1. Introduction
Retinopathy of prematurity (ROP) is a vascular
abnormal proliferative retinopathy in premature infants. ROP is
preventable and treatable; early screening enables timely
implementation of optimal therapeutic interventions for affected
premature infants. The World Health Organization Vision 2020
project identified ROP as the leading cause of childhood blindness
(1,2). Advances in neonatal medicine have
markedly decreased the rate of preterm births worldwide; an
artificial intelligence-based epidemiological assessment in India
showed that the proportion of infants with moderate to severe ROP
has significantly decreased over two time periods (August 2015 to
October 2017 and March 2019 to December 2020) in South India
(3). However, the incidence of
ROP-associated blindness is increasing worldwide, particularly in
low- and middle-income countries. Despite improved conditions and
facilities in neonatal intensive care units (NICUs) in low- and
middle-income countries, many NICUs need more resources and
equipment to monitor and prevent ROP. It is estimated that ~50,000
children worldwide are permanently blinded by ROP each year, 4,000
of which are in China; China accounts for 1.9% of all children
under the age of five who become blind due to ROP globally
(4,5). Therefore, it is key to study the
pathogenesis and influencing factors of ROP. Several factors affect
development of ROP, however pathogenesis and risk factors for ROP
are not fully understood. In addition to preterm birth, low birth
weight and high postnatal oxygen uptake, which have been recognized
as risk factors worldwide, other factors include mode of delivery,
multiple births, maternal pregnancy factors, neonatal
bronchopulmonary dysplasia, use of surfactants, arterial duct
inactivity, necrotizing enterocolitis, race and Apgar scores
(6). In recent years, several
hematological indicators have been suggested to influence the
development and clinical course of ROP (7-11).
The present review summarizes the impact of hematological
indicators on ROP.
2. Pathogenesis of ROP
Fetal retinal vascularization begins at 16 weeks
gestation and reaches the nasal ora serrata at ~36 weeks gestation.
By contrast, the temporal ora serrata is not fully vascularized
until ~40 weeks of corrected gestational age. Therefore, retinal
blood vessels in preterm infants are not yet fully developed and
need to continue to develop after birth. It is hypothesized that
the pathogenesis of ROP is mainly due to an imbalance between pro-
and antiangiogenic factors. Vascular endothelial growth factor
(VEGF) is involved in the development of ROP. VEGF is a family of
polypeptides that exerts its effects primarily through binding to
tyrosine kinase receptors, which play an essential role in ROP. The
expression of VEGF in humans is closely associated with blood
oxygen saturation (SpO2), with hypoxia upregulating VEGF
and hyperoxia downregulating VEGF (12). Studies have shown that the
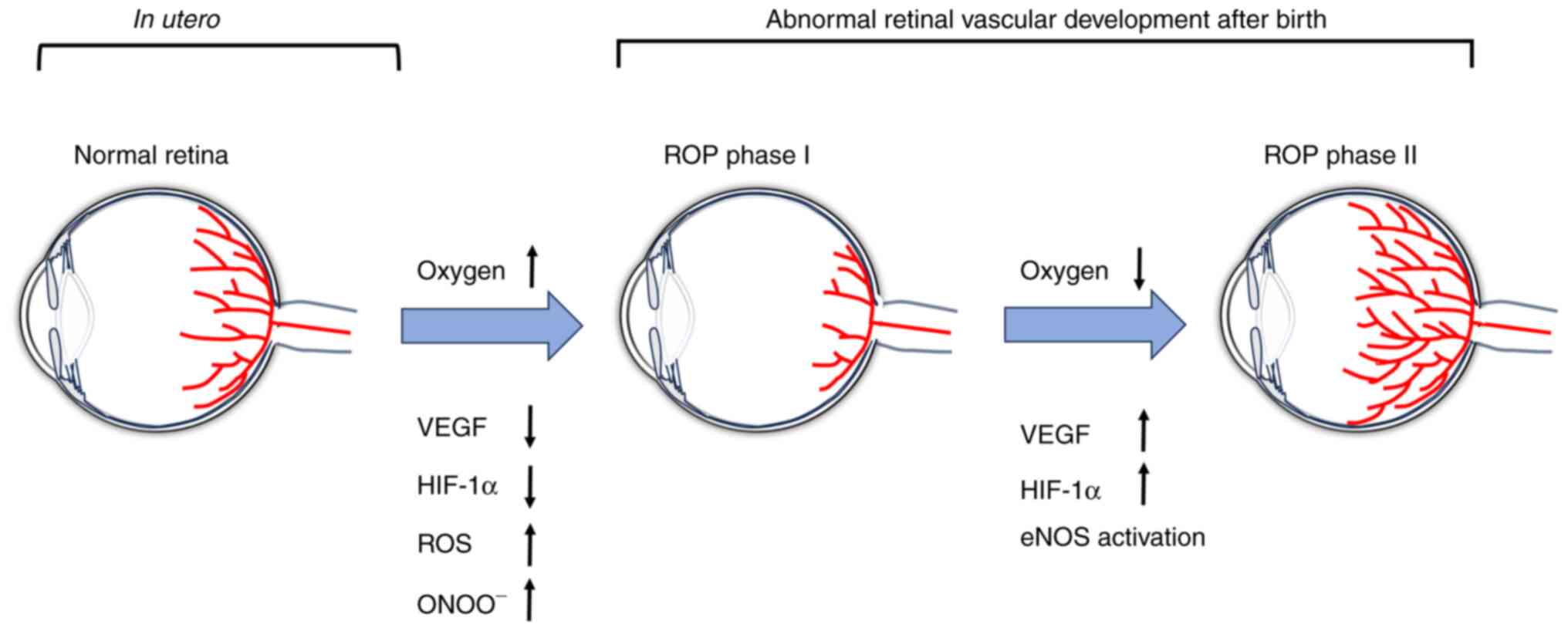
pathogenesis of ROP is divided into two phases (12,13).
Phase I is characterized by vaso-obliteration, which commences at
premature birth as premature infants are born into a relatively
hyperoxic environment [the partial pressure of oxygen
(PaO2) in the extra-uterine environment is close to 100
mmHg, whereas PaO2 is #x003C;35 mmHg in utero];
hyperoxia inhibits VEGF production and the sudden decrease in VEGF
disrupts normal retinal development. Phase II is characterized by
vaso-proliferation. During this phase, disrupted vascular
development results in insufficient blood supply to the retina, a
consequence of its gradual maturation. This insufficiency leads to
retinal hypoxia, which increases VEGF expression. If the avascular
region is minimal, heightened VEGF levels can promote blood vessel
growth to finalize retinal vascularization, meaning ROP can resolve
spontaneously. When avascularity is extensive, VEGF prompts
neovascularization in the retina. Furthermore, insulin-like growth
factor (IGF)-1 collaborates with VEGF to modulate this
neovascularization process (13,14).
