1. Introduction
Foot-and-mouth disease (FMD) has induced disastrous
effects on world livestock production and the general economy for
>100 years. It is one of the most feared diseases in cattle and
pigs. It infects primarily cloven-hoofed wild animals and domestic
animals. In cattle viremia, anorexia with dullness are observed
following a high fever (40 to 41˚C). Subsequently, blisters are
observed on the tongue, lips, cheeks, gums and dental pads. Ropy
saliva, smacking of the lips, lameness, and loss of movement were
the conditions observed in cattle (1). The FMD virus (FMVD) belongs to the
family Picornaviridae, genus Aphthovirus. FMDV is a
non-enveloped virus with a single stranded RNA genome. There are
seven serotypes of FMDV. These are subject to a high number of
mutations, continuously generating new FMDV variants (1). All these serotypes cause similar
clinical symptoms with a duration of 2-14 days after being
infected.
The largest FMD outbreak in Japanese history
occurred in 2010. From April to July of 2010, a total of 221,608
cattle, goats and pigs were collected from 292 farms in the
Miyazaki Prefecture (Table I). The
virus was identified as a serotype O prototype (Mya-98 lineage).
The first case of FMD was reported on April 20, 2010. On May 19,
widespread vaccination was recommended, which began on May 25.
Following vaccination, a decrease in the suspected animals awaiting
culling was observed. The peak of suspected animals awaiting
culling occurred on May 25 (~70,000 animals). On June 22, the last
animal was sacrificed, and on July 27, all restrictions were
lifted. Finally, on February 5, 2011, Japan restored its World
Organisation for Animal Health (WOAH) status as an FMD-free
country, where vaccination was not practiced. However, the Japanese
government keeps a stock of antiviral agent T-1105 (1 ton/year) for
use in any eventuality (1).
 | Table INumber of FMD cases in Miyazaki
during the 2010 epidemic. |
Table I
Number of FMD cases in Miyazaki
during the 2010 epidemic.
| No. of
outbreaks | No. of
infected/suspected animals |
|---|
| 292 | Cattle | 37,412 |
| |
Beef
cattle | 36,284 |
| |
Dairy
cattle | 1,128 |
| | Water buffalo | 42 |
| | Pig | 174,132 |
| | Goat | 14 |
| | Sheep | 8 |
| | Total | 211,608 |
The symptoms exhibited by the animals infected with
FMDV are presented in Table II.
Cattle dribbled foamy saliva continuously, had fevers, and the
tongues and pads were hard with blisters and erosions. Foot lesions
in cattle were rarely observed (2).
However, foot erosions and hemorrhages, causing lameness, were
commonly observed in pigs. A total of >90% of infected pigs in
farms developed lesions on their legs. In breeding farms, the sows
often presented with blisters and erosions on the teats. In total,
14 goats exhibited symptoms of FMD in Miyazaki, Japan (Table I). The goats were seropositive for
FMDV and only displayed small erosions in the nasal cavity.
 | Table IIClinical signs observed in the FMD
cases in Miyazaki during the 2010 epidemic. |
Table II
Clinical signs observed in the FMD
cases in Miyazaki during the 2010 epidemic.
| Clinical signs | Cattle | Pig |
|---|
| Fever | O | O |
| Anorexia | O | O |
| Excessive
salivation | O | Δ |
| Vesicular condition
(blisters, ruptured and erosion) of the feet, buccal mucosa and, in
females, the mammary glands | O | O |
| Lameness | Δ | O |
Most outbreaks (279/292) were observed within a
small area of ~20 km, from south to north, located in the central
region of Miyazaki Prefecture (2).
In addition, this area is a narrow plain situated between mountains
to the west and the sea to the east. Several sporadic epicenters of
infection were observed at distant locations.
Broad-spectrum replication inhibitors and mutagenic
nucleotide analogs exert anti-FMDV effects. These include
fluorouracil (3), 5-azacytidine
(4), 6-azauridine (5), ribavirin (6), favipiravir (T-705) (7) and its derivative (T-1105) (8). Fluorouracil (3), 5-azacytidine (4) and 6-azauridine (5) were primarily studied in the early
2000s. Recently, ribavirin, favipiravir (T-705) (7), and its derivative (T-1105) (8) have been widely studied. Among them,
T-1105 (1,9) has exhibited the greatest promise in
vivo. T-1105 is a favipiravir derivative. Despite its higher
dose (200-400 mg/kg/day), it was demonstrated to exert significant
suppression of symptoms and clinical signs, such as fever, in pigs
and guinea pigs (8). In addition,
newly developed antiviral chemical compounds, such as brequinar
(BQR) (10), mizoribine (11), merimepodib (12) and vesatolimod (13), have been discussed as potential
agents against FMD. The present review describes several
developments in antiviral agents and disinfectants following the
large 2010 outbreak of endemic FMD in Japan.
In the last 13 years, the Japanese Ministry of
Agriculture, Forestry, and Fisheries have invested a substantial
sum of money to develop viral replication inhibitors against FMDV.
The National Institute of Animal Health in Japan is responsible to
update the scientific information of anti-FMDV chemical compounds
especially T-1105 and T-705 (1,7-9).
The aim of the present review was to increase awareness of the
antiviral chemical compounds and disinfectants developed by the
Japanese Government Institution and Universities from 2010.
2. Antiviral chemical compounds
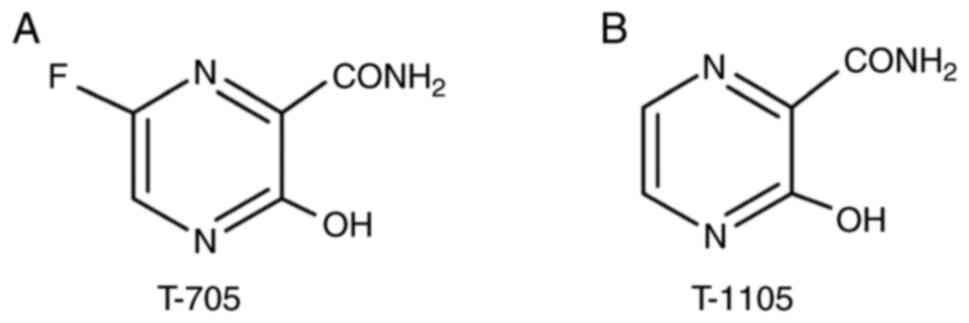
In this section, favipiravir, that was recommended
by the Japanese Government for treatment against FMDV, is mainly
described and discussed. In addition, ribavirin, BQR, mizorbine,
VX-497 and GS-9620, potential candidate chemical compounds which
may be used in the future, are also described.
Favipiravir. Favipiravir (T-705,
6-fluoro-3-hydroxypyrazine-2-carboxamide) was developed for use
against influenza viruses (14).
This agent hinders ribonucleic acid (RNA)-dependent RNA polymerase
within the virus (15). In
addition, this agent prevents the replication of arenaviruses,
bunyaviruses, flaviviruses, alphaviruses and noroviruses (9,16), and
is a candidate for the Ebola virus and SARS-CoV-2 (17,18)
(Fig. 1).
