Introduction
Proton pump inhibitors (PPIs) are potent drugs used
to suppress gastric acid secretion and are widely used worldwide.
Despite several PPIs being available over the counter, the
prevalence of prescription PPIs increased from an estimated 3.9% in
1999-2000 to 7.8% in 2011-2012(1).
Studies have shown that patients are often prescribed PPIs for
inappropriate indications or are administered high doses of PPIs
for extended periods, which is contrary to clinical guidelines
(2,3). Additionally, it has been reported that
patients are discharged from hospitals with PPIs that are not
indicated for their condition (3).
Although PPI therapy is an effective treatment strategy for a range
of conditions, including gastroesophageal reflux disease (GERD),
peptic ulcer disease and Zollinger-Ellison syndrome (which are
generally deemed safe for treatment), evidence of potential
long-term complications of PPI therapy is emerging: These
complications include chronic kidney disease (4), dementia (5), bone fracture (6), myocardial infarction (7), infection (8), micronutrient deficiencies (9) and gastrointestinal malignancy
(10).
To the best of our knowledge, the majority of
research examining the association between exposure to PPIs and
liver disease has focused on patients with cirrhosis (11-13).
In both retrospective and prospective investigations, PPI exposure
has demonstrated inconsistent associations with severe infection,
hepatic decompensation, hepatocellular carcinoma and liver-related
mortality (11-13).
The prevailing hypothesis suggests that PPIs may increase the
likelihood of complications by altering the intestinal microbiota
via the suppression of gastric acid, thus resulting in bacterial
overgrowth in the small intestine and an increase in bacterial
translocation (14,15). To the best of our knowledge,
however, there is limited research available regarding the effects
of PPIs on the development and progression of liver fibrosis and
hepatic steatosis in a general population cohort (11-13).
From January 2017 to March 2020, the National Health
and Nutrition Examination Survey (NHANES) incorporated
vibration-controlled transient elastography (VCTE) measurements to
assess prevalence of steatosis and fibrosis in a representative
sample of the US population (16).
VCTE is a non-invasive technique utilized for quantifying the
severity of hepatic steatosis, determined by the controlled
attenuation parameter (CAP) score, and assessing the degree of
fibrosis via liver stiffness measurement (LSM) (16-18).
The inclusion of LSM and CAP within a nationally representative
dataset presents opportunity to investigate the association between
PPI therapy and the risk of liver steatosis and fibrosis at a
population level. To investigate the association between PPI
therapy and risk of liver steatosis and fibrosis, the present study
conducted a large, nationally representative cross-sectional study
using data from the NHANES.
Materials and methods
Study population
The present study analyzed pre-pandemic data from
the NHANES January 2017-March 2020, which is a nationally
representative survey conducted by the National Center for Health
Statistics (NCHS) to evaluate the health and nutritional status of
adults and children in the US. Comprehensive participant data,
encompassing demographic profiles, examination records (including
liver ultrasound transient elastography), laboratory analyses and
questionnaire responses, were gathered by well-trained examiners
(cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?Cycle=2017-2020).
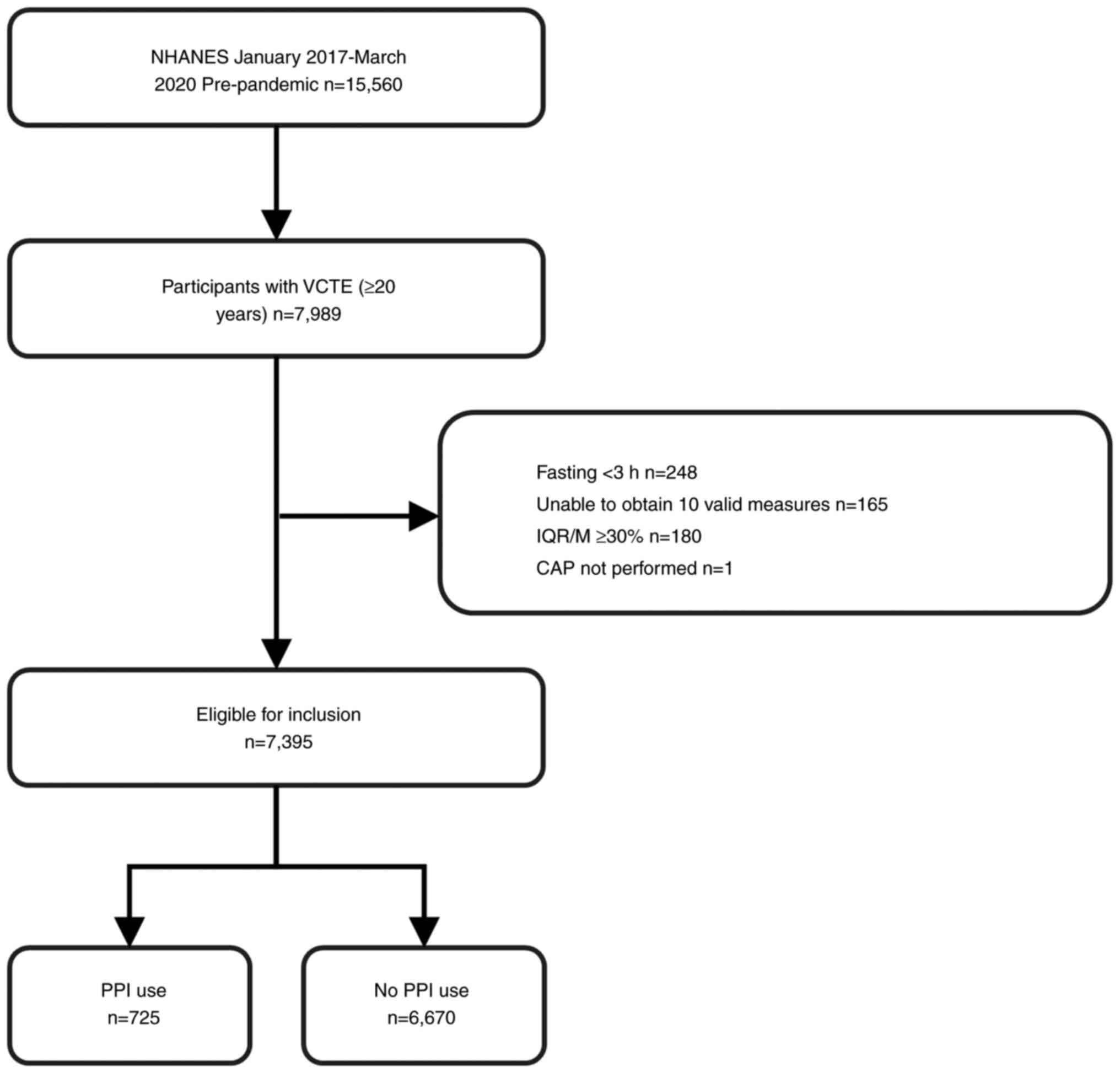
Of the 15,560 patients included in the NHANES, 7,395 aged ≥20 years
completed hepatic VCTE examinations. Exclusion criteria were as
follows: i) aged <20 years (n=6,328); ii) missing elastography
examination data (n=1,243) and iii) invalid elastography
examination data (fasting <3 h, unable to obtain 10 valid
measures, interquartile range/median >30% and CAP not performed)
(n=594). Fig. 1 illustrates the
sample selection flowchart. Researchers at the Centers for Disease
Control and Prevention approved the NHANES (approval nos. #2011-17
and #2018-01) and all participants provided informed consent.
