Introduction
β-defensins (BDs) are cationic peptides which
present broad-spectrum antimicrobial activities against bacteria,
fungi and viruses (1). To date, 6
BDss, human BD (hBD)-1 (2), hBD-2
(3), hBD-3 (4,5), hBD-4
(6), hBD-5 and hBD-6 (7) have been identified and characterized in
humans. hBD-2 is expressed in a wide range of cell types, such as
epithelial cells, keratinocytes and monocytes/macrophages (8,9). It is
expressed rarely or lowly under normal circumstances, and is
upregulated in response to infectious and inflammatory stimuli
through multiple signaling pathways, such as the NF-κB pathway
(10,11). Previous studies have indicated that BD2
has multiple functions in the immune system, including
antibacterial activity (12–14), connection between epithelial innate and
adaptive immunity (15,16), as well as chemotactic function
(16,17).
ATP is a well-known energy source inside cells, it
also functions as an essential messenger between cells. Activation
of P2 receptors, P2X and P2Y, by ATP may initiate the release of
sodium and calcium ions, producing different cellular effects
(18). ATP has a wide range of
functions in the human body, including the development of pain, the
heart and blood vessel disorders, secretion of digestive enzymes,
as well as tumor-killing effects (18). In addition, ATP serves an important
role in inflammation and infection. ATP activation of the P2×7
receptor results in the processing and secretion of mature
interleukin (IL)-1β, which is a potent cytokine capable of inducing
inflammation (19,20). Moreover, active ATP release and
adenosine receptor signaling are required for activation of the NLR
pyrin domain containing 3 (NLRP3) inflammasome that is a major
component of the innate immune system (21). Nonetheless, little is known regarding
the relationship between BD2 and ATP, as well as their roles in the
acute pneumonia of rats.
In the present study, the authors evaluated the
impact of ATP on the expression of rat BD-2 (rBD-2) in a
time-dependent manner, as well as their protective effect in the
acute pneumonia of rat caused by Pseudomonas aeruginosa (PA)
(Xinjiang Uygur Autonomous Region Food and Drug Administration,
Urumqi, China). The completion of the study provides some novel
findings on rBD-2 in acute pneumonia which will be useful to guide
further investigation and targeted therapy for this disease.
Materials and methods
Animal housing and treatment
Given the homology between rBD-2 and hBD-2, such as
6 cysteine and other lysine and arginine residues (22), the rat was chosen as a model to study
the function of hBD-2 in vivo. BALB/c rats (4–6 weeks old,
male/female ratio, 1:1; 20–30 g), provided by Medical School of
Tongji University (Shanghai, China), were housed under controlled
conditions. Procedures involving animals and their care were
approved in accordance with the guidelines of the Experimental
Animal Ethics Committee of Medical School of Tongji University
(Shanghai, China; no. 2010–1-1). A total of 60 rats were randomly
divided into treatment and control groups with 30 rats in each. A
control group was administrated with normal saline at 250 µl/100 g
body weight, treatment group was injected with ATP at 0.12 mg/10 g
body weight through the tail vein. Rats were sacrificed at 12, 24
and 36 h, respectively after treatment, and the lung tissues were
isolated from each rat and stored in liquid nitrogen.
Determination of rBD-2 mRNA in rat
lungs by RT-qPCR
Total RNA was isolated from the whole lung tissue
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and reversely transcribed into cDNA using M-MLV
Reverse Transcriptase (Takara Bio, Inc., Otsu, Japan). The
following primers were used for PCR amplification of rBD-2:
Forward, 5′-GAACTTGACCACTGCCACACC-3′; and reverse,
5′-GCTCTAGATTATCATTTCATGTACTTGCACC-3′. β-actin was used as a
reference to normalize rBD-2 mRNA expression levels using the
2−∆∆Cq method (23). The
RT-qPCR primers used for β-actin in the present study were:
Forward, 5′-AACAGTCCGCCTAGAAGCAC-3′; and reverse,
5′-GGTTGACATCCGTAAAGACC-3′. Each RNA sample was run in triplicate.
