Introduction
The rising prevalence of obesity is associated with
an increase in the consumption of high fat diets (HFDs), which
contributes to early structural alterations and increases the risk
for cardiovascular disease (CVD)-associated morbidity and mortality
(1,2).
Thus, obesity-induced vascular impairment is markedly associated
with damage to target organs, including the heart, kidney, liver
and brain (3). For example, aortic
relaxation dysfunction, a type of vascular dysfunction, decreases
diastolic pressure and increases systolic pressure, which increases
cardiac afterload, left ventricular mass and cardiac oxygen demand
(4). In addition, the decrease in
diastolic pressure contributes to reduction of coronary blood flow
in the period of cardiac diastole (4).
Factors, such as increased reactive oxygen species (ROS), release
of proinflammatory apipokines/cytokines, and tissue insulin
resistance are involved in the abnormal vascular pathophysiological
changes and associated CVD (5). The
combination of abnormal eating habits and a sedentary lifestyle
increase the risk factors of obesity and CVD (6). A previous study has demonstrated that
exercise improves vascular function by increasing the vascular
mitochondrial respiratory capacity, bioavailable nitric oxide (NO),
and redox balance in a sedentary rodent model (7). While prolonged sitting in humans prompts
leg endothelial dysfunction, fidgeting improves leg endothelial and
vascular function through the intermittent increases in vascular
shear stress (8). Thus, the underlying
mechanisms and interactions between obesity and exercise in the
regulation of vascular function involve a complicated network of
interacting factors that require investigation. Therefore, the
present study hypothesized that HFD-induced vascular dysfunction is
mediated by dyslipidemia, excessive ROS, increased expression
levels of proinflammatory adipokines and maladaptive immune
responses, and these abnormalities are prevented by swimming
exercise. An improved understanding of the underlying mechanisms of
vascular function obesity and exercise will be of great clinical
significance.
Materials and methods
Animals
A total of 21 male, 6-week-old C57BL/6J mice were
purchased from the Beijing HFK Bioscience Co., Ltd. (Beijing,
China). For the obese and exercise studies, C57BL/6J male mice were
fed a 60% lipid composition HFD with or without swimming exercise
(90 min/swim and 2 swims/day) for 16 weeks. The control group mice
were age-matched, male mice and were fed regular mouse chow during
the same period of time. The mice were ~18 g and individually
housed on a 12-h light/dark cycle (6 a.m., lights on and 6 p.m.,
lights off). The laboratory temperature was 24°C and the humidity
was 20.5±3.0%. All of the animal studies and procedures were
performed in accordance with the Animal Use and Care Committee at
the Xianning Central Hospital and The First Clinical Hospital of
Hubei University of Science and Technology (Xianning, China).
Following completion of the experiments, the mouse aortae were
isolated to investigate the vascular activity and the left aortae
were maintained in a freezer at −80°C.
Plasma lipid profile assays
During blood sample collection, mice underwent
terminal anesthesia, 0.5 ml was collected through cardiac puncture.
After the blood was prepared in the heparin-coated tubes, plasma
was collected by centrifugation at 604 × g for 15 min. The plasma
levels of triglycerides (TG), total cholesterol (TC) and
high-density lipoprotein (HDL) were examined using a plasma lipid
profile kit (cat. no. MAK043; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) according to manufacturer's instructions. Serum leptin,
resistin and adiponectin levels were measured using a mouse serum
adipokine milliplex kit (cat. no. MADKMAG-71K; Shanghai
Biotechnology Co., Ltd., Shanghai, China) according to
manufacturer's instructions.
Aortic oxidative stress
The formation of ROS in the aortae was evaluated by
chemiluminescence. Aortae were homogenized in a glass homogenizer
with sucrose buffer (pH 7.5 protease inhibitor tablet, 0.5 mm EDTA,
50 mm HEPES and 250 mm sucrose) (9).
The homogenates were centrifuged at 1,500 × g for 10 min at 4°C.
Supernatants in the whole homogenate were removed and added to 1.4
ml 50-mm phosphate (KH2PO4) buffer (100 µm NADPH, 1 mm EGTA, 5 µm
lucigenin and 150 mm sucrose) for incubation at room temperature
for 1 h in dark-adapt counting vials (9). Following dark adaptation, samples were
placed in a scintillation counter to count every 30 sec for 10 min
using a spectrophotometer (wavelength, 450 nm) and normalized to
total protein in the whole homogenate. The ROS values were taken as
counts per min per milligram (cpm/mg) of protein (9).
