Introduction
Oxidative stress is generally described as any
biochemical or physiological condition that results in an imbalance
between the rate of reactive oxygen species (ROS) production and
the ability of body antioxidant systems to overcome the disruption
caused by these chemically reactive radicals (1). The most common ROS are hypochlorite ion,
superoxide anion, hydrogen peroxide and in particular hydroxyl
radical, which is considered the most reactive (2). Free radicals typically interact with
polyunsaturated fatty acid residues of cell membrane phospholipids,
forming lipid peroxides that can further produce mutagenic and
carcinogenic malondialdehyde (MDA) in addition to cyclic DNA
adducts (3,4). Additionally, oxidative stress has potent
effects in the pathogenesis of several clinical conditions
including hypertension (5), coronary
heart diseases (6) and both type 1
and type 2 diabetes mellitus (7).
Regarding the nature of ROS, some are notably toxic and are rapidly
detoxicated to less harmful metabolites by a range of cell
mechanisms that are either enzymatic or non-enzymatic (8). This conversion is key in affected cells
to enable normalization of cell function and metabolism (9).
Organophosphate (OP) compounds consist of a variety
of chemicals that are the most toxic among all pesticides to
vertebrates and humans (10).
However, OPs are still widely used in certain third world countries
for agricultural or veterinary interventions as solvents or extreme
pressure additives (11,12). In the last twenty years the improper
use of OPs has led to toxic resistance, which is of major concern
to the environment, animals and human populations (13). Additionally, it has been reported that
residual quantities of OPs may be detected in plants, tissues of
organisms and foods products (7). OPs
are generally rapidly absorbed by the skin or mucous membranes of
the oral cavity during inhalation (8), which mediates the onset of adverse
reactions. Furthermore, OPs are considered to have damaging effects
on the urinary (14) and reproductive
(15) systems as for other vital
organs.
The neurobehavioral development of fetuses and
children is considered to be one of the disorders with an
undetermined association with OP exposure even at very low levels.
OPs are classified into four principal groups, namely as herbicides
(trichlorfon), ophthalmic glaucoma agents, certain types of nerve
gases (VX and sarin) and most notably, insecticides, for which
there is a marked number (including diazinon, malathion and
parathion) (16). Although it has
been demonstrated that OPs inhibit the activity of
acetylcholinesterase (AChE), the main enzyme responsible for
acetylcholine (ACh) degradation (17), further study revealed that the toxic
consequences of OP exposure may be due to induction of oxidative
stress (18). The acute toxicity of
OPs manifests as cholinergic damage, which is associated with
irreversible covalent inhibition of cholinesterase activity in the
central nervous system (19). As a
result, accumulated ACh is not degraded and nicotinic receptors are
activated, and consequently cell death is prompted (20).
Diazinon
[O,O-diethyl-O-(2-isopropyl-6-methyl-4-pyrimidinyl)phosphorothioate]
is a colorless liquid and also a thiophosphoric acid derivative
that is ordinarily used to control pests and insects (21). There is a range of data indicating the
association between diazinon toxicity and altered liver enzyme
function and biochemical and hematological parameters (22–24). As
mentioned, the main mechanism by which diazinon induces acute
intoxication is AChE inhibition, which causes an abnormal
accumulation of ACh in the synaptic cleft (25). Exposure to sub-lethal doses of
diazinon is associated with chronic toxicity, and ultimately
elevated lipid peroxidation and decreased antioxidant enzyme
activity (10,26). Furthermore, since diazinon is fat
soluble, prolonged toxicity should be considered if a substantial
quantity of diazinon is stored in fatty tissues (27). By contrast, it has been established
that antioxidants as scavenger substances are capable of
neutralizing ROS or inhibiting free radical formation, and thus
effectively protect cells against oxidative stress-inducing agents
including OPs (28). There are two
major types of antioxidants: Enzymatic and non-enzymatic. Over
previous years, vitamins C and E and numerous other natural and
synthetic compounds functioning as non-enzymatic antioxidants have
been used against cellular oxidative stress induction caused by OP
substances (29).
There are a number of synthetic antioxidants that
are permitted by the US Food and Drug Administration for use in
food products (30). Among edible
synthetic antioxidants, tert-butylhydroquinone (TBHQ) is primarily
used as a food additive for unsaturated vegetable oils (31). TBHQ is considered to mainly function
via induction of nuclear translocation of the transcription factor
NF-E2-related factor 2 (Nrf2), which in turn potently regulates the
gene expression of certain antioxidant enzymes, including heme
oxygenase-1 and glutathione S-transferase (32).
Despite the importance of OP toxicity as a health
issue and the risk of constant exposure of farmers to OP
substances, particularly diazinon, there is no sufficient data
regarding the combined effects of diazinon and TBHQ on
hematological indices alteration. Therefore, the present study
aimed to evaluate the effects of sub-lethal doses of the OP
insecticide diazinon on hematological parameters, and to
investigate the possible attenuative role of TBHQ against ROS
production in Wistar rats.
Materials and methods
Chemicals
All chemicals used were of pure analytical grade.
Diazinon, purity 99%, was obtained from Merck KGaA (Darmstadt,
Germany) and TBHQ was supplied by Sigma-Aldrich (Merck KGaA).
