Introduction
Osteoporosis (OP) is one of the most common
metabolic bone diseases and is characterized by loss of trabecular
bone, destruction of the bone microarchitecture and decreased bone
strength, and presents an increasing prevalence in postmenopausal
women (1). In a survey in 2017-2018
in China, the prevalence of OP in postmenopausal women was 32.1%
(2). According to another survey in
the 27 countries of the European Union (EU27), 22 million women
were estimated to have OP (3).
Postmenopausal OP (PMOP) is mainly attributed to a lack of estrogen
and is associated with iron overload and oxidative stress. These
conditions affect bone homeostasis and lead to chronic loss of
trabecular bone, increased bone fragility and a high risk of
fracture (4). As the most severe
clinical outcome of OP, osteoporotic fractures are associated with
high morbidity and mortality, and are emerging as a major global
public health problem (5). The
probability of women at the age of 50 years developing osteoporotic
fractures during their remaining lifespan is 46%. It was estimated
that in the EU27 in 2010 the number of deaths directly attributable
to fracture was at 24 per 100,000 people at risk (aged ≥50 years)
(3). Current treatment options such
as selective estrogen receptor modulators and other therapeutic
approaches for OP, have limitations, including causing other
illness and adverse reactions (6).
With increased demand for alternative medicine, medicinal plants
have been reported as potential therapeutic options for the
prevention and treatment of OP (7).
Ferroptosis is a form of programmed cell death that
is characterized by iron-dependent accumulation of lipid
peroxidation, and impaired cell membrane structure and function
(8). In terms of morphology, cells
are round, small and scattered; mitochondria are small, with
increased mitochondrial membrane density, reduction or loss of
mitochondrial cristae and ruptured mitochondrial outer membrane
(9). Furthermore, the
cystine-glutamate antiporter (System Xc-)/glutathione peroxidase 4
(GPX4) axis serves an essential role in inhibiting ferroptosis
(10). Previous studies have
suggested that ferroptosis is related to the regulation of bone
metabolism via regulation of the metabolism of osteocytes (11,12).
It has also been reported that iron overload is positively
associated with the pathogenesis of OP (13). Postmenopausal women often suffer
from iron overload, and decreasing iron intake or administering
iron chelator treatment can improve bone microstructure and prevent
bone loss (4). Furthermore, it has
been reported that in women aged ≥45 years, serum ferritin
concentrations are inversely associated with lumbar bone mineral
density (BMD), femur neck BMD and total femur BMD (14). Furthermore, iron overload promotes
the formation of reactive oxygen species (ROS), which exert further
stress on bones (11). Therefore,
ferroptosis could markedly influence the pathogenesis of PMOP.
Fructus Ligustri Lucidi (FLL) has been used
in Traditional Chinese Medicine for the treatment of bone diseases
and is still used in the treatment of OP (15). Pharmacological studies have reported
that FLL can promote differentiation of osteoblasts (16), inhibit osteoclastogenesis (17), regulate estrogen levels (18), and regulate BMD and bone properties
via certain pathways, such as the Wnt/β-catenin (19), NADPH oxidase 4/ROS/NF-κB (20) and TGF-β1/Smads signaling pathways
(21). Furthermore, FLL also
inhibits oxidative stress (22,23)
and FLL has been reported to be associated with iron overload
(24). The nuclear factor erythroid
2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) signaling pathway
is an important pathway against oxidative stress and regulates iron
and lipid metabolism (25).
Salidroside is the active ingredient found in FLL, which may
protect against OP by promoting osteogenesis via the upregulation
of Nrf2(26). Nevertheless, whether
the protective effect of FLL against PMOP is mediated by inhibiting
ferroptosis through the Nrf2/HO-1 signaling pathway remains
unclear. In the present study, a rat ovariectomy (OVX) model was
established to assess the role of ferroptosis in PMOP and treatment
with FLL in PMOP, thereby identifying potential targets for the
prevention and treatment of OP.
Materials and methods
Experimental animals
A total of 15 female Sprague-Dawley rats (age, 9-10
weeks; weight, 250±20 g) were purchased from Sipeifu (Beijing)
Biotechnology Co., Ltd. (animal certificate no. SCXK(Jing)
2021-0010). The rats were raised in conventional controlled
conditions (22±1˚C; 50±5% relative humidity and 12 h light-dark
cycle) and allowed free access to food and water. After acclimation
for 1 week, the rats were randomly divided into three groups (n=5)
as follows: SHAM, OVX and FLL group. Subsequently, surgery was
performed on the rats under anesthesia using 3% pentobarbital
sodium (45 mg/kg) by intraperitoneal injection. Bilateral ovaries
were removed and ligatured in rats in the OVX and FLL groups. In
the SHAM group, fat around the bilateral ovaries was ligatured.
After recovering from the procedure, the animals were treated for
12 weeks. Rats in the SHAM and OVX groups were given water orally,
while the FLL group were given FLL (1.56 g/kg/day) orally. After 12
weeks of treatment, the rats were anesthetized with 3%
pentobarbital sodium (45 mg/kg) by intraperitoneal injection,
followed by euthanasia by exsanguination (blood samples were
collected from the abdominal aorta). After that, death was
confirmed by no vital signs (pulse and breath). The uterus was
removed and weighed. The uterus coefficient was calculated as
follows: Uterus coefficient=uterus weight (mg)/body weight (g). The
femurs were removed and preserved in 4% paraformaldehyde at room
temperature for 48 h, and serum and tibia samples were maintained
at -80˚C for use in further experiments.
Micro-CT
The right femur of all groups was scanned after
removing the muscles and tissues. The scan area was set from the
top of the femur to the lowest point of the lateral femoral knee
growth plate using an X-ray energy of 70 kV, a current of 200 µA
and a spatial resolution of 10.2 µm with a 2,016x1,344 image
matrix, using a Skyscan 1276 micro-CT system (Bruker Corporation).
