Introduction
Breast cancer (BC) is one of the most common
malignant tumors in women (1).
Triple-negative BC (TNBC) is a subtype of BC (2,3). In
TNBC, the results of immunohistochemical staining of BC tissues
have revealed that these are negative for estrogen receptors,
progesterone receptors and HER2 (4,5). In
addition, TNBC is usually highly malignant, aggressive and prone to
recurrence and metastasis (6). Due
to the estrogen and progesterone receptors being negative, the
patient cannot receive endocrine therapy (7). Additionally, since the HER2 gene is
negative, the patient cannot receive anti-HER2 targeted therapy
(4,8). At present, there is no adequate
treatment for TNBC. Therefore, it is important to explore novel
treatments for patients with TNBC.
Long non-coding RNAs (lncRNAs) are non-coding RNAs
(9,10). It has been reported that abnormal
expression or function of lncRNAs is important in BC (11-13).
For example, low lncRNA HOTAIR expression can induce the apoptosis
of BC cells (11). Overexpression
of LINC02273 promotes the migration and metabolism of BC cells
(12). Furthermore, Wang et
al (13) reported that
lncRNA-ABHD11-AS1 was aberrantly highly expressed in BC. The
present study demonstrated that ABHD11-AS1 may serve as an
important role in the progression of BC. However, the detailed
roles of ABHD11-AS1 in BC are unknown.
MicroRNAs (miRNAs/miRs) are non-coding RNAs with a
size of 20-25 nucleotides (14,15).
Previous studies have demonstrated that lncRNAs can interact with
miRNAs as competing endogenous RNAs (16,17).
For instance, knockdown of lncRNA BCRT1 could inhibit the
progression of BC via upregulation of miR-1303(16). Moreover, miR-199a-5p was considered
as a vital mediator in tumor progression. For instance, miR-199a-5p
could promote the tumorigenesis of oral squamous cell carcinoma
through regulating SMARCA4(18); Xu
et al (19) suggested lnc
CDKN2B-AS1 was implicated in the development and progression of
liver cancer by suppressing cell autophagy through targeting
miR-199a-5p. Meanwhile, miR-199a-5p was involved in lncRNA
TINCR-mediated progression of BC (20). However, the detailed relation
between ABHD11-AS1 and miR-199a-5p in TNBC remains unclear.
Based on the aforementioned previous studies, the
detailed functions of ABHD11-AS1 in TNBC was explored. The present
study aimed to confirm the mechanism underlying the function of
ABHD11-AS1 in TNBC. This research could potentially lead to
discovering new strategies against TNBC.
Materials and methods
Cell culture
TNBC cells (MDA-MB-231) were provided by Shanghai
Zhongqiao Xinzhou Biotechnology Co., Ltd. (cat. no. ZQ0118).
MDA-MB-231 cells were maintained in 89% L15 medium (Invitrogen;
Thermo Fisher Scientific, Inc.). The medium contained 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.), and 1% penicillin and 1%
streptomycin, and cells were cultured at 37˚C with 5%
CO2.
293T cells were obtained from American Type Culture
Collection. Cells were cultured in RPMI-1640 medium (Invitrogen;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Invitrogen; Thermo Fisher Scientific, Inc.) and 2 mM glutamine
(Sigma-Aldrich; Merck KGaA) and placed at 37˚C in a humidified
incubator containing 5% CO2.
Cell transfection
First, MDA-MB-231 cells were maintained in 89% L15
medium at 37˚C with 5% CO2 for 24 h. Subsequently, cells
were transfected with miR-199a-5p mimic (50 nM; Shanghai GenePharma
Co., Ltd.; 5'-CCCAGUGUUAGACUACCUGUUC-3'), miR-199a-5p inhibitor
(100 nM, Genepharma, 5'-GAACAGGUAGUCUGAACACUGGG-3') or negative
control (100 nM, Genepharma, 5'-CAGUACUUUUGUGUAGUACAA-3') by using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 6 h at 37˚C according to the manufacturer's
instruction. Next, the culture was replaced with fresh L15 medium
at 37˚C with 5% CO2 for 48 h, and then reverse
transcription-quantitative PCR (RT-qPCR) analysis was performed to
verify the transfection efficiency of miR-199a-5p mimic or
inhibitor.
RT-qPCR
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to extract RNA from cells and
tumor samples. Cells were treated with 200 µl chloroform (Shanghai
Richjoint Chemical Reagents Co., Ltd.) for 10 min. Next, isopropyl
alcohol (Shanghai Richjoint Chemical Reagents Co., Ltd.) was added
to the supernatant for 10 min. The supernatant was removed by
centrifugation at 20 x g at 4˚C for 10 min. The precipitate was
washed twice with 1 ml 75% ethanol. Subsequently, miScript Reverse
Transcriptase Mix (Qiagen China Co., Ltd.) was used to generate
cDNA according to the manufacturer's protocol. Afterwards, 2X
QuantiTect SYBR Green PCR Master Mix (Qiagen China Co., Ltd.) was
used for qPCR (conditions for amplification: 95˚C for 30 sec, 40
cycles of 95˚C for 10 sec, 58˚C for 30 sec and 72˚C for 30 sec).
The expression levels were normalized to those of β-actin using the
2-ΔΔCq method (21). The
primers used were as follows: miR-199a-5p forward,
5'-CGCGCCCAGTGTTCAGACTAC-3' and reverse,
5'-AGTGCAGGGTCCGAGGTATT-3'; ABHD11-AS1 forward,
5'-TCCAGACAAGACTTGGTCGC-3' and reverse, 5'-CAGCTGGTTGTGTGGCTTTC-3';
β-actin forward, 5'-CTGGAACGGTGAAGGTGACA-3' and reverse,
5'-CGGCCACATTGTGAACTTTG-3'; and U6 forward,
5'-CTCGCTTCGGCAGCACAT-3' and reverse,
5'-AACGCTTCACGAATTTGCGT-3'.