Animals
A total of 28 adult male Wistar rats weighing
250–300 g (mean age, 18±2 weeks old), purchased from the central
animal house facility at Mashhad University of Medical Sciences
(Mashhad, Iran), were utilized for the current cross-sectional
experimental study. The rats were caged (with a mean number of 4
animals per cage; maximum, n=6) with paddy husk bedding under a
12-h light/dark cycle (light period from 6.30 a.m. to 6.30 p.m.),
23±2°C and 60% humidity. The rats were acclimatized for at least 7
days prior to randomizing into experimental groups. The animals
were permitted access to a commercial pellet diet (manufactured in
Javaneh Khorasan, Mashhad, Iran) and tap water ad libitum.
All animals received human care and the protocols of the experiment
were approved by the Animal Welfare Committee of the School of
Veterinary Medicine, Ferdowsi University of Mashhad (Mashhad,
Iran).
Experimental protocol and drugs
The 28 male Wistar rats were categorized randomly in
4 equal-sized groups (n=7 per group) comprising of a control group
and three experimental groups, as follows: Group 1 (Control), which
received corn oil as a vehicle for both TBHQ and diazinon, once a
day via gavage tube; group 2 (diazinon-treated group), which was
administrated diazinon (10 mg/kg/day) in corn oil, once a day via
gastric tube, with this selected dose having been used in previous
studies and demonstrated efficacy in altering the biochemical and
hematological parameters of male Wistar rats (24,29); group
3 (TBHQ-treated group), in which TBHQ was orally administrated at a
dose of 0.028 g/kg of diet (in corn oil), which has previously been
indicated to have antioxidant effects (33); and group 4 (TBHQ+diazinon), in which
diazinon and TBHQ (also in corn oil) were orally administrated at
the aforementioned doses (10 mg/kg/day of diazinon and 0.028 g/kg
of diet of TBHQ) once a day via gavage tube.
In attempt to approximate the average daily diet
consumption in grams, the food intake of rats was assessed over the
7 days of acclimatization by calculating the difference between
intact pre-weighed food and the quantity of food remaining after 24
h in cages. All rats were non-fasted by the time of oral
administration (between 10 and 11 a.m. in the morning). The first