Subsequently, detailed changes in femoral trabeculae were
identified using histomorphometry. Using the lowest point of the
lateral femoral knee growth plate as the baseline, an area with a
thickness of 3 mm was selected as the region of interest to
reconstruct 3D images using N-Recon (version 2.0.0.1; Bruker
Corporation). The BMD, trabecular number (Tb.N), trabecular
thickness (Tb.Th), trabecular bone separation (Tb.Sp), relative
bone volume over total volume (BV/TV) and structure model index
(SMI) were quantified using CTAn (version 1.5.6.2; Bruker
Corporation).
Western blotting
Whole tibia samples were ground into powder in
liquid nitrogen and were then lysed in RIPA protein lysis buffer
(cat. no. C1053; Applygen Technologies, Inc.) with 2% protease
inhibitor (cat. no. P1261; Beijing Solarbio Science &
Technology Co., Ltd.) in a high-speed low-temperature tissue
grinding machine (Wuhan Servicebio Technology Co., Ltd.). The
supernatant was collected by centrifugation at 10,000 x g for 10
min at 4˚C, and the protein concentration of the supernatant was
quantified using a BCA protein quantification kit (cat. no.
20201ES; Shanghai Yeasen Biotechnology Co., Ltd.). The supernatant
was diluted according to the results of the BCA assay, mixed with
loading buffer and then boiled for 10 min for denaturation. Protein
samples (~40 µg/lane) were separated by 10% SDS-PAGE and
transferred to a polyvinylidene fluoride membrane. Afterwards, the
membranes were placed in TBS with 0.1% Tween-20, blocked with 5%
skimmed milk at room temperature for 1 h and probed with primary
antibodies against Nrf2 (1:5,000; cat. no. 16396-1-AP; Proteintech
Group, Inc.), GPX4 (1:2,000; cat. no. 67763-1-Ig; Proteintech
Group, Inc.) and β-actin (1:10,000; cat. no. 66009-1-Ig;
Proteintech Group, Inc.) for 14 h at 4˚C. β-actin was used as the
loading control. Subsequently, the protein bands were incubated at
room temperature for 1 h with the HRP-conjugated goat anti-mouse
(1:5,000; cat. no. ZB-2305; OriGene Technologies, Inc.) and
anti-rabbit (1:5,000; cat. no. ZB-2301; OriGene Technologies, Inc.)
secondary antibodies. Finally, the protein expression was
visualized using a super sensitive ECL luminescent reagent (cat.
no. MA0186; Dalian Meilun Biology Technology Co., Ltd.), assessed
using a ChemiDoc XRS+ Gel Imaging System (Bio-Rad Laboratories,
Inc.) and analyzed using Image Lab (version 6.1.0; Bio-Rad
Laboratories, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from tibia samples using
TRIzol Reagent (Takara Biotechnology Co., Ltd.) and the Nanodrop
8000 (Thermo Fisher Scientific, Inc.) was used to quantify the RNA.
A total of 3 µg total RNA was utilized to synthesize complementary
DNA sequences. Firstly, the oligo (dT)18 primer (cat.
no. 3806; Takara Biotechnology Co., Ltd.) and mRNA were incubated
at 65˚C for 5 min and cooled on ice. Afterwards, the reaction
buffer (cat. no. 2680Q; Takara Biotechnology Co., Ltd.), reverse
transcriptase (cat. no. 2641A; Takara Biotechnology Co., Ltd.),
dNTP (cat. no. 4030; Takara Biotechnology Co., Ltd.) and murine
RNase inhibitor (cat. no. 10603ES10; Shanghai Yeasen Biotechnology
Co., Ltd.) were added and samples were incubated at 42˚C for 1 h
and at 70˚C for 5 min, then preserved at 4˚C temporarily. qPCR was
performed using Hieff qPCR SYBR Green Master Mix (cat. no.
11203ES08; Shanghai Yeasen Biotechnology Co., Ltd.) with the
following thermocycling conditions: Pre-denaturation at 95˚C for 10
min, followed by 40 cycles of denaturation at 95˚C for 5 sec,
annealing at 60˚C for 30 sec and extension at 60˚C for 10 sec. The
relative mRNA expression levels for each target gene were evaluated
using the CFX Opus Real-Time PCR System (Bio-Rad Laboratories,
Inc.). The mRNA expression levels of the target genes were
standardized using GAPDH and quantified using the 2-ΔΔCq
method (27). The primers used for
RT-qPCR are presented in Table
I.
 | Table ISequences of primers used for reverse
transcription-quantitative RT-qPCR. |
Table I
Sequences of primers used for reverse
transcription-quantitative RT-qPCR.
| Gene | Sequence
(5'-3') |
|---|
| GAPDH | F:
CATCTCCCTCACAATTCCATCC |
| | R:
GAGGGTGCAGCGAACTTTAT |
| RUNX2 | F:
CTCCAGGAAGCCTTTGATACTC |
| | R:
TAGGAGGGCTGGATCTTATGT |
| OSX | F:
CCTACTTACCCGTCTGACTTTG |
| | R:
CAACTGCCTTGGGCTTATAGA |
| Nrf2 | F:
ACGTGATGAGGATGGGAAAC |
| | R:
TATCTGGCTTCTTGCTCTTGG |
| HO-1 | F:
GCATGAACTCTCTGGAGATGAC |
| | R:
CAGCTCCTCAAACAGCTGAA |
| SLC7A11 | F:
CCTCTGTTCATCCCAGCATTAT |
| | R:
CCCAGTCAAGGTGATAAGGAAG |
Fe2+ assay
Tibia samples (60 mg) were lysed and the supernatant
was collected according to the aforementioned method for western
blotting. The tissue total iron content colorimetric assay kit
(cat. no. E1050; Applygen Technologies, Inc.) was used according to
the manufacturer's instructions. Briefly, 100 µl mixed 4.5%
potassium permanganate and buffer (1:1) solution was added to 100
µl supernatant and incubated at 60˚C for 1 h. The samples were then
allowed to cool. A total of 30 µl detection solution was added,
mixed and incubated at room temperature for 30 min. The solution
was then centrifuged at 12,000 x g for 5 min at room temperature. A
total of 200 µl supernatant was collected, and then the supernatant
(200 µl per well) was added to a 96-well plate and quantified at
550 nm using a microplate reader.
