|
1
|
Sakamoto K, Ohashi S, Yamazoe R, Takahashi
K and Furuta Y: FAO report of the European commission for the
control of foot-and mouth disease, pp414-420, 2006. Available at:
https://www.fao.org/ag/againfo/commissions/docs/research_group/pahpos/App64.pdf.
Accessed October 11, 2022.
|
|
2
|
Muroga N, Hayama Y, Yamamoto T, Kurogi A,
Tsuda T and Tsutsui T: The 2010 foot-and-mouth disease epidemic in
Japan. J Vet Med Sci. 74:399–404. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pariente N, Sierra S, Lowenstein PR and
Domingo E: Efficient virus extinction by combinations of a mutagen
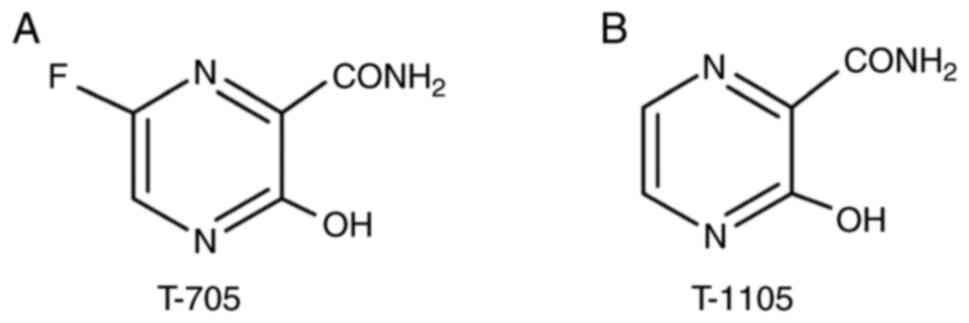
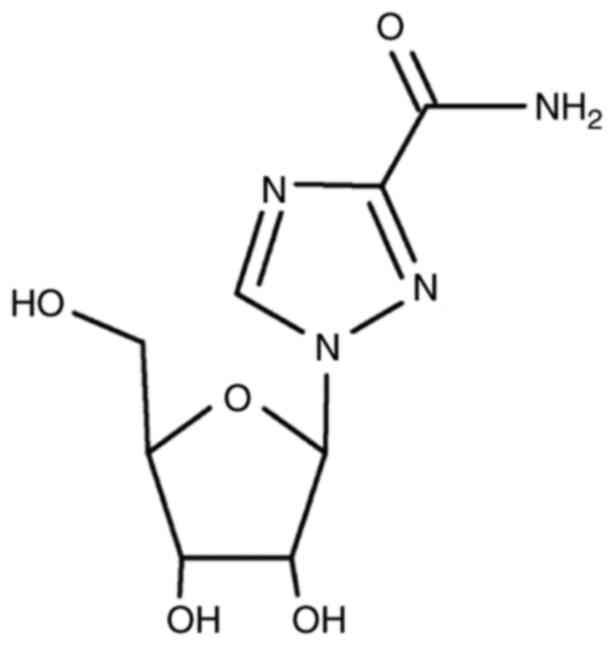
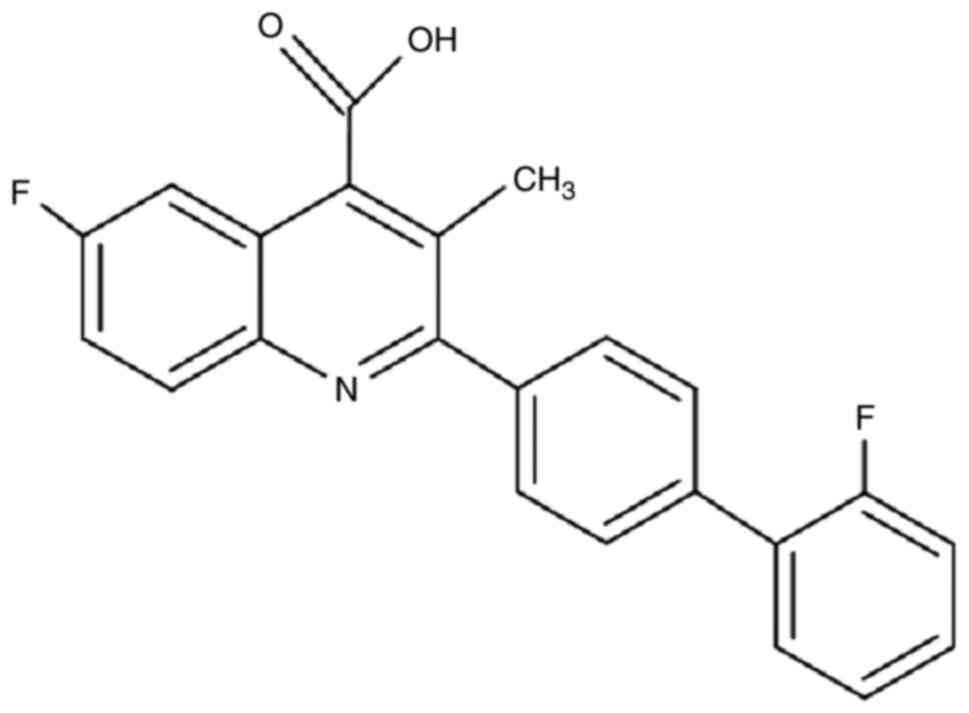
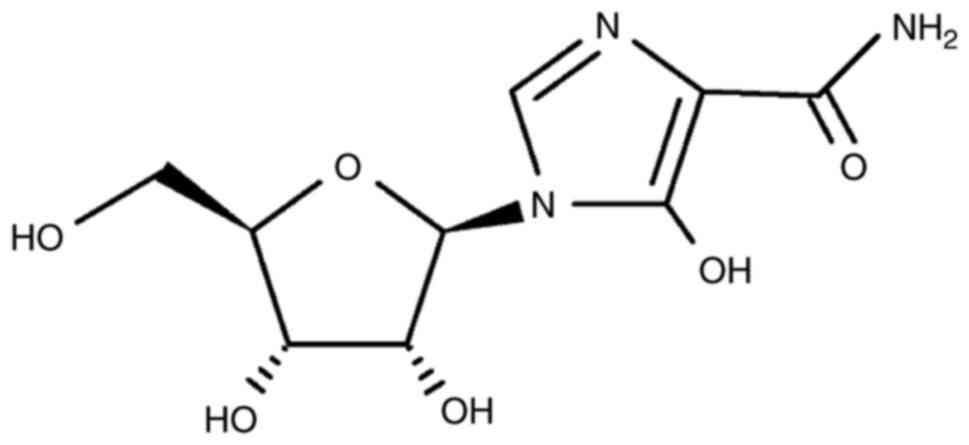
and antiviral inhibitors. J Virol. 75:9723–9730. 2001.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Golde WT, Pacheco JM, Duque H, Doel T,
Penfold B, Ferman GS, Gregg DR and Rodriguez LL: Vaccination
against foot-and-mouth disease virus confers complete clinical
protection in 7 days and partial protection in 4 days: Use in
emergency outbreak response. Vaccine. 23:5775–5782. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rada B and Dragún M: Antiviral action and
selectivity of 6-azauridine. Ann N Y Acad Sci. 284:410–417.
1977.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Goris N, De Palma A, Toussaint JF, Musch
I, Neyts J and De Clercq K: 2'-C-methylcytidine as a potent and