treatment day was appointed the first day of the experiment. The
volume of treatment solution was equal for all the groups.
At the end of day 49, animals were euthanized. Whole
blood samples were immediately collected through cardiac puncture
(up to 7 ml was drawn from each rat). Using a Bayer-Advia 120
hematology analyzer (Bayer Corporation, Whippany, NJ, USA), the
blood samples with anti-coagulant EDTA were instantly analyzed to
evaluate the main hematological indices, namely red blood cell
(RBC) count, hemoglobin (Hb) concentration, hematocrit (Hct)
levels, white blood cell (WBC) count, mean corpuscular Hb (MCH),
mean corpuscular volume (MCV), mean corpuscular Hb concentration
(MCHC) and platelet count.
Statistical analysis
Statistical analysis was conducted using SPSS
version 22 (IBM Corp., Armonk, NY, USA). One-way analysis of
variance with post hoc Tukey's tests were used to determine the
significance of differences, with P<0.05 considered to indicate
statistical significance. The F-test was performed to determine the
normal distribution of variances between groups. The results are
presented as the mean ± standard deviation obtained from the 7
animals in each group.
Results
Comparison of hematological
parameters
The results of hematological parameters in the
current study were assessed according to three comparisons: i)
Compared with the untreated control group; ii) compared with the
TBHQ-treated group; and iii) compared with the diazinon-treated
group.
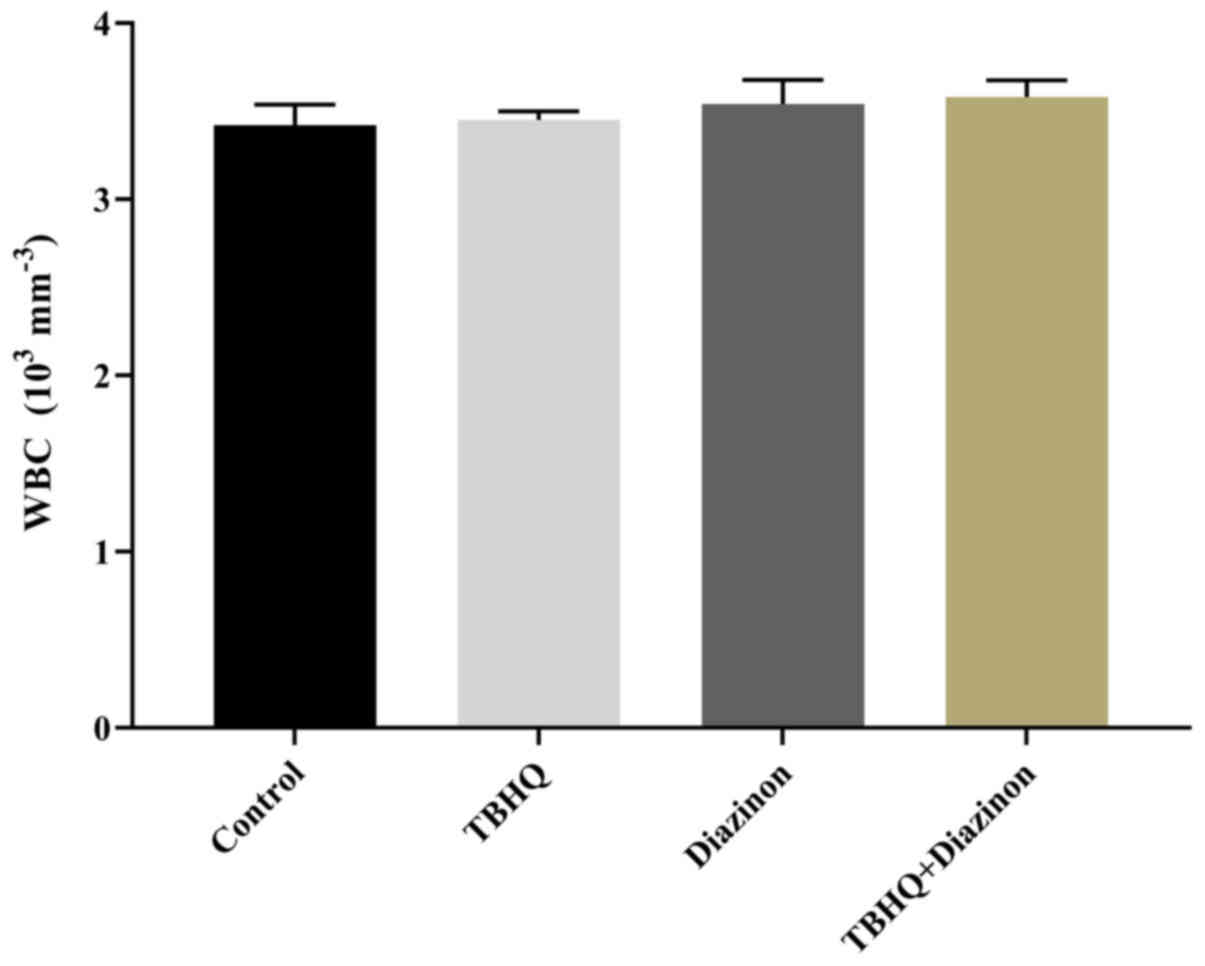
WBC count
Comparison of WBC count between the four groups in
the current study identified no statistically significant
differences, although TBHQ led to a marginal increase in WBC count
compared with the untreated control group. This enhancement was
observed in the diazinon and combination groups with increased
intensity (Fig. 1).
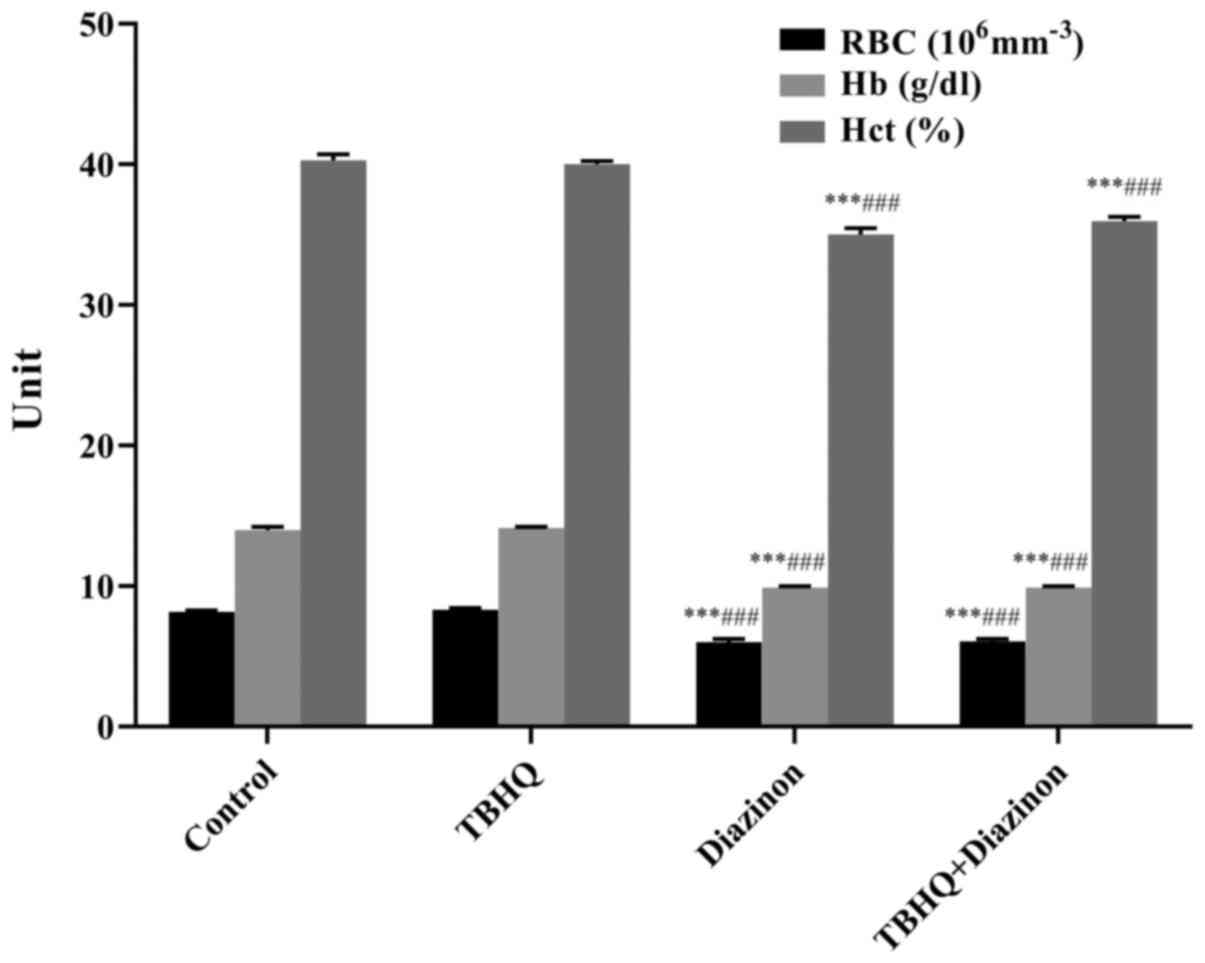
RBC, Hb and Hct
Notable changes were observed in RBC count, Hb
content and Hct levels in erythrocytes. The number of RBCs was
significantly decreased in the diazinon and TBHQ+ diazinon groups
compared with in the untreated control and TBHQ groups
(P<0.001). Hb and Hct levels were also significantly decreased
in the diazinon and TBHQ+diazinon groups compared with in the
control and TBHQ groups (P<0.001; Fig.