Malondialdehyde (MDA) assay
Tibia samples were ground in liquid nitrogen and
lysed in a pre-made cell lysis buffer for Western and IP (cat. no.
P0013; Beyotime Institute of Biotechnology), then subjected to a
lipid peroxidation MDA assay kit (cat. no. S0131S; Beyotime
Institute of Biotechnology) for analysis. Briefly, the supernatant
was collected according to the aforementioned method used for
western blotting. Subsequently, 200 µl MDA working solution was
added to 100 µl supernatant and the mixture was boiled for 15 min.
After cooling to room temperature, the solution was centrifuged at
1,000 x g for 10 min at room temperature, and supernatant was
collected and added to a 96-well plate (200 µl per well).
Subsequently, quantification was performed at 532 nm using a
microplate reader. Total protein concentration was measured using a
BCA protein assay kit to calculate the content of MDA (µmol MDA/mg
protein).
Statistical analysis
Data are presented as the mean ± standard deviation
(n=5). All data were checked for normality using the
Kolmogorov-Smirnov test. Data were assessed using one-way ANOVA
followed by Tukey's test using GraphPad Prism 9.00 (Dotmatics).
P<0.05 was considered to indicate a statistically significant
difference.
Results
FLL decreases the weight and increases
the uterus coefficient in OVX rats
The body weight of all groups increased markedly
between weeks 0 and 6 and remained steady in weeks 8-12. The OVX
and FLL groups exhibited higher weight gain than the SHAM group,
while the FLL group exhibited lower weight gain compared with the
OVX group (Fig. 1A). The uterus
coefficient of the OVX group was significantly lower compared with
that of the SHAM group, while FLL slightly increased the uterus
coefficient of the OVX rats; however, this was not statistically
significant. Furthermore, the female rats exhibited atrophied uteri
after OVX, which indicated that menopause was successfully induced
(Fig. 1B).
FLL improves the bone architecture of
OVX rats
The severity of OP is directly reflected in the bone
architecture, which was analyzed in the present study by micro-CT.
The CT images showed fractured and decreased trabeculae of uneven
thickness in the OVX group in comparison with the SHAM group, while
the FLL group exhibited increased density and an increased number
of trabeculae after 12 weeks of treatment (Fig. 2A). Furthermore, BMD, Tb.N, Tb.Th,
Tb.Sp, BV/TV and SMI were analyzed to assess detailed changes in
bone structure. The BMD and Tb. N data indicated significant bone
loss in the OVX rats. Compared with the OVX group, BMD and Tb.N
were significantly increased following FLL treatment, Tb.Th and
BV/TV increased markedly but showed no statistical difference, and
Tb.Sp and SMI decreased but showed no statistical difference. In
addition, there was a significant difference in BMD, Tb.N, Tb.Sp,
BV/TV and SMI when compared between the SHAM and FLL groups, which
indicated that the FLL treatment partially restored bone structure
(Fig. 2B-G).
 | Figure 2FLL improves bone architecture in OVX
rats. (A) Micro-CT analysis of the distal femur region. (B) BMD,
(C) Tb.N, (D) Tb.Sp, (E) Tb.Th, (F) BV/TV and (G) SMI.
*P<0.05 vs. OVX; #P<0.05 vs. SHAM. BMD,
bone mineral density; BV/TV, relative bone volume over total
volume; FLL, Fructus Ligustri Lucidi; ns, not significant;
OVX, ovariectomy; SMI, structure model index; Tb.N, trabecular
number; Tb.Sp, trabecular bone separation; Tb.Th, trabecular
thickness. |
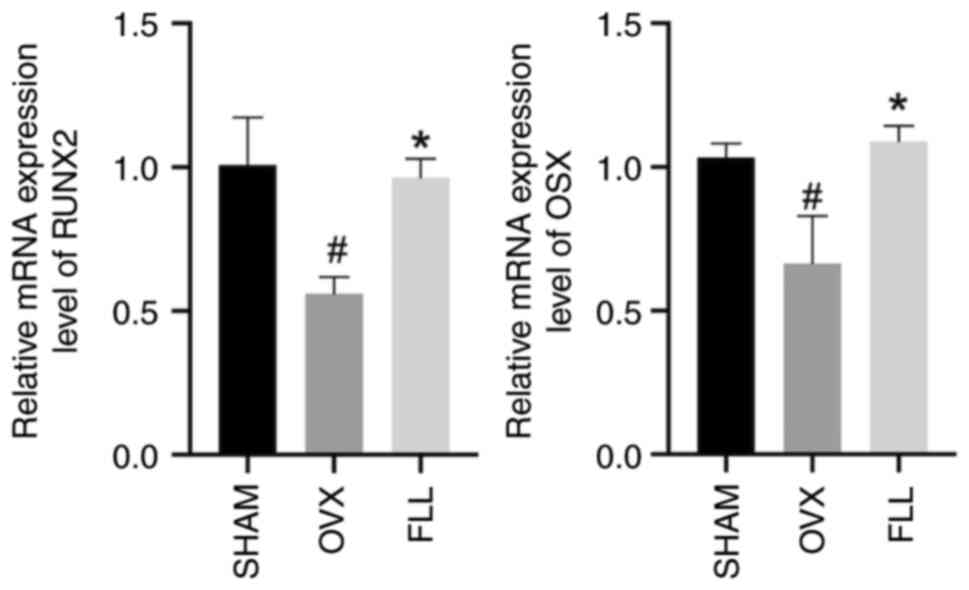
FLL increases the expression levels of
runt-related transcription factor 2 (RUNX2) and osterix (OSX) in
OVX rats
Previous studies have reported that ferroptosis
serves a crucial role in modulating bone metabolism by regulating
osteoblasts (28,29). Thus, bone formation was analyzed in
OVX and FLL rats. Osteoblasts greatly affect bone formation
(30). The transcription factor
runt-related protein family promotes the differentiation of
osteoblasts from bone marrow mesenchymal stem cells (31). OSX is an osteoblast-specific
transcription factor that is required for bone formation and was
first reported to be a bone morphogenetic protein-2-inducible gene
in mesenchymal stem cells (32). As
shown in Fig. 3, the
osteogenesis-related gene expression of RUNX2 and OSX was
significantly decreased in the OVX group compared with the SHAM
group, which was reversed after FLL administration, with a
significant increase in the FLL group compared with the OVX group.