PCR parameters were set as follows: 39 cycles of denaturation at
95°C for 20 sec, annealing at 59°C for 30 sec and extension at 72°C
for 30 sec.
Establishment of acute pneumonia model
of rat, animal grouping and treatment
Rats were randomly divided into four treatment
groups and one control group, with eight rats each. Rats were
anesthetized with ether, 1 cm skin incisions were made along the
middle of the mouse's neck, and muscle tissues were bluntly
dissected exposing the trachea and thyroid. A total of 0.2 ml PA
(6×108 CFU/ml, Xinjiang Uygur Autonomous Region Food and
Drug Administration) was injected into each rat trachea in
treatment groups, while, the control group was injected with the
same dose of sterile phosphate buffered saline, then incisions were
sutured. After three days, treatment groups were divided into four
different groups: PA infected group, group treated with ATP at 16
mg/kg or cephalosporins at 0.4 g/kg through the tail vein, group
treated with both ATP at 16 mg/kg and cephalosporins at 0.4 g/kg
through tail vein. Rats were sacrificed 24 h following treatment,
lung tissues and serum were collected and stored in liquid nitrogen
for subsequent experiments.
Pathological examination
The grouping and treatment were the same as
described above, lung tissues were isolated from rats 24 h
following ATP and cephalosporin intervention. Fresh lung specimens
were fixed in 4% paraformaldehyde, embedded in paraffin, cut and
heated, and subjected to hematoxylin and eosin (H&E) staining.
The morphological changes of H&E-stained tissues were analyzed
by Leica digital microscopy.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue sections of
lung tissues were obtained from treatment and control groups as
described above in animal grouping and treatment. The expression of
rBD-2 was detected following the immunohistochemistry protocol of
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). The sections were
incubated with primary rabbit polyclonal antibody rBD-2 (YY3068R;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 1:200 dilution
overnight at 4°C and secondary antibody [SP9000D; DAB Detection kit
(Streptavidin-biotin); ZSGB-BIO, Beijing, China] for another 2 h at
room temperature. Finally, visualization of the sections was
conducted and images were taken with a Leica confocal laser
scanning microscope (Leica Microsystems, Wetzlar, Germany).
Determination of IL-6 and tumor necrosis factor
(TNF)-α expression in lung tissues and peripheral blood. At 24 h
following ATP and cephalosporin intervention, rats from control and
treatment groups were sacrificed and lung tissues were isolated,
centrifuged at 2,045 × g for 20 min at room temperature followed by
collection of supernatants. The peripheral blood samples were also
collected. IL-6 and TNF-α expression was evaluated using ELISA
assay kits in accordance with the instructions (Invitrogen; Thermo
Fisher Scientific, Inc.).
Statistical analyses
Data were presented as mean ± standard deviation.