An overproduction or disproportion of these elements can result in
ROP (12-14).
The pathogenesis of ROP is associated with reactive
oxygen species (ROS) and nitric oxide synthase (NOS) (15). Premature infants are exposed to
relatively high oxygen levels after birth and have an
underdeveloped antioxidant system. This leads to excessive
production of ROS, triggering the oxidative stress-related
signaling pathways, which causes apoptosis in endothelial cells.
This leads to retinal vascular occlusion that disrupts the
development of normal retinal blood vessels. NOS, an isoenzyme
located in neuronal cells, neurophagocytes, macrophages and
endothelial cells, exists in three isoforms: Neuronal, inducible
and endothelial-type NOS (eNOS). In hyperoxia, eNOS-derived NO
participates in the superoxide reaction to form peroxynitrite
(ONOO-), an essential mediator of hyperoxia-induced
vascular occlusion. Hypoxia induces activation of NOS, activates
the JAK/STAT3 pathway and mediates neovascularization. In addition,
hypoxia induces an increase in VEGF expression, which activates
eNOS through the Akt signaling pathway (15,16).
The NO produced by eNOS impairs integrity of vascular endothelial
cell adhesion junctions, leading to enhanced vascular permeability.
In addition, NO serves as a vasodilator and exhibits anti-occlusive
and proangiogenic properties. Under normal oxygen levels,
hypoxia-inducible factor-1α (HIF-1α) is hydroxylated in the
cytoplasm by the prolyl hydroxylase structural domain (PHD). Both
oxygen and iron serve as essential cofactors for PHD, and hypoxia
inhibits PHD enzyme activity, which increases the stability and
levels of HIF-1α, subsequently elevating VEGF production.
Conversely, hyperoxia inhibits HIF-1α, resulting in a decrease in
VEGF and vascular occlusion. In summary, stage I of ROP is
associated with increased ROS and ONOO- and decreased
levels of VEGF and HIF-1α, whereas stage II is associated with the
activation of eNOS and increased levels of HIF-1α and VEGF
(Fig. 1) (15,17).
Retinal detachment occurs in severe cases of ROP, leading to
permanent blindness. However, if existing blood vessels continue to
grow normally and complete peripapillary retinal vascularization,
ROP may be healed spontaneously (15).
3. Effect of hematological indicators on
ROP
In recent years, an increasing number of studies
(7-11)
have revealed that various laboratory markers in children,
including hemoglobin (Hb), platelet count, blood glucose (BG) and
inflammatory cells, may be linked to the development of ROP
(Table I). These findings
demonstrate the etiology of ROP and its association with systemic
reactions. Hematological indicators may be used as indicators of
risk factors and prognosis outcomes for ROP.
 | Table IAssociation of hematological
indicators with ROP and possible pathophysiological mechanisms. |
Table I
Association of hematological
indicators with ROP and possible pathophysiological mechanisms.
| Biochemical
index | Potential
mechanisms | Research
indicators | Association with
ROP | (Refs.) |
|---|
| Blood PLT | Granules in PLTs
store angiogenic regulatory factors | PLT count | Negative | (18,25) |
| | | | None | (7,19,27) |
| | | MPV | Positive (phase
II) | (20,29) |
| Hb | Lower levels result
in less oxygen being carried | Hb | Negative | (33,34) |
|
Post-transfusion | Shift in the oxygen
dissociation curve | HbF | Negative | (8,32) |
| Hb | caused by
replacement of HbF by HbA results in a higher proportion of
dissolved oxygen in plasma | HbA | Positive | (8,32) |
| Blood GLU | Hyperglycemia
stimulates VEGF production via activation of protein kinase C | GLU | Positive | (41) |
| | | | None | (47) |
| | | HbA1C | Negative
(non-proliferative ROP) | (49) |
| | | | Positive
(proliferative ROP) | (49) |
| Inflammation | Inflammation
activates microglia; infection and inflammation damage vascular
endothelium; retinal perfusion is compromised in infected infants
due to hypotension and changes in oxygenation levels | NEUT
(chorioamnionitis, sepsis) | Positive | (31,51,52,56) |
| | | NLR | Positive | (10) |
| | | | None | (60) |
| | | PLR | None | (60) |
| | | SII | Positive | (56) |
| | | CAR | Positive | (65) |
| | | IL-6, IL-8 | Positive | (66,67,68,73) |
| Blood lipids | Retina is rich in
lipids and lipid oxidation is a key source of energy for the
retina; dyslipidemia can affect pathological angiogenesis in the
retina and inhibit neovascularization by regulating serum levels of
VEGF | Total cholesterol,
LDL, triglycerides | Positive | (11) |
| | | Lipocalin | Negative | (75,76) |
Effect of platelets (PLTs) on ROP
The role of PLTs in ROP is not yet fully understood.
PLTs are cytoplasmic clumps without nuclei produced by
megakaryocytes and have active physiological properties that
regulate neovascularization, fibrin formation and deposition
(18,19). Studies (19-22)
have shown that PLTs locally promote or inhibit angiogenesis.
Platelets contain granules that store regulators of angiogenesis,
including VEGF and angiogenesis inhibitors (such as endothelial
inhibitors), which are released, thus regulating angiogenesis
(23). Most studies (24,25)
have shown that a reduced PLT count is associated with ROP. Cakir
et al (18) discovered that
thrombocytopenia at the corrected gestational age of >30 weeks
is independently associated with severe ROP that requires
treatment. In addition, they found that low PLT count during the
neovascularization phase of ROP (phase II) is significantly
associated with development of severe ROP.
Şahinoğlu et al (25) also found that a low PLT count is
independently associated with severe ROP. Therefore, low PLT count
may increase the risk of ROP in preterm infants. Parrozzani et
al (23) demonstrated that PLTs
act as scavengers in neovascularization by clearing VEGF. A
decrease in PLT count results in reduced VEGF elimination, leading
to an increase in VEGF and subsequent massive neovascularization.
Most studies (21,23,26)
have found an association between thrombocytopenia and development
or severity of ROP; however, a few studies (7,27)
detected no association between low PLT levels and ROP. Choręziak
et al (27) found no
difference in the early postnatal PLT count between patients with
ROP that do and do not require treatment. However, a decrease in
PLT count before the diagnosis of ROP is key for the development of
ROP. This may be attributed to the immaturity of the blood system
at birth. A retrospective study by Özkaya (7) showed no significant difference in PLT
between children with ROP requiring treatment and those not
requiring treatment. However, the number of children with
thrombocytopenia (PLT count #x003C;150x109/l) was higher
in the group that needed treatment compared with the group that did
not. This may be because the study did not exclude subjects based
on number of PLT transfusions or the duration of thrombocytopenia.