These results indicated that FLL could recover the reduced
osteogenic capacity induced by OVX to a certain extent.
FLL inhibits ferroptosis in OVX
rats
Previous studies have reported a connection between
ferroptosis and OP (29,33,34).
In the present study, ferroptosis and decreased osteogenic function
were observed in ovariectomized rats (Figs. 3 and 4). Excess iron promotes the generation of
ROS during lipid peroxidation. Furthermore, activation of GPX4 and
solute carrier family 7 member 11 (SLC7A11) inhibits ferroptosis by
suppressing lipid peroxidation (35). The western blotting results showed
significantly lower protein expression levels of GPX4 in the OVX
group compared with the SHAM group (Fig. 4A). The RT-qPCR results demonstrated
significantly lower mRNA expression levels of SLC7A11 in the OVX
group compared with the SHAM group (Fig. 4B). Furthermore, the content of
Fe2+ and MDA in bone tissues was significantly increased
in the OVX group compared with the SHAM group, but significantly
decreased compared with the OVX rats after FLL treatment (Fig. 4C). These results indicated the
presence of ferroptosis in OVX rats and that FLL effectively
inhibited ferroptosis.
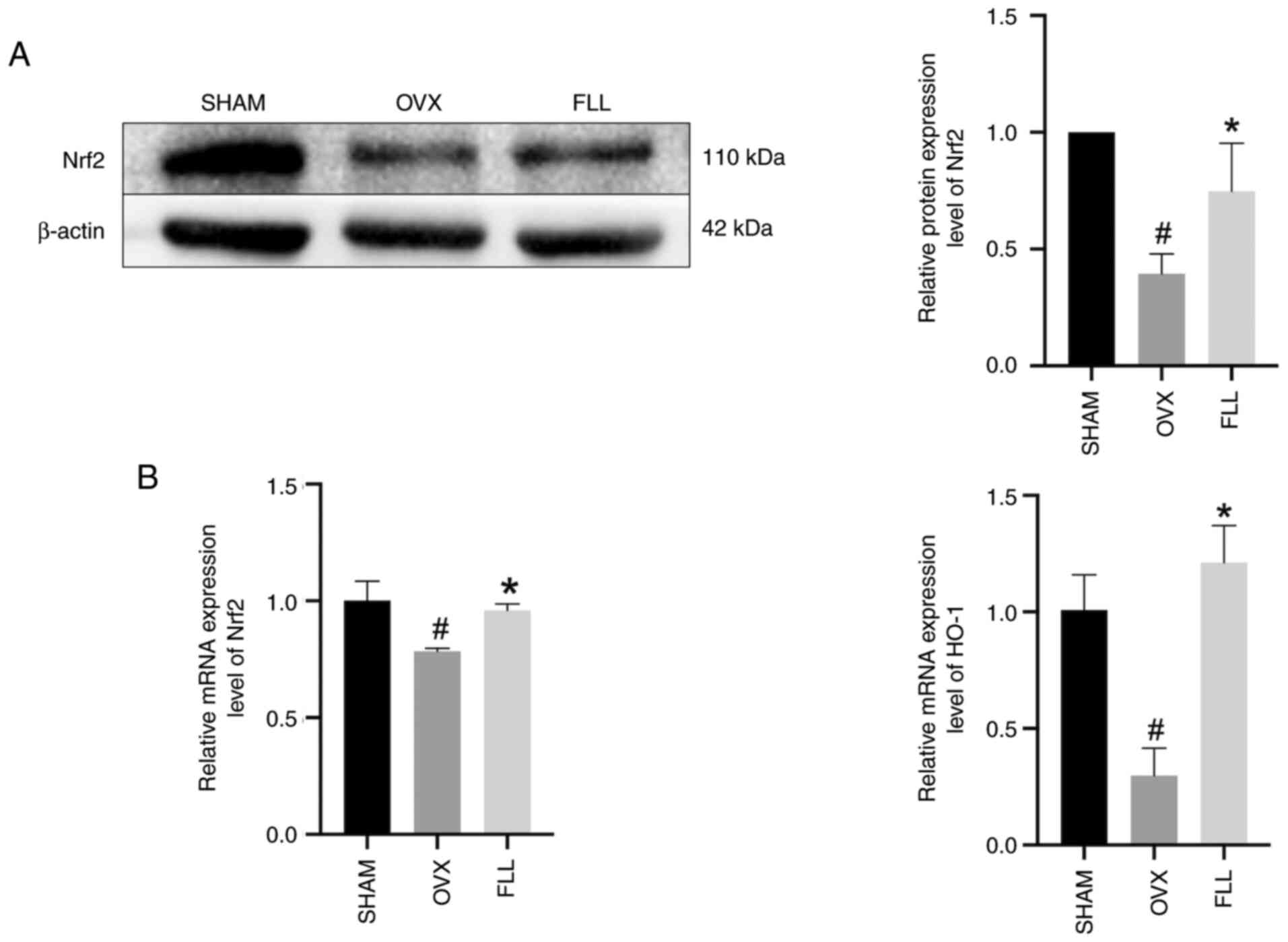
FLL promotes the Nrf2/HO-1 signaling
pathway in OVX rats
As a vital regulator of lipid peroxidation and
ferroptosis, Nrf2 modifies downstream targets of GPX4 and SLC7A11.