In a previously created and described guinea pig
model, the animals were administered T-1105 (400 mg/kg/day) orally,
twice daily for five days. Following the first administration of
T-1105, FMDV [O1 Manisa:100 guinea pig (GP) ID50] was
inoculated into the intraplantar area 1 h later. A total of 14
T-1105-treated animals were completely protected from footpad
lesions. A total of four days post infection (dpi), viral RNA was
detected by reverse transcription-polymerase chain reaction
(RT-PCR) in serum, organs and oral swabs of half of the animals
(19). In total, 10 animals
received a single dose (2 ml) of the commercial double-oil
emulsion-inactivated O1 ELISA vaccine. Three weeks later, the
immunized animals were inoculated with FMDV. All vaccinated animals
were protected from footpad lesions. At 4 dpi, only one out of the
ten immunized and inoculated animals exhibited FMDV in the sera,
without clinical symptoms The mean viral RNA levels of the animals
treated and vaccinated were significantly lower than those of the
controls not treated with T-1105 and non-vaccinated (P<0.01).
These results demonstrated the suitability of T-1105 to combat FMD
outbreaks (19).
In an in vivo study of pigs, animals were
injected with 106 TCID50 of FMDV O/JPN/2000 on the right
side of the footpad. The T-1105-treated group was administered 200
mg/kg T-1105 orally mixed with food for 1 h before viral injection.
Following the first administration, the same doses were
administered twice a day for six days. The treated and
non-T-1105-treated groups consisted of six and two pigs,
respectively (1). For virological
testing, plasma and nasal swab samples were collected for virus
titration [plaque-forming units (PFU)] and quantitative PCR (qPCR).
For immunological testing, liquid-phase blocking ELISA and virus
neutralization tests were performed (1).
Typical clinical symptoms of FMD, such as fever,
anoxia, feet blisters and lameness (Table II), were observed in control pigs
(without T-1105). No clinical FMD symptoms were observed in
T-1105-treated pigs throughout the experiments. In real-time qPCR,
the Cq value of the treated animals reached >40 at 6 dpi in
plasma, while the Cq value of the untreated animals was 20-30 at
1-3 dpi. Non-treated animals exhibited FMDV in plasma at least
three days earlier than the treated animals (1).
The excretion of FMDV from the nasal cavity was
assessed using a viral plaque assay (reported in PFU) and qPCR. The
virus was not detected in the nasal swabs nor in the viral plaque
assay in animals treated with T-1105. Aberrant Cq values were not
observed in five out of the six T-1105-treated animals. Notably,
one out of the six T-1105-treated animals had a Cq value of 36 at 1
dpi. However, these swabs did not isolate FMDV using a viral plaque
assay. Cq values of the untreated control animals were 29-38 at 2-3
dpi on nasal swabs. Untreated control animals exhibited FMDV in
nasal swab samples at 102-104 PFU/ml at 2-4
dpi (1).
ELISA antibody testing revealed that untreated
controls had increased antibody levels from 4 days post infection
(dpi) to >1:360 at 5-6 dpi. Serum neutralizing antibody levels
reached 1:128 at 6 dpi. Conversly, the animals treated with T-1105
exhibited ELISA antibody levels of <1:45 and neutralizing
antibody levels of <1:32(1).
In a previous in vitro study, T-1105 was more
effective than T-705 in treating FMDV (20). T-1105 exerted an antiviral effect,
inhibiting viral RNA-dependent RNA polymerase (20). T-705 inhibited influenza virus RNA
polymerase, and T-1105 is a derivative of T-705(20). FMDV is excreted in the early phase
of infection in aerosols and in oral or nasal droplets from
animals. This occurs before the animals produce antibodies to
protect themselves against infection. Combined with the FMDV
vaccine, T-1105 and chemical disinfectants could provide more
efficient protection against FMD outbreaks. More detailed studies
are necessary to determine the most effective time point of
administration and dose of T-1105 in pigs.
Recently, Nishi et al conducted a detailed
study using T-1105, 6 h after oral infection with FMDV in pigs
(21). In the aforementioned study,
105.5 TCID50 O/HKN/1/2015 FMDV was inoculated
orally into pigs. As stated in a previously mentioned study
(1), the pigs were treated orally
with 200 mg/kg T-1105, which mixed with feed twice daily for 6 days
(1), starting at 1 h before virus
inoculation. In another group of pigs, 105.5
TCID50 O/HKN/1/2015 FMDV was inoculated orally, and 200
mg/kg T-1105 was administered orally to pigs twice daily for 6
days, starting from 6 h after infection. As previously mentioned,
all untreated and infected control pigs exhibited clinical signs of
FMD, FMDV-specific genes, and serum antibodies (1). Through the use of
immunohistochemistry, the FMDV antigen was observed in the pig
tissues of all untreated and infected controls. No clinical signs,
FMDV-specific genes, serum antibodies, or tissue antigens with FMDV
were observed in pigs treated with T-1105 twice daily for 6 days,
starting at 6 h after or 1 h before infection. The results of
FMDV-infected high-risk groups in the field, with T-1105 treatment
or without T-1105 treatment require further investigation.
In the next section, the antiviral effects of
ribavirin, BQR, mizoribine, merimepodib and vesatolimod are
described as possible candidates during an FMDV pandemic.
Currently, a limited number of studies have reported antiviral
chemical compounds for FMD (6,22).
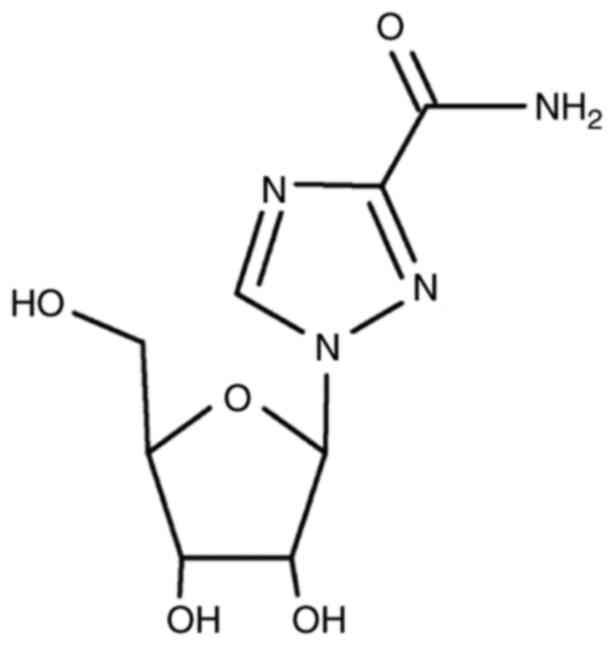
Ribavirin. Ribavirin is an antiviral agent
(synthetic purine nucleoside analog) that acts against various RNA
and DNA viruses (6,22) (Fig.