Western blotting
Total protein was extracted using the RIPA buffer
(Beyotime Institute of Biotechnology) from MDA-MB-231 cells by two
rounds of centrifugation at 20 x g for 10 min. Subsequently, the
protein concentration was assessed using a bicinchoninic acid (BCA)
kit (Beyotime Institute of Biotechnology). Total protein (40
µg/lane) was electrophoresed by 10% SDS-PAGE and transferred onto
nitrocellulose membranes. The membranes were blocked with 5 ml
confining liquid (5% skim milk in TBS-0.1% Tween 20) for 1 h at
room temperature and then incubated with anti-N-Cadherin (1:1,000;
cat. no. ab76011; Abcam), anti-Snail (1:1,000; cat. no. ab216347;
Abcam) and anti-E-Cadherin (1:5,000; 20874-1-AP; Proteintech Group,
Inc.) overnight at 4˚C. Subsequently, the membranes were washed
with TBS-0.1% Tween 20 buffer, and then incubated with a secondary
antibody (HRP-conjugated anti-rabbit IgG; 1:5,000; cat. no.
ab288151; Abcam) at room temperature for 2 h. Finally, protein was
visualized using an enhanced chemiluminescence kit. β-actin
(1:1,000; cat. no. ab8226; Abcam) was used as the internal
standard. The protein expressions were evaluated using a
densitometric semiquantitative analysis with the Image-Pro Plus 6.0
(Media Cybernetics, Inc.) software.
Transwell assay
Cells (105 cells) were seeded into the
upper chamber. The lower chamber contained 700 µl L15 medium
supplemented with 10% FBS. After 24 h of incubation at 37˚C, the
migratory cells were stained with 1% crystal violet solution for 10
min at room temperature. Finally, images were captured, and
migratory cells were counted in 3 random fields under a light
microscope.
Cell viability assay
MDA-MB-231 cells (5x103 cells) were
seeded in 96-well plates. Subsequently, cells were treated with
ABHD11-AS1 short hairpin RNA (shRNA)1, miR-199a-5p mimic,
miR-199a-5p inhibitor and ABHD11-AS1 shRNA1 + miR-199a-5p
inhibitor. Next, cells were treated with 10 µl of Cell Counting
Kit-8 reagent (Beyotime Institute of Biotechnology) at 37˚C.
Finally, absorbance at 450 nm was determined using a microplate
reader.
Lentiviral infection
The lentiviral vector LV3 (H1/GFP&Puro) and
shRNAs were provided by Shanghai GenePharma Co., Ltd. The sequence
of ABHD11-AS1 shRNAs was as follows: ABHD11-AS1 shRNA1,
ACCGGTcacctgacagcaacatcaaTTCAAGAGATTGATGTTGCTGTCAGGTGTTTTTTGAATTC;
ABHD11-AS1 shRNA2,
ACCGGTggaccaagtcctccaggaaTTCAAGAGATTCCTGGAGGACTTGGTCCTTTTTTGAATTC;
and ABHD11-AS1 shRNA3,
ACCGGTggagctgcagagatgcaaaTTCAAGAGATTTGCATCTCTGCAGCTCCTTTTTTGAATTC.
The 293T cells were maintained in serum-free L15 medium containing
ABHD11-AS1 shRNA1, ABHD11-AS1 shRNA2 or ABHD11-AS1 shRNA3, plasmid
(1 µg/µl), and packaging plasmid (pAX2, 1 µg/µl), envelope plasmid
(pMD2.G, 1 µg/µl; 2nd-generation lentiviral packaging system) and
the transfection reagent polyethylenimine (Polysciences, Inc.) for
6 h at 37˚C. Next, the culture was replaced with fresh L15 medium
containing 10% FBS and cells were then incubated for 72 h at 37˚C.
After that, the virus-containing supernatant was collected by
centrifugation at 1,500 x g at 4˚C for 30 min. Subsequently,
MDA-MB-231 cells were incubated with the concentrated virus at
multiplicity of infection (MOI)=40 and polybrene (5 µg/ml; Shanghai
Yeasen Biotechnology Co., Ltd.) for 24 h. After transduction, these
MDA-MB-231 cells in the second passage were incubated with
puromycin (2 µg/ml, Gibco; Thermo Fisher Scientific, Inc.) for 72
h.
Xenograft tumor model
To investigate the effect of ABHD11-AS1 on the
progression and development of TNBC, a xenograft animal model was
established. BALB/c female nude mice (n=8; weight, 18-22 g; 6-8
weeks-old) were provided by Beijing Vital River Laboratory Animal
Technology Co., Ltd. All animal experiments were approved (approval
no. 20200527-SJ01) by the Ethics Committee of Huzhou University.
The temperature was maintained at 18-22˚C, the humidity was 50-60%
and a 12-h light/dark cycle. Drinking water and a food supplement
were provided 3-4 times a week. The behavior and food intake of the
mice were monitored every day to maintain their health. MDA-MB-231
cells (5x106 cells/mouse in 100 µl PBS) were
subcutaneously injected into the right flanks of mice. When the
tumor volumes reached 150 mm3, a total of 8 mice were
randomly divided into two groups: ABHD11-AS1 shRNA1-control and
ABHD11-AS1 shRNA1. Each group contained 4 mice. The mice were
weighed, and tumor size was calculated weekly for 5 weeks.
Additionally, mice were intraperitoneally injected with 50 mg/kg
pentobarbital sodium for 10 min. Subsequently, the mice in the
ABHD11-AS1 shRNA1 group were intratumorally injected with
ABHD11-AS1 shRNA1 twice a week. After 5 weeks of treatment, all
mice were euthanized using CO2 at a displacement rate of
40% of the chamber volume/min (CO2 flow rate, 2.5
l/min), and animal death was confirmed by cessation of heartbeat.
The tumor was separated and weighed. The humane endpoints were
judged by a 15% reduction of body weight, no mice were sacrificed
due to meeting the endpoint.