VCTE is a non-invasive imaging method widely used to
assess liver fibrosis and steatosis in patients with liver disease
(16-18).
The present study utilized VCTE to evaluate the extent of liver
fibrosis and steatosis. According to the American Association for
the Study of Liver Diseases Practice Guidance on the Clinical
Assessment and Management of Non-alcoholic Fatty Liver Disease
(NAFLD) (17), steatotic liver
disease is diagnosed histologically or through imaging techniques
such as ultrasound, FibroScan (CAP), Computed Tomography, and
Magnetic Resonance Imaging-Derived Proton Density Fat Fraction. Due
to limitations in the available data from NHANES 2017-2020, the
present study only used CAP for quantifying the severity of hepatic
steatosis. Consequently, patients who did not undergo VCTE were
excluded from the analysis.
Definition of PPI exposure
During the household sample person interview, survey
participants were asked if they had taken prescription medications
in the last 30 days. Those who answered ‘yes’ were requested to
show the interviewer the pill containers for all utilized products.
Participants in the survey were also asked when they took the drug
and why they did so. The use of PPIs or H2-receptor antagonists
(H2RAs) in 1 month preceding the interview was defined as exposure.
The length of use was categorized as <0.5, 0.5-2.0 and >2.0
years. The PPIs included omeprazole, pantoprazole, esomeprazole and
other (such as lansoprazole, rabeprazole and dexlansoprazole). The
indication for prescribing PPI was based on the American
Gastroenterological Association Clinical Practice Guidelines
(2). The H2RAs included ranitidine
and famotidine.
Steatosis and fibrosis assessment
FibroScan® model 502 V2 Touch, equipped
with either a medium (M) or extra-large (XL) wand (probe), was used
to obtain elastography measurements within the NHANES Mobile
Examination Center. A complete examination was defined as having a
fasting time ≥3 h, obtaining ≥10 complete stiffness measurements
and a liver stiffness interquartile range/median <30%. According
to previous studies (19,20), an optimal CAP cut-off of ≥274 dB/m
(sensitivity, 90%) is indicative of hepatic steatosis, whereas an
optimal LSM cut-off of ≥9.7 kPa (sensitivity, 71%; specificity,
75%) is suggestive of advanced fibrosis (Metavir Fibrosis Stage
≥F3) (21).
Covariates
Based on the literature (22-25),
the following covariates were included: Age, sex, race/ethnicity,
educational level, ratio of family income to poverty (calculated by
dividing family or individual income by the poverty guidelines
specific to the survey year), smoking status, notable alcohol
consumption and vigorous activity. To avoid overadjustment, obesity
was not included as a covariate, as previously described (22-25).
Race/ethnicity was classified into five groups, including
non-Hispanic White, Hispanic, non-Hispanic Black, non-Hispanic
Asian and other (including multiracial). Education level was
categorized into four groups: Less than high school, high school
graduate, some college or associate's degree and college or above.
According to the ratio of family income to poverty, family income
was categorized as low (<1.3), medium (1.3-4.9), or high (≥5.0).
Smoking status was categorized into three groups, including never
smoked, former smoker and current smoker. Notable alcoholic
consumption was defined as >2 or 3 standard units)/day on
average for female and male participants, respectively. Vigorous
activity was defined as engaging in activity with a metabolic
equivalent (ratio of the rate at which a person expends energy,
relative to the mass) of ≥6, at least three times/week.
Statistical analysis
Continuous variables are expressed as the mean ± SD;
categorical data are expressed as count and percentages. To compare
clinical characteristics, linear regression for continuous
variables and χ2 test for categorical variables were
used. To assess the effect of PPIs and H2RAs on the presence of
steatosis and fibrosis, multivariable linear and logistic
regression analyses were conducted. The multivariate test used
three models: 1, no variables adjusted; 2, adjusted for age, sex
and race/ethnicity and 3, further adjusted for education, the ratio
of family income to poverty, smoking status, notable alcohol
consumption and vigorous activity.
Three sensitivity tests were conducted. First,
hepatic steatosis was defined by using a cut-off value of CAP ≥285
dB/m, which was chosen to optimize sensitivity and specificity
(26). Second, the target
population was adjusted to include only patients with NAFLD
(n=5,897) to eliminate the influence of certain factors (alcohol
consumption, steatogenic medications,) on steatosis (27). Patients with hepatitis B (n=40) or C
(n=165), notable alcoholic consumption (n=1,198), or use of
steatogenic medications for >6 months (n=95) were excluded. The
target population was refined to focus specifically on individuals
with metabolic dysfunction-associated steatotic liver disease
(MASLD; n=5,355). The diagnosis of MASLD was based on hepatic
steatosis when no other underlying cause is identified and at least
one of the cardiometabolic risk factors (general obesity, central
obesity, diabetes, prediabetes, dyslipidemia, hypertension) is
present (28).