Analysis of vascular function
The thoracic aorta was dissected and sliced in 2 mm
long aortic rings that were suspended in 95% O2/5%
CO2 aerated organ chambers filled with modified
Krebs-Ringer bicarbonate solution (118 mM NaCl, 1.2 mM MgCl2, 4.7
mM KCl, 11.2 mM NaH2PO4, 2.5 mM
CaCl2, 1.2 mM Na2SO4, 25 mM
NaHCO3, 10 mM glucose; pH 7.4; Sigma-Aldrich; Merck
KGaA) (10). Aortic contractile state
was ascertained by KCl (80 mM). Rings were pre-constricted with
norepinephrine and relaxations to acetylcholine (Ach,
10−9-10−4 M) or sodium nitroprusside (SNP;
10−9-10−4 M; Sigma-Aldrich; Merck KGaA) were
obtained in a cumulative fashion.
Western blot analysis
After the mice were under deep anesthesia with
sodium pentobarbital (50 mg/kg) intraperitoneal injection, a
scalpel cut through the skin all around and just above the ankle,
the gastrocnemius muscles were removed from mouse hind limbs and
lysed with protein lysis buffer (Sigma-Aldrich; Merck KGaA). After
the protein concentration of the lysate was determined by Bio-Rad
protein assay (Bio-Rad Laboratories Ltd., Shanghai, China). The
proteins (20 µg) were separated on 10% SDS-PAGE gels and
transferred onto nitrocellulose membranes at 90 V for 90 min
(Sigma-Aldrich; Merck KGaA). Membranes were incubated overnight at
4°C with blocking solution containing antibodies targeting vascular
endothelial growth factor (VEGF; cat. no. 07-1420, 1:1,000
dilution; Sigma-Aldrich; Merck KGaA). The membranes were incubated
with horseradish peroxidase (HRP)-conjugated secondary antibody
(cat. no. A9542, 1:5,000 dilution; Sigma-Aldrich; Merck KGaA) at
room temperature for 1 h. The HRP activity was detected using an
enhanced chemiluminescence kit (cat. no. 1705060; Bio-Rad
Laboratories Ltd.). GAPDH was run in parallel and served as a
loading control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The genes associated with expression of
pro-inflammatory cytokines were examined by (RT-qPCR) (11). Total RNA was isolated from the aortae
using RNAzol (Sigma-Aldrich; Merck KGaA) and cDNA synthesis was
completed by using 1 µg total RNA with 5X reaction buffer,
oligo(dT) (1 µg), RNAse inhibitor, MgCl2, dNTP mix, and ImProm II
reverse transcriptase as per the ImProm II reverse transcription
kit (cat. no. 11939823001; Sigma-Aldrich; Merck KGaA). Following
first strand cDNA synthesis, qPCR was performed using 8 µl cDNA, 10
µl SYBR-Green PCR master mix (Sigma-Aldrich; Merck KGaA) and
forward and reverse primers (10 pM/µl; Sigma-Aldrich; Merck KGaA)
using a real-time PCR system (CFX96; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The following primer sequences were used:
Forward, 5′-GCGATAGTTAATGCAACACT-3′ and reverse,
5′-TTGGTTACCGGGTGCAGCAC-3′ for monocyte chemoattractant protein 1
(MCP1); forward, 5′-ACACAGTGTGCTCACGCATGA-3′ and reverse,
5′-GCAAAGTTATATGCCGACAGC-3′ for interleukin (IL)6; forward,
5′-CGGAATTGGGGTTCATTTGT-3′ and reverse, 5′-ACTTGCAGTCCGATGCAAGC-3′
for IL8; forward, 5′-GGTAATTAACGCCAAGTCTGA-3′ and reverse,
5′-TGGTCAGCCTAGCTGAGGA-3′ for 18S. The PCR cycling conditions for
the gene expression were as follows: 5 min at 95°C for initial
denaturation, 39 cycles of 30 sec at 95°C, 30 sec at 58°C and 30
sec at 72°C. The qPCR data were obtained from five different
samples in triplicate. Calculations of relative normalized gene
expression were performed according to the ΔCt method as described
before (7). The data were normalized
to housekeeping gene, 18S ribosomal RNA.
Statistical analysis
All data are reported as the mean ± standard error.
Statistical analysis was performed using one-way analysis of
variance (ANOVA) and analyzed variance using the IBM software SPSS
v22.0 (IBM Corp., Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of HFD and exercise on body
weight, visceral fat weight and plasma lipid profiles
As hypothesized, HFD induced a 2.75-fold increase in
body weight, an ~3.5-fold increase in visceral fat body weight
compared with the control C57BL/6J mice that were fed regular mouse
chow (P<0.05; Table I). The levels
of plasma TC and TG were higher in the HFD group than those mice
fed with regular mouse chow. However, swimming exercise prevented
HFD-induced increases in visceral fat weight, plasma TC and TG
levels (Table I). Notably, no
significant difference in body weight was noted between HFD alone
and HFD with exercise. In addition, no significant difference in
HDL level was observed between the three groups of mice.
 | Table I.Effects of exercise on characteristics
of mice fed with a HFD. |
Table I.
