erythema to slight eschar
formation.
Phototoxicity was subsequently evaluated by
analyzing the skin exposed to UV irradiation. Following fur
removal, guinea pig skin was treated with DHFAME and 8-MOP, and the
degree of erythema was determined using the aforementioned scale.
For up to 4 h after UV irradiation, similar erythema symptoms were
observed. After 24 h, the DHFAME-treated groups showed no symptoms
of toxicity in the skin, whereas the 8-MOP group (0.1% as a
positive control) showed moderate to severe erythema (Fig. 2). To measure edema, the following
scale was used: 0, no edema; 1, extremely slight; 2, well-defined;
3, moderate to severe; and 4, severe edema. The results showed that
DHFAME did not cause erythema or eschar, whereas 8-MOP resulted in
slight edema (Fig. 2). A final
score was then determined by assessing the total scores for
erythema, edema and crust as follows: 0.0–0.5, almost no phototoxic
resistance; 0.6–1.2, weakly phototoxic; 1.3–2.5, clearly and highly
phototoxic; and 2.6–5.0, highly and severely phototoxic. As shown
in Table I, the three samples (10,
30 and 100 mg/ml) were associated with scores of only 0.0–0.5,
suggesting that the agent tested in the experiment was
non-irritating. However, treatment with 8-MOP was a clearly
irritating compound that resulted in erythema, eschar, and edema
(Fig. 2). After 2 to 4 h of UV
irradiation, a slight redness was observed in all agent-treated
groups, but this redness disappeared after 24 h. Conversely, the
groups treated with 8-MOP developed erythema and edema, indicating
that the overall condition of the phototoxicity test was achieved.
Taken together, these findings indicate that 8-MOP treatment
induced erythema, edema, and/or eschar in a concentration-dependent
manner, whereas DHFAME had no effect.
 | Table IComparison of the phototoxicity test
evaluating the effects of S-(−)-10,11-dihydroxyfarnesic acid methyl
ester (DHFAME) produced by Beauveria bassiana KACC46831. |
Table I
Comparison of the phototoxicity test
evaluating the effects of S-(−)-10,11-dihydroxyfarnesic acid methyl
ester (DHFAME) produced by Beauveria bassiana KACC46831.
| | | DHFAME, mg/ml | |
|---|
| | |
| |
|---|
| Criteria | Total scores | Distilled water | 10 | 30 | 100 | 0.1% 8-MOP |
|---|
| Non-irritating | 0.0–0.5 | Yes | Yes | Yes | Yes | |
| Minimally
irritating | 0.6–1.2 | | | | | |
| Obviously
irritating | 1.3–2.5 | | | | | Yes |
| Extremely
irritating | 2.6–5.0 | | | | | |
In summary, the present study investigated whether
DHFAME has the potential to cause skin phototoxicity. None of the
investigated concentrations of DHFAME were found to irritate the
skin or were phototoxic, indicating that DHFAME may be useful in
the cosmetic or cosmeceutical industry and for other applications.
Although DHFAME was derived from an entomopathogenic fungus, its
potential mode of action and toxicity require further
evaluation.
Acknowledgements
The present study was supported by the Bio-Green21
Agenda Project (grant no. PJ009608012013). The authors would like
to thank Mr. Dong-Yoon Nam and Mr. Yong-Soo Cha for their technical
assistance.
References
|
1
|
Ownley BH, Griffin MR, Klingeman WE, Gwinn
KD, Moulton JK and Pereira RM: Beauveria bassiana:
endophytic colonization and plant disease control. J Invertebr
Pathol. 98:267–270. 2008. View Article : Google Scholar
|
|
2
|
Pemberton RW: Insects and other arthropods
used as drugs in Korean traditional medicine. J Ethnopharmacol.
65:207–216. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Figueira L, Pinheiro D, Moreira R, Pinto
E, Simões J, Camisa E, Torrão L, Palmares J and Falcão-Reis F:
Beauveria bassiana keratitis in bullous keratopathy:
antifungal sensitivity testing and management. Eur J Ophthalmol.