All statistical analyses were performed by using R
version 4.3.0 (R Foundation for Statistical Computing). and
EmpowerStats (version 4.1) software (https://www.empowerstats.net/cn/index.php#). Two-sided
P-values were utilized. P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline characteristics
Out of the 15,560 participants who were included in
the NHANES January 2017-March 2020 pre-pandemic database, 8,195
individuals were excluded because they were aged <20 years or
had missing or invalid elastography examination data. This resulted
in a final sample size of 7,395 patients. Among these adults, the
mean age was 50.59 years, with 3,656 being male. The M probe was
used to evaluate 5,381 adults (72.77%), whereas the XL probe was
used for 2,014 adults (27.23%). The baseline characteristics of
individuals based on current use of PPIs are presented in Table I. Compared with individuals who did
not use PPIs, patients who took PPIs were significantly older, more
likely to be female and Non-Hispanic White and had higher rates of
obesity and comorbidities. Furthermore, PPI users demonstrated a
greater prevalence of advanced liver fibrosis (7.72 vs. 5.73%) and
hepatic steatosis (52.14 vs. 42.56%).
 | Table IFeatures of the study populations
according to current PPI use. |
Table I
Features of the study populations
according to current PPI use.
| Characteristic | Total
(n=7,395) | No PPI use
(n=6,670) | PPI use
(n=725) | P-value |
|---|
| Mean age,
years | 50.59±17.27 | 49.26±17.10 | 62.82±13.72 | <0.01 |
| Male (%) | 3,656 (49.44) | 3,332 (49.96) | 324 (44.69) | <0.01 |
| Race/ethnicity
(%) | | | | |
|
Non-Hispanic
White | 2,510 (33.94) | 2,167 (32.49) | 343 (47.31) | <0.01 |
|
Hispanic | 1,648 (22.29) | 1,510 (22.64) | 138 (19.03) | |
|
Non-Hispanic
Black | 1,967 (26.60) | 1,803 (27.03) | 164 (22.62) | |
|
Non-Hispanic
Asian | 904 (12.22) | 855 (12.82) | 49 (6.76) | |
|
Other | 366 (4.95) | 335 (5.02) | 31 (4.28) | |
| Education level
(%) | | | | <0.01 |
|
Less than
high school | 1,357 (16.35) | 1,190 (17.84) | 167 (23.03) | |
|
High school
graduate | 1,781 (24.08) | 1,598 (23.96) | 183 (25.24) | |
|
Some college
or associate's degree | 2,401 (32.47) | 2,152 (32.26) | 249 (34.34) | |
|
College or
above | 1,847 (24.98) | 1,722 (25.82) | 125 (17.24) | |
| Body mass index,
kg/m2 (%) | | | | <0.01 |
|
Underweight
(<18.5) | 101 (1.37) | 95 (1.42) | 6 (0.83) | |
|
Normal
weight (18.5-24.9) | 1,810 (24.48) | 1,692 (25.37) | 118 (16.28) | |
|
Overweight
(25.0-29.9) | 2,389 (32.31) | 2,157 (32.34) | 232 (32.00) | |
|
Obese
(>30.0) | 3,028 (40.96) | 2,673 (40.07) | 355 (48.97) | |
| Ratio of family
income to poverty (%) | | | | 0.72 |
|
<1.3 | 1,772 (23.96) | 1,598 (23.96) | 174 (24.00) | |
|
1.3-4.9 | 2,492 (33.70) | 2,240 (33.58) | 252 (34.76) | |
|
≥5.0 | 993 (13.43) | 1,941 (29.10) | 197 (27.17) | |
| Smoking status
(%) | | | | <0.01 |
|
Never
smoked | 4,325 (58.49) | 3,957 (59.33) | 368 (50.76) | |
|
Former