Nrf2 binds kelch like ECH associated protein 1 (Keap1) which is
responsible for the cytosolic sequestration of Nrf2 under
physiological conditions. Under stress conditions, Nrf2 is released
from Keap1 and translocates to the nucleus, where Nrf2 exhibits a
short half-life of ~200 min and is then degraded (25). HO-1, the enzyme responsible for
catalyzing the conversion of heme into biliverdin, is upregulated
by Nrf2(36). Following OVX, Nrf2
and HO-1 were both significantly downregulated compared with the
SHAM group, which was significantly reversed by FLL treatment
(Fig. 5).
Discussion
The relationship between ferroptosis and OP has
received growing attention, but only a small number of published
papers have explored the relationship between ferroptosis and PMOP
(10,29,33,37).
The present study determined the presence of ferroptosis in
OVX-induced OP in rats. In addition, the OVX rats were treated with
FLL, and the results indicated that FLL protected against OP by
inhibiting ferroptosis via the Nrf2/HO-1 signaling pathway.
In the bone remodeling process, the activities of
osteoblasts and osteoclasts are in dynamic balance to maintain the
homeostasis and integrity of bone tissues. Osteoblasts are mainly
associated with bone reconstruction, involving the formation,
mineralization and secretion of bone cells (30). The femur of OVX rats exhibited a low
BMD, destroyed bone microarchitecture and downregulated expression
of the transcription factors RUNX2 and OSX. These findings
indicated dysfunctional bone formation in OVX-induced OP.
Furthermore, micro-CT indicated that FLL promoted bone formation,
increased the amount of trabecular bone and bone density, and
improved bone histomorphology, which was consistent with previous
reports (17,18). FLL treatment effectively recovered
the expression of RUNX2 and OSX to similar levels compared with the
SHAM group, which suggested that the therapeutic effect of FLL
could be attributed to the promotion of bone formation.
The pathogenesis of PMOP is a complicated process
and is still not fully understood. Previous studies have reported
that ferroptosis serves a crucial role in OP (29,33,37).
Ferroptosis, a form of programmed cell death, is characterized by
the accumulation of intracellular iron and lipid ROS. Ferroptosis
is mediated by the toxicity of free iron, the depletion of the
antioxidant glutathione (GSH) and the accumulation of oxidative
damage to membrane lipids (38).
Although iron is essential for maintaining cell function,
intracellular iron overload induces ROS formation through the
Fenton reaction, generating deleterious lipid peroxides that damage
the cellular structure and function. Among reactive lipid species
produced by lipid peroxidation, 4-hydroxy-2-nonenal, MDA and other
products can cause harm to cells (39). Furthermore, the System Xc-/GPX4 axis
has been reported to serve a vital role in inhibiting ferroptosis.
Firstly, cystine, the precursor of cysteine, is transported into
the cell via System Xc-. Then, GSH is produced from cysteine,
glutamate and glycine by enzymatic catalysis (40). SLC7A11 is a subunit of the solute
transport family System Xc-transporter (41). GSH is a critical antioxidant in
cells and is catalyzed by GPX4 to oxidize GSH during lipid
peroxidation. Thereafter, H+ is released to scavenge
intracellular oxygen radicals and inhibit lipid
peroxidation-induced ferroptosis (42). It has been previously reported that,
in mice, ferroptosis exerts a marked impact on bone loss induced by
a high-fat diet (29). Another
study reported ferroptosis and increased mitochondrial ferritin in
type 2 diabetic osteoporotic rats (37). The presence of iron has been
reported to suppress the expression of RUNX2, the key transcription
factor related to osteogenic differentiation (43). The anti-ferroptosis effect of
‘Qing'e Pills’ has been reported to be mediated by the upregulation
of System Xc- and GPX4 in OVX rats (44). In essence, ferroptosis is mediated
by lipid peroxidation and reduces expression of the core factors
GPX4 and SLC7A11 in response to external stimuli, thereby impairing
the antioxidant system. In the present study, increased levels of
Fe2+ and MDA were observed in rat bone tissues 12 weeks
after OVX, which indicated increased lipid peroxidation, leading to
impaired cell metabolism (45).
Western blotting indicated decreased protein expression levels of
GPX4 and RT-qPCR indicated decreased mRNA expression levels of
SLC7A11, which was consistent with a previous study (44). These results indicated that OVX
could induce ferroptosis. A previous study reported that FLL could
protect against OP by inhibiting oxidative stress (23). Significantly decreased
Fe2+ and MDA levels and significantly increased levels
of the marker factors GPX4 and SLC7A11 were observed in OVX rats
treated with FLL compared with the OVX group. These findings
suggested that FLL promoted osteogenesis and protected against PMOP
by inhibiting ferroptosis.