Immunohistochemical staining
Tumor tissues were fixed in 4% paraformaldehyde for
24 h at 4˚C, embedded in paraffin and then cut into 4-µm sections.
Next, the sections were heated at 60˚C, dewaxed using the xylene
reagent and rehydrated (100, 100, 95, 90, 80 and 70% alcohol for 5
min, respectively). Next, 3% H2O2 solution
was dropped onto the slices for 10 min. Subsequently, the tissues
were blocked in 5% BSA (Roche Diagnostics) for 20 min at room
temperature, and then incubated with primary antibodies, including
anti-N-Cadherin (1:100), anti-Snail (1:100; cat. no. ab224731;
Abcam) and anti-E-Cadherin (1:100; cat. no. ab40772; Abcam) at 4˚C
overnight. Next, the tissues were incubated with secondary antibody
(HRP-conjugated anti-rabbit IgG; 1:500;) at 37˚C for 50 min.
Immunostaining was detected by adding 3,3'-diaminobenzidine for 30
sec. Images were observed using a light microscope.
Statistical analysis
The statistically significant differences among
multiple groups were analyzed by one-way ANOVA followed by Tukey's
post hoc tests. GraphPad Prism software (version 7.0; Dotmatics)
was used to analyze data. The data are presented as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Knockdown of ABHD11-AS1 inhibits the
epithelial-mesenchymal transition (EMT) process and migration of
TNBC cells
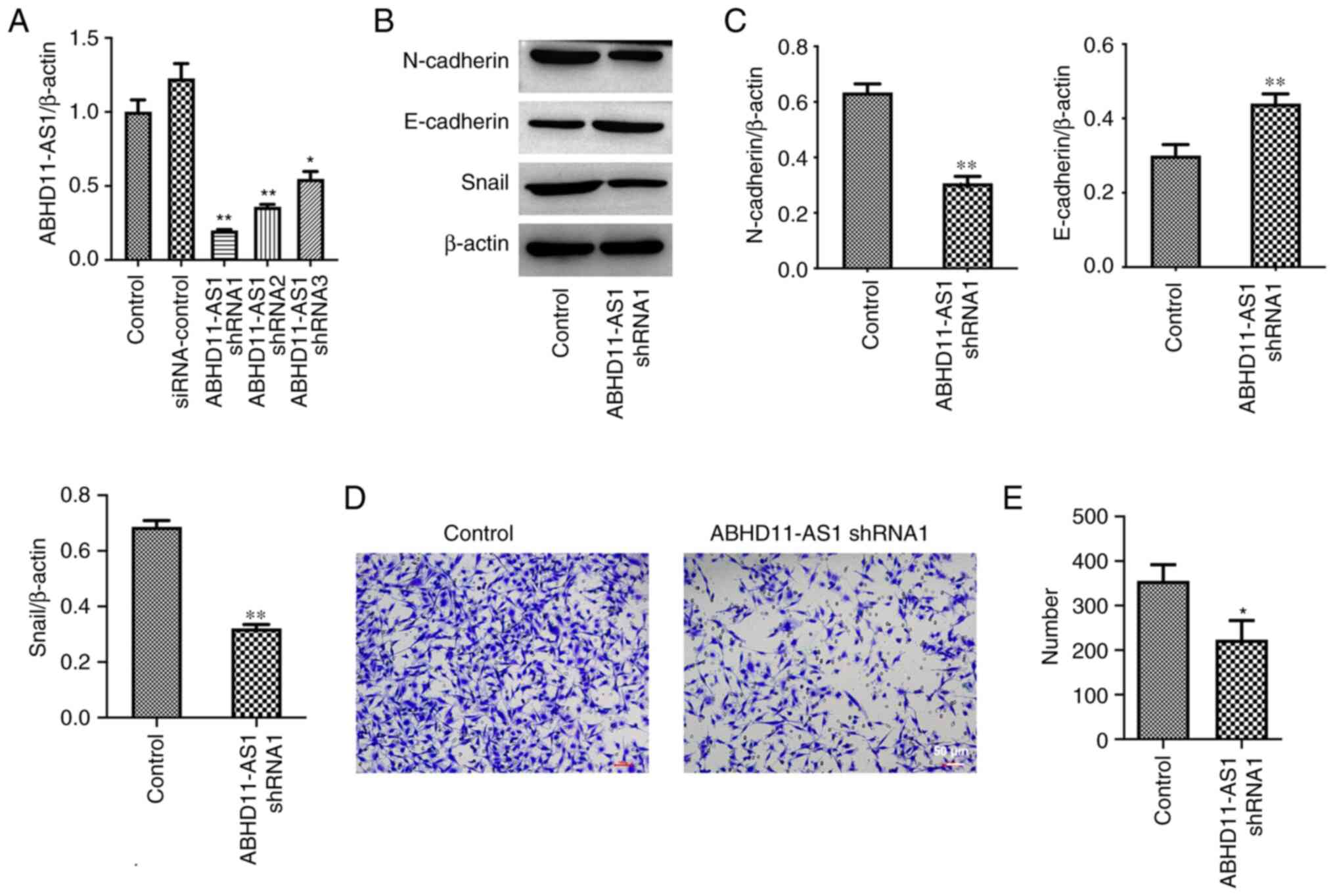
ABHD11-AS1 is aberrantly highly expressed in BC
(13). In order to explore the role
of ABHD11-AS1 in BC, ABHD11-AS1 expression in MDA-MB-231 cells was
first knocked down. The results of RT-qPCR indicated that
ABHD11-AS1 shRNAs significantly inhibited ABHD11-AS1 expression in
MDA-MB-231 cells (Fig. 1A). Since
ABHD11-AS1 shRNA1 exhibited the largest effect, ABHD11-AS1 shRNA1
was used in the subsequent experiments (Fig. 1A). In addition, ABHD11-AS1 shRNA1
downregulated the expression levels of N-Cadherin and Snail, and
upregulated the expression levels of E-Cadherin in MDA-MB-231 cells
(Fig. 1B and C). Furthermore, ABHD11-AS1 shRNA1
significantly suppressed the migration of MDA-MB-231 cells
(Fig. 1D and E). Overall, knockdown of ABHD11-AS1 could
inhibit the EMT process and migration of BC cells.