2). Different serotypes of FMDV ribavirin have been
demonstrated, only in vitro, to have inhibitory effects
(22). Ribavirin is a promising
antiviral compound. Similar to favipiravir, ribavirin interferes
with viral polymerase (22) and RNA
capping (23). Suckling mice have
been used to investigate FMDV in lethal animal models. The FMDV A
strain (A/IND/40/2000) was used in C57BL/6 suckling mice (22). This viral strain was revealed to be
highly virulent in suckling mice with acute flaccid paralysis and
was lethal within 72 h after infection (24).
The antiviral efficiency of ribavirin against FMDV A
was also determined. A single dose of ribavirin (50 mg/kg) was
administered 12 and 6 h before and after treatment, respectively. A
drug toxicity study was observed using 5- to 6-day-old C57BL/6 mice
(18). Ribavirin (up to 80 mg/kg)
was administered to mice once a day for five days. In another
study, the drug was administered intraperitoneally to old
ICR-suckling mice (25). Both
studies reported that no mice has succumbed 15 days after
administration. Therefore, ribavirin can be used in the early phase
of infection or before infection with vaccine treatment. However,
further testing is required in pigs.
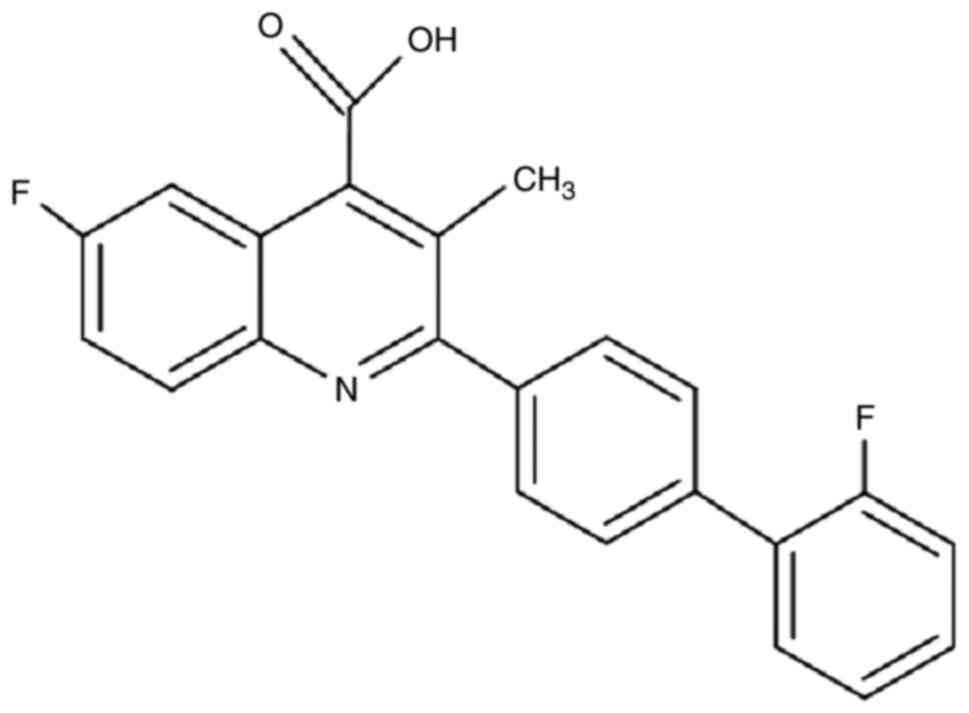
BQR. A mechanism of action of BQR is the
inhibition of dihydroorotate dehydrogenase (DHODH) (Fig. 3). The rate-limiting enzyme in the
de novo synthesis of pyrimidines is DHODH (10). Thus, BQR, as a DHODH inhibitor, was
capable of depleting the cellular pyrimidine pool. The pyrimidine
pool is essential for RNA and DNA production. BQR has exerted
virucidal effects against numerous types of viruses, including
dengue virus (26), Ebola virus
(27), and hepatitis E virus
(28). Nearly all of these studies
were conducted in vitro.
BQR against FMDV has been studied in vitro
and in vivo (29). Mice were
infected with 100 LD50 FMDV. All mice in the non-BQR
group were treated 60 h after infection. This study included 3- to
4-day-old mice. The FMDV dose LD50 (O/MYA98/BY/2010) was
determined using the Reed-Muench method with 10-fold serial
dilutions. BQR (50 µg) was injected intraperitoneally into suckling
mice, 2 h before virus challenge. The suckling mice were inoculated
intraperitoneally with 100 µl of 100 LD50 FMDV, and the
animals were observed for five days. After five days, 25% of the
mice in the BQR-treated group had survived and histopathological
changes in the hearts were observed under a microscope. Treatment
with BQR significantly reduced the severity of lesions in the heart
muscles of FMDV-infected mice (P<0.05) (29). Thus, BQR may be used as a vaccine
before infection, however further studies are required in pigs and
cows.
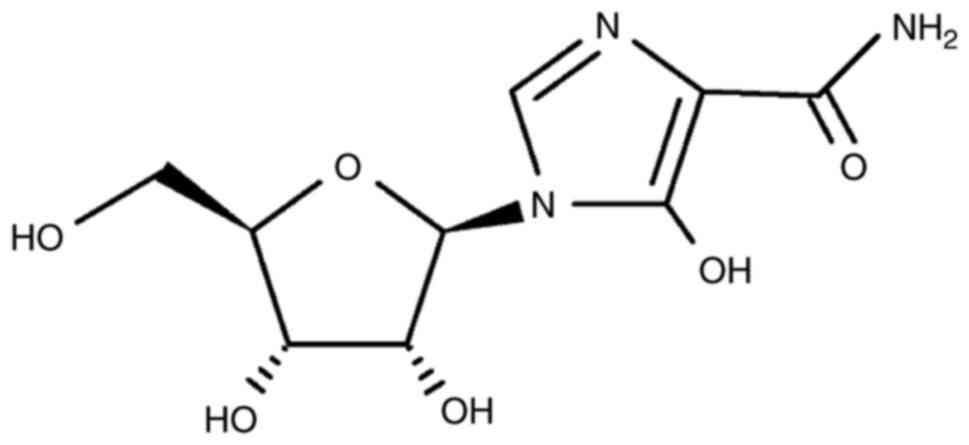
Mizoribine. Mizoribine is an imidazole
nucleoside (11) (Fig. 4) that inhibits the replication of
the hepatitis C virus at a 50% inhibition concentration
(IC50) of approximately 100 µM (30). Mizoribine was originally developed
as an immunosuppressant for transplantation immunity without
obvious side effects (30) and as
an inhibitor of inosine 5'-monophosphate dehydrogenase (IMPDH).
This inhibition is useful in renal transplantation, autoimmune
diseases, and steroid-resistant nephrotic syndrome (31). Mizoribine was produced by culturing
Eupenicillium brefeldianum M-2166 molds in culture medium
(32).