Student's t-test or SNK-q test was used to determine the
statistical significance between each two groups, differences among
multiple groups were analyzed using an analysis of variance
(ANOVA). The SNK-q test was used for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
ATP enhanced expression of rBD-2 mRNA
in rat lungs
Expression of rBD-2 in lung tissues of rats in
response to ATP has not yet been investigated. Therefore, the
authors determined induction of rBD-2 expression by ATP in the lung
tissues of rat. RT-qPCR was conducted to analyze the expression
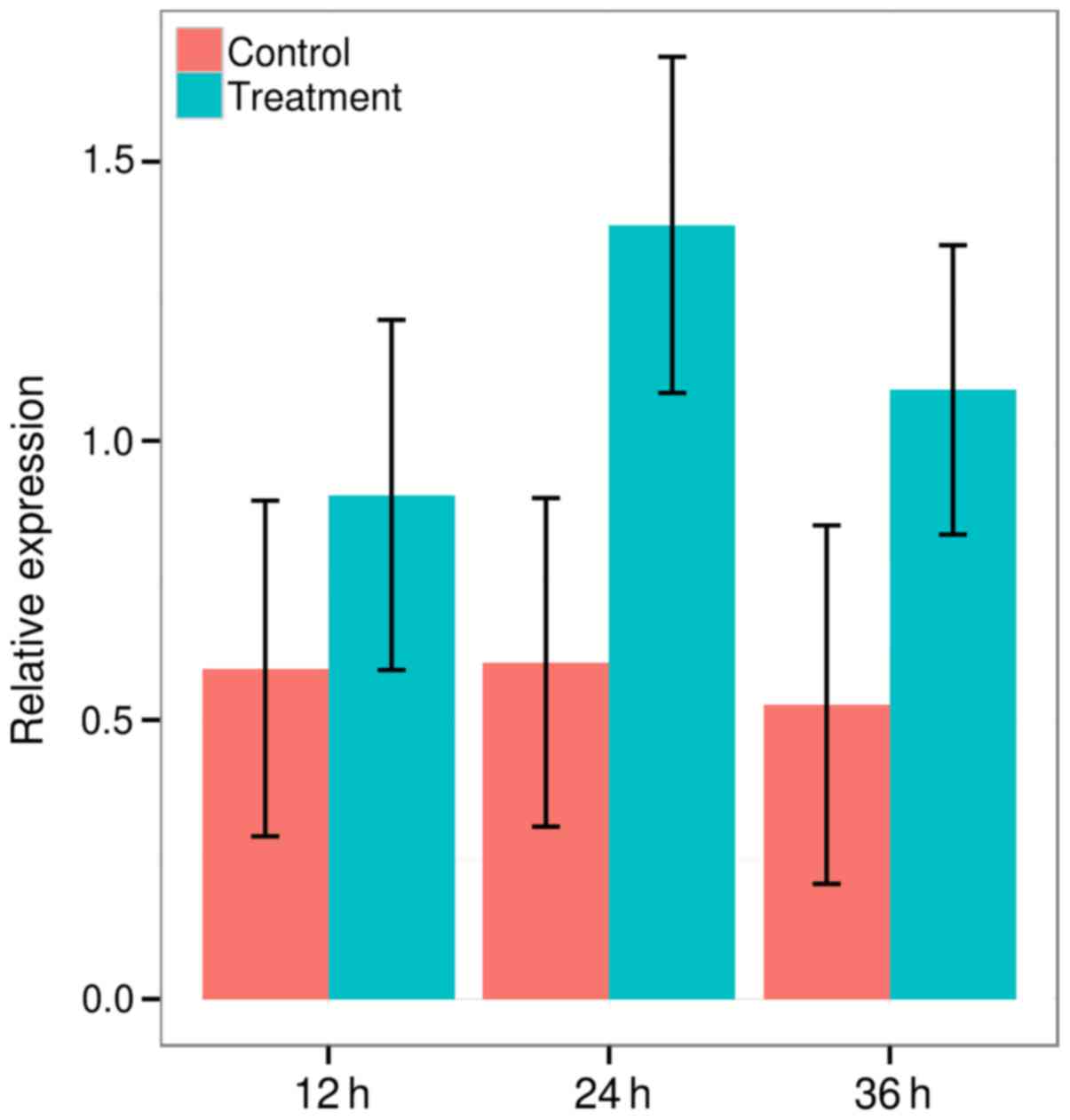
pattern of rBD-2 following ATP stimulation. The expression of rBD-2
gene was upregulated in ATP-treated groups at 12 h (0.5921±0.3007
vs. 0.9029±0.3136, P=0.001, t-test), 24 h (0.6031±0.2943 vs.
1.3867±0.3011, P<0.001, t-test) and 36 h (0.5273±0.3211 vs.
1.0912±0.2591, P<0.001, t-test; Fig.
1). It increased greatly at 12 h, reached highest peak at 24 h
and gradually declined by ~20% at 36 h following ATP treatment,
however, significant differences of the expression of rBD-2 were
observed between each control and treatment group at 12, 24 and 36
h. Therefore, the authors confirmed that ATP could upregulate rBD-2
mRNA expression in rat lungs.