Lim et al (19) studied mean
weekly PLT during the first 6 weeks of life. After adjusting for
covariates, there was no significant difference between mean PLT
count of preterm infants in the group with ROP and without ROP.
Decreased PLT count is not directly linked to ROP, but an effect of
other risk factors such as sepsis, blood transfusion and
bronchopulmonary dysplasia (19).
ROP is influenced by various factors and due to different research
and statistical methods, the results can also vary. Recent research
(22) examining the impact of stage
I and II PLT parameters on ROP has shown no differences in stage I
platelet parameters. However, an increase in PLT in stage II with
more VEGF release results in increased neovascularization, which
contradicts the findings of Parrozzani et al (23). Therefore, the effect of increased or
decreased PLT count on ROP is inconclusive, and the pathological
mechanisms of PLT count on ROP need further study.
An association has been found between mean PLT
volume (MPV) and ROP. MPV reflects PLT size and activity and is a
marker of PLT reactivity. During the second stage of ROP, the
retina is in a state of hypoxia. The continued increase in VEGF
expression stimulates formation of multiple new blood vessels, in
which PLTs serve a role in regulating neovascularization and
transporting neovascularization factors (28). PLTs in peripheral blood are
hyperfunctional, large in size and have a relatively high MPV
(28,29). Clinical studies (20,29)
have shown a statistically significant difference in MPV levels
between children with ROP and controls. Increase in MPV volume
raises the risk of ROP by a factor of 1.94(29). MPV levels are associated with
diabetic retinopathy, suggesting a link between activated PLTs and
proliferative retinopathy (30,31).
To the best of our knowledge, there is a lack of research on the
association between PLT parameters and ROP; effect of PLTs on VEGF
homeostasis and the severity and pathogenesis of ROP requires
further investigation.
Effect of Hb, post-transfusion adult
Hb (HbA) and fetal Hb (HbF) on ROP. Effect of Hb on ROP
Reduced Hb levels result in less oxygen being
transported. At stage I of ROP, infants with lower Hb levels are
unable to meet the increased oxygen demand of the developing retina
and retinal vascularization is delayed. At stage II, hypoxia
increases VEGF levels, thereby promoting neovascularization
(32). Lundgren et al
(33) conducted a retrospective
study of 227 preterm infants aged ≤28 weeks. Hb levels in the first
week of life were significantly lower in infants with ROP requiring
treatment than those not requiring treatment. Logistic regression
analysis demonstrated the duration of anemia in the first week of
life in infants with ROP requiring treatment is an independent risk
factor for ROP treatment. Therefore, early prevention of anemia
decreases risk of developing ROP. Akyüz et al (34) retrospectively analyzed complete
blood count (CBC) parameters in 150 preterm infants; mean
corpuscular Hb (MCH) of erythrocytes was the most significant
predictor among all parameters. MCH represents the average value of
Hb in erythrocytes. The association between MCH and ROP may be
associated with the NO pathway. Excess NO can cause vasodilation,
capillary leakage and edema, while Hb scavenges NO, resulting in
the formation of Hb-NO complexes (34). The aforementioned studies indicate
the importance of sufficient Hb in preventing ROP.
Effect of HbA and HbF on ROP after
transfusion. HbF serves as the primary oxygen transport protein
during fetal development and is involved in transporting oxygen
from maternal blood to fetal organs and tissue. In preterm infants,
HbF is converted to HbA after birth. HbF has a higher oxygen
affinity than HbA. The oxygen dissociation curve of HbF is shifted
to the left compared with HbA, thus HbF has a higher oxygen
affinity than HbA at any oxygen partial pressure (32). The substitution of HbF for HbA
causes the oxygen dissociation curve to shift left, leading to a
higher proportion of dissolved oxygen in plasma and an increase in
tissue exposure to oxygen (8,35).
Studies have shown that low levels of HbF are independently
associated with development of ROP and that maintaining higher
levels of HbF may protect against ROP (8,32).
Therefore, different transfusion regimens may affect outcome of
ROP. Children with anemia who receive transfusions of adult red
blood cells have a higher incidence of ROP compared with those who
receive transfusions of autologous cord blood red cells. In
addition, an association is found between the number of blood
transfusions and ROP (36). A study
suggests that performing ≥3 transfusions before 32 weeks gestation
raises risk of ROP (37). The
substitution of HbF with HbA during transfusion could contribute to
the progression of ROP (38). In a
prospective cohort study, Stutchfield et al (39) found a negative association between
mean Hb concentration and severity of ROP in postnatal fetuses.
They also found that replacing HbF with HbA during transfusion
accelerates the development of ROP. This is because transfusing
infants with HbA can suddenly deliver a large amount of oxygen to
the retina, which downregulates VEGF and leads to stagnation in the
development of the retinal vasculature. It has also been suggested
that the sudden increase in free iron in blood following the
transfusion of adult blood may induce the Fenton reaction, which
generates free hydroxyl radicals that damage the retina, thereby
inducing retinopathy (40).
Moderate blood transfusions are administered to raise Hb levels,
which increases oxygen concentration and decreases hypoxic damage
to the body. However, excessive adult blood transfusions increase
the risk of ROP (39). Therefore,
studies in preterm neonates have highlighted that a balance must be
found between toxic effects of high-oxygen saturation and the
damage caused by hypoxia; during transfusion, it is essential to
monitor blood oxygen saturation to prevent anemia and potential
damage to the retina from excessive transfusion (16,39).
Therefore, prospective research is required to evaluate the
predictive value of early anemia detection in preterm infants for
ROP.
Influence of glycaemia on ROP. Elevated BG
causes biological changes in the retina and most studies (41,42)
have found a positive association between BG levels and development
of ROP. Mohsen et al (41)
conducted a prospective study of 65 children to investigate the
association between BG and ROP. They found that maximum and mean BG
concentrations were significantly higher in children with than
those without ROP. Using logistic regression analysis, they
concluded that increase in mean BG concentration in the first week
of life is an independent risk factor for developing ROP, which was
in accordance with the findings of Vannadil et al (42). In studies (43,44) on
the mechanisms by which elevated BG levels affect ROP, it has been
found that BG levels are primarily associated with VEGF and IGF-1
and act synergistically. VEGF protein expression in cultured
retinal Müller cells is increased at higher glucose concentrations
(45), as hyperglycemia can
stimulate VEGF production by activating protein kinase C (46). Low serum levels of IGF-1 promote
pathological neovascularization in the retina. Studies (43,42)
have shown that low levels of IGF-1 inhibit development of normal
retinal vasculature, leading to local ischemia and the production
of large amounts of VEGF, which leads to hyperproliferative changes
in the retinal vasculature. Furthermore, IGF-1 is hypothesized to
mitigate insulin resistance. Thus, a deficiency in IGF-1 may result
in fluctuating BG levels, potentially contributing to onset of ROP.