2).
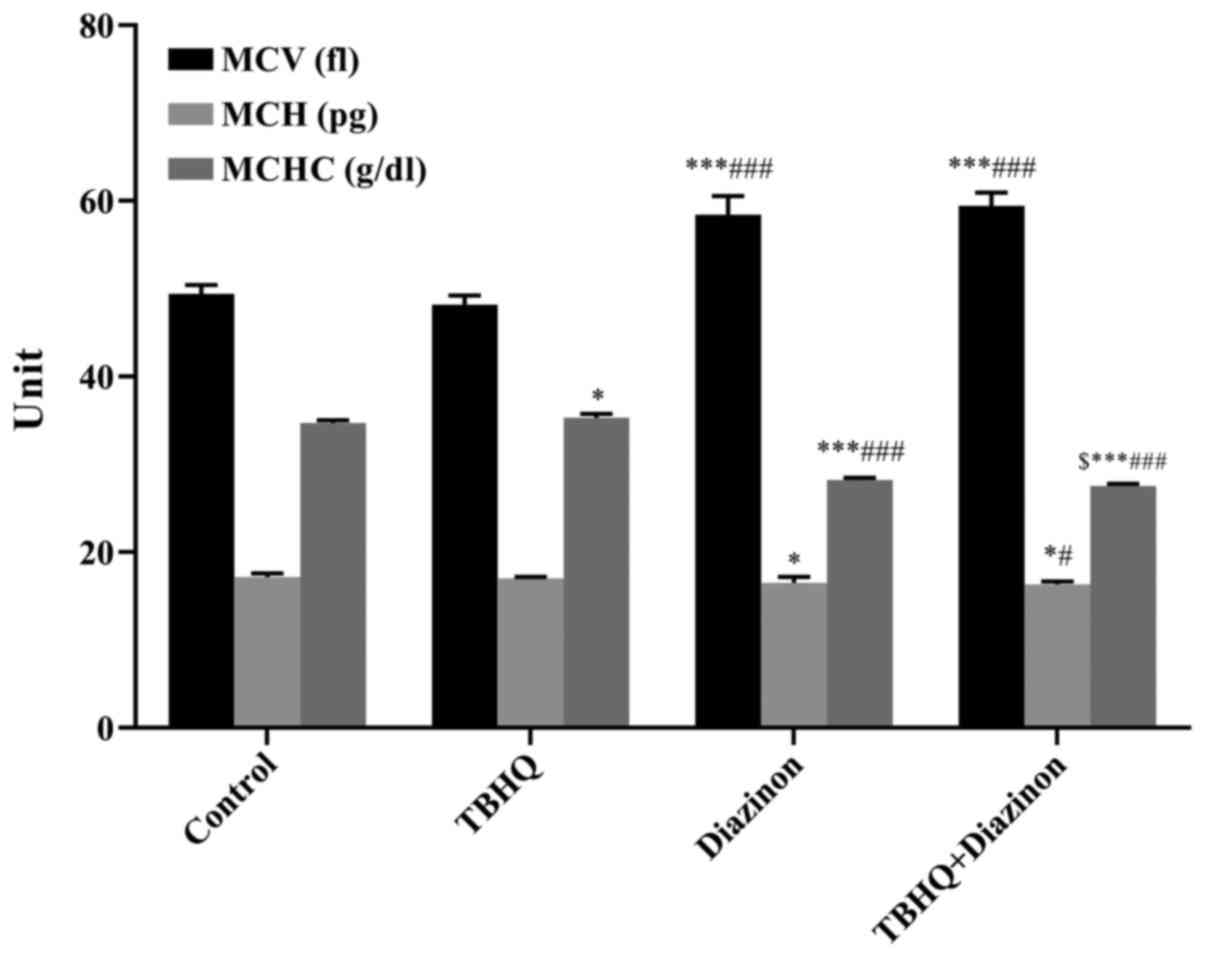
MCV, MCH and MCHC
Comparing the untreated control group with the other
groups, significant alterations were identified in MCV [diazinon
group vs. control group (P<0.001) and TBHQ+diazinon group vs.
control group (P<0.001)], MCH [diazinon group vs. control group
(P<0.05) and TBHQ+diazinon group vs. control group (P<0.05)]
and MCHC [TBHQ group vs. control group (P<0.05), diazinon group
vs. control group (P<0.001) and TBHQ+diazinon group vs. control
group (P<0.001)]. Significant differences were also identified
between the diazinon groups (single and combined treatments) and
TBHQ group concerning MCV [diazinon group vs. TBHQ group
(P<0.001) and TBHQ+diazinon group vs. TBHQ group (P<0.001)],
MCH [TBHQ+diazinon group vs. TBHQ group (P<0.05)] and MCHC
[diazinon group vs. TBHQ group (P<0.001) and TBHQ+diazinon group
vs. TBHQ group (P<0.001)]. MCHC was the only parameter that
significantly differed between the TBHQ+diazinon and diazinon
groups (P<0.05), being significantly decreased in the
TBHQ+diazinon group (Fig. 3).