ATP increases expression of rBD-2
protein in rat lungs
As expression of rBD-2 mRNA was upregulated by ATP
in rat lungs, the next question concerned whether ATP promotes the
production of rBD-2 protein in vivo. In the present study,
the authors reported that the expression of the rBD-2 protein was
slightly increased in the PA infected group, an enhanced expression
of the rBD-2 protein was observed in the ATP or cephalosporin
treatment group and the cephalosporin and ATP treatment group, in
comparison with that in PA infected group and control group,
suggesting ATP could induce rBD-2 expression at both mRNA and
protein levels in rat lungs (Fig.
2).
ATP alleviates histological changes in
rat lungs
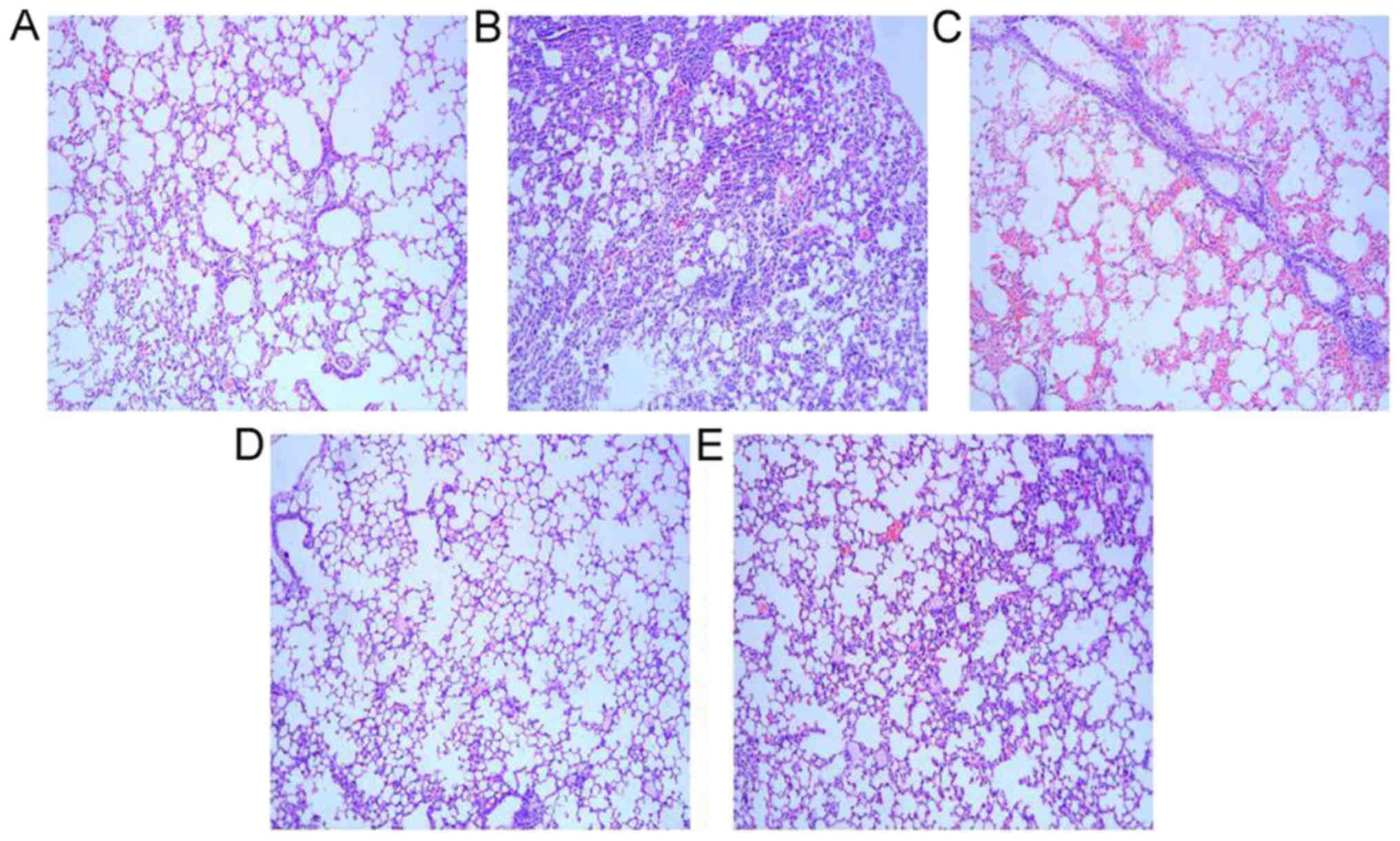
Microcopy indicated that alveolar and interstitial
lung structures were normal, alveolar inflatable was good, no
exudation of red blood cells was observed, part of bronchial and
alveolar walls were infiltrated with a small amount of inflammatory
cells in the lung tissues of the control group. The PA infected
group presented alveolar wall edema, infiltration of a large number
of inflammatory cells (neutrophils and lymphocytes) on bronchial
and alveolar walls, red blood cells and cellulose exudation in the
alveolar space. ATP or ceftazidime intervention groups presented an
improved lung edema, less inflammatory cell infiltration, thicker
partial alveolar wall and a small amount of red blood cells as
compared with the PA infected group. The results demonstrated that
ceftazidime, ATP and ceftazidime + ATP treatment groups improved