Effects of exercise on characteristics
of mice fed with a HFD.
| Parameter | Control | HFD | HFD + exercise |
|---|
| Body weight (g) |
4.12±0.28 |
11.34±1.08a |
9.58±1.66a |
| Visceral fat weight
(g) |
0.49±0.08 |
1.71±0.31a |
1.08±0.11a,b |
| Total cholesterol
(mg/dl) |
99.34±8.32 |
148.23±6.77a |
120.08±7.11a,b |
| Triglycerides
(mg/dl) |
70.11±5.28 |
97.13±6.25a |
80.71±4.01a,b |
| High-density
lipoprotein (mg/dl) |
63.65±8.63
(4) |
62.81±7.56 |
64.22±7.93 |
Exercise prevented HFD-induced
impairment of vascular function
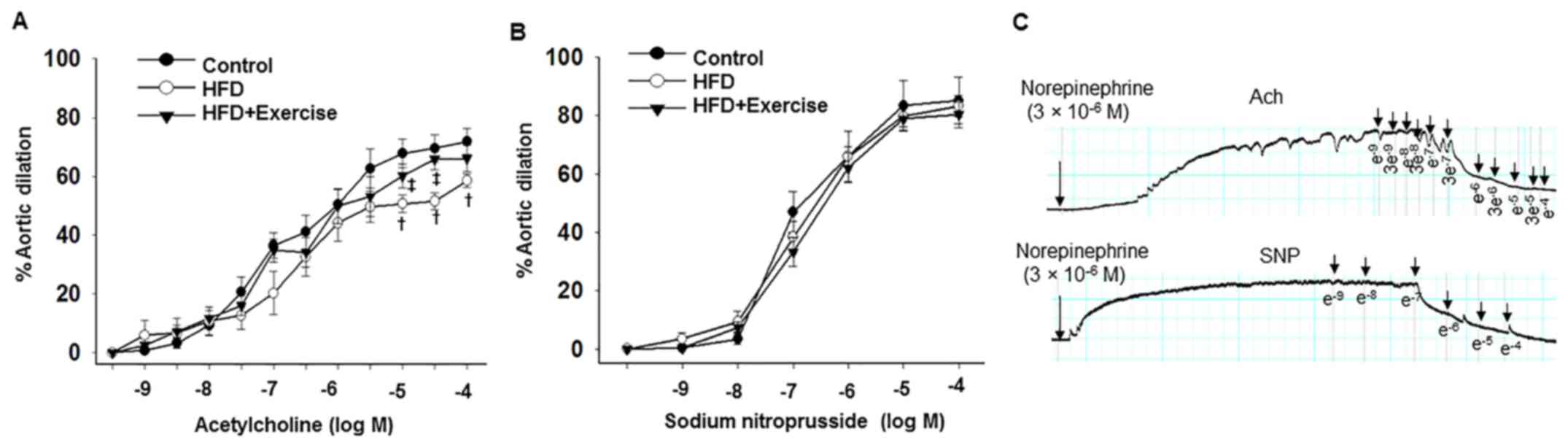
Aortic endothelium-dependent dilation responses to
acetylcholine were decreased in the HFD mice compared with the
control C57BL/6J mice fed with regular mouse chow, and these
abnormalities were prevented in the HFD with swimming exercise
group (Fig. 1A and Table II). However, no differences in sodium
nitroprusside-induced aortic endothelium-independent dilation
responses were observed between the three groups (Fig. 1B and Table
II), indicating that exercise prevented HFD-induced impairment
of the aortic endothelium-dependent relaxation function.
 | Table II.Exercise improved aortic relaxation
(%) in mice fed with a HFD. |
Table II.