The transcription factor Nrf2, one of the crucial
regulators of the cellular antioxidant response, serves an
essential role in protecting against oxidative stress (39). The antioxidant defense systems are
closely associated with lipid accumulation (45). Due to its protective effect on
maintaining cell function, inhibition of Nrf2 or a number of its
downstream target genes results in decreased responsiveness to
cellular dysfunction and increased cell death (39). Following Nrf2 activation, HO-1 and
other ROS-detoxifying enzymes are also upregulated (25). In the presence of oxidative stress,
Nrf2 in the nucleus induces transcription of HO-1 and upregulates
HO-1 gene and protein expression (46,47).
However, downregulation of Nrf2 is associated with diseases
associated with lipid peroxidation and ferroptosis (48). Previous studies have reported that
the Nrf2/HO-1 signaling pathway is involved in the regulation of
ferroptosis (34,49-51).
Activation of the Nrf2/HO-1 signaling pathway may suppress the
generation of intracellular lipid ROS and increase GPX4 activity,
thereby protecting MC3T3-E1 cells against ferroptosis (34). Panaxydol has been reported to
inhibit lipopolysaccharide-induced ferroptosis and inflammation
through Keap1-Nrf2/HO-1 signaling (49). In conclusion, as the Nrf2/HO-1
pathway is activated, ferroptosis and cell injury are inhibited,
restoring normal lipid metabolism (48). Consistent with the aforementioned
reports, the present study demonstrated that Nrf2 and HO-1 were
downregulated in OVX rats and signs of ferroptosis were observed in
OVX rats, which indicated that the Nrf2/HO-1 signaling pathway may
participate in OVX-induced ferroptosis. Salidroside, the active
ingredient in FLL, has been previously reported to provide
protection against OP by upregulating Nrf2(26). Furthermore, FLL treatment
significantly increased the expression levels of Nrf2, HO-1, GPX4
and SLC7A11, and Fe2+ and MDA content. Ferroptosis and
lipid peroxidation were inhibited as the Nrf2/HO-1 signaling
pathway was activated by FLL. These results indicated that the
Nrf2/HO-1 signaling pathway could be an important factor in the
regulation of OVX-induced OP and that the deactivation of the
Nrf2/HO-1 signaling pathway corresponds with OVX-induced
ferroptosis and OP.
In summary, the present study demonstrated that
ferroptosis serves an essential role in the development of PMOP and
may be a potential therapeutic target. The therapeutic effects of
FLL in OP could be mediated by the activation of the Nrf2/HO-1
signaling pathway to inhibit ferroptosis. These findings may
provide pharmacological and material evidence for clinical
therapies for PMOP and form a foundation for further studies on the
mechanism of iron-dependent ferroptosis and OP. However, the study
had some limitations. The effect of FLL on OP may be achieved
through complicated mechanisms, such as regulation of bone
resorption (17). The study did not
explore the detailed mechanism of FLL. Except for the Nrf2/HO-1
pathway, other pathways that may affect ferroptosis should also be
explored. There are three main pathways that inhibit ferroptosis by
acting on peroxide, including the System Xc-/GPX4,
NADPH/ferroptosis suppressor protein 1/coenzyme Q10 and GTP
cyclohydrolase 1/tetrahydrobioterin/phospholipid axes (40). The study only investigated the
System Xc-/GPX4 axis. Experiments exploring the mechanism of
ferroptosis in OVX-induced OP in detail should be conducted.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the China Academy of
Chinese Medical Sciences Innovation Fund (grant no. CI 2021A00107),
the National Natural Science Foundation of China (grant no.
82074297) and the Fundamental Research Funds for the Central Public
Welfare Research Institutes (grant no. YZX-202244).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PL was responsible for project administration, data
curation, development and design of the methodology, and writing
the original draft of the manuscript. YW was responsible for data
curation, data interpretation and project administration. QY was
responsible for data curation, and the development and design of
the methodology. YY was responsible for the development and design
of the methodology, data validation, and data interpretation. RZ
was responsible for project administration, data acquisition and
data analysis. JM was responsible for data acquisition and data
analysis. YC was responsible for supervision, data acquisition,
data analysis and data validation. HL was responsible for funding
acquisition, study design, supervision and writing the original
draft of the manuscript. ZZ was responsible for conceptualization,
funding acquisition, supervision, and reviewing and editing the
manuscript. PL and HL confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Ethics Committee of the Institute of Basic Theory of Chinese
Medicine, China Academy of Chinese Medical Sciences (ethical
approval no. IBTCMCACMS21-2202-02; Beijing, China).
Patient consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing
interests.
References
|
1
|
Song S, Guo Y, Yang Y and Fu D: Advances
in pathogenesis and therapeutic strategies for osteoporosis.
Pharmacol Ther. 237(108168)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang L, Yu W, Yin X, Cui L, Tang S, Jiang
N, Cui L, Zhao N, Lin Q, Chen L, et al: Prevalence of osteoporosis
and fracture in China: The China osteoporosis prevalence study.
JAMA Netw Open. 4(e2121106)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hernlund E, Svedbom A, Ivergård M,
Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B and Kanis
JA: Osteoporosis in the European Union: Medical management,
epidemiology and economic burden. A report prepared in
collaboration with the international osteoporosis foundation (IOF)
and the European federation of pharmaceutical industry associations
(EFPIA). Arch Osteoporos. 8(136)2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chen B, Li GF, Shen Y, Huang XI and Xu YJ:
Reducing iron accumulation: A potential approach for the prevention
and treatment of postmenopausal osteoporosis. Exp Ther Med.
10:7–11. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fink HA, MacDonald R, Forte ML, Rosebush
CE, Ensrud KE, Schousboe JT, Nelson VA, Ullman K, Butler M, Olson
CM, et al: Long-term drug therapy and drug discontinuations and
holidays for osteoporosis fracture prevention: A systematic review.