Mizoribine was demonstrated to inhibit SARS-CoV
(31). Additionally, mizoribine
inhibited FMDV replication in suckling mice. The suckling mice were
inoculated with a mizoribine solution (50 µg/0.1 ml) by
subcutaneous injection into the neck. After 2 h, FMDV was
inoculated intradermally (11) at
100 LD50 (serotype O O/MY98/BY/2010). The animals were
observed for five days. Within 60 h after FMDV infection, all
control mice [phosphate-buffered saline (PBS) solvent-treated]
succumbed. In the FMDV-infected group treated with mizoribine, a
48-h delay in death was observed (11). A significant difference in the
mortality curve was observed between the mizoribine-treated and
control groups (P=0.0014) (11).
The findings of the mizoribine study warrant further investigation
using the natural host of FMDV. Mizoribine could be used to
complement vaccine treatment in the future.
Merimepodib (VX-497). Merimepodib is an oral
drug with broad antiviral activities (12) (Fig.
5). In mouse experiments, suckling mice were administered 30 µg
of merimepodib intranasally. After administration, the nasally
administered drug was moved to the stomach. Adult mice were
administered orally with merimepodib. Similar to mizoribine, this
drug is an oral inhibitor of IMPDH. Merimepodib has an
immunosuppressive and anti-keratinocyte effect (33). This immunosuppressive effect of
merimepodib disappears within 48 h after the end of administration
(34). Numerous viruses, such as
herpes simplex virus-1, parainfluenza-3 virus, bovine viral
diarrhea virus, Venezuelan equine encephalitis virus, and dengue
virus, have been demonstrated to be suppressed by merimepodib in an
in vitro study (12). This
drug is used regularly at concentrations ranging from 6 to 19 µM
(34).
Oral administration of merimepodib in mice inhibits
the response to the primary IgM antibody (33). Decker et al reported the
effects of merimepodib on transplant immunology and treatment of
graft-vs.-host disease (35). Li
et al reported the antiviral activity of merimepodib using
FMDV in mice in vivo via intranasal merimepodib
administration (12). BALB/c mice,
three days old were treated with 100 LD50 FMDV
O/MYA98/BY/200. The mice were divided into three groups. One group
received 100 µl of PBS containing 10% dimethyl sulfoxide (DMSO) and
5% Tween-80 as a control group. The other group received 30 µg of
merimepodib dissolved in 100 µl of PBS containing 10% DMSO and 5%
Tween-80. After 2 h, 100 µl of diluted FMDV was subcutaneously
injected into the dorsal cervical area of each mouse. The untreated
and virus-infected mice began to succumb at 36 h after infection,
and at 60 h, all mice had succumbed. In the merimepodib-treated and
virus-infected groups, mice began to succumb 48 h after infection
and all mice in this group succumbed within 108 h. The survival
rate of mice administered merimepodib was significantly higher than
that of mice not administered after infection with 100
LD50 FMDV (P<0.0001). Merimepodib may be used as an
oral drug in the treatment of FMD (12). In future, more experiments are
required to test this hypothesis in pigs.
GS-9620 (vesatolimod). Chimpanzees have been
used as animal models to study the chronic hepatitis B virus
isolated from humans (13). GS-9620
(Fig. 6) is a potent and selective
orally active small molecule agonist of Toll-like receptor 7 (TLR7)
developed in studies of hepatitis B virus (13). Orally administered GS-9620 has been
used as an immunostimulant to enhance innate immunity (36). Furthermore, GS-9620 is an inducer of
type 1 interferon, which induces an antiviral state in host cells
via TLR7. Administration of TLR7 agonists has been attempted using
various routes as adjuvants. Bhagchandani et al attempted
oral, intravenous, topical, and intracutaneous administration
(37). Lee et al attempted
intramuscular administration of ISA 206 adjuvant (38). Moreover, the adjuvant effect of the
TLR7 agonist, GS-9620, is as an antiviral agent in the early phase
of FMD. Mice were injected intraperitoneally with 0.1 ml of
GS-9620, ribavirin, or PBS (negative control) for 16, 24 and 72 h
prior to FMDV infection. Mice were challenged with intraperitoneal
injection of mouse-adapted FMDV (250 LD50 of
O/VIT/2013). GS-9620 was injected at a dose of 5 or 10 mg/kg body
weight. Ribavirin was injected at a dose of 10 mg/kg of body
weight. A total of five mice were used and observed for six days
after infection in each group. Both GS-9620 and ribavirin
treatments increased the survival rate of drug-treated mice after
FMDV infection. The survival rate of mice treated with GS-9620 (10
mg/kg) was higher than that of mice treated with ribavirin
(38). Further confirmation is
necessary using natural hosts for FMDV, such as pigs, cows or
goats.
3. Disinfectants for use against
environmental contaminants
This section discusses recently developed
disinfectants against FMDV, including CAC-717, accelerated hydrogen
peroxide, and Virkon S, which can be used against environmental
contaminants.
CAC-717. Sodium carbonate solution (4%) and
several commercial products are widely used for disinfection of
FMDV in Japan (39). In the United
States, 3% sodium hypochlorite, 4-5% acetic acid, Virkon S
(potassium peroxymonosulfate and sodium chlorite), 4% sodium
carbonate, and 2% sodium hydroxide are recommended (40). To guarantee food safety, all
surfaces must be washed or treated after sterilization before
consumption. The heat-denatured egg white lysozyme is known to
inactivate FMDV (39). Fukai et
al observed that the heat-denatured lysozyme reduced the RNA
content of FMDV O/Taiwan/1977 by 2.7log10 after
treatment (39). However, the
mechanisms underlying these virucidal effects are unclear. Usually,
a heat-denatured lysozyme is more hydrophobic and the pH increases.
This increased pH results in a virucidal effect on murine norovirus
(41). Similar virucidal mechanisms
may be involved in FMDV. Using FMDVsA/TAI/46-1/2015 and
Asia1/Shamir (ISR/3/89), the virucidal effect of the heat-denatured
lysozyme is unclear (39). Further
studies are required to elucidate the virucidal effects of this
enzyme.
Harada et al evaluated the virucidal
efficiency of 13 commercially available products in Japan (42). These products included five
alcohol-based disinfectants, two hand soaps, two alkaline cleaners,
two quaternary ammonium compound sanitizers, and two chlorine
disinfectants with short exposure times (42). Because FMDV is inactivated in acidic
environments, acidic ethanol disinfectants Vir Stera and Alpet NV
significantly reduced FMDV infectivity by at least
3.75log10 within 30 sec of exposure. Since FMDV is also
inactivated under strongly alkalic conditions (pH >12), an
alkaline cleaner named Degreaser FII and Start Clean significantly
reduced FMDV infectivity by at least 3.5log10 in 30 sec
of exposure. However, strongly alkaline conditions can be harmful
to human skin. A chlorine disinfectant named Zia Knock (a sodium
hypochlorite product; 200 ppm) reduced the infectivity by at least
3.5log10 in 30 sec of exposure. The virucidal effect of
chlorine is rapidly lost under dirty conditions (42). Therefore, hypochlorous acid solution
should be used for virucidal effects in areas free of organic
substances.