It is not clear whether hyperglycemia is a clinical manifestation
of low IGF-1 levels or the cause of ROP.
Contrary to the findings of Mohsen et al
(41) and Vannadil et al
(42), Nicolaeva et al
(47) found no significant
difference in mean BG levels between children with and without ROP
and those with spontaneous remission of ROP, ignoring the effect of
duration of hyperglycemia on ROP. A meta-analysis by Lei et
al (48) revealed a significant
association between the duration of hyperglycemia and ROP after
adjusting for ORs (odds ratio, but no significant association
between average glucose levels and the incidence of ROP. The
aforementioned studies therefore suggest that the risk factors for
ROP are associated with the duration of hyperglycemia rather than
the average glucose levels.
In addition, association between glycated Hb (HbA1c)
and ROP has been studied. Movsas and Muthusamy (49) showed that low HbA1c levels are
associated with non-proliferative ROP (NP-ROP), while high levels
of HbA1c are associated with P-ROP. This may be associated with
fetal glucose exposure and metabolic conversion of HbA1c in
utero and suggests HbA1c levels in preterm infants as a
potential biomarker of ROP. Large-scale prospective studies are
needed to confirm the association between levels of HbA1c and
ROP.
Risk factors for ROP do not act independently.
Instead, multiple factors interact and synergize. For example, it
has been shown that hyperglycemia is closely linked to MPV
(50). In diabetic patients,
vascular endothelial disease accelerates PLT activation and
increases peripheral blood MPV, which in turn accelerates the
progression of retinopathy (31).
The impact of neonatal hyperglycemia on ROP development is still
unclear and requires further investigation.
Effect of inflammatory cells and
factors on ROP. Influence of inflammatory cells on ROP
Both maternal systemic inflammation during pregnancy
and neonatal inflammatory exposure increase the risk for ROP
(51). In addition, maternal
inflammatory response during the antenatal period and that of the
preterm infant during the postnatal period are associated with the
development of ROP. Prenatal inflammation is predominantly
histological chorioamnionitis (HCA) and the newborn postnatal
inflammation is primarily due to prematurity-related sepsis. HCA is
a prevalent inflammatory condition during the perinatal period. HCA
is a maternal reaction to infection of the chorionic and placental
membranes of the amniotic membrane in the uterus, with bacterial
infection as the primary cause of chorioamnionitis (52). The inflammatory response in both the
mother and the fetus is the primary risk factor for severe ROP
(51). A meta-analysis indicated
that maternal chorioamnionitis increases risk of developing ROP
(53).
Sepsis is a type of systemic inflammatory response
syndrome (SIRS) caused by infection by pathogens (including
bacteria, viruses and protozoa). A meta-analysis revealed a
substantial association between sepsis and the progression of ROP
(54). Early-onset neonatal sepsis
exhibits a stronger connection with severe ROP compared with
late-onset sepsis. In addition, the majority of cases of
early-onset neonatal sepsis are associated with intrauterine
infection, indicating a potential association between maternal
systemic inflammation and an increased risk of ROP in their
offspring (55). Early severe SIRS
has been observed in C57BL/6 wild-type mice model to result in
abnormal retinal vascular development and increased vascular
anastomoses, which are associated with microglia activation
(56). Childhood sepsis is
associated with the development of ROP and is significantly
associated with development of severe ROP (55). A meta-analysis by Wang et al
(54) also drew a similar
conclusion that sepsis is strongly associated with the degree of
ROP at any stage, particularly severe ROP (stages III-V) and sepsis
increases the risk of ROP in preterm infants. This may result from
damage caused by pathogenic microorganisms and their toxins to the
vascular endothelium. This increases the likelihood of leukocytes
adhering to vessel walls and forming microthrombi within the small
retinal vessels. These microthrombi may obstruct blood flow and
cause vascular leakage. Secondly, infected infants may experience
pulmonary and respiratory failure as blood pressure drops and
hypoxia and changes in blood flow occur (54). In addition, post-sepsis hypotension
and changes in oxygen saturation may affect retinal perfusion and
worsen retinal ischemia (9).
Neutrophil count is the most direct indicator of inflammation.
Study has shown that preterm infants with ROP have a higher
neutrophil count in their first month than non-ROP preterm infants
(57).
Neutrophil-to-lymphocyte ratio (NLR) and
platelet-to-lymphocyte ratio (PLR) are potential inflammatory
markers for diagnosing and prognosing medical conditions, such as
acute myocardial infarction and cancer (58,59).
Recent studies (10,60) have explored their association with
ROP. In a study of CBC in preterm infants 1 week after birth, it
was found that NLR is higher in premature infants who develop ROP
compared with those who do not and that NLR is independently
associated with development of ROP (10). However, a study has not found an
association between NLR and the development of ROP but hypothesize
that NLR may serve as a risk factor for ROP requiring treatment
(60). Contrary to these findings,
Ozturk et al (61) found
that neither NLR nor PLR are predictive risk factors for treatment
in children with ROP. This may be due to the effect of selecting
cases of CBC from preterm infants within 24 h of birth and the
immature neonatal immune system. Systemic Immunoinflammatory Index
(SII) is a relatively new indicator, initially used as a prognostic
indicator for hepatocellular carcinoma; however, it is now also
used in inflammatory disease (62,63).
SII is calculated from lymphocyte, neutrophil and PLT counts,
representing a homeostatic balance between inflammatory, immune and
thrombotic states (64). SII is
calculated as follows: PLT count x neutrophil count/lymphocyte
count (65). Akdogan et al
(57) first identified SII as an
independent predictor of ROP development. They found that preterm
infants with ROP have a significantly higher SII than preterm
infants without ROP. A recent study (66) proposed that the C-reactive
protein/albumin ratio (CAR) may be a marker for the development of
ROP. During the first month of life, CAR is significantly higher in
preterm infants with ROP than in those without ROP and CAR was also
significantly higher in the treated than in the untreated group,
suggesting that there may be an association between postnatal
inflammation and ROP severity.
In conclusion, inflammation and infection are
associated, and maternal chorioamnionitis and sepsis in preterm
infants have a strong association with the development of ROP.