the histological destruction of lung tissues caused by PA (Fig. 3).
ATP decreases expression of TNF-α and
IL-6 levels in rat lungs
Following this, the authors next investigated
mechanisms by which enhancement of rBD-2 by ATP presented a
beneficial effect on the PA infection in rat lungs. In order to
clarify whether rBD-2 regulated the secretion of proinflammatory
cytokines, IL-6 and TNF-α expression was analyzed by ELISA.
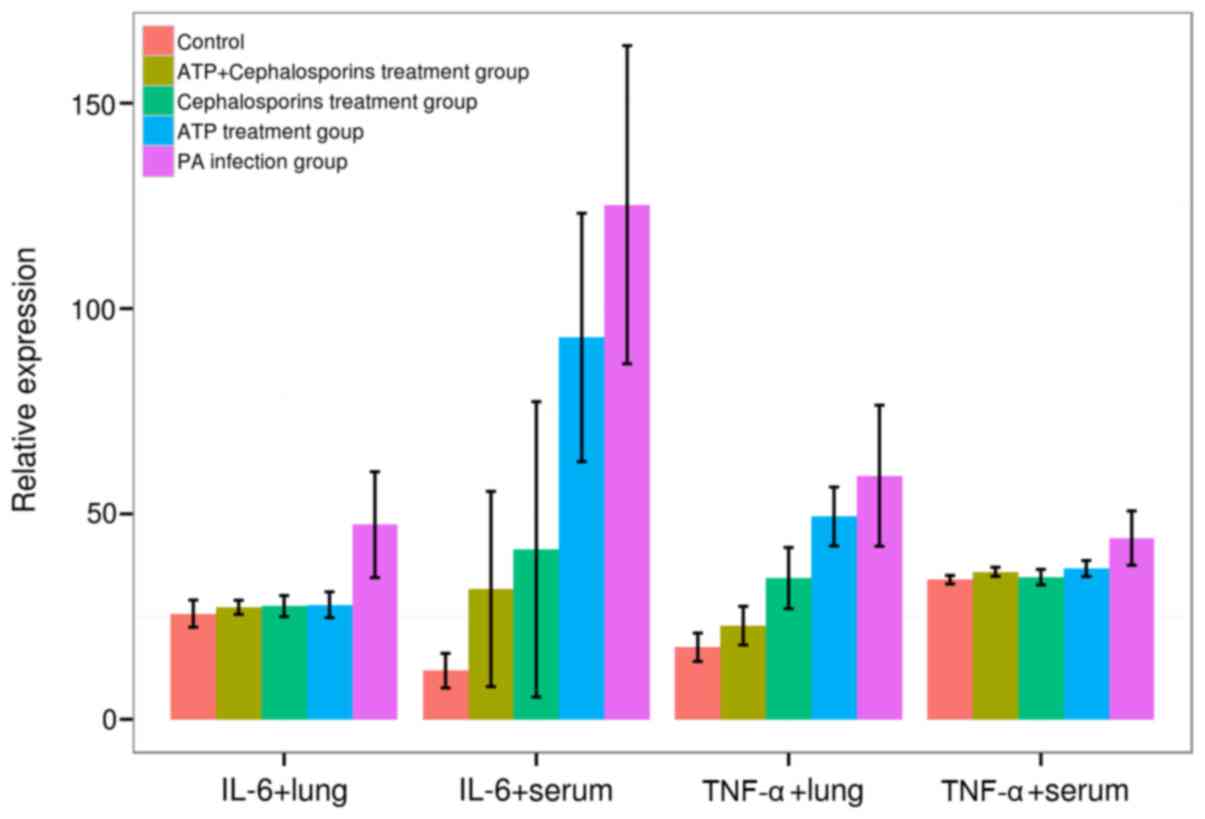
Significant differences of IL-6 and TNF-α expression were
identified in both lung and serum among five groups (F:
12.4864–28.3971, v=4, P=0.0000, ANOVA), PA induced a significant
increase in TNF-α and IL-6 expression levels in rat lungs and serum
of treatment groups as compared with the control group (TNF-α,
44.1250±6.6235 vs. 33.9750±1.0403; IL-6, 125.2813±38.7227 vs.
11.8438±4.2075 in serum; TNF-α, 59.3000±17.1713 vs. 17.5438
±3.4582; IL-6, 47.3875±12.8895 vs. 25.7250±3.3139 in rat lung,
P<0.05 for all cases, SNK-q test). Moreover, IL-6 and TNF-α
expression in the rat lung and serum was reduced in ceftazidime,
ATP and ceftazidime + ATP treatment groups relative to the
PA-infected group (P<0.05 for all cases, SNK-q test). No
significant difference was observed for TNF-α expression in serum
and IL-6 in lungs among ceftazidime, ATP and ceftazidime + ATP
treatment groups (P>0.05 for all cases, SNK-q test). However,
the ceftazidime + ATP treatment group presented a decreased
expression of TNF-α in lungs and IL-6 in serum relative to
ceftazidime or ATP treatment groups (P<0.05 for all cases, SNK-q
test; Fig. 4).
Discussion
There has been an increasing body of evidence that