22:814–818. 2012.
|
|
4
|
Zhou X, Gong Z, Su Y, Lin J and Tang K:
Cordyceps fungi: natural products, pharmacological functions
and developmental products. J Pharm Pharmacol. 61:279–291. 2009.
View Article : Google Scholar
|
|
5
|
Fernandes ÉK, Bittencourt VR and Roberts
DW: Perspectives on the potential of entomopathogenic fungi in
biological control of ticks. Exp Parasitol. 130:300–305.
2012.PubMed/NCBI
|
|
6
|
Madsen AM: Occupational exposure to
microorganisms used as biocontrol agents in plant production. Front
Biosci (Schol Ed). 3:606–620. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seo ST, Lee JS, Park JH, Han KS and Jang
HI: Investigation of antibiotic susceptibility of some plant
pathogenic bacteria. Kor J Food Sci Technol. 23:495–498. 2005.
|
|
8
|
Wang Q and Xu L: Beauvericin, a bioactive
compound produced by fungi: a short review. Molecules.
17:2367–2377. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nam SH, Yoon CS, Jeon JY, Lee SH, Lee KG,
Yeo JH and Hwang JS: Composition exhibiting melanin-inhibiting
activity. Republic of Korea KR Patent 10-1239631. Filed March 28,
2011; issued Feb 27, 2013.
|
|
10
|
Nigam PK: Adverse reactions to cosmetics
and methods of testing. Indian J Dermatol Venereol Leprol.
75:10–18. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clothier RH: Phototoxicity and acute
toxicity studies conducted by the FRAME Alternatives Laboratory: a
brief review. Altern Lab Anim. 35:515–519. 2007.PubMed/NCBI
|
|
12
|
Goebel C, Aeby P, Ade N, Alépée N, Aptula
A, Araki D, Dufour E, Gilmour N, Hibatallah J, Keller D, Kern P,
Kirst A, Marrec-Fairley M, Maxwell G, Rowland J, Safford B,
Schellauf F, Schepky A, Seaman C, Teichert T, Tessier N, Teissier
S, Weltzien HU, Winkler P and Scheel J: Guiding principles for the
implementation of non-animal safety assessment approaches for
cosmetics: skin sensitisation. Regul Toxicol Pharmacol. 63:40–52.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tavaszi J, Budai P, Pálovics A and
Kismányoki A: An alternative test battery in detecting ocular
irritancy of agrochemicals. Commun Agric Appl Biol Sci. 73:891–895.
2008.PubMed/NCBI
|
|
14
|
Scott L, Eskes C, Hoffmann S, et al: A
proposed eye irritation testing strategy to reduce and replace in
vivo studies using Bottom-Up and Top-Down approaches. Toxicol In
Vitro. 24:1–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Osborne R, Perkins MA and Roberts DA:
Development and intralaboratory evaluation of an in vitro human
cell-based test to aid ocular irritancy assessments. Fundam Appl
Toxicol. 28:139–153. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nolan KA and Marmur ES: Over-the-counter
topical skincare products: a review of the literature. J Drugs
Dermatol. 11:220–224. 2012.PubMed/NCBI
|
|
17
|
Zimmermann M: Ethical guidelines for
investigations of experimental pain in conscious animals. Pain.
16:109–110. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nam SH, Yoon CS and Lee SH: Final report
of development on bioactive compounds derived from entomopathogenic
fungi. Rural Development Agency of Korea; pp. 1–100. 2011,
http://lib.rda.go.kr/newliburi.
|
|
19
|
Peters B and Holzhütter HG: In vitro
phototoxicity testing: development and validation of a new
concentration response analysis software and biostatistical
analyses related to the use of various prediction models. Altern
Lab Anim. 30:415–432. 2002.
|
|
20
|
Neumann NJ, Blotz A, Wasinska-Kempka G,
Rosenbruch M, Lehmann P, Ahr HJ and Vohr HW: Evaluation of
phototoxic and photoallergic potentials of 13 compounds by
different in vitro and in vivo methods. J Photochem Photobiol B.
79:25–34. 2005. View Article : Google Scholar : PubMed/NCBI
|