Neutrophils, NLR, PLR, SII and CAR serve as indicators of
inflammatory response in ROP, but the results of NLR in patients
with ROP are controversial, which may be associated with time of
collection of blood specimens from the children. There are fewer
studies on the association between inflammatory factors such as
NLR, PLR, SII, CAR and ROP, which needs to be confirmed by a large
number of studies.
Effect of inflammatory factors on ROP. The
study of inflammatory factors has improved the understanding of the
pathogenesis and etiology of ROP (67-70).
IL-6 is a proinflammatory cytokine that serves roles in
inflammation and immune responses and is a key early indicator of
inflammation. IL-8 has multiple functions, including recruiting
neutrophils to inflammation sites, cell adhesion, tumor growth,
angiogenesis, neuronal protection and brain development (70). Several eye diseases show high levels
of IL-8 and IL-6, highlighting the importance of inflammation in
these conditions, such as age-related macular degeneration and
retinal vein obstruction (71,72).
Hellgren et al (73)
explored the association between inflammatory factors, insulin-like
growth factor I (IGF-I) levels and ROP, demonstrating that
inflammatory factors directly or indirectly influence the
development of ROP. Elevated postnatal proinflammatory cytokine
concentrations are associated with decreased IGF-I levels and ROP
since inflammatory factors may inhibit certain components of the
IGF-I pathway. Many cytokines share signaling components with
IGF-I, such as extracellular signal-regulated kinase 1/2 and
mitogen-activated protein kinase. Studies have found that the
inflammatory factors IL-6 and IL-8 in amniotic fluid are
independently associated with an increased risk of ROP development
and progression (67,74). This is consistent with the results
of a previous study, which suggested that elevated cord plasma IL-6
levels may be used as an independent predictor of severe ROP and
laser treatment (68). Elevated
IL-6 is significantly associated with increased risk of developing
stage ≥II ROP, suggesting its potential as a biomarker for ROP risk
prediction (69). Preterm infants
with ROP who require treatment exhibit significantly higher levels
of IL-8 compared with those who do not require treatment. However,
AUROC (area under the receiver operating characteristic) curves are
not effective at identifying serum IL-8 (67,70).
The aforementioned studies suggest that the pathophysiological
factors that predispose preterm infants to ROP are present
prenatally. Therefore, therapeutic strategies to decrease the risk
of ROP (specific treatment with antibiotics, anti-inflammatory
and/or anti-angiogenic drugs) can be implemented during pregnancy.
In conclusion, both prenatal and postnatal inflammatory factors
contribute to etiology of ROP.
However, a study (75) did not find that cytokine levels in
cord blood (IL-1b, IL-4, IL-6, IL-8, IL-10 and TNF-a) were
associated with the risk of ROP, suggesting that measurement of
cytokine levels in cord blood samples from preterm infants may be
of little value in predicting ROP. Overall, the aforementioned
studies highlight the need for monitoring inflammatory markers in
preterm infants and future studies should focus on larger,
multicenter cohorts and explore the mechanistic pathways between
inflammation and ROP.
Effect of blood lipids on ROP
There are limited studies (11,76) on
the effect of lipid metabolism disorders on ROP. Yang et al
(11) used mass spectrometry to
investigate the link between metabolic changes and the disease.
They found significant metabolic disturbances in plasma of the
children in the ROP group compared with the non-ROP group,
including elevated lipid levels and hyperactivity of lipid
metabolism. The retina is rich in lipids and lipid oxidation is a
key energy source for the retina. Dyslipidemia can affect
pathological angiogenesis in the retina (11). Abnormal activation of lipid
metabolic pathways in ROP may lead to lactate accumulation and
ketone body production. Dyslipidemia may manifest as elevated serum
total cholesterol, low-density lipoprotein and triglyceride levels
or decreased serum high-density lipoprotein concentrations
(11).
Association between adiponectin (APN) and ROP
development has also been investigated (76-78).
Adipocytes produce endogenous bioactive protein lipocalin, which
regulates lipid and glucose metabolism by promoting fatty acid
oxidation and inhibiting lipid synthesis, thus decreasing
triglyceride and cholesterol levels in blood. Studies have shown
that preterm infants with ROP have lower levels of APN compared
with those without ROP (76,77).
Lipocalin may inhibit neovascularization by regulating serum levels
of TNF-α, IGF-1, ω-3 long-chain polyunsaturated fatty acids (ω-3
LCPUFAs) and VEGF, thereby inhibiting occurrence and development of
ROP. Furthermore, preterm birth results in a decreased supply of
factors such as IGF-1, ω-3 LCPUFAs and APN from the
maternal-placental interface to the fetus. This leads to metabolic
disorders, insulin system immaturity and insulin resistance in
preterm infants (79). There are
few studies (11,76,77) on
the effect of lipids and lipocalin levels on ROP in children with
ROP and further research is necessary to validate this.
4. Association between plasma metabolic
pathways and ROP
In recent years, mass spectrometry has emerged as a
prominent analytical technique in metabolomic studies (11,80) of
ROP. Yang et al (11)
identified the plasma metabolic pathways in infants with ROP. These
metabolic pathways include glycolysis, redox homeostasis and the
arginine pathway. The aforementioned study observed elevated levels
of glycolytic intermediates in plasma of infants with ROP, which
suggests that aerobic glycolysis is hyperactive in ROP. The aerobic
glycolytic pathway is responsible for the production of ATP, which
is essential for the migration of vascular endothelial cells. This
is consistent with notable proliferation of endothelial cells
involved in neovascularization in ROP. The oxidized pentose
phosphate pathway (oxPPP) also serves an important role in
endothelial cell activity and migration. oxPPP has increased levels
of NADPH compared with other oxidative decomposition pathways,
which produces large amounts of ROS, which may lead to a decrease
in the production of associated metabolites (such as creatinine).
The arginine pathway is associated with development of ROP, as
evidenced by significantly lower plasma ornithine levels observed
in children with ROP. This may be associated with decreased
arginase activity. Inhibition of arginase activity may result in
activation of NOS. Metabolomic profiling of ROP may provide
insights for development of new therapeutic approaches, although
further validation is required to ascertain its clinical
significance (11).
5. Conclusion
In conclusion, the study of blood markers has
enhanced understanding of the etiology of ROP, indicating that ROP
is not only a retinal disease, but also associated with systemic
responses. The etiology of ROP is multifaceted, with numerous
contributing factors. Thus, it is key to explore a minimally
invasive, effective and unbiased examination method for diagnosing
and treating ROP. Hematological markers suggest potential risk
factors for ROP development and further studies are necessary to
clarify its exact pathogenesis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Hebei Province
Medical Applicable Technology Tracking Project (grant no.