selective inhibitor of the replication of foot-and-mouth disease
virus. Antiviral Res. 73:161–168. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lefebvre DJ, De Vleeschauwer AR, Goris N,
Kollanur D, Billiet A, Murao L, Neyts J and De Clercq K: Proof of
concept for the inhibition of foot-and-mouth disease virus
replication by the anti-viral drug 2'-C-methylcytidine in severe
combined immunodeficient mice. Transbound Emerg Dis. 61:e89–e91.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Furuta Y, Takahashi K, Shiraki K, Sakamoto
K, Smee DF, Barnard DL, Gowen BB, Julander JG and Morrey JD: T-705
(favipiravir) and related compounds: Novel broad-spectrum
inhibitors of RNA viral infections. Antiviral Res. 82:95–102.
2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
De Vleeschauwer AR, Lefebvre DJ, Willems
T, Paul G, Billiet A, Murao LE, Neyts J, Goris N and De Clercq K: A
refined guinea pig model of foot-and-mouth disease virus infection
for assessing the efficacy of antiviral compounds. Transbound Emerg
Dis. 63:e205–e212. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen SF, Perrella FW, Behrens DL and Papp
LM: Inhibition of dihydroorotate dehydrogenase activity by
brequinar sodium. Cancer Res. 52:3521–3527. 1992.PubMed/NCBI
|
|
11
|
Li SF, Gong MJ, Sun YF, Shao JJ, Zhang YG
and Chang HY: In vitro and in vivo antiviral activity of mizoribine
against foot-and-mouth disease virus. Molecules.