Exercise improved aortic relaxation
(%) in mice fed with a HFD.
|
| Control | HFD | HFD + exercise |
|---|
|
|
|
|
|
|---|
| Dose (mol) | Ach | SNP | Ach | SNP | Ach | SNP |
|---|
|
1×10−9 |
0.78±0.53 |
0.45±0.35 |
5.93±5.16 |
3.52±1.97 |
2.49±1.26 |
0.42±0.21 |
|
3×10−9 |
3.29±1.76 |
|
6.72±4.91 |
|
7.13±2.18 |
|
|
1×10−8 |
9.36±3.33 |
3.39±1.74 |
10.73±4.87 |
9.36±3.68 |
11.72±2.59 |
7.48±1.69 |
|
3×10−8 |
20.67±5.03 |
|
12.61±4.63 |
|
15.99±3.40 |
|
|
1×10−7 |
36.51±4.22 |
46.92±6.91 |
20.27±7.41 |
38.39±5.38 |
34.83±3.95 |
33.31±5.03 |
|
3×10−7 |
41.16±5.71 |
|
32.61±6.46 |
|
34.14±4.92 |
|
|
1×10−6 |
50.45±5.45 |
66.06±8.64 |
44.18±6.30 |
65.91±
3.45 |
49.84±5.82 |
62.07±5.14 |
|
3×10−6 |
62.67±6.66 |
|
49.59±5.28 |
|
53.10±6.83 |
|
|
1×10−5 |
67.80±4.90 |
83.44±8.50 |
50.70±3.05a |
79.67±3.64 |
60.17±4.06b |
78.81±4.23 |
|
3×10−5 |
69.52±4.73 |
|
51.55±2.83a |
|
65.84±3.55b |
|
|
1×10−4 |
71.85±4.36 |
85.18±8.00 |
58.53±2.20a |
83.21±3.72 |
66.10±4.49 |
80.28±4.36 |
Exercise prevented HFD-induced
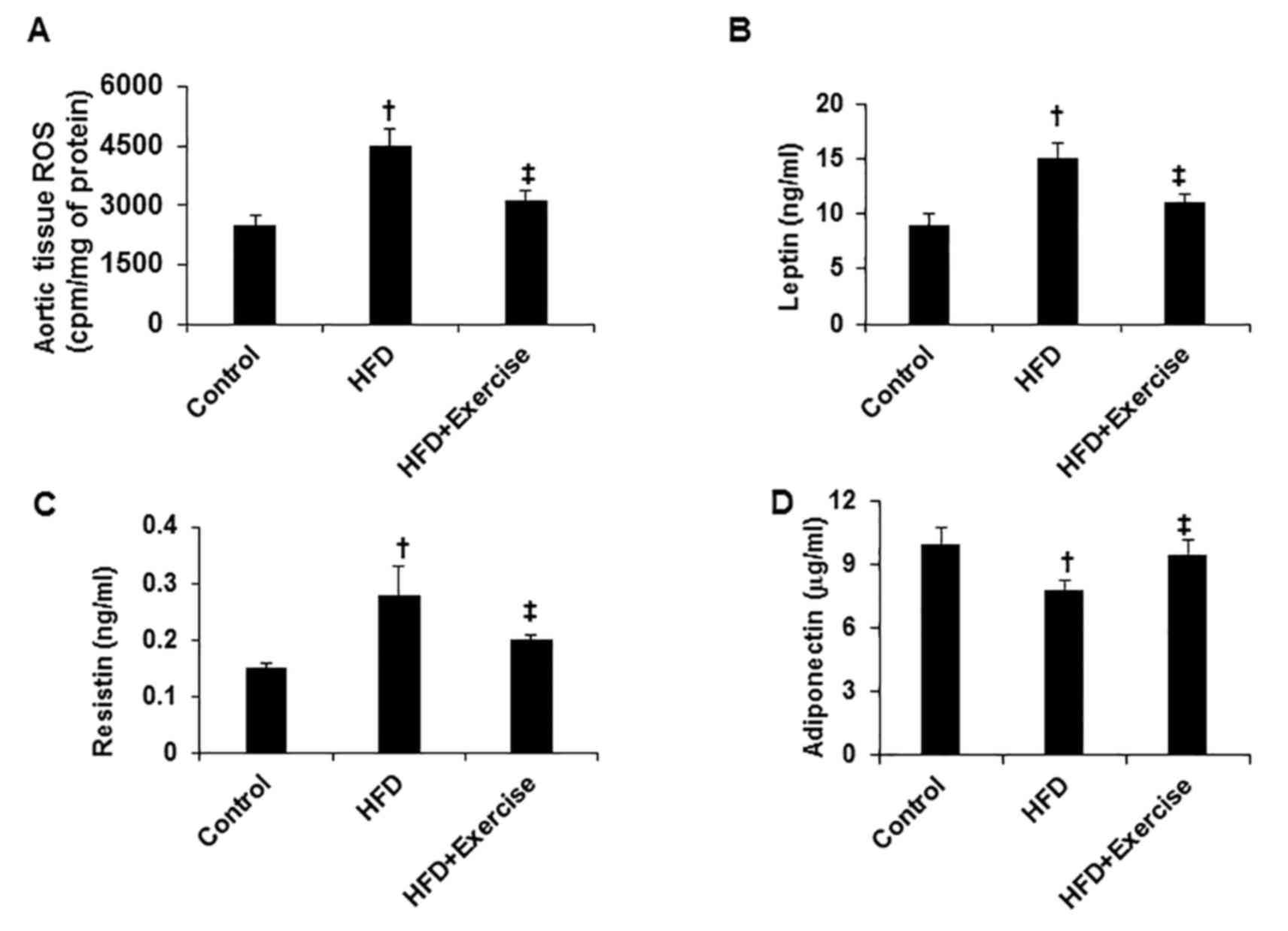
oxidative stress and proinflammatory adipokine expression
HFD induced increases in oxidant stress, assessed by
ROS assay in the aortic tissues compared with the control C57BL/6J
mice fed with regular mouse chow (Fig.