ATP serves a protective role in the immune system. ATP promotes a
wide range of epithelial responses through the activation of P2X
and P2Y receptors in response to infectious stimuli and injuries.
For instance, ATP mediates the activation of the NADPH oxidase
DUOX1 through the P2Y receptor, phospholipase C and intracellular
calcium signaling in the airway epithelium, leading to the
activation of ERK1/2 and NF-κB pathways, IL-8 release and the
production of H2O2 in response to bacterial
stimulations or tissue injury (24,25). In
addition, ATP modulates the activities of the NLRP3 inflammasome
during bacterial infection by activation of the purinergic receptor
P2X7, resulting in IL-1β maturation and secretion of chemokines
(26,27). Here, believed to be for the first time,
the authors reported that ATP upregulated the expression of rBD-2
mRNA and enhanced the production of rBD-2 protein in response to
the bacterial stimulation of PA in rat lungs. A possible mechanism
underlying this process is that ATP activates the NF-κB pathway,
which is critical to the production of rBD-2 (11,28) through
either the P2X or P2Y receptor, however, further studies are still
required in the future.
In addition, enhanced rBD-2 expression by ATP
reduced inflammatory damage was caused by PA in lung tissues,
indicating that rBD-2 ameliorates the infection of PA. These
findings are consistent with studies conducted by Wu et al
(12) and Hu et al (29). The primary reasons may lie in the
antibacterial function of increased rBD-2 and its regulation of
inflammatory factors during bacterial infection and inflammation.