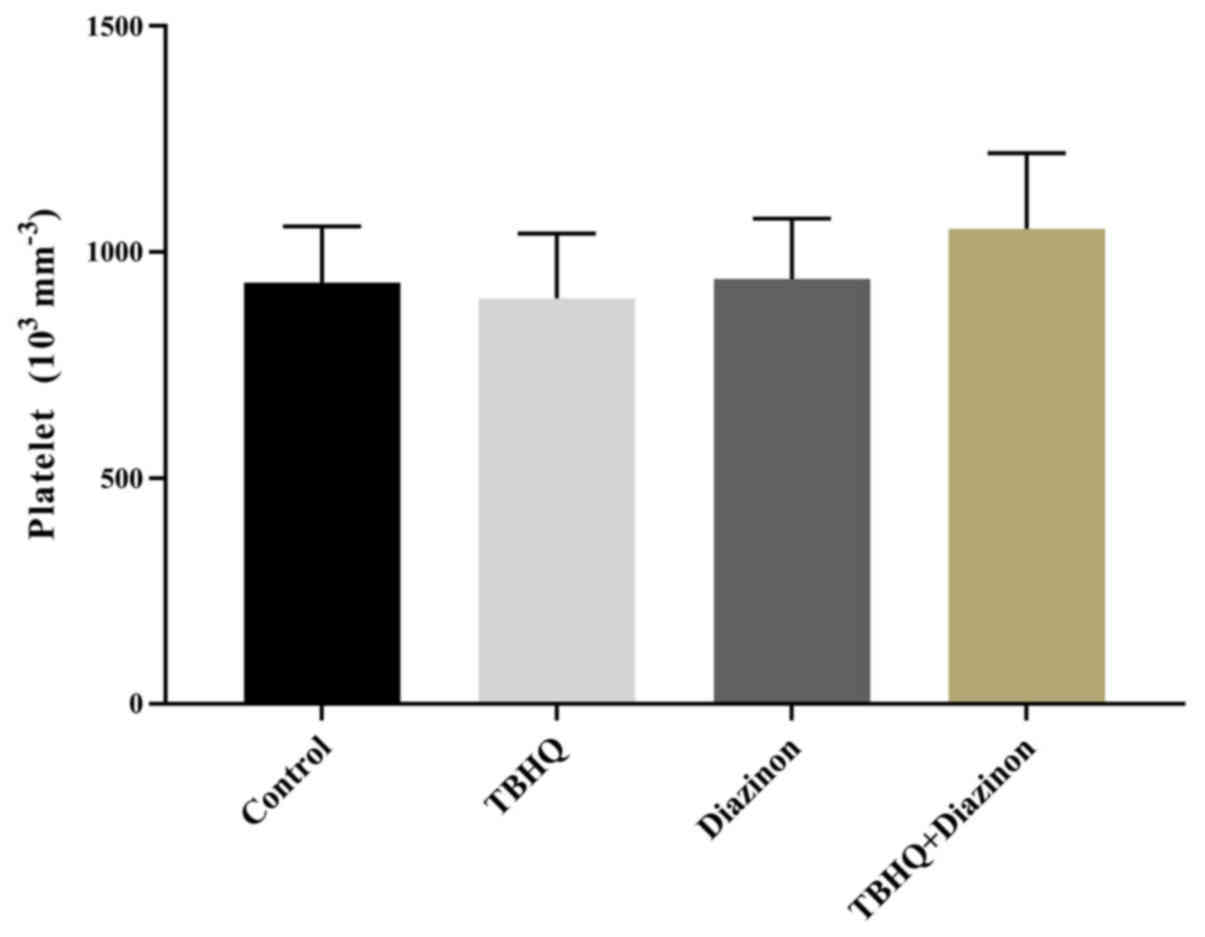
Platelet count
There were no significant differences in platelet
number between all four groups (P>0.05; Fig. 4).
Discussion
OP toxicity is considered to be among the most
important challenges currently faced by agricultural environments,
and biological environments in general. Over previous years, a
number of types of pesticides have been used for pest and insect
control, and the use of OPs has been reported to have among the
most potent effects (34). In this
regard, OPs are essential for sufficient agricultural yield, and
thus their application still continues in certain developing
countries (35). Diazinon is widely
used as an insecticide for plant protection, and also serves as an
ectoparasitic agent for parasitic control in sheep (36). As a consequence, broad distribution of
OPs including diazinon in the environment correlates with onset of
toxic effects in land and marine animals (37,38).
Alongside the natural antioxidants, permitted concentrations of
synthetic antioxidants have been demonstrated to exert protective
effects against the oxidative stress-induced cytotoxicity (39). In the present study, the attenuative
effect of TBHQ against diazinon-induced toxicity with regard to
hematological indices was evaluated in 28 male Wistar rats, in
which mild oxidative damage was observed following exposure to
sub-lethal levels of diazinon, indicated by reductions in RBC
count, Hb concentration and Hct level.