smoker | 1,732 (23.42) | 1,482 (22.22) | 250 (34.48) | |
|
Current
smoker | 1,338 (16.09) | 1,231 (18.46) | 107 (14.76) | |
| Alcohol abuse
(%) | 1,236 (16.71) | 1,144 (17.15) | 92 (12.69) | <0.01 |
| Vigorous activity
(%) | 2,636 (36.65) | 2,424 (36.34) | 212 (29.24) | <0.01 |
| Laboratory
features | | | | |
|
Total
cholesterol, mmol/l | 4.82±1.05 | 4.84±1.05 | 4.67±1.07 | <0.01 |
|
HDL-cholesterol,
mmol/l | 1.38±0.41 | 1.39±0.41 | 1.36±0.40 | 0.15 |
|
Triglycerides,
mmol/l | 1.57±1.23 | 1.55±1.22 | 1.77±1.35 | <0.01 |
|
Glycohemoglobin,
% | 5.85±1.10 | 5.81±1.08 | 6.14±1.20 | <0.01 |
|
AST,
U/l | 21.96±14.44 | 21.98±14.66 | 21.82±12.26 | 0.89 |
|
ALT,
U/l | 22.48±18.86 | 22.59±19.21 | 21.48±15.19 | 0.20 |
|
GGT,
U/l | 32.18±45.78 | 31.46±44.64 | 38.84±54.81 | <0.01 |
|
Total
bilirubin, µmol/l | 7.84±4.72 | 7.86±4.74 | 7.65±4.50 | 0.21 |
|
Albumin,
g/l | 40.67±3.30 | 40.76±3.28 | 39.85±3.32 | <0.01 |
|
Creatinine,
µmol/l | 79.51±40.21 | 78.62±38.36 | 87.75±53.79 | <0.01 |
|
Uric acid,
µmol/l | 321.48±87.08 | 320.61±86.92 | 329.51±88.21 | 0.02 |
|
Platelet
count, x109/µl | 246.13±65.17 | 246.32±64.51 | 244.38±70.99 | 0.15 |
|
hsCRP,
mg/l | 3.98±8.27 | 3.78±7.00 | 5.86±15.62 | <0.01 |
|
LSM≥9.7 KPa
(%) | 438 (5.92) | 382 (5.73) | 56 (7.72) | 0.03 |
|
CAP≥274 dB/m
(%) | 3,217 (43.50) | 2,839 (42.56) | 378 (52.14) | <0.01 |
| Comorbidities
(%) | | | | |
|
Diabetes | 1,390 (18.80) | 1,139 (17.08) | 251 (34.62) | <0.01 |
|
Hypertension | 2,807 (37.96) | 2,348 (35.20) | 459 (63.31) | <0.01 |
|
Hypercholesterolemia | 2,658 (35.84) | 2,229 (33.42) | 429 (59.17) | <0.01 |
|
Congestive
heart failure | 202 (2.73) | 156 (2.34) | 46 (6.34) | <0.01 |
| Coronary heart
disease | 292 (3.95) | 226 (3.39) | 66 (9.10) | <0.01 |
|
Angina | 171 (2.31) | 129 (1.93) | 42 (5.79) | <0.01 |
|
Stroke | 342 (4.62) | 259 (3.88) | 83 (11.45) | <0.01 |
|
Asthma | 1,144 (15.47) | 986 (14.78) | 158 (21.79) | <0.01 |
|
COPD | 618 (8.36) | 472 (7.08) | 146 (20.14) | <0.01 |
|
Thyroid
condition | 849 (11.49) | 704 (10.55) | 145 (20.00) | <0.01 |
|
Arthritis | 2,180 (29.48) | 1,733 (25.98) | 447 (61.66) | <0.01 |
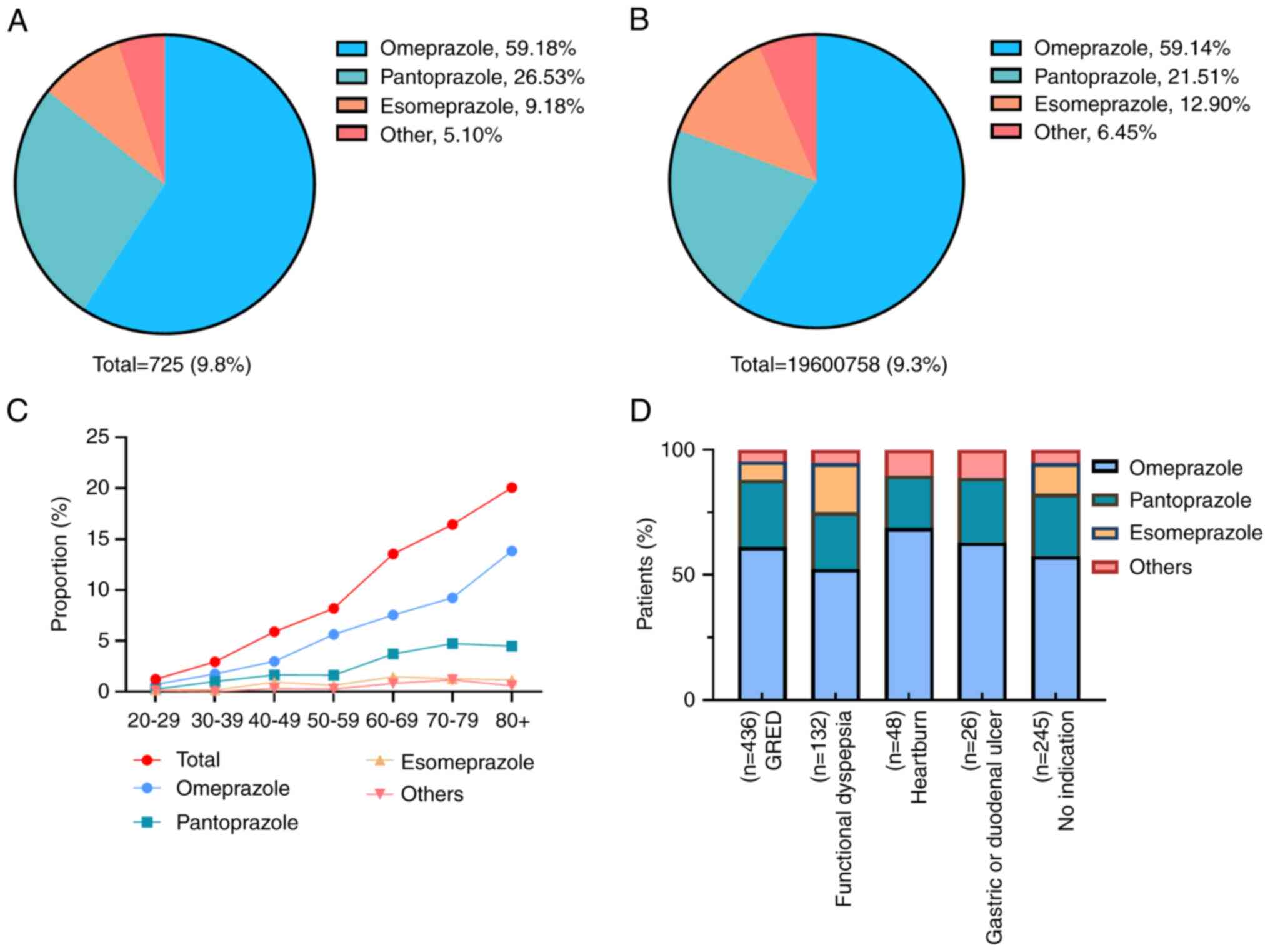
A total of 9.8% of individuals were prescribed PPIs
(Fig. 2). Among the prescribed
PPIs, omeprazole was the most commonly prescribed medication,
accounting for 59.18% of prescriptions, followed by pantoprazole
(26.53%), esomeprazole (9.18%) and other PPIs such as lansoprazole
and rabeprazole (5.10%). The prevalence of prescription PPIs varied
across age groups, with an estimated 1.25% of individuals aged
20-29 years having a prescription, compared with 20.08% of
individuals aged >80 years. The primary indication for PPI use
was gastroesophageal reflux disease (60.14%), followed by
functional dyspepsia (18.21%), heartburn (6.62%) and gastric or
duodenal ulcers (3.59%). According to the American
Gastroenterological Association Clinical Practice (2), 245 patients (33.79%) exhibited no
indication for prescription.