Ann Intern Med. 171:37–50. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Kanis JA, Cooper C, Rizzoli R and
Reginster JY: Scientific Advisory Board of the European Society for
Clinical and Economic Aspects of Osteoporosis (ESCEO) and the
Committees of Scientific Advisors and National Societies of the
International Osteoporosis Foundation (IOF). European guidance for
the diagnosis and management of osteoporosis in postmenopausal
women. Osteoporos Int. 30:3–44. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Peng Z, Xu R and You Q: Role of
traditional Chinese medicine in bone regeneration and osteoporosis.
Front Bioeng Biotechnol. 10(911326)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liang C, Zhang X, Yang M and Dong X:
Recent progress in ferroptosis inducers for cancer therapy. Adv
Mater. 31(e1904197)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu P, Wang W, Li Z, Li Y, Yu X, Tu J and
Zhang Z: Ferroptosis: A new regulatory mechanism in osteoporosis.
Oxid Med Cell Longev. 2022(2634431)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jiang Z, Wang H, Qi G, Jiang C, Chen K and
Yan Z: Iron overload-induced ferroptosis of osteoblasts inhibits
osteogenesis and promotes osteoporosis: An in vitro and in vivo
study. IUBMB Life. 74:1052–1069. 2022.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Xie Q, Sun Y, Xu H, Chen T, Xiang H, Liu
H, Wang R, Tan B, Yi Q, Tian J and Zhu J: Ferrostatin-1 improves
BMSC survival by inhibiting ferroptosis. Arch Biochem Biophys.
736(109535)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li Y, Bai B and Zhang Y: Expression of
iron-regulators in the bone tissue of rats with and without iron
overload. Biometals. 31:749–757. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kim BJ, Lee SH, Koh JM and Kim GS: The
association between higher serum ferritin level and lower bone
mineral density is prominent in women ≥45 years of age (KNHANES
2008-2010). Osteoporos Int. 24:2627–2637. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cao M, Wu J, Peng Y, Dong B, Jiang Y, Hu
C, Yu L and Chen Z: Ligustri Lucidi Fructus, a traditional
Chinese medicine: Comprehensive review of botany, traditional uses,
chemical composition, pharmacology, and toxicity. J Ethnopharmacol.
301(115789)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kong Y, Ma X, Zhang X, Wu L, Chen D, Su B,
Liu D and Wang X: The potential mechanism of Fructus Ligustri
Lucidi promoting osteogenetic differentiation of bone marrow
mesenchymal stem cells based on network pharmacology, molecular
docking and experimental identification. Bioengineered.
13:10640–10653. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ma Z, Tang X, Chen Y, Wang H, Li Y, Long Y
and Liu R: Epimedii Folium and Ligustri Lucidi Fructus
promote osteoblastogenesis and inhibit osteoclastogenesis against
osteoporosis via acting on osteoblast-osteoclast communication.
Oxid Med Cell Longev. 2023(7212642)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tang YQ, Li C, Sun XJ, Liu Y, Wang XT, Guo
YB, Wang LL, Ma RF, Niu JZ, Fu M, et al: Fructus Ligustri
Lucidi modulates estrogen receptor expression with no
uterotrophic effect in ovariectomized rats. BMC Complement Altern
Med. 18(118)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu H, Guo Y, Zhu R, Wang L, Chen B, Tian
Y, Li R, Ma R, Jia Q, Zhang H, et al: Fructus Ligustri
Lucidi preserves bone quality through induction of canonical
Wnt/β-catenin signaling pathway in ovariectomized rats. Phytother
Res. 35:424–441. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Wang L, Ma R, Guo Y, Sun J, Liu H, Zhu R,
Liu C, Li J, Li L, Chen B, et al: Antioxidant Effect of Fructus
Ligustri Lucidi aqueous extract in ovariectomized rats is
mediated through Nox4-ROS-NF-κB pathway. Front Pharmacol.
8(266)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang Y, Nian H, Tang X, Wang X and Liu R:
Effects of the combined Herba Epimedii and Fructus Ligustri
Lucidi on bone turnover and TGF-β1/Smads pathway in GIOP rats.
J Ethnopharmacol. 201:91–99. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li L, Chen B, Zhu R, Li R, Tian Y, Liu C,
Jia Q, Wang L, Tang J, Zhao D, et al: Fructus Ligustri
Lucidi preserves bone quality through the regulation of gut
microbiota diversity, oxidative stress, TMAO and Sirt6 levels in
aging mice. Aging (Albany NY). 11:9348–9368. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wu Y, Hu Y, Zhao Z, Xu L, Chen Y, Liu T
and Li Q: Protective effects of water extract of Fructus
Ligustri Lucidi against oxidative stress-related
osteoporosis in vivo and in vitro. Vet Sci. 8(198)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Seo HL, Baek SY, Lee EH, Lee JH, Lee SG,
Kim KY, Jang MH, Park MH, Kim JH, Kim KJ, et al: Liqustri lucidi
Fructus inhibits hepatic injury and functions as an antioxidant by
activation of AMP-activated protein kinase in vivo and in vitro.
Chem Biol Interact. 262:57–68. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Loboda A, Damulewicz M, Pyza E, Jozkowicz
A and Dulak J: Role of Nrf2/HO-1 system in development, oxidative
stress response and diseases: An evolutionarily conserved
mechanism. Cell Mol Life Sci. 73:3221–3247. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang YF, Chang YY, Zhang XM, Gao MT, Zhang
QL, Li X, Zhang L and Yao WF: Salidroside protects against
osteoporosis in ovariectomized rats by inhibiting oxidative stress
and promoting osteogenesis via Nrf2 activation. Phytomedicine.
99(154020)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhao Y, Du Y, Gao Y, Xu Z, Zhao D and Yang
M: ATF3 regulates osteogenic function by mediating osteoblast
ferroptosis in type 2 diabetic osteoporosis. Dis Markers.