Nakashima et al (43) reported that the calcium bicarbonate
solution (CAC-717) (43,44) did not decrease the virucidal
activity against the influenza virus after incubation with bovine
serum albumin (10 mg/ml). However, mixing the medium with organic
materials (fetal bovine serum; protein concentration >7.0 mg/ml,
final concentration) weakly reduced the effect of CAC-717 against
bovine adenovirus-7(45). As fetal
bovine serum contains various inhibitors other than albumin,
further studies are necessary to address these issues. Furthermore,
CAC-717 works most efficiently (reduction of
>3.5log10 TCID50) against Asia-1, O and A
type strains of FMDV compared to other reported disinfectants such
as heat-denatured lysozyme (39).
In heat-denatured lysozyme treatment Type O and Asia 1 FMDV were
not inactivated efficiently, although Type A virus was inactivated.
CAC-717 can be used internationally (Fig. 7 and Table III).
 | Table IIIVirucidal effects of CAC-717 on the
three FMDV serotypes. |
Table III
Virucidal effects of CAC-717 on the
three FMDV serotypes.
| | Solution |
|---|
| FMDV serotype | CAC-717 60 min | Tap water 60
min | Maintenance medium
60 min |
|---|
| Type A | ≤0.5a | 5.00±0.13 | 5.38±0.13 |
| Type O | ≤0.5b | 4.25±0.00 | 4.00±0.00 |
| Type Asia 1 | ≤0.5a | 4.00±0.00 | 4.63±0.13 |
Several studies have shown that sodium hypochlorous
solutions can be used to inactivate various viruses (46-49),
including FMDV (40). However,
these solutions must be stored in cool and dark conditions to
maintain their virucidal effects (49). Conversely, Kirisawa et al
demonstrated that the virucidal effect of CAC-717 did not decrease
after storage for four years at room temperature without direct
sunlight (45).
Accelarated hydrogen peroxide (AHP). The
virucidal effects of AHP on wet films were assessed against FMDV
and swine vesicular disease virus (SVDV) (50). The AHP solution was commercially
named ACCEL TB (51). This solution
was based on 0.5% accelerated H2O2 at a pH of
3.0. In the United States, this solution has been patented (patent
no. 6.346.279) as a new generation disinfectant. To observe the
effectiveness of this disinfectant, SVDV was spread on a film by
spraying a 0.2-ml suspension inside the bottle, as previously
described (51). The test plates
inside the bottles were dried for 20 min at 20±1˚C (relative
humidity, 42%). The test substances were added to 2 ml of the
solution at 20˚C for the desired contact time. After scraping the
test surfaces of the films, the samples were tested for infectious
viruses, as previously reported (51).
AHP was effective against FMDV at a 1/40 dilution,
resulting in a >5log10 reduction in the virus titer
(50). The virucidal effect was
tested immediately after reconstitution with AHP (1/20 dilution in
distilled water). Subsequently, the diluted AHP was tested every
two weeks at a contact time of 10 min. The results revealed that
the disinfectant maintained its effectiveness for at least six
weeks when kept in a sealed bottle at room temperature. A toxicity
test in animals demonstrated that AHP is non-toxic, non-irritating
to the skin and eyes, and immunologically non-sensitizing (50). Therefore, AHP could be useful in the
field of FMDV outbreaks.
Virkon S. Virkon S (potassium
peroxymonosulfate and sodium chlorite) was selected as a
disinfectant for porous concrete surfaces, because the United
States Environmental Protection Agency registered this chemical as
broad-spectrum disinfectant and virucide that is effective against
FMDV (52,53). Little has been published on the
virucidal effects on agricultural porous surfaces. Wood is also a
common construction material used on farms. Modeling virus
decontamination in agricultural facilities is important for FMDV
control (54). Virkon S has been
reported to deactivate FMDV (50).
The FMDV strains A/24/Cruzeiro/BRA/55 and 01 Campos
were identified in a porcine kidney cell line. To assess virus
survival and recovery, 10 µl of virus inoculum was deposited on
stainless steel and concrete surfaces and dried under desiccation
for ~1 h. Carrier disinfection tests were also performed (52). A total of 50 µl (stainless steel) or
100 µl (concrete) of Virkon S were spread on the dried virus for 5
or 10 min (+3 sec). The samples were then eluted with 10 ml of
medium (DMEM + 2% FBS). Quantitative disinfection experiments were
performed using FMDV samples on stainless steel and concrete to
determine the virucidal effects of Virkon S, and the results
revealed adequate virucidal effects (52).
4. Foot-and-mouth disease and environmental
contamination
CAC-717, AHP, and Virkon S are novel disinfectants
that can be used to treat FMDV. Sodium hypochlorite (NaOCl) is
widely used for disinfection in public places. The United States
Environmental Protection Agency has listed it as one of the most
used disinfectants to combat COVID-19(55). During the COVID-19 outbreak,
disinfectants containing NaOCl were widely used. However, NaOCl can
negatively affect the environment, sewage water, and farm
irrigation (56). A recent report
showed that NaOCl waste threatens fish, seafood and food safety
(57). Therefore, there are
concerns about using NaOCl-based solutions for future FMDV
outbreaks. Zhang et al reported that China has been using
chlorine disinfectants extensively indoors and outdoors. China was
reported to have consumed 2,000 tons of chlorine to combat COVID-19
in Wuhan. This chemical has been identified in sewage and drinking
water (57). The widespread use of
chlorine during outbreaks can negatively impact the environment
(57) because chlorine can react
with nitrogen to produce chloramine or N-nitrosodimethylamine
(58). Furthermore, these chemicals
are notorious for their role in carcinogenesis (59). Therefore, risk assessments are
necessary in aquatic environments prior to use. In addition,
non-chlorine disinfectants that can be used during outbreaks,
including FMD, must be developed.
5. Discussion
Government reports have shown that FMDV was probably
introduced to Japan from other Asian countries by people or goods
(19). In Miyazaki, the main route
for developing the FMD cluster is the movement of people or
transportation. A total of 403 disinfection posts for vehicles were
established in the Miyazaki Prefecture (2). These findings were summarized in
several reports (Interim Report, October 2010; Supplement Report,
March 2013, Epidemiological Investigation of FMD; OIE Symposium in
Tokyo, November 13, 2014) (19).
The diagnosis and containment were performed in the
event of an outbreak. All laboratory tests for FMD diagnosis in
Japan were conducted at the National Institute of Animal Health in
Kodaira (Tokyo, Japan) (Fig. 8). To
contain the spread of FMD, stamping out, disinfection, movement
restrictions (a radius of 10 km around an infected farm), and tests
of surrounding farms were performed. Furthermore, when the presence
of FMD was confirmed, all infected and suspected animals were
culled. Common burial sites were used due to the rapid increase in
infected farms. This made it difficult to identify the appropriate
sites on all farms. Clinical and serological surveillance was
conducted on all susceptible animals within the movement
restriction zone. All cases and related materials were discarded. A
liquid phase blocking ELISA was performed on sera of animals within
a radius of 3 km of infected and related farms (Fig. 8).