Association between PPI use and
hepatic steatosis
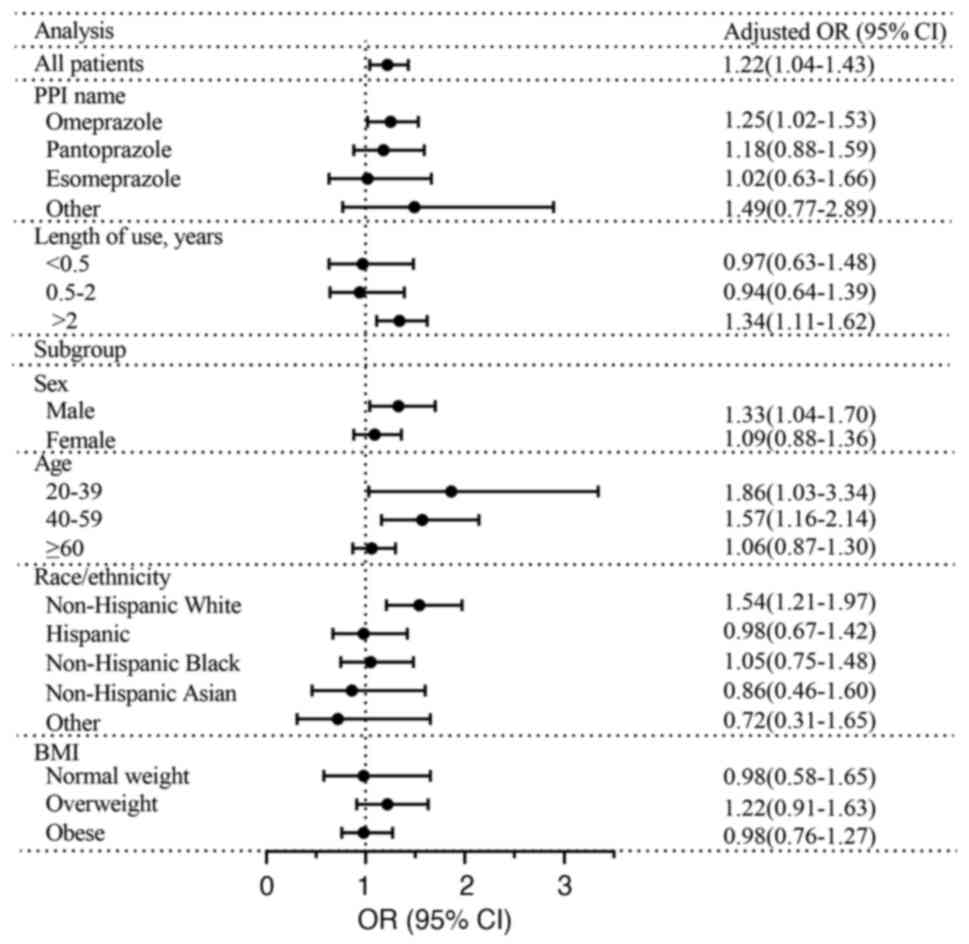
Table II and
Fig. 3 show the results of
multivariable logistic regression analysis. According to model 1,
PPI use was significantly associated with hepatic steatosis (OR,
1.47; 95% CI, 1.26-1.71). This association was unchanged even after
adjusting for multiple confounding factors (model 3) (OR, 1.22; 95%
CI, 1.04-1.43), particularly regarding omeprazole (OR, 1.25; 95%
CI, 1.02-1.53). Furthermore, use of PPIs for >2 years exhibited
a significant positive association with hepatic steatosis (OR,
1.34; 95% CI, 1.11-1.62). The use of PPIs was associated with
hepatic steatosis in individuals aged 20-39 (OR, 1.86; 95% CI,
1.03-3.34) and 40-59 years (OR, 1.57; 95% CI, 1.16-2.14), as well
as in males (OR, 1.33; 95% CI, 1.04-1.70) and non-Hispanic whites
(OR, 1.54; 95% CI, 1.21-1.97). Furthermore, use of PPIs and CAP
demonstrated similar outcomes in the modelling of steatosis
severity using multivariable linear regression (Table SI).
 | Table IIAssociation between PPI use and
hepatic steatosis. |
Table II
Association between PPI use and
hepatic steatosis.
| | Model 1 | Model 2 | Model 3 |
|---|
| Characteristic | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| PPI use | | | | | | |
|
No | 1 | | 1 | | 1 | |
|
Yes | 1.47
(1.26-1.71) | <0.01 | 1.24
(1.05-1.45) | 0.01 | 1.22
(1.04-1.43) | 0.02a |
| PPI | | | | | | |
|
None | 1 | | 1 | | 1 | |
|
Omeprazole | 1.54
(1.27-1.88) | <0.01 | 1.26
(1.03-1.54) | 0.03 | 1.25
(1.02-1.53) | 0.03a |
|
Pantoprazole | 1.36
(1.02-1.82) | 0.04 | 1.21
(0.90-1.62) | 0.22 | 1.18
(0.88-1.59) | 0.28 |
|
Esomeprazole | 1.20
(0.74-1.94) | 0.46 | 1.05
(0.64-1.71) | 0.85 | 1.02
(0.63-1.66) | 0.94 |
|
Other | 1.77
(0.92-3.40) | 0.09 | 1.57
(0.81-3.05) | 0.18 | 1.49
(0.77-2.89) | 0.24 |
| Length of PPI use,
years | | | | | | |
|
Not
applicable | 1 | | 1 | | 1 | |
|
<0.5 | 1.06
(0.70-1.60) | 0.79 | 0.96
(0.63-1.47) | 0.85 | 0.97
(0.63-1.48) | 0.87 |
|
0.5-2.0 | 1.07
(0.73-1.56) | 0.74 | 0.96
(0.66-1.42) | 0.85 | 0.94
(0.64-1.39) | 0.77 |
|
>2.0 | 1.66
(1.39-1.99) | <0.01 | 1.36
(1.13-1.65) | <0.01 | 1.34
(1.11-1.62) |
<0.01a |
| Sex | | | | | | |
|
Male | 1.59
(1.26-2.00) | <0.01 | 1.35
(1.06-1.72) | 0.01 | 1.33
(1.04-1.70) | 0.02a |
|
Female | 1.44
(1.17-1.78) | <0.01 | 1.12
(0.90-1.39) | 0.31 | 1.09
(0.88-1.36) | 0.42 |
| Age, years | | | | | | |
|
20-39 | 1.72
(0.97-3.03) | 0.06 | 1.87
(1.04-3.36) | 0.04 | 1.86
(1.03-3.34) | 0.04a |
|
40-59 | 1.57
(1.16-2.12) | <0.01 | 1.60
(1.18-2.18) | <0.01 | 1.57
(1.16-2.14) |
<0.01a |
|
≥60 | 1.11
(0.91-1.35) | 0.30 | 1.08
(0.88-1.32) | 0.46 | 1.06
(0.87-1.30) | 0.57 |
| Race/ethnicity | | | | | | |
|
Non-Hispanic
White | 1.81
(1.44-2.28) | <0.01 | 1.60
(1.26-2.03) | <0.01 | 1.54
(1.21-1.97) |
<0.01a |
|
Hispanic | 1.19
(0.84-1.70) | 0.32 | 0.99
(0.68-1.43) | 0.95 | 0.98
(0.67-1.42) | 0.91 |
|
Non-Hispanic
Black | 1.28
(0.92-1.78) | 0.14 | 1.07
(0.76-1.49) | 0.71 | 1.05
(0.75-1.48) | 0.76 |
|
Non-Hispanic
Asian | 0.96
(0.53-1.74) | 0.90 | 0.86
(0.47-1.59) | 0.64 | 0.86
(0.46-1.60) | 0.63 |
|
Other | 1.12
(0.53-2.35) | 0.77 | 0.78
(0.36-1.71) | 0.54 | 0.72
(0.31-1.65) | 0.44 |
| BMI | | | | | | |
|
Normal
weight | 1.51
(0.92-2.47) | 0.10 | 0.99
(0.59-1.66) | 0.96 | 0.98
(0.58-1.65) | 0.94 |
|
Overweight | 1.42
(1.08-1.87) | 0.01 | 1.22
(0.91-1.63) | 0.18 | 1.22
(0.91-1.63) | 0.18 |
|
Obese | 1.14
(0.90-,1.45) | 0.29 | 0.98
(0.761.27) | 0.90 | 0.98
(0.76-1.27) | 0.89 |
Association between PPIs and advanced
fibrosis
The present study subsequently examined the
association between PPIs and advanced fibrosis (Table III). According to model 1, a
significant association was found between PPI use and advanced
fibrosis (OR, 1.38; 95% CI, 1.03-1.84). However, in model 3, there
was no association between any PPI use and advanced fibrosis (OR,
0.98; 95% CI, 0.72-1.33). In the subgroup analyses, stratified by
sex, age, race/ethnicity, and body mass index, an insignificant
association between use of PPIs and advanced fibrosis was observed
in model 3. This was further supported by the multivariable linear
regression, which showed no association between the use of any PPI
and advanced fibrosis in all subgroups. (Table SII).