Therefore, the authors analyzed the secretion of cytokines, TNF-α
and IL-6 following stimulation of ATP and ceftazidime. It was
identified that PA infection significantly increased
pro-inflammatory factors, such as TNF-α and IL-6 in the rats, and
overexpression of rBD-2 by ATP inhibited the increase of these
inflammatory factors. These results are in line with a previous
report by Wu et al (12),
indicating that rBD-2 accelerated the weakening process of
inflammation.
In summary, these results demonstrated that ATP
induces rBD-2 mRNA and rBD-2 protein expression in rat lungs and
decreases the expression of proinflammatory cytokines TNF-α and
IL-6, suggesting ATP has protective effects against infection of
PA. These results may provide a better understanding of the
therapeutic values of ATP and hBD-2 in infectious diseases.
Acknowledgements
The present study was made possible with financial
support from Youth Foundation of Shanghai Municipal Health Bureau
(grant no. 20124y113) to H.L.
References
|
1
|
Schutte BC and McCray PB Jr:
[beta]-defensins in lung host defense. Annu Rev Physiol.
64:709–748. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bensch KW, Raida M, Mägert HJ,
Schulz-Knappe P and Forssmann WG: hBD-1: A novel β-defensin from
human plasma. FEBS Lett. 368:331–335. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harder J, Bartels J, Christophers E and
Schröder JM: A peptide antibiotic from human skin. Nature.
387:8611997. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harder J, Bartels J, Christophers E and
Schröder JM: Isolation and characterization of human β-defensin-3,
a novel human inducible peptide antibiotic. J Biol Chem.
276:5707–5713. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jia HP, Schutte BC, Schudy A, Linzmeier R,
Guthmiller JM, Johnson GK, Tack BF, Mitros JP, Rosenthal A, Ganz T,
et al: Discovery of new human beta-defensins using a genomics-based
approach. Gene. 263:211–218. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yanagi S, Ashitani J, Ishimoto H, Date Y,
Mukae H, Chino N and Nakazato M: Isolation of human beta-defensin-4
in lung tissue and its increase in lower respiratory tract
infection. Respir Res. 6:1302005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamaguchi Y, Nagase T, Makita R, Fukuhara
S, Tomita T, Tominaga T, Kurihara H and Ouchi Y: Identification of
multiple novel epididymis-specific beta-defensin isoforms in humans
and mice. J Immunol. 169:2516–2523. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lehrer RI, Lichtenstein AK and Ganz T:
Defensins: Antimicrobial and cytotoxic peptides of mammalian cells.
Annu Rev Immunol. 11:105–128. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ganz T: Defensins: Antimicrobial peptides
of innate immunity. Nat Rev Immunol. 3:710–720. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Méndez-Samperio P, Miranda E and Trejo A:
Regulation of human β-defensin-2 by Mycobacterium bovis bacillus
Calmette-Guérin (BCG): Involvement of PKC, JNK, and PI3K in human
lung epithelial cell line (A549). Peptides. 29:1657–1663. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsutsumi-Ishii Y and Nagaoka I: NF-kappa
B-mediated transcriptional regulation of human beta-defensin-2 gene
following lipopolysaccharide stimulation. J Leukoc Biol.
71:154–162. 2002.PubMed/NCBI
|
|
12
|
Wu M, McClellan SA, Barrett RP and Hazlett
LD: Beta-defensin-2 promotes resistance against infection with P.
aeruginosa. J Immunol. 182:1609–1616. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dalcin D and Ulanova M: The Role of Human
Beta-Defensin-2 in Pseudomonas aeruginosa Pulmonary Infection in
Cystic Fibrosis Patients. Infect Dis Ther. 2:159–166. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guaní-Guerra E, Negrete-García MC,
Montes-Vizuet R, Asbun-Bojalil J and Terán LM: Human β-defensin-2
induction in nasal mucosa after administration of bacterial
lysates. Arch Med Res. 42:189–194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krisanaprakornkit S, Kimball JR, Weinberg
A, Darveau RP, Bainbridge BW and Dale BA: Inducible expression of
human beta-defensin 2 by Fusobacterium nucleatum in oral epithelial
cells: Multiple signaling pathways and role of commensal bacteria
in innate immunity and the epithelial barrier. Infect Immun.