2022(9872243)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhu R, Wang Z, Xu Y, Wan H, Zhang X, Song
M, Yang H, Chai Y and Yu B: High-fat diet increases bone loss by
inducing ferroptosis in osteoblasts. Stem Cells Int.
2022(9359429)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ponzetti M and Rucci N: Osteoblast
differentiation and signaling: Established concepts and emerging
topics. Int J Mol Sci. 22(6651)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Vimalraj S and Sekaran S: RUNX family as a
promising biomarker and a therapeutic target in bone cancers: A
review on its molecular mechanism(s) behind tumorigenesis. Cancers
(Basel). 15(3247)2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang C: Molecular mechanisms of
osteoblast-specific transcription factor osterix effect on bone
formation. Beijing Da Xue Xue Bao Yi Xue Ban. 44:659–665.
2012.PubMed/NCBI
|
|
33
|
Li M, Yang N, Hao L, Zhou W, Li L, Liu L,
Yang F, Xu L, Yao G, Zhu C, et al: Melatonin inhibits the
ferroptosis pathway in rat bone marrow mesenchymal stem cells by
activating the PI3K/AKT/mTOR signaling axis to attenuate
steroid-induced osteoporosis. Oxid Med Cell Longev.
2022(8223737)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ma H, Wang X, Zhang W, Li H, Zhao W, Sun J
and Yang M: Melatonin suppresses ferroptosis induced by high
glucose via activation of the Nrf2/HO-1 signaling pathway in type 2
diabetic osteoporosis. Oxid Med Cell Longev.
2020(9067610)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Stockwell BR, Friedmann Angeli JP, Bayir
H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK,
Kagan VE, et al: Ferroptosis: A regulated cell death nexus linking
metabolism, redox biology, and disease. Cell. 171:273–285.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang Q, Liu J, Duan H, Li R, Peng W and
Wu C: Activation of Nrf2/HO-1 signaling: An important molecular
mechanism of herbal medicine in the treatment of atherosclerosis
via the protection of vascular endothelial cells from oxidative
stress. J Adv Res. 34:43–63. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang X, Ma H, Sun J, Zheng T, Zhao P, Li H
and Yang M: Mitochondrial ferritin deficiency promotes osteoblastic
ferroptosis via mitophagy in type 2 diabetic osteoporosis. Biol
Trace Elem Res. 200:298–307. 2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bertrand RL: Iron accumulation,
glutathione depletion, and lipid peroxidation must occur
simultaneously during ferroptosis and are mutually amplifying
events. Med Hypotheses. 101:69–74. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Dodson M, Castro-Portuguez R and Zhang DD:
NRF2 plays a critical role in mitigating lipid peroxidation and
ferroptosis. Redox Biol. 23(101107)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Duan JY, Lin X, Xu F, Shan SK, Guo B, Li
FX, Wang Y, Zheng MH, Xu QS, Lei LM, et al: Ferroptosis and its
potential role in metabolic diseases: A curse or revitalization?
Front Cell Dev Biol. 9(701788)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chai D, Zhang L, Xi S, Cheng Y, Jiang H
and Hu R: Nrf2 activation induced by Sirt1 ameliorates acute lung
injury after intestinal ischemia/reperfusion through NOX4-mediated
gene regulation. Cell Physiol Biochem. 46:781–792. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Bachhawat AK and Yadav S: The glutathione
cycle: Glutathione metabolism beyond the γ-glutamyl cycle. IUBMB
Life. 70:585–592. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Balogh E, Tolnai E, Nagy B Jr, Nagy B,
Balla G, Balla J and Jeney V: Iron overload inhibits osteogenic
commitment and differentiation of mesenchymal stem cells via the
induction of ferritin. Biochim Biophys Acta. 1862:1640–1649.
2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hao J, Bei J, Li Z, Han M, Ma B, Ma P and
Zhou X: Qing'e pill inhibits osteoblast ferroptosis via ATM
serine/threonine kinase (ATM) and the PI3K/AKT pathway in primary
osteoporosis. Front Pharmacol. 13(902102)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Gaschler MM and Stockwell BR: Lipid
peroxidation in cell death. Biochem Biophys Res Commun.
482:419–425. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Baird L, Swift S, Llères D and
Dinkova-Kostova AT: Monitoring Keap1-Nrf2 interactions in single
live cells. Biotechnol Adv. 32:1133–1144. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Baird L, Llères D, Swift S and
Dinkova-Kostova AT: Regulatory flexibility in the Nrf2-mediated
stress response is conferred by conformational cycling of the
Keap1-Nrf2 protein complex. Proc Natl Acad Sci USA.
110:15259–15264. 2013.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Dong H, Qiang Z, Chai D, Peng J, Xia Y, Hu
R and Jiang H: Nrf2 inhibits ferroptosis and protects against acute
lung injury due to intestinal ischemia reperfusion via regulating
SLC7A11 and HO-1. Aging (Albany NY). 12:12943–12959.
2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Li J, Lu K, Sun F, Tan S, Zhang X, Sheng
W, Hao W, Liu M, Lv W and Han W: Panaxydol attenuates ferroptosis
against LPS-induced acute lung injury in mice by Keap1-Nrf2/HO-1
pathway. J Transl Med. 19(96)2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Chen Y, Zhang P, Chen W and Chen G:
Ferroptosis mediated DSS-induced ulcerative colitis associated with
Nrf2/HO-1 signaling pathway. Immunol Lett. 225:9–15.
2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Dang R, Wang M, Li X, Wang H, Liu L, Wu Q,
Zhao J, Ji P, Zhong L, Licinio J and Xie P: Edaravone ameliorates
depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4
pathway. J Neuroinflammation. 19(41)2022.PubMed/NCBI View Article : Google Scholar
|