 | Table IIIAssociation between PPI use and
advanced fibrosis. |
Table III
Association between PPI use and
advanced fibrosis.
| | Model 1 | Model 2 | Model 3 |
|---|
| Characteristic | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| PPI use | | | | | | |
|
No | 1 | | 1 | | 1 | |
|
Yes | 1.38
(1.03-1.84) | 0.03 | 1.02
(0.75-1.38) | 0.91 | 0.98
(0.72-1.33) | 0.89 |
| PPI | | | | | | |
|
None | 1 | | 1 | | 1 | |
|
Omeprazole | 1.32
(0.91-1.92) | 0.15 | 0.95
(0.65-1.39) | 0.79 | 0.91
(0.62-1.35) | 0.65 |
|
Pantoprazole | 1.52
(0.90-2.57) | 0.12 | 1.15
(0.68-1.96) | 0.60 | 1.09
(0.64-1.85) | 0.76 |
|
Esomeprazole | 1.03
(0.37-2.84) | 0.96 | 0.83
(0.30-2.32) | 0.73 | 0.83
(0.30-2.32) | 0.73 |
|
Other | 2.00
(0.70-5.66) | 0.19 | 1.47
(0.51-4.22) | 0.47 | 1.47
(0.51-4.23) | 0.47 |
| Length of PPI use,
years | | | | | | |
|
Not
applicable | 1 | | 1 | | 1 | |
|
<0.5 | 0.76
(0.28-2.07) | 0.59 | 0.66
(0.24-1.83) | 0.43 | 0.68
(0.25-1.88) | 0.46 |
|
0.5-2.0 | 1.45
(0.73-2.90) | 0.29 | 1.22
(0.61-2.45) | 0.57 | 1.16
(0.57-2.33) | 0.68 |
|
>2.0 | 1.48
(1.07-2.06) | 0.02 | 1.04
(0.74-1.46) | 0.81 | 1.00
(0.71-1.40) | 0.98 |
| Sex | | | | | | |
|
Male | 1.34
(0.90-1.99) | 0.15 | 0.98
(0.65-1.48) | 0.94 | 0.95
(0.63-1.44) | 0.81 |
|
Female | 1.52
(0.99-2.33) | 0.06 | 1.05
(0.67-1.63) | 0.85 | 1.00
(0.64-1.57) | 0.99 |
| Age, years | | | | | | |
|
20-39 | | | | | | |
|
40-59 | 1.41
(0.80-2.51) | 0.24 | 1.37
(0.77-2.44) | 0.29 | 1.32
(0.73-2.37) | 0.36 |
|
≥60 | 0.97
(0.68-1.37) | 0.85 | 0.99
(0.69-1.40) | 0.9 | 0.96
(0.67-1.37) | 0.83 |
| Race/ethnicity | | | | | | |
|
Non-Hispanic
White | 1.12
(0.73-1.73) | 0.61 | 0.93
(0.60-1.46) | 0.76 | 0.87
(0.56-1.37) | 0.55 |
|
Hispanic | 2.55
(1.48-4.38) | <0.01 | 1.67
(0.95-2.95) | 0.08 | 1.69
(0.95-3,00) | 0.08 |
|
Non-Hispanic
Black | 0.89
(0.431.87) | 0.76 | 0.65
(0.31-1.39) | 0.27 | 0.64
(0.30-1.37) | 0.25 |
|
Non-Hispanic
Asian | 0.54
(0.07-4.00) | 0.54 | 0.46
(0.06-3.49) | 0.45 | 0.33
(0.04-2.72) | 0.31 |
|
Other | 1.69
(0.47-6.03) | 0.42 | 1.62
(0.42-6.19) | 0.48 | 1.62
(0.42-6.19) | 0.53 |
| BMI | | | | | | |
|
Normal
weight | 1.66
(0.58-4.76) | 0.34 | 0.87
(0.29-2.56) | 0.80 | 1.08
(0.35-3.29) | 0.89 |
|
Overweight | 1.22
(0.60-2.48) | 0.58 | 0.72
(0.35-1.50) | 0.38 | 0.62
(0.29-1.30) | 0.21 |
|
Obese | 1.09
(0.76-1.55) | 0.64 | 0.89
(0.61-1.28) | 0.52 | 0.88
(0.61-1.28) | 0.51 |
Sensitivity testing
Table SIII presents
a summary of the findings of the sensitivity analyses. Following
utilization of CAP ≥285 dB/m as a criterion for defining hepatic
steatosis, the association between PPI use and hepatic steatosis
was significant (OR, 1.19; 95% CI, 1.01-1.40). To mitigate the
potential influence of other factors contributing to hepatic
steatosis, the present study focused exclusively on patients with
NAFLD. In logistic regression sensitivity analysis, use of PPIs was
significantly associated with hepatic steatosis (OR, 1.50; 95% CI,
1.16-1.93; Table SIV).
Furthermore, upon modifying the target population to individuals
with MASLD and controlling for confounding variables, the
association between the use of PPIs and hepatic steatosis persisted
(OR, 1.26; 95% CI, 1.05-1.52; Table
SV).