68:2907–2915. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang D, Chertov O, Bykovskaia SN, Chen Q,
Buffo MJ, Shogan J, Anderson M, Schröder JM, Wang JM, Howard OM, et
al: Beta-defensins: Linking innate and adaptive immunity through
dendritic and T cell CCR6. Science. 286:525–528. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boniotto M, Jordan WJ, Eskdale J, Tossi A,
Antcheva N, Crovella S, Connell ND and Gallagher G: Human
beta-defensin 2 induces a vigorous cytokine response in peripheral
blood mononuclear cells. Antimicrob Agents Chemother. 50:1433–1441.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khakh BS and Burnstock G: The double life
of ATP. Sci Am. 301:84–92. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stoffels M, Zaal R, Kok N, van der Meer
JW, Dinarello CA and Simon A: ATP-Induced IL-1β Specific Secretion:
True Under Stringent Conditions. Front Immunol. 6:542015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ferrari D, Chiozzi P, Falzoni S, Dal
Susino M, Melchiorri L, Baricordi OR and Di Virgilio F:
Extracellular ATP triggers IL-1 beta release by activating the
purinergic P2Z receptor of human macrophages. J Immunol.
159:1451–1458. 1997.PubMed/NCBI
|
|
21
|
Baron L, Gombault A, Fanny M, Villeret B,
Savigny F, Guillou N, Panek C, Le Bert M, Lagente V, Rassendren F,
et al: The NLRP3 inflammasome is activated by nanoparticles through
ATP, ADP and adenosine. Cell Death Dis. 6:e16292015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jia HP, Mills JN, Barahmand-Pour F,
Nishimura D, Mallampali RK, Wang G, Wiles K, Tack BF, Bevins CL and
McCray PB Jr: Molecular cloning and characterization of rat genes
encoding homologues of human beta-defensins. Infect Immun.
67:4827–4833. 1999.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boots AW, Hristova M, Kasahara DI, Haenen
GR, Bast A and van der Vliet A: ATP-mediated activation of the
NADPH oxidase DUOX1 mediates airway epithelial responses to
bacterial stimuli. J Biol Chem. 284:17858–17867. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Oliveira S, López-Muñoz A, Candel S,
Pelegrín P, Calado  and Mulero V: ATP modulates acute inflammation
in vivo through dual oxidase 1-derived H2O2 production and NF-κB
activation. J Immunol. 192:5710–5719. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiang Y, Wang X, Yan C, Gao Q, Li SA, Liu
J, Zhou K, Guo X, Lee W and Zhang Y: Adenosine-5′-triphosphate
(ATP) protects mice against bacterial infection by activation of
the NLRP3 inflammasome. PLoS One. 8:e637592013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Di Virgilio F: Liaisons dangereuses:
P2X(7) and the inflammasome. Trends Pharmacol Sci. 28:465–472.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wada A, Ogushi K, Kimura T, Hojo H, Mori
N, Suzuki S, Kumatori A, Se M, Nakahara Y, Nakamura M, et al:
Helicobacter pylori-mediated transcriptional regulation of the
human beta-defensin 2 gene requires NF-kappaB. Cell Microbiol.
3:115–123. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu Q, Zuo P, Shao B, Yang S, Xu G, Lan F,
Lu X, Xiong W, Xu Y and Xiong S: Administration of nonviral gene
vector encoding rat beta-defensin-2 ameliorates chronic Pseudomonas
aeruginosa lung infection in rats. J Gene Med. 12:276–286.
2010.PubMed/NCBI
|