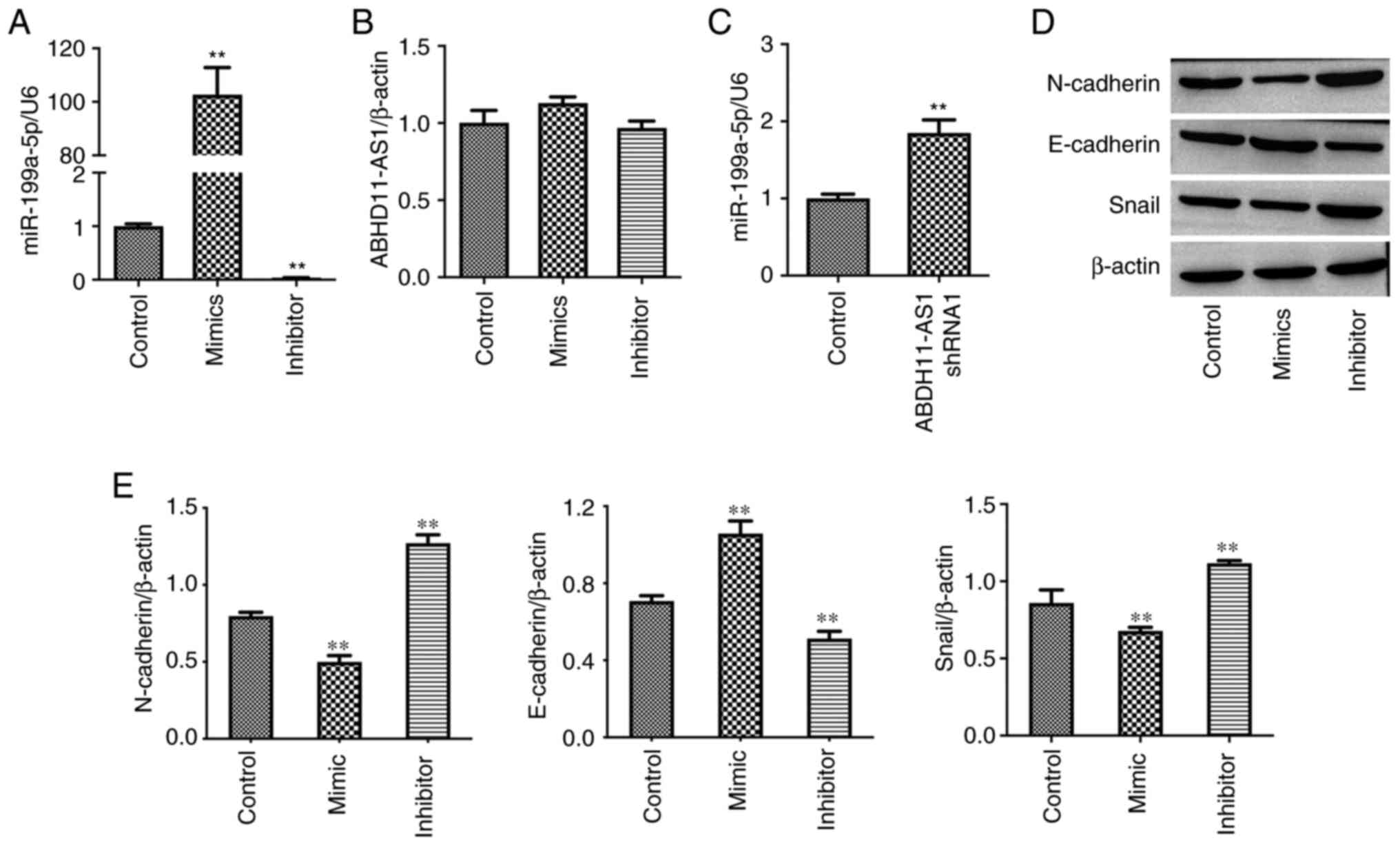
miR-199a-5p mimics inhibit the EMT
process of TNBC cells
Previous evidence has indicated that ABHD11-AS1
could regulate the levels of miR-199a-5p (22). miR-199a-5p mimics increased the
expression levels of miR-199a-5p in MDA-MB-231 cells, while
miR-199a-5p inhibitor exerted the opposite effect (Fig. 2A). Additionally, neither miR-199a-5p
mimics nor miR-199a-5p inhibitor affected the expression levels of
ABHD11-AS1 (Fig. 2B). By contrast,
ABHD11-AS1 shRNA1 upregulated the levels of miR-199a-5p in cells
(Fig. 2C). Furthermore, miR-199a-5p
mimics decreased the expression levels of N-Cadherin and Snail, and
increased the expression levels of E-Cadherin (Fig. 2D and E). Compared with miR-199a-5p mimics,
miR-199a-5p inhibitor exerted the opposite effects on these
proteins (Fig. 2D and E).