Primary hematological indices include WBC, RBC and
platelet counts. Other parameters including Hb, Hct, MCV, MCH and
MCHC provide specific information on RBC characteristics such as
size and morphology in vertebrates (40). In the current study, TBHQ, Diazinon,
or their combination had no significant effect on WBC and platelet
numbers, although the compounds had marked influence on RBC-related
indices. Notably, RBC count was decreased in male Wistar rats
following exposure to diazinon alone or in combination with TBHQ,
compared with in the control and TBHQ groups. Reductions in Hb and
Hct were also observed. Other parameters of RBCs including MCV, MCH
and MCHC were also influenced following exposure of the rats to the
compounds: MCV was increased following treatment with Diazinon and
TBHQ+diazinon compared with in the untreated and TBHQ groups; MCH
was marginally decreased in the diazinon group compared with in the
untreated group, and in the TBHQ+diazinon groups compared with in
the untreated and TBHQ groups; and MCHC was significantly lower in
the untreated group vs. TBHQ group and TBHQ+diazinon group vs.
diazinon group. Additionally, MCHC was reduced in the diazinon and
TBHQ+diazinon groups compared with in the untreated and TBHQ
groups. A possible mechanism for the reduction in RBC count is that
chronic intoxication induced by diazinon leads to oxidation of the
membrane phospholipids of the RBC cells, subsequently decreasing Hb
and Hct and effecting related indices including MCV, MCH, and MCHC;
while the antioxidant of the present study (TBHQ) did not
efficiently protect the morphology of RBCs against 10 mg/kg/day
diazinon. Regarding WBC and PLT counts, the related mechanism
cannot be concluded due to contradictory results of previous
studies mentioned below.
Many investigations have been conducted concerning
the effect of diazinon toxicity on hematological indices, which
have yielded contradictory data (41,42);
nevertheless, the current results do not contradict the findings of
most previous investigations mentioned above. However, to the best
of our knowledge, there is no published report on utilization of
TBHQ as an antioxidant substance and its combination with diazinon
in effecting hematological parameters.
According to previous experiments performed by
Kalender et al (22), all RBC
parameters tested except MCH were altered in rats exposed to
diazinon compared with in an untreated group at 1, 4 and 7 weeks
after treatment. A study on the effects of Basudin 600 EW (an
active substance of diazinon) in common carp fish (Cyprinus
carpio L.) demonstrated that this compound did not exert
significant effect on MCV, MCH or MCHC, but significantly decreased
leukocyte and lymphocyte count while increasing the absolute count
of developmental forms of neutrophile granulocytes including
myelocytes and metamyelocytes (41).
Another similar study conducted by Ahmad (43) highlighted the effect of diazinon
exposure toxicity in common carp, manifested as increases in RBC,
Hb and Hct. Effects of diazinon on the hematological parameters of
fry rainbow trout (Oncorhynchus mykiss) have also been
revealed, in that sub-lethal doses of the OP substance led to a
significant decrease in RBC and Hb, and increased MCV and MCH in a
diazinon-exposed group in comparison with untreated groups
(42). The results of a study by
Yassa et al (44) indicated a
significant decrease in Hb concentration and RBC number in a
diazinon group from the third week of the experiment compared with
levels in untreated rats. Banaee et al (45) identified that diazinon treatment
caused an increase in RBC, Hb and Hct parameters while decreasing
MCV and MCH compared with in an untreated group in common carp.
Various doses of diazinon led to reduction in RBC count, Hb
concentration and other indices including Hct, MCV, MCH and MCHC in
fingerling European catfish (Silurus glanis L.) in an
investigation performed by Köprücü et al (46). Additionally, other studies have also
demonstrated alterations in hematological indices in different
species including rat (47), African
catfish (Clarias gariepinus) (48) and Indian carp [Cirrhinus
mrigala (Hamilton)] (49).
Regarding improvements to the current study,
extending the number of rats in each experimental group, using
higher doses of TBHQ, and assessment of oxidative stress-related
markers by methods including monitoring of their gene expression
patterns may be useful in further investigations to acquire more
comprehensive and conclusive results.
Nevertheless, the present data suggest that TBHQ as
a novel synthetic antioxidant could not efficaciously attenuate the
effects of sub-lethal exposure of diazinon on hematological indices
in male Wistar rats, despite attenuating certain red cell indices
affected by diazinon. The use of higher doses of TBHQ may be
necessary to acquire a more convincing outcome.
Acknowledgements
The authors are thankful to Dr Mostafa Heidarzadegan
for his assistance with the study.
References
|
1
|
Sharma P, Jha AB, Dubey RS and Pessarakli
M: Reactive oxygen species, oxidative damage, and antioxidative
defense mechanism in plants under stressful conditions. J Botany.
2012:2170372012. View Article : Google Scholar
|
|
2
|
Machlin LJ and Bendich A: Free radical
tissue damage: Protective role of antioxidant nutrients. FASEB J.
1:441–445. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ayala A, Muñoz MF and Argüelles S: Lipid
peroxidation: production, metabolism, and signaling mechanisms of
malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev.
2014:3604382014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beckman KB and Ames BN: Oxidative decay of
DNA. J Biol Chem. 272:19633–19636. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bokelmann I and Mahlknecht U: Valproic
acid sensitizes chronic lymphocytic leukemia cells to apoptosis and
restores the balance between pro- and antiapoptotic proteins. Mol
Med. 14:20–27. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Higashi Y, Noma K, Yoshizumi M and Kihara
Y: Endothelial function and oxidative stress in cardiovascular
diseases. Circ J. 73:411–418. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stadler K: Oxidative stress in
diabetesDiabetes: An Old Disease, a New Insight. Ahmad S:
Springer-Verlag; New York, NY: pp. 272–287. 2013
|
|
8
|
Apel K and Hirt H: Reactive oxygen
species: Metabolism, oxidative stress, and signal transduction.