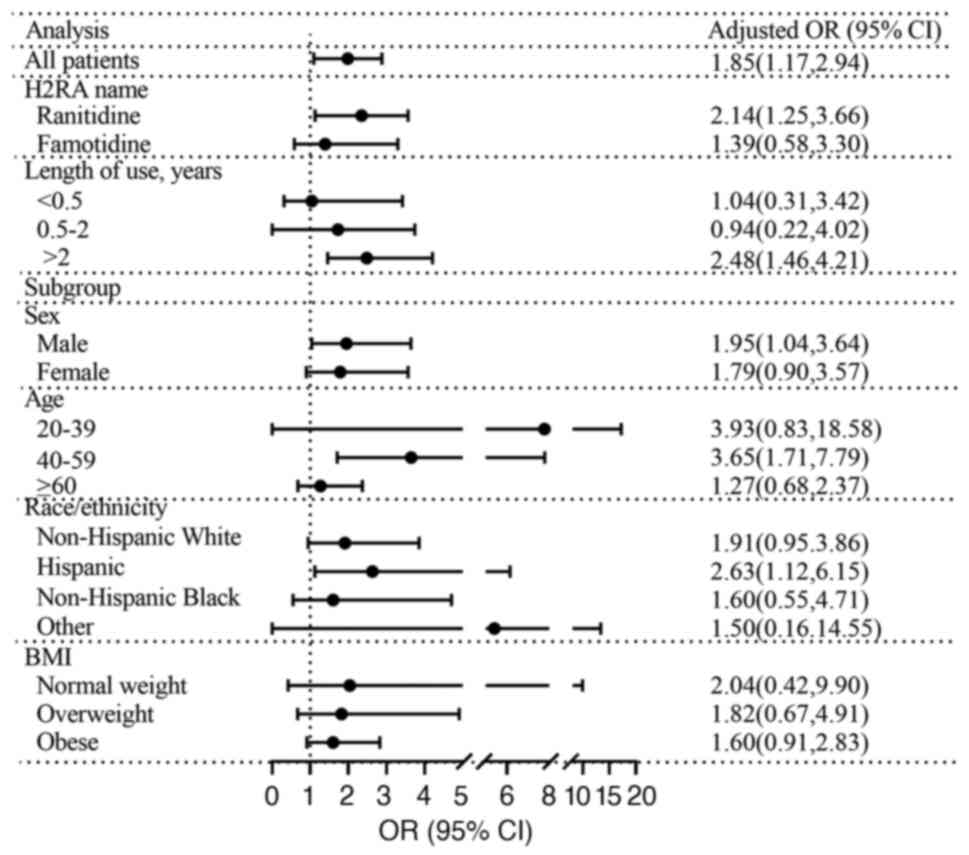
Associations between H2RAs and hepatic
steatosis or advanced fibrosis
The findings are presented in Fig. 4 and Tables SVI and SVII. There was no significant difference
in incidence of hepatic steatosis among users of H2RAs (OR, 1.20;
95% CI, 0.88-1.62; Table SVI).
However, use of ranitidine was significantly associated with
hepatic steatosis (OR, 1.56; 95% CI, 1.07-2.27; Table SVI). Furthermore, after adjusting
for potential confounding factors (model 3), the use of H2RAs was
significantly associated with an 85% greater incidence of advanced
fibrosis, particularly in the case of ranitidine use and a duration
of use >2 years (Fig. 4). There
was a significant association between use of H2RAs and an elevated
risk of advanced fibrosis in males (OR, 1.95; 95% CI, 1.04-3.64;
Table SVII), individuals aged
40-59 years (OR, 3.65; 95% CI, 1.71-7.79; Table SVII) and individuals of Hispanic
ethnicity (OR, 2.63; 95% CI, 1.12-6.15; Table SVII).
Discussion
In the present nationally representative
cross-sectional study, use of PPIs was linked to hepatic steatosis.
This association was observed after adjusting for various factors,
with omeprazole showing a particularly strong association.
Furthermore, prolonged use of PPIs was found to significantly
increase risk of developing hepatic steatosis. This association
persisted when hepatic steatosis was redefined using a cut-off
value of CAP ≥285 dB/m and when focusing specifically on
individuals with NAFLD or MASLD. The subgroup analysis demonstrated
a heightened OR in the relationship between the use of PPIs and
hepatic steatosis among male individuals aged 20 to 59 years, as
well as those who were Non-Hispanic White. By incorporating the
NHANES design to acquire national estimates for the US, the present
results may exhibit generalizability to the adult outpatient
population.
NAFLD is the liver component of a cluster of
diseases associated with metabolic dysfunction and it has emerged
as being the predominant etiology of chronic liver disease on a
global scale, with a prevalence of 25.04% (29). The ‘multiple hit’ hypothesis is
gaining increasing support as a comprehensive explanation for the
progression of NAFLD (17,27,28).
This hypothesis encompasses factors such as genetic predisposition,
insulin resistance, lipid metabolism imbalance, oxidative and
endoplasmic reticulum stress, inflammation and dysbiosis of the gut
microbiota. These factors serve key roles in the development of
NAFLD across diverse pathogenic stages (30,31).
Long-term PPI use has been reported to be associated
with the risk of NAFLD, but this association remains controversial
(32-35).
Shen and Liangpunsakul (32)
reported no significant association between the use of PPIs and the
incidence of NAFLD (adjusted OR, 0.97; 95% CI, 0.87-1.29). However,
the diagnosis of NAFLD relied solely on serum aminotransferase
levels and was not confirmed by ultrasonography or liver biopsy.
Consequently, certain study participants may have been
misclassified as either having NAFLD or not. By contrast, a cohort
study from South Korea reported that PPI use is associated with an
increased risk of fatty liver disease [adjusted hazard ratio (aHR),
1.50; 95% CI, 1.44-1.57] (33).
However, the aforementioned study investigated a single ethnic
group (Koreans) (33) and future
research should encompass diverse ethnicities. Furthermore, use of
CAP for diagnosing steatosis may offer greater accuracy compared
with diagnostic codes (which have a high rate of underdiagnosis in
NAFLD). Moreover, Llorente et al (34) discovered that individuals who use
PPIs are at a significantly greater risk of developing alcoholic
liver disease than previous users (aHR, 1.37; 95% CI, 1.00-1.88) or
those who never used PPIs (aHR, 1.52; 95% CI, 1.21–1.91). Llorente
et al (34) focused on the
relationship between PPI use and ALD and underlying mechanism
(inducing overgrowth of intestinal Enterococcus). Huang
et al (35) indicated that
taking PPIs was associated with increased risk of NAFLD, especially
severe hepatic steatosis (OR, 1.451, 95% CI, 1.034-2.036). The
aforementioned study did not assess the dose-response effect of PPI
treatment on the risk of NAFLD due to a lack of data on dosage and
frequency of PPI use. In conclusion, the aforementioned studies
were limited by a single ethnic group, inability to control for
intricate confounding factors, inaccurate measures for categorizing
liver disease such as diagnostic codes or indirect estimations of
liver disease such as liver enzyme levels, absence of indications
and categories for PPI use or inability to assess the degree of
steatosis. Therefore, the present study conducted a large,
nationally representative cross-sectional study using data from the
NHANES.