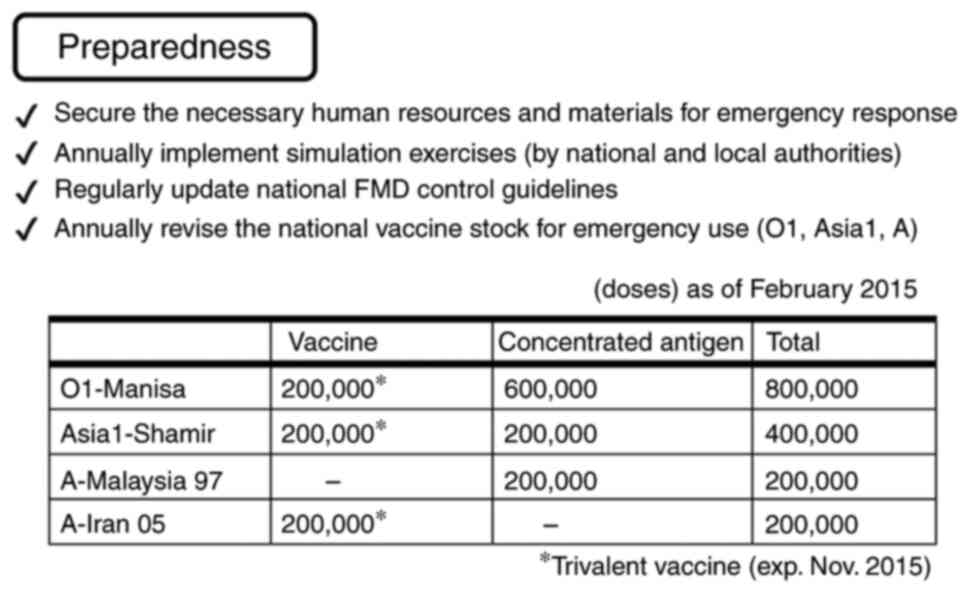
To prepare for future FMD outbreaks, the Japanese
government has secured vaccines (Fig.
9) and T-1105. Furthermore, national and local authorities
perform simulation exercises annually. The government also
regularly updates national FMD control guidelines and annually
reviews the national vaccine stock for emergency use (O1, Asia1 and
A; Fig. 9).
6. Conclusion
Various disinfectants, such as 3% sodium
hypochlorite, 4-5% acetic acid, Virkon S, 4% sodium carbonate, and
2% sodium hydroxide have been used to deal with FMD. Furthermore,
broad-spectrum replication inhibitors and mutagenic nucleotide
analogs (T-1105) are stored on Kyushu Island if necessary for
future outbreaks. Recently, there has been a trend to identify
antiviral agents to complement vaccination. Therefore, T-1105 may
be an important factor in enhancing the effects of FMD vaccination.
A multilevel approach for disinfection and viral replication
inhibitors is necessary to prepare for an FMD outbreak.
In addition, a 4% sodium carbonate solution and
several commercial products are commonly used to observe the
virucidal effect on FMDV. Occasionally, the effects of these
disinfectants are reduced when they are mixed with organic
materials. The effectiveness of surveillance and containment
systems is a common concern for diseases transmitted by aerosols,
such as FMD and COVID-19.
An epidemiological investigation of the 2010 FMD
outbreak in Japan concluded that FMD was introduced by goods or
people from countries affected by FMD in Asia. Local spread was
presumed in areas highly affected by aerosols. To avoid the
introduction and recurrence of FMD, various FMD control activities
have been implemented. To address the aerosol transmission of FMDV,
it is necessary to assess the environmental risks of using
chlorite. Prompt diagnosis and containment are critical during the
early stages of large-scale endemic disease outbreaks.
Environmental epidemiological information is valuable in this age
of global pandemics. The ‘One Health’ approach (60) should be shared between public health
services, and the veterinary fields, with the aim that viral
outbreaks, including those due to FMD, can be better managed using
social distancing, antiviral chemical compounds, environmental
viral decontamination and quarantine.
Acknowledgements
The authors would like to thank Professor Ken-ichi
Sakamoto (Miyazaki University, Miyazaki, Japan; former Director
General of the National Institute of Animal Health, Tsukuba,
Japan), for reviewing the manuscript.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
TO, AS and RK contributed to the conceptualization
of the study. TO contributed to the writing of the original draft
of the manuscript. TO, AS, KS, MH, KF and RK wrote, reviewed and
edited the original draft of the manuscript. Data authentication is
not applicable. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
KF was employed by the Mineral Activation Technical
Research Center (Tamana, Japan). The remaining co-authors declare
that they have no competing interests.
References
|
1
|
Sakamoto K, Ohashi S, Yamazoe R, Takahashi
K and Furuta Y: FAO report of the European commission for the
control of foot-and mouth disease, pp414-420, 2006. Available at:
https://www.fao.org/ag/againfo/commissions/docs/research_group/pahpos/App64.pdf.
Accessed October 11, 2022.
|
|
2
|
Muroga N, Hayama Y, Yamamoto T, Kurogi A,
Tsuda T and Tsutsui T: The 2010 foot-and-mouth disease epidemic in
Japan. J Vet Med Sci. 74:399–404. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pariente N, Sierra S, Lowenstein PR and
Domingo E: Efficient virus extinction by combinations of a mutagen
and antiviral inhibitors. J Virol. 75:9723–9730. 2001.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Golde WT, Pacheco JM, Duque H, Doel T,
Penfold B, Ferman GS, Gregg DR and Rodriguez LL: Vaccination
against foot-and-mouth disease virus confers complete clinical
protection in 7 days and partial protection in 4 days: Use in
emergency outbreak response. Vaccine. 23:5775–5782. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rada B and Dragún M: Antiviral action and
selectivity of 6-azauridine. Ann N Y Acad Sci. 284:410–417.
1977.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Goris N, De Palma A, Toussaint JF, Musch
I, Neyts J and De Clercq K: 2'-C-methylcytidine as a potent and
selective inhibitor of the replication of foot-and-mouth disease
virus. Antiviral Res. 73:161–168. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lefebvre DJ, De Vleeschauwer AR, Goris N,
Kollanur D, Billiet A, Murao L, Neyts J and De Clercq K: Proof of
concept for the inhibition of foot-and-mouth disease virus
replication by the anti-viral drug 2'-C-methylcytidine in severe
combined immunodeficient mice. Transbound Emerg Dis. 61:e89–e91.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Furuta Y, Takahashi K, Shiraki K, Sakamoto
K, Smee DF, Barnard DL, Gowen BB, Julander JG and Morrey JD: T-705
(favipiravir) and related compounds: Novel broad-spectrum
inhibitors of RNA viral infections. Antiviral Res. 82:95–102.