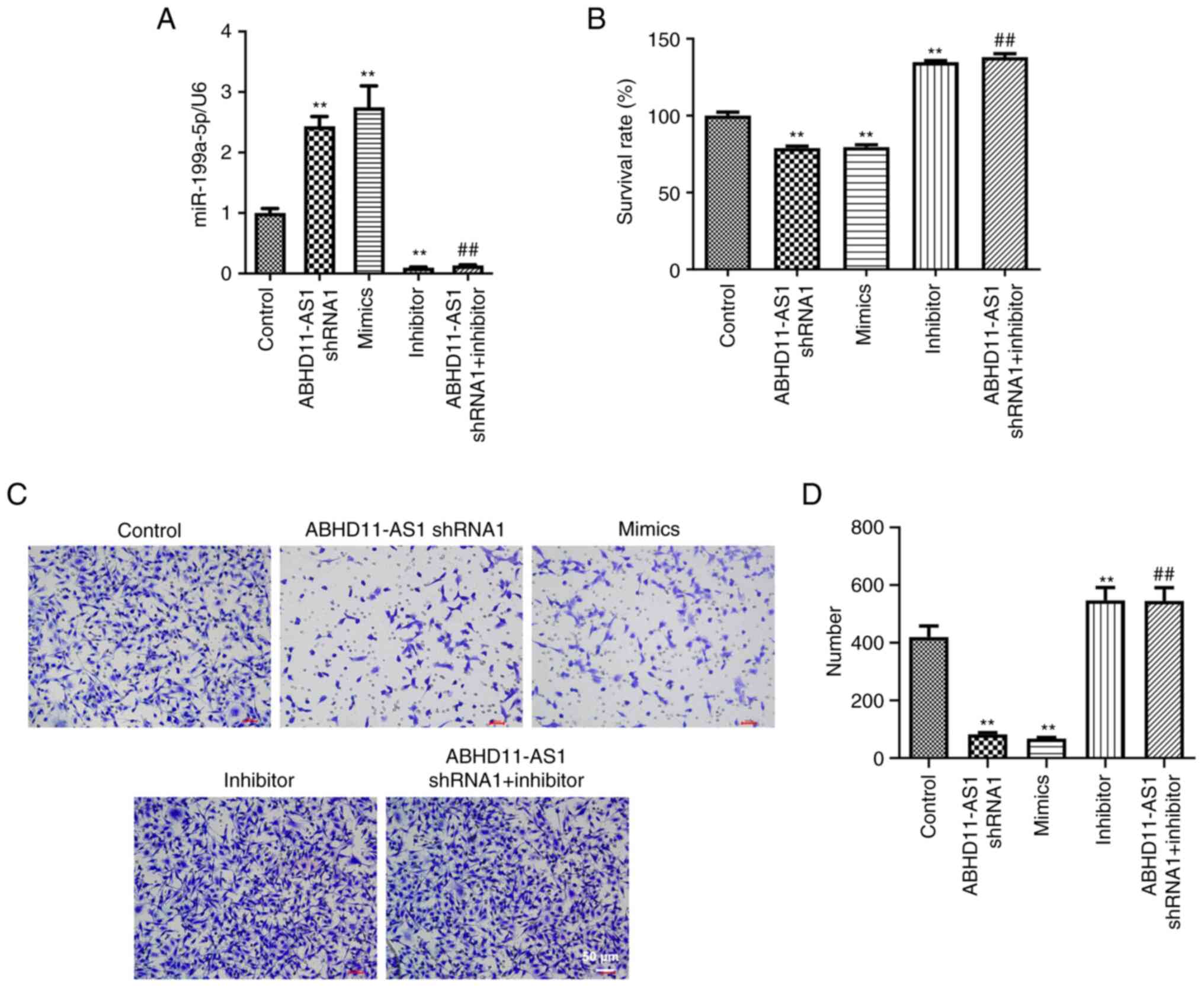
Knockdown of ABHD11-AS1 inhibits the
viability and migration of TNBC cells by upregulating
miR-199a-5p
To further investigate the relationship between
ABHD11-AS1 and miR-199a-5p in BC cells, RT-qPCR was conducted. As
indicated in Fig. 3A, ABHD11-AS1
shRNA1 or miR-199a-5p mimics significantly upregulated the
expression levels of miR-199a-5p in MDA-MB-231 cells, while
miR-199a-5p inhibitor suppressed the expression levels of
miR-199a-5p. Additionally, the effect of ABHD11-AS1 shRNA1 on
miR-199a-5p levels was completely reversed by miR-199a-5p inhibitor
(Fig. 3A). ABHD11-AS1 shRNA1 or
miR-199a-5p mimics inhibited the viability and migration of
MDA-MB-231 cells; however, miR-199a-5p inhibitor and miR-199a-5p
inhibitor + ABHD11-AS1 shRNA1 promoted the viability and migration
of MDA-MB-231 cells (Fig.
3B-D).
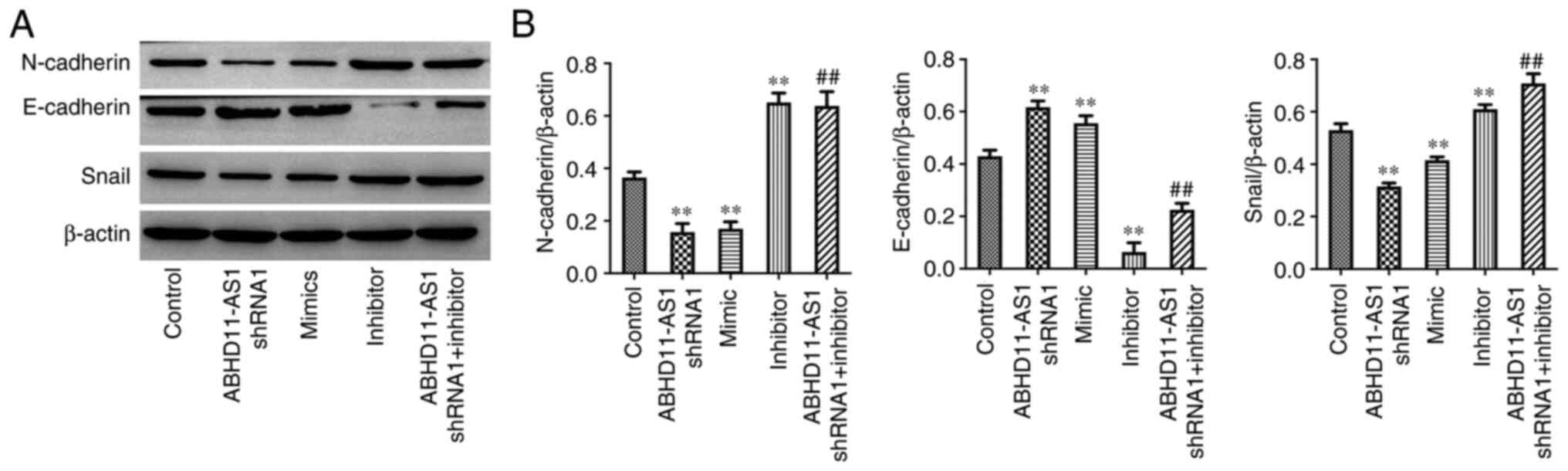
Knockdown of ABHD11-AS1 inhibits the
EMT process of TNBC cells by upregulating miR-199a-5p
As demonstrated in Fig.