Annu Rev Plant Biol. 55:373–399. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
El-Shenawy NS, El-Salmy F, Al-Eisa RA and
El-Ahmary B: Amelioratory effect of vitamin E on organophosphorus
insecticide diazinon-induced oxidative stress in mice liver. Pestic
Biochem Physiol. 96:101–107. 2010. View Article : Google Scholar
|
|
10
|
Shah MD and Iqbal M: Diazinon-induced
oxidative stress and renal dysfunction in rats. Food Chem Toxicol.
48:3345–3353. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eddleston M, Street JM, Self I, Thompson
A, King T, Williams N, Naredo G, Dissanayake K, Yu LM, Worek F, et
al: A role for solvents in the toxicity of agricultural
organophosphorus pesticides. Toxicology. 294:94–103. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Soltaninejad K and Shadnia S: History of
the use and epidemiology of organophosphorus poisoningBasic and
Clinical Toxicology of Organophosphorus Compounds. Springer; New
York, NY: pp. 25–43. 2014, View Article : Google Scholar
|
|
13
|
Casas E, Bonilla E, Ducolomb Y and
Betancourt M: Differential effects of herbicides atrazine and
fenoxaprop-ethyl, and insecticides diazinon and malathion, on
viability and maturation of porcine oocytes in vitro. Toxicol In
Vitro. 24:224–230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rodrigo L, Hernández AF, López-Caballero
JJ, Gil F and Pla A: Immunohistochemical evidence for the
expression and induction of paraoxonase in rat liver, kidney, lung
and brain tissue. Implications for its physiological role. Chem
Biol Interact. 137:123–137. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sargazi Z, Nikravesh MR, Jalali M,
Sadeghnia H, Anbarkeh FR and Mohammadzadeh L: Gender-related
differences in sensitivity to diazinon in gonads of adult rats and
the protective effect of vitamin E. Int J Womens Health Reprod Sci.
3:40–47. 2015. View Article : Google Scholar
|
|
16
|
Aluigi MG, Angelini C, Falugi C, Fossa R,
Genever P, Gallus L, Layer PG, Prestipino G, Rakonczay Z, Sgro M,
et al: Interaction between organophosphate compounds and
cholinergic functions during development. Chem Biol Interact.
157–158:305–316. 2005. View Article : Google Scholar
|
|
17
|
Buyukokuroglu ME, Cemek M, Yurumez Y,
Yavuz Y and Aslan A: Antioxidative role of melatonin in
organophosphate toxicity in rats. Cell Biol Toxicol. 24:151–158.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu XT, Ma Y, Wang C, Zhang XF, Jin DQ and
Huang CJ: Cytotoxicity and DNA damage of five organophosphorus
pesticides mediated by oxidative stress in PC12 cells and
protection by vitamin E. J Environ Sci Health B. 47:445–454. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Colović MB, Krstić DZ, Lazarević-Pašti TD,
Bondžić AM and Vasić VM: Acetylcholinesterase inhibitors:
Pharmacology and toxicology. Curr Neuropharmacol. 11:315–335. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aygun D, Erenler AK, Karatas AD and Baydin
A: Intermediate syndrome following acute organophosphate poisoning:
Correlation with initial serum levels of muscle enzymes. Basic Clin
Pharmacol Toxicol. 100:201–204. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kalender S, Uzun FG, Durak D, Demir F and
Kalender Y: Malathion-induced hepatotoxicity in rats: The effects
of vitamins C and E. Food Chem Toxicol. 48:633–638. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kalender Y, Uzunhisarcikli M, Ogutcu A,
Acikgoz F and Kalender S: Effects of diazinon on
pseudocholinesterase activity and haematological indices in rats:
The protective role of vitamin E. Environ Toxicol Pharmacol.
22:46–51. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Banaee M, Sureda A, Mirvaghefi A and
Ahmadi K: Effects of diazinon on biochemical parameters of blood in
rainbow trout (Oncorhynchus mykiss). Pestic Biochem Physiol.
99:1–6. 2011. View Article : Google Scholar
|
|
24
|
Kalender S, Ogutcu A, Uzunhisarcikli M,
Açikgoz F, Durak D, Ulusoy Y and Kalender Y: Diazinon-induced
hepatotoxicity and protective effect of vitamin E on some
biochemical indices and ultrastructural changes. Toxicology.
211:197–206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rush T, Liu XQ, Hjelmhaug J and Lobner D:
Mechanisms of chlorpyrifos and diazinon induced neurotoxicity in
cortical culture. Neuroscience. 166:899–906. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sargazi Z, Nikravesh MR, Jalali M,
Sadeghnia HR, Anbarkeh Rahimi F and Mohammadzadeh L:
Diazinon-induced ovarian toxicity and protection by vitamins E.
Iranian J Toxicol. 8:1130–1135. 2014.
|
|
27
|
Oruç EÖ and Usta D: Evaluation of
oxidative stress responses and neurotoxicity potential of diazinon
in different tissues of Cyprinus carpio. Environ Toxicol
Pharmacol. 23:48–55. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gokalp O, Buyukvanlı B, Cicek E, Ozer MK,
Koyu A, Altuntas I and Koylu H: The effects of diazinon on
pancreatic damage and ameliorating role of vitamin E and vitamin C.