2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
De Vleeschauwer AR, Lefebvre DJ, Willems
T, Paul G, Billiet A, Murao LE, Neyts J, Goris N and De Clercq K: A
refined guinea pig model of foot-and-mouth disease virus infection
for assessing the efficacy of antiviral compounds. Transbound Emerg
Dis. 63:e205–e212. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen SF, Perrella FW, Behrens DL and Papp
LM: Inhibition of dihydroorotate dehydrogenase activity by
brequinar sodium. Cancer Res. 52:3521–3527. 1992.PubMed/NCBI
|
|
11
|
Li SF, Gong MJ, Sun YF, Shao JJ, Zhang YG
and Chang HY: In vitro and in vivo antiviral activity of mizoribine
against foot-and-mouth disease virus. Molecules.
24(1723)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li SF, Gong MJ, Shao JJ, Sun YF, Zhang YG
and Chang HY: Antiviral activity of merimepodib against foot and
mouth disease virus in vitro and in vivo. Mol Immunol. 114:226–232.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lanford RE, Guerra B, Chavez D, Giavedoni
L, Hodara VL, Brasky KM, Fosdick A, Frey CR, Zheng J, Wolfgang G,
et al: GS-9620, an oral agonist of Toll-like receptor-7, induces
prolonged suppression of hepatitis B virus in chronically infected
chimpanzees. Gastroenterology. 144:1508–1517, 1517.e1-e10.
2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
de Avila AI, Moreno E, Perales C and
Domingo E: Favipiravir can evoke lethal mutagenesis and extinction
of foot-and-mouth disease virus. Virus Res. 233:105–112.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Furuta Y, Gowen BB, Takahashi K, Shiraki
K, Smee DF and Barnard DL: Favipiravir (T-705), a novel viral RNA
polymerase inhibitor. Antiviral Res. 100:446–454. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jin Z, Tucker K, Lin X, Kao CC, Shaw K,
Tan H, Symons J, Behera I, Rajwanshi VK, Dyatkina N, et al:
Biochemical evaluation of the inhibition properties of favipiravir
and 2'-C-methyl-cytidine triphosphates against human and mouse
norovirus RNA polymerases. Antimicrob Agents Chemother.
59:7504–7516. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Smither SJ, Eastaugh LS, Steward JA,
Nelson M, Lenk RP and Lever MS: Post-exposure efficacy of oral
T-705 (Favipiravir) against inhalational Ebola virus infection in a
mouse model. Antiviral Res. 104:153–155. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Malik P, Jain S, Jain P, Kumawat J,
Dwivedi J and Kishore D: A comprehensive update on the structure
and synthesis of potential drug targets for combating the
coronavirus pandemic caused by SARS-CoV-2. Arch Pharm (Weinheim).
355(e2100382)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Takehisa T: FMD status and control
strategy in Japan. https://www.maff.go.jp/j/syouan/douei/pdf/japan.pdf.
Accessed October 11, 2022.
|
|
20
|
Furuta Y, Takahashi K, Kuno-Maekawa M,
Sangawa H, Uehara S, Kozaki K, Nomura N, Egawa H and Shiraki K:
Mechanism of action of T-705 against influenza virus. Antimicrob
Agents Chemother. 49:981–986. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nishi T, Fukai K, Masujin K, Kawaguchi R,
Ikezawa M, Yamada M, Nakajima N, Komeno T, Furuta Y, Sugihara H, et
al: Administration of the antiviral agent T-1105 fully protects
pigs from foot-and-mouth disease infection. Antiviral Res.
208(105425)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nikunjkumar P, Tamil Selvan RP and
Bhanuprakash V: Ribavirin as a curative and prophylactic agent
against foot and mouth disease virus infection in C57BL/6 suckling
and adult mice model. Virusdisease. 32:737–747. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Choi JH, Jeong K, Kim SM, Ko MK, You SH,
Lyoo YS, Kim B, Ku JM and Park JH: Synergistic effect of ribavirin
and vaccine for protection during early infection stage of
foot-and-mouth disease. J Vet Sci. 19:788–797. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Platt H: A study of the pathological
changes produced in young mice by the virus of foot-and-mouth
disease. J Pathol Bacteriol. 72:299–312. 1956.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang G, Zhou F, Gu B, Ding C, Feng D, Xie
F, Wang J, Zhang C, Cao Q, Deng Y, et al: In vitro and in vivo
evaluation of ribavirin and pleconaril antiviral activity against
enterovirus 71 infection. Arch Virol. 157:669–679. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Qing M, Zou G, Wang QY, Xu HY, Dong H,
Yuan Z and Shi PY: Characterization of dengue virus resistance to
brequinar in cell culture. Antimicrob Agents Chemother.
54:3686–3695. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Luthra P, Naidoo J, Pietzsch CA, De S,
Khadka S, Anantpadma M, Williams CG, Edwards MR, Davey RA, Bukreyev
A, et al: Inhibiting pyrimidine biosynthesis impairs Ebola virus
replication through depletion of nucleoside pools and activation of
innate immune responses. Antiviral Res. 158:288–302.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang Y, Wang W, Xu L, Zhou X, Shokrollahi
E, Felczak K, van der Laan LJ, Pankiewicz KW, Sprengers D, Raat NJ,
et al: Cross talk between nucleotide synthesis pathways with
cellular immunity in constraining hepatitis E virus replication.
Antimicrob Agents Chemother. 60:2834–2848. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li SF, Gong MJ, Sun YF, Shao JJ, Zhang YG
and Chang HY: Antiviral activity of brequinar against
foot-and-mouth disease virus infection in vitro and in vivo. Biomed
Pharmacother. 116(108982)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ishikawa H: Mizoribine and mycophenolate
mofetil. Curr Med Chem. 6:575–597. 1999.PubMed/NCBI
|
|
31
|
Saijo M, Morikawa S, Fukushi S, Mizutani
T, Hasegawa H, Nagata N, Iwata N and Kurane I: Inhibitory effect of
mizoribine and ribavirin on the replication of severe acute
respiratory syndrome (SARS)-associated coronavirus. Antiviral Res.
66:159–163. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Naka K, Ikeda M, Abe K, Dansako H and Kato
N: Mizoribine inhibits hepatitis C virus RNA replication: Effect of
combination with interferon-alpha. Biochem Biophys Res Commun.