4A and B, ABHD11-AS1 shRNA1 or
miR-199a-5p mimics significantly decreased the expression levels of
N-Cadherin and Snail, and increased the expression levels of
E-Cadherin. Notably, miR-199a-5p inhibitor exerted the opposite
effects on these proteins, even in the presence of ABHD11-AS1
shRNA1. Therefore, it was deduced that knockdown of ABHD11-AS1
inhibited the EMT process of BC cells by upregulating
miR-199a-5p.
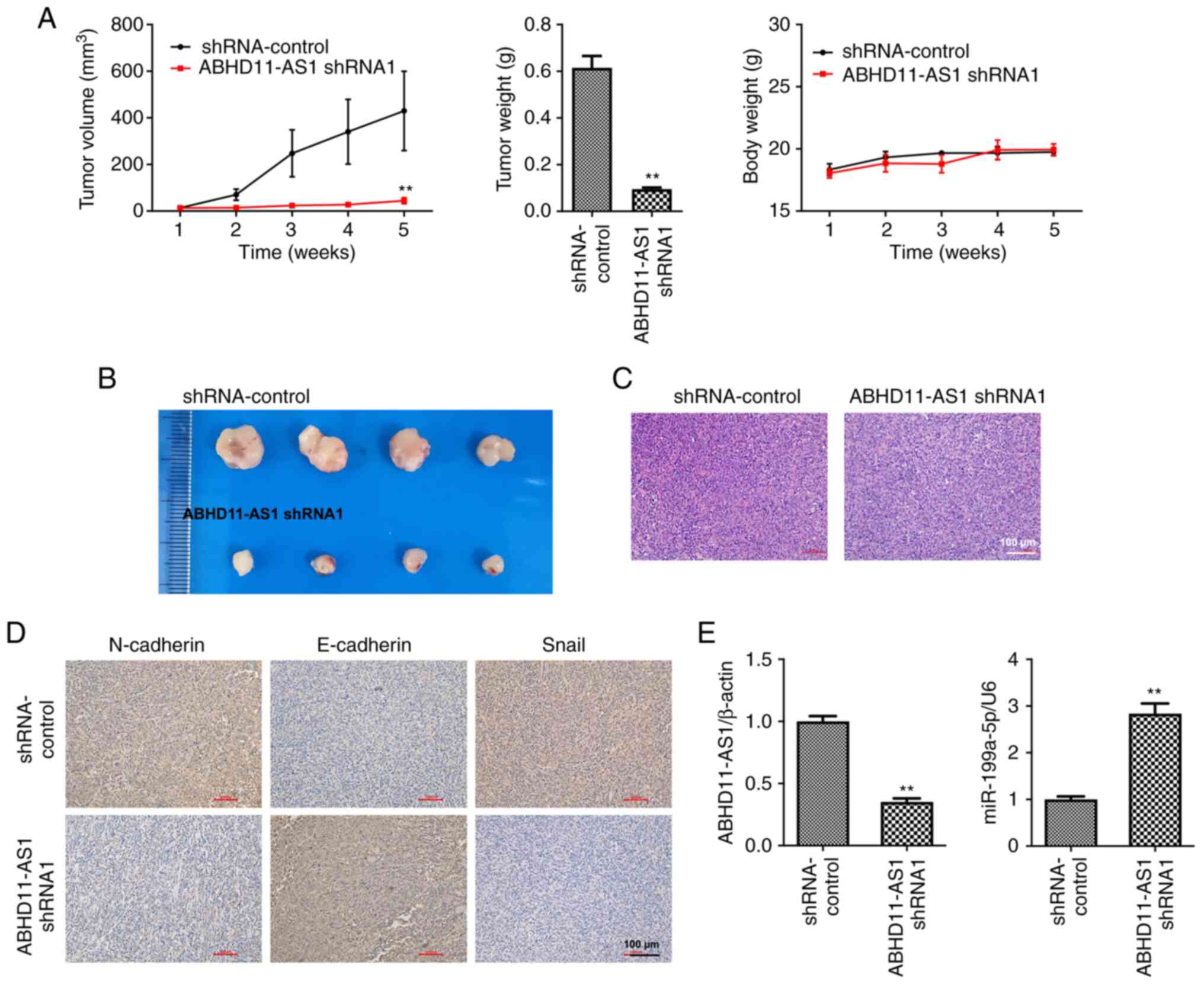
Knockdown of ABHD11-AS1 suppresses
TNBC tumor growth by upregulating miR-199a-5p
To further investigate the effect of ABHD11-AS1 on
the progression and development of TNBC, an in vivo animal
model was established. The results of the animal experiment
revealed that ABHD11-AS1 shRNA1 significantly inhibited TNBC tumor
growth (Fig. 5A and B). ABHD11-AS1 shRNA1 had no significant
effect on the body weight of mice (Fig.