24(1723)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li SF, Gong MJ, Shao JJ, Sun YF, Zhang YG
and Chang HY: Antiviral activity of merimepodib against foot and
mouth disease virus in vitro and in vivo. Mol Immunol. 114:226–232.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lanford RE, Guerra B, Chavez D, Giavedoni
L, Hodara VL, Brasky KM, Fosdick A, Frey CR, Zheng J, Wolfgang G,
et al: GS-9620, an oral agonist of Toll-like receptor-7, induces
prolonged suppression of hepatitis B virus in chronically infected
chimpanzees. Gastroenterology. 144:1508–1517, 1517.e1-e10.
2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
de Avila AI, Moreno E, Perales C and
Domingo E: Favipiravir can evoke lethal mutagenesis and extinction
of foot-and-mouth disease virus. Virus Res. 233:105–112.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Furuta Y, Gowen BB, Takahashi K, Shiraki
K, Smee DF and Barnard DL: Favipiravir (T-705), a novel viral RNA
polymerase inhibitor. Antiviral Res. 100:446–454. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jin Z, Tucker K, Lin X, Kao CC, Shaw K,
Tan H, Symons J, Behera I, Rajwanshi VK, Dyatkina N, et al:
Biochemical evaluation of the inhibition properties of favipiravir
and 2'-C-methyl-cytidine triphosphates against human and mouse
norovirus RNA polymerases. Antimicrob Agents Chemother.
59:7504–7516. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Smither SJ, Eastaugh LS, Steward JA,
Nelson M, Lenk RP and Lever MS: Post-exposure efficacy of oral
T-705 (Favipiravir) against inhalational Ebola virus infection in a
mouse model. Antiviral Res. 104:153–155. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Malik P, Jain S, Jain P, Kumawat J,
Dwivedi J and Kishore D: A comprehensive update on the structure
and synthesis of potential drug targets for combating the
coronavirus pandemic caused by SARS-CoV-2. Arch Pharm (Weinheim).
355(e2100382)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Takehisa T: FMD status and control
strategy in Japan. https://www.maff.go.jp/j/syouan/douei/pdf/japan.pdf.
Accessed October 11, 2022.
|
|
20
|
Furuta Y, Takahashi K, Kuno-Maekawa M,
Sangawa H, Uehara S, Kozaki K, Nomura N, Egawa H and Shiraki K:
Mechanism of action of T-705 against influenza virus. Antimicrob
Agents Chemother. 49:981–986. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nishi T, Fukai K, Masujin K, Kawaguchi R,
Ikezawa M, Yamada M, Nakajima N, Komeno T, Furuta Y, Sugihara H, et
al: Administration of the antiviral agent T-1105 fully protects
pigs from foot-and-mouth disease infection. Antiviral Res.
208(105425)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nikunjkumar P, Tamil Selvan RP and
Bhanuprakash V: Ribavirin as a curative and prophylactic agent
against foot and mouth disease virus infection in C57BL/6 suckling
and adult mice model. Virusdisease. 32:737–747. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Choi JH, Jeong K, Kim SM, Ko MK, You SH,
Lyoo YS, Kim B, Ku JM and Park JH: Synergistic effect of ribavirin
and vaccine for protection during early infection stage of
foot-and-mouth disease. J Vet Sci. 19:788–797. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Platt H: A study of the pathological
changes produced in young mice by the virus of foot-and-mouth
disease. J Pathol Bacteriol. 72:299–312. 1956.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang G, Zhou F, Gu B, Ding C, Feng D, Xie
F, Wang J, Zhang C, Cao Q, Deng Y, et al: In vitro and in vivo
evaluation of ribavirin and pleconaril antiviral activity against
enterovirus 71 infection. Arch Virol. 157:669–679. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Qing M, Zou G, Wang QY, Xu HY, Dong H,
Yuan Z and Shi PY: Characterization of dengue virus resistance to
brequinar in cell culture. Antimicrob Agents Chemother.
54:3686–3695. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Luthra P, Naidoo J, Pietzsch CA, De S,
Khadka S, Anantpadma M, Williams CG, Edwards MR, Davey RA, Bukreyev
A, et al: Inhibiting pyrimidine biosynthesis impairs Ebola virus
replication through depletion of nucleoside pools and activation of
innate immune responses. Antiviral Res. 158:288–302.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang Y, Wang W, Xu L, Zhou X, Shokrollahi
E, Felczak K, van der Laan LJ, Pankiewicz KW, Sprengers D, Raat NJ,
et al: Cross talk between nucleotide synthesis pathways with
cellular immunity in constraining hepatitis E virus replication.