GZ2020094) and Projects of Medical Science Research of Health
Commission of Hebei Province of China (grant no. 20210725).
Availability of data and materials
Not applicable.
Authors' contributions
QM and WT conceived the study and wrote the
manuscript. WT, YZ, HZ, KL, ZZ, HM, XJ, ZJ and QM critically
revised the manuscript for intellectual content. All authors have
read and approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kósa E and Grasselly M: Severe visual
impairment and blindness in children in the past 15 years in county
Vas-in the context of vision 2020. Orv Hetil. 147:205–209.
2006.PubMed/NCBI(In Hungarian).
|
|
2
|
Ugurlu A: Frequency of retinopathy of
prematurity (ROP) in infants screened for ROP: Two years follow-up
results of a single center in Turkey. Biomedicine (Taipei).
11:38–42. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
deCampos-Stairiker MA, Coyner AS, Gupta A,
Oh M, Shah PK, Subramanian P, Venkatapathy N, Singh P,
Kalpathy-Cramer J, Chiang MF, et al: Epidemiologic evaluation of
retinopathy of prematurity severity in a large telemedicine program
in india using artificial intelligence. Ophthalmology. 130:837–843.
2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gilbert C: Retinopathy of prematurity: A
global perspective of the epidemics, population of babies at risk
and implications for control. Early Hum Dev. 84:77–82.
2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu CG, Cao JK, Wang YH, Wang D, Han T, Li
QP and Feng ZC: A bibliometric analysis and visualization of
retinopathy of prematurity from 2001 to 2021. Eur Rev Med Pharmacol
Sci. 28:477–501. 2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kim SJ, Port AD, Swan R, Campbell JP, Chan
RVP and Chiang MF: Retinopathy of prematurity: A review of risk
factors and their clinical significance. Surv Ophthalmol.
63:618–637. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Özkaya D: The role of thrombocyte
parameters in retinopathy of prematurity development. Int J Clin
Pract. 2022(7518533)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Prasad N, Kumar K and Dubey A: Fetal
hemoglobin, blood transfusion, and retinopathy of prematurity in
preterm infants: An observational, prospective study. Indian J
Ophthalmol. 71:2803–2807. 2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rasoulinejad SA and Kiyamehr P: The
determinative role of cytokines in retinopathy of prematurity. Curr
Mol Med. 23:36–43. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Fevereiro-Martins M, Santos AC,
Marques-Neves C, Guimarães H and Bicho M: GenE-ROP Study Group.
Complete blood count parameters as biomarkers of retinopathy of
prematurity: A portuguese multicenter study. Graefes Arch Clin Exp
Ophthalmol. 261:2997–3006. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang Y, Yang Q, Luo S, Zhang Y, Lian C, He
H, Zeng J and Zhang G: Comparative analysis reveals novel changes
in plasma metabolites and metabolomic networks of infants with
retinopathy of prematurity. Invest Ophthalmol Vis Sci.
63(28)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Quimson SK: Retinopathy of prematurity:
Pathogenesis and current treatment options. Neonatal Netw.
34:284–287. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kim CB, D'Amore PA and Connor KM:
Revisiting the mouse model of oxygen-induced retinopathy. Eye
Brain. 8:67–79. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mezu-Ndubuisi OJ: In vivo angiography
quantifies oxygen-induced retinopathy vascular recovery. Optom Vis
Sci. 93:1268–1279. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fevereiro-Martins M, Marques-Neves C,
Guimarães H and Bicho M: Retinopathy of prematurity: A review of
pathophysiology and signaling pathways. Surv Ophthalmol.
68:175–210. 2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hartnett ME: Pathophysiology of
retinopathy of prematurity. Annu Rev Vis Sci. 9:39–70.
2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Strube YNJ and Wright KW: Pathophysiology
of retinopathy of prematurity. Saudi J Ophthalmol. 36:239–242.
2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cakir B, Liegl R, Hellgren G, Lundgren P,
Sun Y, Klevebro S, Löfqvist C, Mannheimer C, Cho S, Poblete A, et
al: Thrombocytopenia is associated with severe retinopathy of
prematurity. JCI Insight. 3(e99448)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lim ZD, Pheng E, Min ETL, Van Rostenberghe
H and Shatriah I: Comparison of mean platelet counts in preterm
infants with and without retinopathy of prematurity. Int J Environ
Res Public Health. 18(3783)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yolcu U and Civan DY: Relationship between
mean platelet volume and retinopathy of prematurity. Graefes Arch
Clin Exp Ophthalmol. 253(2337)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hengartner T, Adams M, Pfister RE, Snyers
D, McDougall J, Waldvogel S, Held-Egli K, Spring L, Rogdo B, Riedel
T, et al: Associations between red blood cell and platelet
transfusions and retinopathy of prematurity. Neonatology. 117:1–7.
2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Parrozzani R, Marchione G, Fantin A,
Frizziero L, Salvadori S, Nardo D and Midena G: Thrombocytopenia as
type 1 ROP biomarker: A longitudinal study. J Pers Med.
11(1120)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Parrozzani R, Nacci EB, Bini S, Marchione
G, Salvadori S, Nardo D and Midena E: Severe retinopathy of
prematurity is associated with early post-natal low platelet count.
Sci Rep. 11(891)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Reddy RM, Bhandary SV, Rao KA, Lewis LE,
Lal SM and Rachel NM: Assessment of role of platelet indices in the
occurrence of retinopathy of prematurity. Middle East Afr J
Ophthalmol. 29:91–95. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Şahinoğlu Keşkek N, Gülcan H, Yılmaz G and
Akkoyun İ: Impact of platelet count in retinopathy of prematurity.
Turk J Ophthalmol. 50:351–355. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Vinekar A, Hegde K, Gilbert C, Braganza S,
Pradeep M, Shetty R and Shetty KB: Do platelets have a role in the
pathogenesis of aggressive posterior retinopathy of prematurity?
Retina. 30 (4 Suppl):S20–S23. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Choręziak A, Szpecht D,
Chmielarz-Czarnocińska A, Pawłowska I and Gotz-Więckowska A: The
association of platelet counts with development and treatment for
retinopathy of prematurity-is thrombocytopenia a risk factor? Arch
Med Sci. 18:400–405. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chu SG, Becker RC, Berger PB, Bhatt DL,
Eikelboom JW, Konkle B, Mohler ER, Reilly MP and Berger JS: Mean
platelet volume as a predictor of cardiovascular risk: A systematic
review and meta-analysis. J Thromb Haemost. 8:148–156.