2A). In addition, HFD increased the proinflammatory adipokine
expression of leptin and resistin, and inhibited the
anti-inflammatory adipokine expression of adiponectin (Fig. 2B-D). However, swimming exercise
significantly prevented these HFD-induced abnormalities (Fig. 2).
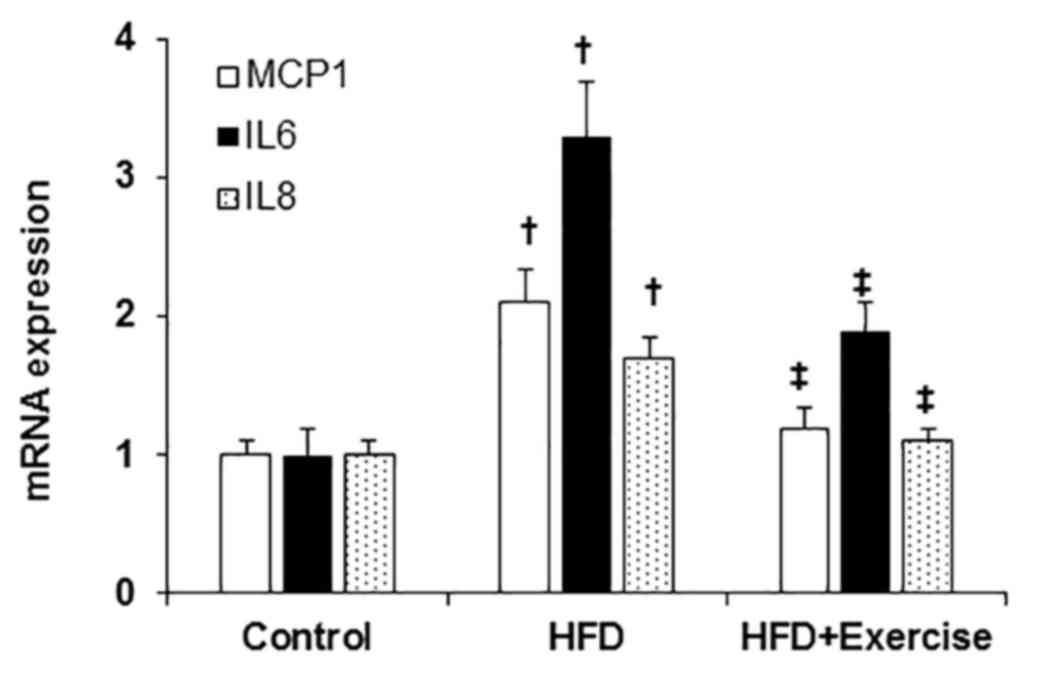
Exercise prevented HFD-induced
proinflammatory response
The levels of the pro-inflammatory transcripts,
MCP1, IL6 and IL8 were elevated in the aortic samples from HFD mice
compared with those of the control mice fed with regular mouse chow
(Fig. 3). However, swimming exercise
significantly decreased the HFD-induced aortic proinflammatory
response demonstrated by decreased expression levels of MCP1, IL6
and IL8 (Fig. 3).