330:871–879. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jain J, Almquist SJ, Shlyakhter D and
Harding MW: VX-497: A novel, selective IMPDH inhibitor and
immunosuppressive agent. J Pharm Sci. 90:625–637. 2001.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Markland W, McQuaid TJ, Jain J and Kwong
AD: Broad-spectrum antiviral activity of the IMP dehydrogenase
inhibitor VX-497: A comparison with ribavirin and demonstration of
antiviral additivity with alpha interferon. Antimicrob Agents
Chemother. 44:859–866. 2000.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Decker CJ, Heiser AD, Chaturvedi PR, Faust
TJ, Ku G, Moseley S and Nimmesgern E: The novel IMPDH inhibitor
VX-497 prolongs skin graft survival and improves graft versus host
disease in mice. Drugs Exp Clin Res. 27:89–95. 2001.PubMed/NCBI
|
|
36
|
Fosdick A, Zheng J, Pflanz S, Frey CR,
Hesselgesser J, Halcomb RL, Wolfgang G and Tumas DB:
Pharmacokinetic and pharmacodynamic properties of GS-9620, a novel
Toll-like receptor 7 agonist, demonstrate interferon-stimulated
gene induction without detectable serum interferon at low oral
doses. J Pharmacol Exp Ther. 348:96–105. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bhagchandani S, Johnson JA and Irvine DJ:
Evolution of Toll-like receptor 7/8 agonist therapeutics and their
delivery approaches: From antiviral formulations to vaccine
adjuvants. Adv Drug Deliv Rev. 175(113803)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lee G, Kang HR, Kim A, Park JH, Lee MJ and
Kim SM: Antiviral effect of vesatolimod (GS-9620) against
foot-and-mouth disease virus both in vitro and in vivo. Antiviral
Res. 205(105384)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fukai K, Inoue K, Takeuchi A and Yamakawa
M: New possibilities for egg white lysozyme: Heat-denatured
lysozyme partially inactivates select foot-and-mouth disease virus
strains. Sci Rep. 11(526)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
USDA. National Emergency Response to a
Highly Contagious Animal Disease. Executive Summary, March 30,
2001. https://www.uvm.edu/sites/default/files/media/fmd_disinfectants.pdf?fbclid=IwAR3qbqvIPH2vVntcnsTrNb1PkA_ldovV5Vcyx95WMHM4PX2iw-dp-iTSCsM.
Accessed October 15, 2022.
|
|
41
|
Takahashi M, Takahashi H, Okakura Y,
Ichikawa M, Kuda T and Kimura B: Impact of pH and protein
hydrophobicity on norovirus inactivation by heat-denatured
lysozyme. PLoS One. 15(e0237888)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Harada Y, Lekcharoensuk P, Furuta T and
Taniguchi T: Inactivation of foot-and-mouth disease virus by
commercially available disinfectants and cleaners. Biocontrol Sci.
20:205–208. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Nakashima R, Kawamoto M, Miyazaki S,
Onishi R, Furusaki K, Osaki M, Kirisawa R, Sakudo A and Onodera T:
Evaluation of calcium hydrogen carbonate mesoscopic crystals as a
disinfectant for influenza A viruses. J Vet Med Sci. 79:939–942.
2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Onodera T, Sakudo A, Iwamaru Y, Yokoyama
T, Haritani M, Sugiura K, Shimakura H, Haga T, Onishi R and
Furusaki K: Calcium bicarbonate as an antimicrobial, antiviral, and
prion-inhibiting agent (review). Biomed Rep. 17(57)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kirisawa R, Kato R, Furusaki K and Onodera
T: Universal virucidal activity of calcium bicarbonate mesoscopic
crystals that provides an effective and biosafe disinfectant.
Microorganisms. 10(262)2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ishihara M, Murakami K, Fukuda K, Nakamura
S, Kuwabara M, Hattori H, Fujita M, Kiyosawa T and Yokoe H:
Stability of weakly acidic hypochlorous acid solution with
microbicidal activity. Biocontrol Sci. 22:223–227. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Horiuchi I, Kawata H, Nagao T, Imaohji H,
Murakami K, Kino Y, Yamasaki H, Koyama AH, Fujita Y, Goda H and
Kuwahara T: Antimicrobial activity and stability of weakly
acidified chlorous acid water. Biocontrol Sci. 20:43–51.
2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sato Y, Ishihara M, Nakamura S, Fukuda K,
Kuwabara M, Takayama T, Hiruma S, Murakami K, Fujita M and Yokoe H:
Comparison of various disinfectants on bactericidal activity under
organic matter contaminated environments. Biocontrol Sci.
24:103–108. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Goda H, Yamaoka H, Nakayama-Imaohji H,
Kawata H, Horiuchi I, Fujita Y, Nagao T, Tada A, Terada A and
Kuwahara T: Microbicidal effects of weakly acidified chlorous acid
water against feline calicivirus and Clostridium difficile spores
under protein-rich conditions. PLoS One.
12(e0176718)2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Hole K, Ahmadpour F, Krishnan J,
Stansfield C, Copps J and Nfon C: Efficacy of accelerated hydrogen
peroxide® disinfectant on foot-and-mouth disease virus,
swine vesicular disease virus and Senecavirus A. J Appl Microbiol.
122:634–639. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Omidbakhsh N and Sattar SA: Broad-spectrum
microbicidal activity, toxicologic assessment, and materials
compatibility of a new generation of accelerated hydrogen
peroxide-based environmental surface disinfectant. Am J Infect
Control. 34:251–257. 2006.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Gabbert LR, Neilan JG and Rasmussen M:
Recovery and chemical disinfection of foot-and-mouth disease and
African swine fever viruses from porous concrete surfaces. J Appl
Microbiol. 129:1092–1101. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Environmental Protection Agency (EPA).
Antimicrobial testing methods and procedures: MB-05-14: AOAC use
dilution method for testing disinfectants. https://www.epa.gov/sites/production/files/2016-08/documents/mb-05-14.pdf.
Accessed December 9, 2019.
|
|
54
|
Krug PW, Larson CR, Eslami AC and
Rodriguez LL: Disinfection of foot-and-mouth disease and African
swine fever viruses with citric acid and sodium hypochlorite on
birch wood carriers. Vet Microbiol. 156:96–101. 2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
EPA. List N advanced search page:
Disinfectant for coronaviruses (COVID-19), 2021. https://www.epa.gov/pesticide-registration/list-n-advanced-search-page-disinfectants-coronavirus-covid-19.
Accessed October 13, 2022.
|
|
56
|
Chen B, Han J, Dai H and Jia P:
Biocide-tolerance and antibiotic-resistance in community
environments and risk of direct transfers to humans: Unintended
consequences of community-wide surface disinfecting during
COVID-19? Environ Pollut. 283(117074)2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhang H, Tang W, Chen Y and Yin W:
Disinfection threatens aquatic ecosystems. Science. 368:146–147.
2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Bei E, Shu Y, Li S, Liao X, Wang J, Zhang
X, Chen C and Krasner S: Occurrence of nitrosamines and their
precursors in drinking water systems around mainland China. Water
Res. 98:168–175. 2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Challis BC and Kyrtopoulos SA: Rapid
formation of carcinogenic N-nitrosamines in aqueous alkaline
solutions. Br J Cancer. 35:693–696. 1977.PubMed/NCBI View Article : Google Scholar
|
|
60
|
World Health Organization (WHO), 2023: One
Health, https://www.who.int/news-room/questions-and-answers/item/one-health,
Accessed, 06 Jul 2023.
|