2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tao Y, Dong Y, Lu CW, Yang W and Li Q:
Relationship between mean platelet volume and retinopathy of
prematurity. Graefes Arch Clin Exp Ophthalmol. 253:1791–1794.
2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhong ZL, Han M and Chen S: Risk factors
associated with retinal neovascularization of diabetic retinopathy
in type 2 diabetes mellitus. Int J Ophthalmol. 4:182–185.
2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ayhan Tuzcu E, Arıca S, Ilhan N, Daglioglu
M, Coskun M, Ilhan O and Ustun I: Relationship between mean
platelet volume and retinopathy in patients with type 2 diabetes
mellitus. Graefes Arch Clin Exp Ophthalmol. 252:237–240.
2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Prasad N, Dubey A, Kumar K and Shrivastava
J: Role of fetal hemoglobin in the development and progression of
retinopathy of prematurity in preterm infants. Indian J Ophthalmol.
71:3478–3483. 2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lundgren P, Athikarisamy SE, Patole S, Lam
GC, Smith LE and Simmer K: Duration of anaemia during the first
week of life is an independent risk factor for retinopathy of
prematurity. Acta Paediatr. 107:759–766. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Akyüz Ünsal A, Key Ö, Güler D, Kurt Omurlu
İ, Anık A, Demirci B and Dündar S: Can complete blood count
parameters predict retinopathy of prematurity? Turk J Ophthalmol.
50:87–93. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Erdöl H, Hacioglu D, Kola M, Türk A and
Aslan Y: Investigation of the effect of hemoglobin F and A levels
on development of retinopathy of prematurity. J AAPOS. 21:136–140.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Raffa LH and Aljohani W: Evaluation of the
effect of blood transfusion on retinopathy of prematurity at a
tertiary care center in Western Saudi Arabia. Cureus.
14(e24495)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Uberos J, Fernandez-Marin E,
Campos-Martínez A, Ruiz-López A and García-Serrano JL: Blood
products transfusion and retinopathy of prematurity: A cohort
study. Acta Ophthalmol. 101:e294–e301. 2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Teofili L, Bianchi M, Baldascino A,
Papacci P and Vento G: Foetal haemoglobin, blood transfusion, and
retinopathy of prematurity. Eye (Lond). 32:1155–1156.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Stutchfield CJ, Jain A, Odd D, Williams C
and Markham R: Foetal haemoglobin, blood transfusion, and
retinopathy of prematurity in very preterm infants: A pilot
prospective cohort study. Eye (Lond). 31:1451–1455. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Dai AI, Demiryürek S, Aksoy SN, Perk P,
Saygili O and Güngör K: Maternal iron deficiency anemia as a risk
factor for the development of retinopathy of prematurity. Pediatr
Neurol. 53:146–150. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mohsen L, Abou-Alam M, El-Dib M, Labib M,
Elsada M and Aly H: A prospective study on hyperglycemia and
retinopathy of prematurity. J Perinatol. 34:453–457.
2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Vannadil H, Moulick PS, Khan MA, Shankar
S, Kaushik J and Sati A: Hyperglycaemia as a risk factor for the
development of retinopathy of prematurity: A cohort study. Med J
Armed Forces India. 76:95–102. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Cakir B, Hellström W, Tomita Y, Fu Z,
Liegl R, Winberg A, Hansen-Pupp I, Ley D, Hellström A, Löfqvist C
and Smith LE: IGF1, serum glucose, and retinopathy of prematurity
in extremely preterm infants. JCI Insight.
5(e140363)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Esmail J, Sakaria RP and Dhanireddy R:
Early hyperglycemia is associated with increased incidence of
severe retinopathy of prematurity in extremely low birth weight
infants. Am J Perinatol: Oct 17, 2023 (Epub ahead of print).
|
|
45
|
Brooks SE, Gu X, Kaufmann PM, Marcus DM
and Caldwell RB: Modulation of VEGF production by pH and glucose in
retinal Müller cells. Curr Eye Res. 17:875–882. 1998.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kim NH, Jung HH, Cha DR and Choi DS:
Expression of vascular endothelial growth factor in response to
high glucose in rat mesangial cells. J Endocrinol. 165:617–624.
2000.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Nicolaeva GV, Sidorenko EI and Iosifovna
AL: Influence of the blood glucose level on the development of
retinopathy of prematurity in extremely premature children. Arq
Bras Oftalmol. 78:232–235. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lei C, Duan J, Ge G and Zhang M:
Association between neonatal hyperglycemia and retinopathy of
prematurity: A meta-analysis. Eur J Pediatr. 180:3433–3442.
2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Movsas TZ and Muthusamy A: Feasibility of
neonatal haemoglobin A1C as a biomarker for retinopathy of
prematurity. Biomarkers. 25:468–473. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Karim F, Akter QS, Khanom A, Haque S and
Nahar S: Mean platelet volume in type 2 diabetes male. Mymensingh
Med J. 29:659–663. 2020.PubMed/NCBI
|
|
51
|
Lynch AM, Berning AA, Thevarajah TS,
Wagner BD, Post MD, McCourt EA, Cathcart JN, Hodges JK, Mandava N,
Gibbs RS and Palestine AG: The role of the maternal and fetal
inflammatory response in retinopathy of prematurity. Am J Reprod
Immunol. 80(e12986)2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Manabu S, Nawa N, Noguchi Y, Taki A,
Kashimada A, Honda I, Koyama A, Okazaki K, Kondo M, Miyahara H, et
al: Stage III chorioamnionitis is associated with reduced risk of
severe retinopathy of prematurity. J Pediatr: 114085, 2024 (Epub
ahead of print).
|
|
53
|
Woo SJ and Park KH, Jung HJ, Kim SN, Choe
G, Ahn J and Park KH: Effects of maternal and placental
inflammation on retinopathy of prematurity. Graefes Arch Clin Exp
Ophthalmol. 250:915–923. 2012.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wang X, Tang K, Chen L, Cheng S and Xu H:
Association between sepsis and retinopathy of prematurity: A
systematic review and meta-analysis. BMJ Open.
9(e025440)2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Huang J, Tang Y, Zhu T, Li Y, Chun H, Qu Y
and Mu D: Cumulative evidence for association of sepsis and
retinopathy of prematurity. Medicine (Baltimore).
98(e17512)2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Tremblay S, Miloudi K, Chaychi S, Favret
S, Binet F, Polosa A, Lachapelle P, Chemtob S and Sapieha P:
Systemic inflammation perturbs developmental retinal angiogenesis
and neuroretinal function. Invest Ophthalmol Vis Sci. 54:8125–8139.