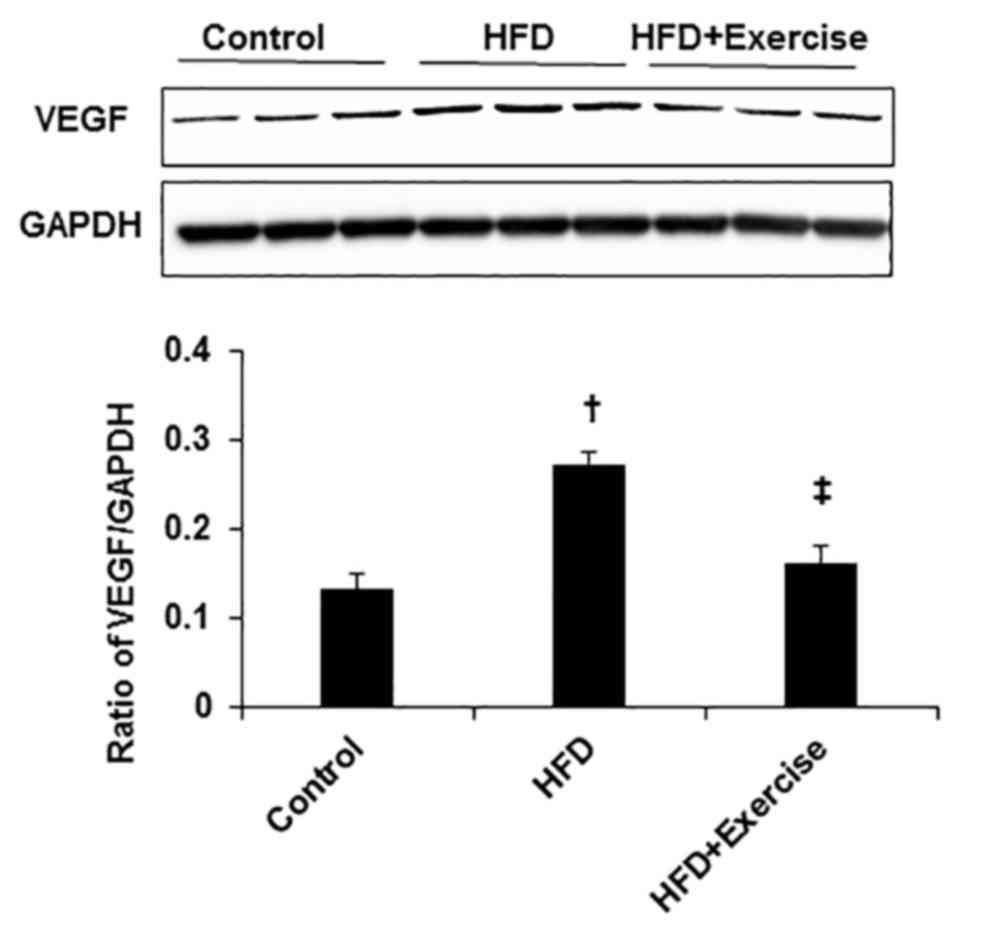
Exercise inhibited HFD-induced
angiogenesis in skeletal muscle
To reveal the effect of HFD on angiogenesis, the
protein expression level of angiogenic factor, VEGF was measured.
The protein expression level of VEGF was greater in the skeletal
muscles of HFD mice compared with the control mice (Fig. 4). However, swimming exercise
significantly decreased HFD-induced VEGF expression levels in the
gastrocnemius skeletal muscle samples (Fig. 4).
Discussion
The primary finding in the current study is that was
that consumption of a HFD for 16 weeks induced increases in plasma
dyslipidemia, oxidative stress, proinflammatory
adipokines/cytokines, as well as skeletal muscle angiogenesis.
These pathophysiological changes contributed to impairment of
aortic endothelium-dependent dilation. Furthermore, swimming
exercise prevented HFD-increased dyslipidemia, ROS, proinflammatory
adipokines/cytokines, skeletal muscle VEGF expression and
associated aortic dysfunction. The current findings are of
considerable translational importance, as obesity and sedentary
lifestyles are strongly associated with vascular dysfunction and
CVD.
Dyslipidemia, one of the pathophysiological
characteristics in obese patients, includes high levels of plasma
TC and TG, low levels of HDL, and is key in the development of CVD
in obese patients (12). For example,
in a large series of 26,000 overweight children, 14.1% of patients
had abnormal TC levels, 15.8% of patients had abnormal LDL-C, 11.1%
of patients had abnormal HDL-C and 14.3% of patients had abnormal
TG levels (13). Consistent with these
data, the present study demonstrated that HFD induced increases in
body weight and visceral fat body weight using abnormal lipid
profile assays. The coexistence of these pathophysiological changes
markedly aggravated the lipid accumulation and impaired aortic
endothelium-dependent dilation in the present study. Thus,
acetylcholine may induce aortic dilation via NO production and
bioavailability, and sodium nitroprusside may induce vascular
smooth muscle cell relaxation via NO/cyclic guanosine
monophosphate/protein kinase G signaling pathways (14,15).