Antimicrob Agents Chemother. 60:2834–2848. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li SF, Gong MJ, Sun YF, Shao JJ, Zhang YG
and Chang HY: Antiviral activity of brequinar against
foot-and-mouth disease virus infection in vitro and in vivo. Biomed
Pharmacother. 116(108982)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ishikawa H: Mizoribine and mycophenolate
mofetil. Curr Med Chem. 6:575–597. 1999.PubMed/NCBI
|
|
31
|
Saijo M, Morikawa S, Fukushi S, Mizutani
T, Hasegawa H, Nagata N, Iwata N and Kurane I: Inhibitory effect of
mizoribine and ribavirin on the replication of severe acute
respiratory syndrome (SARS)-associated coronavirus. Antiviral Res.
66:159–163. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Naka K, Ikeda M, Abe K, Dansako H and Kato
N: Mizoribine inhibits hepatitis C virus RNA replication: Effect of
combination with interferon-alpha. Biochem Biophys Res Commun.
330:871–879. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jain J, Almquist SJ, Shlyakhter D and
Harding MW: VX-497: A novel, selective IMPDH inhibitor and
immunosuppressive agent. J Pharm Sci. 90:625–637. 2001.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Markland W, McQuaid TJ, Jain J and Kwong
AD: Broad-spectrum antiviral activity of the IMP dehydrogenase
inhibitor VX-497: A comparison with ribavirin and demonstration of
antiviral additivity with alpha interferon. Antimicrob Agents
Chemother. 44:859–866. 2000.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Decker CJ, Heiser AD, Chaturvedi PR, Faust
TJ, Ku G, Moseley S and Nimmesgern E: The novel IMPDH inhibitor
VX-497 prolongs skin graft survival and improves graft versus host
disease in mice. Drugs Exp Clin Res. 27:89–95. 2001.PubMed/NCBI
|
|
36
|
Fosdick A, Zheng J, Pflanz S, Frey CR,
Hesselgesser J, Halcomb RL, Wolfgang G and Tumas DB:
Pharmacokinetic and pharmacodynamic properties of GS-9620, a novel
Toll-like receptor 7 agonist, demonstrate interferon-stimulated
gene induction without detectable serum interferon at low oral
doses. J Pharmacol Exp Ther. 348:96–105. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bhagchandani S, Johnson JA and Irvine DJ:
Evolution of Toll-like receptor 7/8 agonist therapeutics and their
delivery approaches: From antiviral formulations to vaccine
adjuvants. Adv Drug Deliv Rev. 175(113803)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lee G, Kang HR, Kim A, Park JH, Lee MJ and
Kim SM: Antiviral effect of vesatolimod (GS-9620) against
foot-and-mouth disease virus both in vitro and in vivo. Antiviral
Res. 205(105384)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fukai K, Inoue K, Takeuchi A and Yamakawa
M: New possibilities for egg white lysozyme: Heat-denatured
lysozyme partially inactivates select foot-and-mouth disease virus
strains. Sci Rep. 11(526)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
USDA. National Emergency Response to a
Highly Contagious Animal Disease. Executive Summary, March 30,
2001. https://www.uvm.edu/sites/default/files/media/fmd_disinfectants.pdf?fbclid=IwAR3qbqvIPH2vVntcnsTrNb1PkA_ldovV5Vcyx95WMHM4PX2iw-dp-iTSCsM.
Accessed October 15, 2022.
|
|
41
|
Takahashi M, Takahashi H, Okakura Y,
Ichikawa M, Kuda T and Kimura B: Impact of pH and protein
hydrophobicity on norovirus inactivation by heat-denatured
lysozyme. PLoS One. 15(e0237888)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Harada Y, Lekcharoensuk P, Furuta T and
Taniguchi T: Inactivation of foot-and-mouth disease virus by
commercially available disinfectants and cleaners. Biocontrol Sci.
20:205–208. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Nakashima R, Kawamoto M, Miyazaki S,
Onishi R, Furusaki K, Osaki M, Kirisawa R, Sakudo A and Onodera T:
Evaluation of calcium hydrogen carbonate mesoscopic crystals as a
disinfectant for influenza A viruses. J Vet Med Sci. 79:939–942.