5C). In addition, ABHD11-AS1 shRNA1 inhibited N-Cadherin and
Snail expression and promoted E-Cadherin expression in tumor
tissues (Fig. 5D). Furthermore,
ABHD11-AS1 shRNA1 significantly downregulated ABHD11-AS1 expression
and upregulated the miR-199a-5p levels in tumors (Fig. 5E). These data suggested that
knockdown of ABHD11-AS1 could suppress TNBC tumor growth by
upregulating miR-199a-5p.
Discussion
BC has the highest occurrence (29%) among all cancer
types in women, and uterine cancer (8%) has the second highest
incidence (23-25).
TNBC is a highly malignant and aggressive subtype of BC (6,26,27).
In addition, EMT is closely related to tumor invasion and
metastasis (28-31).
The EMT of TNBC could be inhibited by lovastatin (28). Lovastatin, a lipid-lowering drug,
could suppress the development of TNBC (28). In addition, the progression of TNBC
could be impaired by Dihydrotanshinone-I via obstruction of the EMT
(32). DHTS is a lipophilic
compound of Salvia miltiorrhiza Bunge (Danshen), which is a
traditional Chinese medicine (32).
The present study revealed that knockdown of ABHD11-AS1 markedly
suppressed the progression of BC by inhibiting the EMT process.
These data confirmed that BC can be suppressed by inhibiting the
EMT process.
lncRNA NR2F1-AS1 could promote the proliferation and
angiogenesis of TNBC cells (33).
Additionally, lncRNA GATA3-AS1 could increase the immune escape in
TNBC (34). Furthermore, ABHD11-AS1
is expressed in BC, indicating that ABHD11-AS1 may serve an
important role in BC (13).
Therefore, the expression levels of ABHD11-AS1 in MDA-MB-231 cells
were knocked down. knockdown of ABHD11-AS1 inhibited the EMT
process, viability and migration of TNBC cells by upregulating
miR-199a-5p. All these confirmed that lncRNAs could serve important
roles in the treatment of BC.
A previous study has indicated that ABHD11-AS1 could
promote the proliferation of papillary thyroid cancer cells by
negatively regulating the expression levels of miR-199a-5p
(22). In order to explore the
mechanism by which ABHD11-AS1 regulates the genesis and development
of BC, miR-199a-5p was meticulously examined and revealed that its
upregulation was successful by knockdown of ABHD11-AS1 thus leading
to the suppression of BC cells progression. These results revealed
that miR-199a-5p could be negatively regulated by ABHD11-AS1 in
different cancer types.
The true aim of this research was as follows: i) To
identify the relation between ABHD11-AS1 and miR-199a-5p in TNBC;
ii) the detailed function of ABHD11-AS1 in TNBC was confirmed.
There are certain limitations to the present study.
For instance, whether ABHD11-AS1 can modulate other targets was not
well studied. In addition, the downstream proteins of miR-199a-5p
remain under investigation. Therefore, further investigations
should be conducted in the future.
In the present study, knockdown of ABHD11-AS1
suppressed the progression of BC cells by upregulating miR-199a-5p.
These data may provide novel directions and a theoretical basis for
the treatment of BC.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Huzhou Science and
Τechnology program (grant no. 2020GZ32) and Suzhou municipal key
clinical disciplines cultivate program (grant no.
SZFCXK202142).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DG made substantial contributions to the conception
of the study. DG and YD designed the study. YD, TZ, SS, XL, PJ and
YG performed the experiments. YD drafted the manuscript. DG
supervised the study and revised the manuscript. All authors read
and approved the final manuscript. All authors confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
All animal experiments were approved (approval no.
20200527-SJ01) by the Ethics Committee of Huzhou University. The
National Institute of Health Guide for the Care and Use of
Laboratory Animals was followed.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fan L, Strasser-Weippl K, Li JJ, St Louis
J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM and Goss PE: Breast
cancer in China. Lancet Oncol. 15:e279–e289. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yin L, Duan JJ, Bian XW and Yu SC:
Triple-negative breast cancer molecular subtyping and treatment
progress. Breast Cancer Res. 22(61)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lyons TG: Targeted therapies for
triple-negative breast cancer. Curr Treat Options Oncol.
20(82)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kwapisz D: Pembrolizumab and atezolizumab
in triple-negative breast cancer. Cancer Immunol Immunother.
70:607–617. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Borri F and Granaglia A: Pathology of
triple negative breast cancer. Semin Cancer Biol. 72:136–145.
2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Garrido-Castro AC, Lin NU and Polyak K:
Insights into molecular classifications of triple-negative breast
cancer: Improving patient selection for treatment. Cancer Discov.
9:176–198. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nagini S: Breast Cancer: Current molecular
therapeutic targets and new players. Anticancer Agents Med Chem.
17:152–163. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li J, Ma M, Yang X, Zhang M, Luo J, Zhou
H, Huang N, Xiao F, Lai B, Lv W and Zhang N: Circular HER2 RNA
positive triple negative breast cancer is sensitive to Pertuzumab.
Mol Cancer. 19(142)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bridges MC, Daulagala AC and Kourtidis A:
LNCcation: lncRNA localization and function. J Cell Biol.
220(e202009045)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kong S, Tao M, Shen X and Ju S:
Translatable circRNAs and lncRNAs: Driving mechanisms and functions
of their translation products. Cancer Lett. 483:59–65.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhao W, Geng D, Li S, Chen Z and Sun M:
LncRNA HOTAIR influences cell growth, migration, invasion, and
apoptosis via the miR-20a-5p/HMGA2 axis in breast cancer. Cancer
Med. 7:842–855. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xiu B, Chi Y, Liu L, Chi W, Zhang Q, Chen
J, Guo R, Si J, Li L, Xue J, et al: LINC02273 drives breast cancer
metastasis by epigenetically increasing AGR2 transcription. Mol
Cancer. 18(187)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang X, Zhao Z, Han X, Zhang Y, Zhang Y,
Li F and Li H: Single-Nucleotide polymorphisms promote
dysregulation activation by essential gene mediated bio-molecular
interaction in breast cancer. Front Oncol.