Increased oxidative stress is one of the underlying
mechanisms in obesity-induced CVD (16). The Framingham study demonstrated that
there was an increase in urinary levels of 8-epi-prostaglandin F2α,
which is a systemic oxidative stress marker and was significantly
linked to body mass index in 2,828 obese individuals (17). ROS are produced during mitochondrial
respiration, as well as from outside the mitochondria, via free
fatty acid β-oxidation, nicotinamide adenine dinucleotide phosphate
(NADPH) oxidase, xanthine oxidase, lipoxygenase and cyclooxygenases
(18,19). As anticipated, HFD increased the level
of aortic ROS, serum leptin and resistin, which further prompted
aortic tissue maladaptive immune response and release of
proinflammatory cytokines, including MCP1, IL6 and IL8. It has been
demonstrated that HFD and high-fructose beverages increased body
weight, leptin, inflammation and insulin resistance that are
associated with steatosis, and oxidative stress in plasma and
tissues, indicating that oxidative stress and inflammation are
important contributors to the pathogenesis of CVD (20,21).
Recent studies have shown that physical activity
decreases the risk of chronic diseases, and improves quality of
life, as well as increasing life expectancy (22,23). For
example, exercise increases atheroprotection by inhibition of
oxidative stress and inflammation through two distinct signaling
pathways (24). Exercise increases
laminar shear stress activation to decrease ROS activity and to
preserve endothelial NO bioavailability (25). Furthermore, exercise increases
expression levels of anti-inflammatory cytokines in adipose tissue
(25). One study indicated that
exercise improves mitochondrial performance, and thus decreases ROS
production and maladaptive immune response (26). Consistent with these studies, the
present study further identified that exercise increased
anti-inflammatory adipokine adiponectin expression levels and
inhibited proinflammatory adipokine expression levels of leptin and
resistin. Therefore, exercise or physical activity exerts
protective effects in the pathologic conditions of over-nutrition
and obesity, as well as other CVDs.
In the present study, HFD increased VEGF expression
levels in skeletal muscle, which is an important marker for
angiogenesis, indicating that HFD prompts mouse obesity and
endothelial function impairment that further induce VEGF expression
and angiogenesis with a feed-back mechanism. Other factors, such as
VEGFs, PDGFs, angiopoietins and fibroblast growth factors are
involved in regulating angiogenesis (27).
In conclusion, these data demonstrate that exercise
prevented HFD-induced impairment of aortic dysfunction, and these
protective effects were accompanied by a reduction in ROS and
expression of inflammatory cytokines. The link between increased
ROS, maladaptive immune responses, and aortic dysfunction offers
the potential for identifying the origins of the impairment of
vascular function and contributes to further development of novel
therapeutic strategies.
Acknowledgements
The present study was supported by Xianning Science
Funding (grant no. XN201516) to Dr Jun Fang.
References
|
1
|
Ramalho L, da Jornada MN, Antunes LC and
Hidalgo MP: Metabolic disturbances due to a high-fat diet in a
non-insulin-resistant animal model. Nutr Diabetes. 7:e2452017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang S, Liu H, Meng N, Li B and Wang J:
Hypolipidemic and Antioxidant effects of Malus toringoides
(Rehd.) hughes leaves in high-fat-diet-induced hyperlipidemic rats.
J Med Food. 20:258–264. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lim S and Meigs JB: Links between ectopic
fat and vascular disease in humans. Arterioscler Thromb Vasc Biol.
34:1820–1826. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Townsend RR: Arterial stiffness:
Recommendations and Standardization. Pulse Basel. 4 Suppl 1:3–7.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamaguchi R, Yamamoto T, Sakamoto A,
Ishimaru Y, Narahara S, Sugiuchi H and Yamaguchi Y: Chemokine
profiles of human visceral adipocytes from cryopreserved
preadipocytes: Neutrophil activation and induction of nuclear
factor-kappa B repressing factor. Life Sci. 143:225–230. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Selcuk A, Bulucu F, Kalafat F, Cakar M,
Demirbas S, Karaman M, Ay SA, Saglam K, Balta S, Demirkol S, et al:
Skinfold thickness as a predictor of arterial stiffness: Obesity
and fatness linked to higher stiffness measurements in hypertensive
patients. Clin Exp Hypertens. 35:459–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park SY, Rossman MJ, Gifford JR, Bharath
LP, Bauersachs J, Richardson RS, Abel ED, Symons JD and Riehle C:
Exercise training improves vascular mitochondrial function. Am J
Physiol Heart Circ Physiol. 310:H821–H829. 2016.PubMed/NCBI
|
|
8
|
Morishima T, Restaino RM, Walsh LK,
Kanaley JA, Fadel PJ and Padilla J: Prolonged sitting-induced leg
endothelial dysfunction is prevented by fidgeting. Am J Physiol
Heart Circ Physiol. 311:H177–H182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li YY, Shi ZM, Yu XY, Feng P and Wang XJ:
Urotensin II-induced insulin resistance is mediated by NADPH
oxidase-derived reactive oxygen species in HepG2 cells. World J
Gastroenterol. 22:5769–5779. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan Q, Liu Q, Zweier JL and Liu X: Potency
of authentic nitric oxide in inducing aortic relaxation. Pharmacol
Res. 55:329–334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang M and Fang J: TNF-α regulates
apoptosis of human vascular smooth muscle cells through gap
junctions. Mol Med Rep. 15:1407–1411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang FL, Xing YQ, Wu YH, Liu HY, Luo Y,
Sun MS, Guo ZN and Yang Y: The prevalence, awareness, treatment,
and control of dyslipidemia in northeast China: A population-based
cross-sectional survey. Lipids Health Dis. 16:612017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raj M: Obesity and cardiovascular risk in
children and adolescents. Indian J Endocrinol Metab. 16:13–19.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamashita T, Kawashima S, Ohashi Y, Ozaki
M, Rikitake Y, Inoue N, Hirata K, Akita H and Yokoyama M:
Mechanisms of reduced nitric oxide/cGMP-mediated vasorelaxation in
transgenic mice overexpressing endothelial nitric oxide synthase.