Pestic Biochem Physiol. 81:123–128. 2005. View Article : Google Scholar
|
|
29
|
Ogutcu A, Uzunhisarcikli M, Kalender S,
Durak D, Bayrakdar F and Kalender Y: The effects of organophosphate
insecticide diazinon on malondialdehyde levels and myocardial cells
in rat heart tissue and protective role of vitamin E. Pestic
Biochem Physiol. 86:93–98. 2006. View Article : Google Scholar
|
|
30
|
Meyer AS and Isaksen A: Application of
enzymes as food antioxidants. Trends Food Sci Technol. 6:300–304.
1995. View Article : Google Scholar
|
|
31
|
Sargazi S, Moghadam-Jafari A and
Heidarpour M: Protective effect of tert butyl hydroquinone on
diazinon-induced oxidative stress in brain and heart of male rats.
Zahedan J Res Med Sci. 18:1–5. 2016.
|
|
32
|
Tasset I, Pérez-De La Cruz V,
Elinos-Calderón D, Carrillo-Mora P, González-Herrera IG, Luna-López
A, Konigsberg M, Pedraza-Chaverrí J, Maldonado PD, Ali SF, et al:
Protective effect of tert-butylhydroquinone on the
quinolinic-acid-induced toxicity in rat striatal slices: Role of
the Nrf2-antioxidant response element pathway. Neurosignals.
18:24–31. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nishizono S, Hayami T, Ikeda I and
Imaizumi K: Protection against the diabetogenic effect of feeding
tert-butylhydroquinone to rats prior to the administration of
streptozotocin. Biosci Biotechnol Biochem. 64:1153–1158. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sanders HO and Cope OB: Toxicities of
several pesticides to two species of cladocerans. Trans Am Fish
Soc. 95:165–169. 1966. View Article : Google Scholar
|
|
35
|
Nasrabadi T, Bidhendi GN, Karbassi A,
Grathwohl P and Mehrdadi N: Impact of major organophosphate
pesticides used in agriculture to surface water and sediment
quality (Southern Caspian Sea basin, Haraz River)Environmental
Earth Sciences. 63. Springer; New York, NY: pp. 873–883. 2011,
View Article : Google Scholar
|
|
36
|
Garfitt SJ, Jones K, Mason HJ and Cocker
J: Exposure to the organophosphate diazinon: Data from a human
volunteer study with oral and dermal doses. Toxicol Lett.
134:105–113. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Castillo LE, Ruepert C and Solis E:
Pesticide residues in the aquatic environment of banana plantation
areas in the north Atlantic zone of Costa Rica. Environ Toxicol
Chem. 19:1942–1950. 2000. View Article : Google Scholar
|
|
38
|
Galloway T and Handy R: Immunotoxicity of
organophosphorous pesticides. Ecotoxicology. 12:345–363. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kahl R and Kappus H: Toxicology of the
synthetic antioxidants BHA and BHT in comparison with the natural
antioxidant vitamin E. Z Lebensm Unters Forsch. 196:329–338.
1993.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hawkey CM, Bennett PM, Gascoyne SC, Hart
MG and Kirkwood JK: Erythrocyte size, number and haemoglobin
content in vertebrates. Br J Haematol. 77:392–397. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Svoboda M, Luskova V, Drastichova J and
Žlabek V: The effect of diazinon on haematological indices of
common carp (Cyprinus carpio L.). Acta Vet Brno. 70:457–465.
2001. View Article : Google Scholar
|
|
42
|
Far MS, Roodsari HV, Zamini A, Mirrasooli
E and Kazemi R: The effects of diazinon on behavior and some
hematological parameters of fry rainbow trout (Oncorhynchus
mykiss). World J Fish Mar Sci. 4:369–375. 2012.
|
|
43
|
Ahmad Z: Acute toxicity and haematological
changes in common carp (Cyprinus carpio) caused by diazinon
exposure. Afr J Biotechnol. 10:13852–13859. 2011. View Article : Google Scholar
|
|
44
|
Yassa VF, Girgis SM and Abumourad IM:
Potential protective effects of vitamin E on diazinon-induced DNA
damage and some haematological and biochemical alterations in rats.
J Mediterr Ecol. 11:31–39. 2011.
|
|
45
|
Banaee M, Mirvaghefi AR, Mojazi AB, Rafiee
GR and Nematdost B: Hematological and histopathological effects of
diazinon poisoning in common carp (Cyprinus carpio). Iranian
J Nat Resources. 64:1–12. 2011.
|
|
46
|
Köprücü SŞ, Köprücü K, Ural MŞ, İspir Ü
and Pala M: Acute toxicity of organophosphorous pesticide diazinon
and its effects on behavior and some hematological parameters of
fingerling European catfish (Silurus glanis L.). Pestic
Biochem Physiol. 86:99–105. 2006. View Article : Google Scholar
|
|
47
|
Messarah M, Amamra W, Boumendjel A, Barkat
L, Bouasla I, Abdennour C, Boulakoud MS and Feki AE: Ameliorating
effects of curcumin and vitamin E on diazinon-induced oxidative
damage in rat liver and erythrocytes. Toxicol Ind Health. 29:77–88.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nwani CD, Okeke OC, Onyishi G, Atama C,
Chinekwu U and Eneje LO: Toxicity and effects of diazinon on
behaviour and some haematological parameters of African catfish
Clarias gariepinus. Zool Ecol. 22:246–253. 2012. View Article : Google Scholar
|
|
49
|
Rauf A and Arain N: Acute toxicity of
diazinon and its effects on hematological parameters in the Indian
carp, Cirrhinus mrigala (Hamilton). Turk J Vet Anim Sci.
37:535–540. 2013. View Article : Google Scholar
|