11(791943)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sabit H, Cevik E, Tombuloglu H,
Abdel-Ghany S, Tombuloglu G and Esteller M: Triple negative breast
cancer in the era of miRNA. Crit Rev Oncol Hematol.
157(103196)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bernardo BC, Ooi JY, Lin RC and McMullen
JR: miRNA therapeutics: A new class of drugs with potential
therapeutic applications in the heart. Future Med Chem.
7:1771–1792. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liang Y, Song X, Li Y, Chen B, Zhao W,
Wang L, Zhang H, Liu Y, Han D, Zhang N, et al: LncRNA BCRT1
promotes breast cancer progression by targeting miR-1303/PTBP3
axis. Mol Cancer. 19(85)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fan CN, Ma L and Liu N: Systematic
analysis of lncRNA-miRNA-mRNA competing endogenous RNA network
identifies four-lncRNA signature as a prognostic biomarker for
breast cancer. J Transl Med. 16(264)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xu M, Zhang J, Lu X, Liu F, Shi S and Deng
X: MiR-199a-5p-Regulated SMARCA4 promotes oral squamous cell
carcinoma tumorigenesis. Int J Mol Sci. 24(4756)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xu L, Wu H, Pan J, Chen Z and Du L:
Significance of lncRNA CDKN2B-AS1 in Interventional therapy of
liver cancer and the mechanism under its participation in tumour
cell growth via miR-199a-5p. J Oncol. 2022(2313416)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang Q, Li G, Ma X, Liu L, Liu J, Yin Y,
Li H, Chen Y, Zhang X, Zhang L, et al: LncRNA TINCR impairs the
efficacy of immunotherapy against breast cancer by recruiting DNMT1
and downregulating MiR-199a-5p via the STAT1-TINCR-USP20-PD-L1
axis. Cell Death Dis. 14(76)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhuang X, Tong H, Ding Y, Wu L, Cai J, Si
Y, Zhang H and Shen M: Long noncoding RNA ABHD11-AS1 functions as a
competing endogenous RNA to regulate papillary thyroid cancer
progression by miR-199a-5p/SLC1A5 axis. Cell Death Dis.
10(620)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
S P: Thermal imaging techniques for breast
screening-a survey. Curr Med Imaging. 16:855–862. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lu G, Li Y, Ma Y, Lu J, Chen Y, Jiang Q,
Qin Q, Zhao L, Huang Q, Luo Z, et al: Long noncoding RNA LINC00511
contributes to breast cancer tumourigenesis and stemness by
inducing the miR-185-3p/E2F1/Nanog axis. J Exp Clin Cancer Res.
37(289)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cedro-Tanda A, Ríos-Romero M,
Romero-Córdoba S, Cisneros-Villanueva M, Rebollar-Vega RG,
Alfaro-Ruiz LA, Jiménez-Morales S, Domínguez-Reyes C,
Villegas-Carlos F, Tenorio-Torres A, et al: A lncRNA landscape in
breast cancer reveals a potential role for AC009283.1 in
proliferation and apoptosis in HER2-enriched subtype. Sci Rep.
10(13146)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lin X, Dinglin X, Cao S, Zheng S, Wu C,
Chen W, Li Q, Hu Q, Zheng F, Wu Z, et al: Enhancer-Driven lncRNA
BDNF-AS induces endocrine resistance and malignant progression of
breast cancer through the RNH1/TRIM21/mTOR Cascade. Cell Rep.
31(107753)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zheng S, Yang L, Zou Y, Liang JY, Liu P,
Gao G, Yang A, Tang H and Xie X: Long non-coding RNA HUMT
hypomethylation promotes lymphangiogenesis and metastasis via
activating FOXK1 transcription in triple-negative breast cancer. J
Hematol Oncol. 13(17)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zheng C, Yan S, Lu L, Yao H, He G, Chen S,
Li Y, Peng X, Cheng Z, Wu M, et al: Lovastatin Inhibits EMT and
metastasis of triple-negative breast cancer stem cells through
dysregulation of cytoskeleton-associated proteins. Front Oncol.
11(656687)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Luo J, Yao JF, Deng XF, Zheng XD, Jia M,
Wang YQ, Huang Y and Zhu JH: 14, 15-EET induces breast cancer cell
EMT and cisplatin resistance by up-regulating integrin αvβ3 and
activating FAK/PI3K/AKT signaling. J Exp Clin Cancer Res.
37(23)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Leng X, Huang G, Li S, Yao M, Ding J and
Ma F: Correlation of breast cancer microcirculation construction
with tumor stem cells (CSCs) and epithelial-mesenchymal transition
(EMT) based on contrast-enhanced ultrasound (CEUS). PLoS One.
16(e0261138)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kumar D, Patel SA, Hassan MK, Mohapatra N,
Pattanaik N and Dixit M: Reduced IQGAP2 expression promotes EMT and
inhibits apoptosis by modulating the MEK-ERK and p38 signaling in
breast cancer irrespective of ER status. Cell Death Dis.
12(389)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kashyap A, Umar SM, Dev JR and Prasad CP:
Dihydrotanshinone-I modulates epithelial mesenchymal transition
(EMT) thereby impairing migration and clonogenicity of triple
negative breast cancer cells. Asian Pac J Cancer Prev.
22:2177–2184. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang Q, Li T, Wang Z, Kuang X, Shao N and
Lin Y: lncRNA NR2F1-AS1 promotes breast cancer angiogenesis through
activating IGF-1/IGF-1R/ERK pathway. J Cell Mol Med. 24:8236–8247.
2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang M, Wang N, Song P, Fu Y, Ren Y, Li Z
and Wang J: LncRNA GATA3-AS1 facilitates tumour progression and
immune escape in triple-negative breast cancer through
destabilization of GATA3 but stabilization of PD-L1. Cell Prolif.
53(e12855)2020.PubMed/NCBI View Article : Google Scholar
|