Hypertension. 36:97–102. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rodriguez-Pascual F, Miras-Portugal MT and
Torres M: Effect of cyclic GMP-increasing agents nitric oxide and
C-type natriuretic peptide on bovine chromaffin cell function:
Inhibitory role mediated by cyclic GMP-dependent protein kinase.
Mol Pharmacol. 49:1058–1070. 1996.PubMed/NCBI
|
|
16
|
Rios FJ, Neves KB, Nguyen Dinh Cat A, Even
S, Palacios R, Montezano AC and Touyz RM: Cholesteryl
ester-transfer protein inhibitors stimulate aldosterone
biosynthesis in adipocytes through Nox-dependent processes. J
Pharmacol Exp Ther. 353:27–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Keaney JF Jr, Larson MG, Vasan RS, Wilson
PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA and
Benjamin EJ: Framingham Study: Obesity and systemic oxidative
stress: Clinical correlates of oxidative stress in the Framingham
Study. Arterioscler Thromb Vasc Biol. 23:434–439. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Csányi G and Miller FJ Jr: Oxidative
stress in cardiovascular disease. Int J Mol Sci. 15:6002–6008.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rocha M, Apostolova N, Hernandez-Mijares
A, Herance R and Victor VM: Oxidative stress and endothelial
dysfunction in cardiovascular disease: Mitochondria-targeted
therapeutics. Curr Med Chem. 17:3827–3841. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lozano I, Van der Werf R, Bietiger W,
Seyfritz E, Peronet C, Pinget M, Jeandidier N, Maillard E,
Marchioni E, Sigrist S, et al: High-fructose and high-fat
diet-induced disorders in rats: Impact on diabetes risk, hepatic
and vascular complications. Nutr Metab (Lond). 13:152016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo X, Li J, Tang R, Zhang G, Zeng H, Wood
RJ and Liu Z: High fat diet alters gut microbiota and the
expression of paneth cell-antimicrobial peptides preceding changes
of circulating inflammatory cytokines. Mediators Inflamm.
2017:94748962017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wong A, Sanchez-Gonzalez M, Kalfon R,
Alvarez-Alvarado S and Figueroa A: The effects of stretching
training on cardiac autonomic function in obese postmenopausal
women. Altern Ther Health Med. 23:20–26. 2017.PubMed/NCBI
|
|
23
|
Toyama K, Sugiyama S, Oka H, Hamada M,
Iwasaki Y, Horio E, Rokutanda T, Nakamura S, Spin JM, Tsao PS, et
al: A pilot study: The beneficial effects of combined
statin-exercise therapy on cognitive function in patients with
coronary artery disease and mild cognitive decline. Intern Med.
56:641–649. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Onat A: Metabolic syndrome: Nature,
therapeutic solutions and options. Expert Opin Pharmacother.
12:1887–1900. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Szostak J and Laurant P: The forgotten
face of regular physical exercise: A ‘natural’ anti-atherogenic
activity. Clin Sci (Lond). 121:91–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peeri M and Amiri S: Protective effects of
exercise in metabolic disorders are mediated by inhibition of
mitochondrial-derived sterile inflammation. Med Hypotheses.
85:707–709. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Silvennoinen M, Rinnankoski-Tuikka R,
Vuento M, Hulmi JJ, Torvinen S, Lehti M, Kivelä R and Kainulainen
H: High-fat feeding induces angiogenesis in skeletal muscle and
activates angiogenic pathways in capillaries. Angiogenesis.
16:297–307. 2013. View Article : Google Scholar : PubMed/NCBI
|