2013.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Akdogan M, Ustundag Y, Cevik SG, Dogan P
and Dogan N: Correlation between systemic immune-inflammation index
and routine hemogram-related inflammatory markers in the prognosis
of retinopathy of prematurity. Indian J Ophthalmol. 69:2182–2187.
2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Liu J, Ao W, Zhou J, Luo P, Wang Q and
Xiang D: The correlation between PLR-NLR and prognosis in acute
myocardial infarction. Am J Transl Res. 13:4892–4899.
2021.PubMed/NCBI
|
|
59
|
Xia WK, Liu ZL, Shen D, Lin QF, Su J and
Mao WD: Prognostic performance of pre-treatment NLR and PLR in
patients suffering from osteosarcoma. World J Surg Oncol.
14(127)2016.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Obata S, Matsumoto R, Kakinoki M, Sawada
O, Sawada T, Saishin Y, Yanagi T, Maruo Y and Ohji M: Blood
neutrophil-to-lymphocyte ratio as a risk factor in treatment for
retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol.
261:951–957. 2023.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Ozturk T, Durmaz Engin C, Kaya M and Yaman
A: Complete blood count parameters to predict retinopathy of
prematurity: When to evaluate and what do they tell us? Int
Ophthalmol. 41:2009–2018. 2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W,
Zhang X, Wang WM, Qiu SJ, Zhou J and Fan J: Systemic
immune-inflammation index predicts prognosis of patients after
curative resection for hepatocellular carcinoma. Clin Cancer Res.
20:6212–6222. 2014.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Dziedzic EA, Gąsior JS, Tuzimek A,
Dąbrowski M and Jankowski P: The association between serum vitamin
D concentration and new inflammatory biomarkers-systemic
inflammatory index (SII) and systemic inflammatory response
(SIRI)-in patients with ischemic heart disease. Nutrients.
14(4212)2022.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Oruz O, Dervişoğulları MS, Öktem ME and
İncekaş C: Predictive role of systemic immune-inflammation index
and neutrophil/lymphocyte ratio values in infants with retinopathy
of prematurity. Graefes Arch Clin Exp Ophthalmol: Apr 24, 2024
(Epub ahead of print).
|
|
65
|
Gur DO, Efe MM, Alpsoy S, Akyüz A, Uslu N,
Çelikkol A and Gur O: Systemic immune-inflammatory index as a
determinant of atherosclerotic burden and high-risk patients with
acute coronary syndromes. Arq Bras Cardiol. 119:382–390.
2022.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
66
|
Ekinci DY, Bezirganoglu H, Okur N and Tas
M: A novel marker for predicting type 1 retinopathy of prematurity:
C-reactive protein/albumin ratio. Int Ophthalmol. 43:3345–3353.
2023.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Woo SJ, Park JY, Hong S, Kim YM, Park YH,
Lee YE and Park KH: Inflammatory and angiogenic mediators in
amniotic fluid are associated with the development of retinopathy
of prematurity in preterm infants. Invest Ophthalmol Vis Sci.
61(42)2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Park YJ, Woo SJ, Kim YM, Hong S, Lee YE
and Park KH: Immune and inflammatory proteins in cord blood as
predictive biomarkers of retinopathy of prematurity in preterm
infants. Invest Ophthalmol Vis Sci. 60:3813–3820. 2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Borțea CI, Enatescu I, Dima M, Pantea M,
Iacob ER, Dumitru C, Popescu A, Stoica F, Heredea RE and Iacob D: A
prospective analysis of the retinopathy of prematurity correlated
with the inflammatory status of the extremely premature and very
premature neonates. Diagnostics (Basel). 13(2105)2023.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Sehgal P, Narang S, Chawla D, Gupta S,
Jain S, Sharma U, Katoch D and Kaur J: Systemic biomarkers of
retinopathy of prematurity in preterm babies. Int Ophthalmol.
43:1751–1759. 2023.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Mimura T, Funatsu H, Noma H, Shimura M,
Kamei Y, Yoshida M, Kondo A, Watanabe E and Mizota A: Aqueous humor
levels of cytokines in patients with age-related macular
degeneration. Ophthalmologica. 241:81–89. 2019.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Li X, Cao X, Zhao M and Bao Y: The changes
of irisin and inflammatory cytokines in the age-related macular
degeneration and retinal vein occlusion. Front Endocrinol
(Lausanne). 13(861757)2022.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Hellgren G, Löfqvist C, Hansen-Pupp I,
Gram M, Smith LE, Ley D and Hellström A: Increased postnatal
concentrations of pro-inflammatory cytokines are associated with
reduced IGF-I levels and retinopathy of prematurity. Growth Horm
IGF Res. 39:19–24. 2018.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Jang JH, Kim JG, Lee YH, Bae JG and Park
JH: The association between amniotic fluid-derived inflammatory
mediators and the risk of retinopathy of prematurity. Medicine
(Baltimore). 101(e29368)2022.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Woo SJ, Park KH, Lee SY, Ahn SJ, Ahn J,
Park KH, Oh KJ and Ryu A: The relationship between cord blood
cytokine levels and perinatal factors and retinopathy of
prematurity: A gestational age-matched case-control study. Invest
Ophthalmol Vis Sci. 54:3434–3439. 2013.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Fu Z, Lofqvist CA, Shao Z, Sun Y, Joyal
JS, Hurst CG, Cui RZ, Evans LP, Tian K, SanGiovanni JP, et al:
Dietary ω-3 polyunsaturated fatty acids decrease retinal
neovascularization by adipose-endoplasmic reticulum stress
reduction to increase adiponectin. Am J Clin Nutr. 101:879–888.
2015.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Fu Z, Yan W, Chen CT, Nilsson AK, Bull E,
Allen W, Yang J, Ko M, SanGiovanni JP, Akula JD, et al:
Omega-3/Omega-6 long-chain fatty acid imbalance in phase I
retinopathy of prematurity. Nutrients. 14(1333)2022.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Fu Z, Lundgren P, Pivodic A, Yagi H,
Harman JC, Yang J, Ko M, Neilsen K, Talukdar S, Hellström A and
Smith LEH: FGF21 via mitochondrial lipid oxidation promotes
physiological vascularization in a mouse model of phase I ROP.
Angiogenesis. 26:409–421. 2023.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Fu Z, Gong Y, Löfqvist C, Hellström A and
Smith LE: Review: Adiponectin in retinopathy. Biochim Biophys Acta.
1862:1392–1400. 2016.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Tomita Y, Usui-Ouchi A, Nilsson AK, Yang
J, Ko M, Hellström A and Fu Z: Metabolism in retinopathy of
prematurity. Life (Basel). 11(1119)2021.PubMed/NCBI View Article : Google Scholar
|