2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Onodera T, Sakudo A, Iwamaru Y, Yokoyama
T, Haritani M, Sugiura K, Shimakura H, Haga T, Onishi R and
Furusaki K: Calcium bicarbonate as an antimicrobial, antiviral, and
prion-inhibiting agent (review). Biomed Rep. 17(57)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kirisawa R, Kato R, Furusaki K and Onodera
T: Universal virucidal activity of calcium bicarbonate mesoscopic
crystals that provides an effective and biosafe disinfectant.
Microorganisms. 10(262)2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ishihara M, Murakami K, Fukuda K, Nakamura
S, Kuwabara M, Hattori H, Fujita M, Kiyosawa T and Yokoe H:
Stability of weakly acidic hypochlorous acid solution with
microbicidal activity. Biocontrol Sci. 22:223–227. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Horiuchi I, Kawata H, Nagao T, Imaohji H,
Murakami K, Kino Y, Yamasaki H, Koyama AH, Fujita Y, Goda H and
Kuwahara T: Antimicrobial activity and stability of weakly
acidified chlorous acid water. Biocontrol Sci. 20:43–51.
2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sato Y, Ishihara M, Nakamura S, Fukuda K,
Kuwabara M, Takayama T, Hiruma S, Murakami K, Fujita M and Yokoe H:
Comparison of various disinfectants on bactericidal activity under
organic matter contaminated environments. Biocontrol Sci.
24:103–108. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Goda H, Yamaoka H, Nakayama-Imaohji H,
Kawata H, Horiuchi I, Fujita Y, Nagao T, Tada A, Terada A and
Kuwahara T: Microbicidal effects of weakly acidified chlorous acid
water against feline calicivirus and Clostridium difficile spores
under protein-rich conditions. PLoS One.
12(e0176718)2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Hole K, Ahmadpour F, Krishnan J,
Stansfield C, Copps J and Nfon C: Efficacy of accelerated hydrogen
peroxide® disinfectant on foot-and-mouth disease virus,
swine vesicular disease virus and Senecavirus A. J Appl Microbiol.
122:634–639. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Omidbakhsh N and Sattar SA: Broad-spectrum
microbicidal activity, toxicologic assessment, and materials
compatibility of a new generation of accelerated hydrogen
peroxide-based environmental surface disinfectant. Am J Infect
Control. 34:251–257. 2006.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Gabbert LR, Neilan JG and Rasmussen M:
Recovery and chemical disinfection of foot-and-mouth disease and
African swine fever viruses from porous concrete surfaces. J Appl
Microbiol. 129:1092–1101. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Environmental Protection Agency (EPA).
Antimicrobial testing methods and procedures: MB-05-14: AOAC use
dilution method for testing disinfectants. https://www.epa.gov/sites/production/files/2016-08/documents/mb-05-14.pdf.
Accessed December 9, 2019.
|
|
54
|
Krug PW, Larson CR, Eslami AC and
Rodriguez LL: Disinfection of foot-and-mouth disease and African
swine fever viruses with citric acid and sodium hypochlorite on
birch wood carriers. Vet Microbiol. 156:96–101. 2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
EPA. List N advanced search page:
Disinfectant for coronaviruses (COVID-19), 2021. https://www.epa.gov/pesticide-registration/list-n-advanced-search-page-disinfectants-coronavirus-covid-19.
Accessed October 13, 2022.
|
|
56
|
Chen B, Han J, Dai H and Jia P:
Biocide-tolerance and antibiotic-resistance in community
environments and risk of direct transfers to humans: Unintended
consequences of community-wide surface disinfecting during
COVID-19? Environ Pollut. 283(117074)2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhang H, Tang W, Chen Y and Yin W:
Disinfection threatens aquatic ecosystems. Science. 368:146–147.
2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Bei E, Shu Y, Li S, Liao X, Wang J, Zhang
X, Chen C and Krasner S: Occurrence of nitrosamines and their
precursors in drinking water systems around mainland China. Water
Res. 98:168–175. 2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Challis BC and Kyrtopoulos SA: Rapid
formation of carcinogenic N-nitrosamines in aqueous alkaline
solutions. Br J Cancer. 35:693–696. 1977.PubMed/NCBI View Article : Google Scholar
|
|
60
|
World Health Organization (WHO), 2023: One
Health, https://www.who.int/news-room/questions-and-answers/item/one-health,
Accessed, 06 Jul 2023.
|