Understanding of the potential mechanisms linking
the use of PPIs to hepatic steatosis remains elusive. Numerous
molecular mechanisms have been suggested for the increased risk of
hepatic steatosis associated with PPI use. Primarily, PPIs may
predispose patients to small intestinal bacterial overgrowth
(8). Various pathogenetic
mechanisms include diminished bacterial elimination, overgrowth of
bacteria in the intestine, modified gastrointestinal motility, and
augmented intestinal permeability (36). Llorente et al (34) conducted a study utilizing data from
mouse models and humans and demonstrated that the use of gastric
acid-suppressive medications contributes to the excessive
proliferation of Enterococcus in the intestine, thus
facilitating the progression of liver disease. Likewise, multiple
studies have indicated the potential role of the gut microbiota in
mediating initiation and progression of NAFLD through the gut-liver
axis (37-40).
The potential underlying mechanisms include hepatic inflammatory
response, impaired bile acid metabolic cycle, impaired choline
metabolism, reduced production of short-chain fatty acids and
endogenous alcohol production. These alterations have the potential
to contribute to hepatic insulin resistance, inflammation
(non-alcoholic steatohepatitis) and fibrosis. Furthermore, the
administration of PPIs has been associated with a decrease in
granulocyte and monocyte count, which is likely due to a decrease
in oxidative bursts. This reduction in immune cell function may
weaken systemic immunity (41).
Several other potential mechanisms have been hypothesized to
establish a connection between use of PPIs and development of
hepatic steatosis. These mechanisms include PPI-induced
hypomagnesemia, which leads to insulin resistance and low-grade
systemic inflammation, decreased levels of insulin-like growth
factor 1 and activation of the pregnane X receptor (42,43).
The present subgroup analysis showed demonstrated
that among patients aged 20-9 years and male and Non-Hispanic White
patients, those receiving PPI therapy had a greater risk of hepatic
steatosis. This requires further investigation. A previous study
reported that the one year change in body weight was 1.52±0.6
kilograms higher in male using PPI compared to men not using PPI,
whereas female PPI users do not exhibit such a trend (44). The precise mechanisms underlying the
association between weight gain and PPI use remain unclear,
although there are no notable disparities in energy intake or
indicators of energy expenditure (44). Here, the primary indication for
prescribing PPIs was GERD. A double-blind randomized trial
demonstrated that female patients with GERD may require lower
dosages of PPIs than male patients (45). Nguyen et al (46) demonstrated a greater prevalence of
Barrett's esophagus among Non-Hispanic Whites than African
Americans. Additionally, Non-Hispanic Whites exhibit a greater
likelihood of being male and using PPIs than African Americans
(46). These findings potentially
explain the outcomes of the present subgroup analysis. However, the
present study had a limited sample size. Consequently, further
well-designed prospective studies are warranted.
The present study examined the potential association
between PPI therapy and VCTE-estimated liver fibrosis and hepatic
steatosis, allowing more accurate determination of the degree of
hepatic fibrosis and steatosis than other assessment methods.
Additionally, the present study included PPI type and therapy
duration as variables. The study sample was representative of the
population at the national level and all of the patients had a
comprehensive medical service utilization history. This contributes
to a high level of generalizability of results and enables subgroup
analyses with high statistical power. Furthermore, the present
study employed rigorous exclusion criteria and effectively
controlled for potential confounding factors by providing
comprehensive data on lifestyles, including educational attainment,
family income-to-poverty ratio, smoking status, alcohol misuse and
vigorous physical activity. Additionally, the robustness of the
present findings was substantiated through confirmation in multiple
subgroups and sensitivity analyses.
The present study was subject to certain limitations
due to its cross-sectional and observational design. First, as a
cross-sectional analysis, it did not allow for the establishment of
causality or identification of associations with clinical outcomes.
Second, the exclusion of individuals who underwent unsuccessful
VCTE examinations may result in the omission of a potentially
significant at-risk group. Another limitation of the present study
was that it used prescription data. Prescription data may not
accurately reflect patient adherence and do not consider
over-the-counter use of acid-suppressive therapies. Finally,
potential influence of other confounding factors could not be
eliminated.
In conclusion, PPI therapy was associated with an
increased risk of hepatic steatosis in a representative sample of
the US population. It may be advisable to prioritize the
appropriate indication for PPIs and administer the lowest feasible
dosage for the shortest possible duration in patients with fatty
liver resulting from any etiology.
Supplementary Material
Association between PPI use and
controlled attenuation parameter.
Association between PPI use and liver
stiffness measurement.
Sensitivity analyses of associations
between PPI use and hepatic steatosis (controlled attenuation
parameter ≥285 dB/m).
Sensitivity analyses of associations
between PPI use and hepatic steatosis in patients with
non-alcoholic fatty liver disease.
Sensitivity analysis of associations
between PPI use and hepatic steatosis in patients with metabolic
dysfunction-associated steatotic liver disease.
Association between H2RA and hepatic
steatosis
Association between H2RA and advanced
fibrosis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grants from Natural
Science Foundation of Guangdong Province for Distinguished Young
Scholar (grant no. 2022B1515020024), National Natural Science
Foundation of China (grant no. 82070574) and Natural Science
Foundation Team Project of Guangdong Province (grant no.
2018B030312009).
Availability of data and materials
The data generated in the present study may be found
in the NHANES database under accession number (NHANES 2017-March
2020 Pre-pandemic) or at the following URL: https://wwwn.cdc.gov/nchs/nhanes/.
Authors' contributions
HY and ML designed the study, performed the
statistical analysis and drafted the manuscript. BL, HS, HJ, ZL and
AS collected and analyzed the data. BW and YY revised the
manuscript, conceived and designed the study and analyzed data. BW
and YY confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
As the National Health and Nutrition Examination
Survey data sets are completely de-identified and publicly
available, this analysis was deemed exempt by the Institutional
Review Board (Third Affiliated Hospital of Sun Yat-Sen University,
Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kantor ED, Rehm CD, Haas JS, Chan AT and
Giovannucci EL: Trends in prescription drug use among adults in the
United States from 1999-2012. JAMA. 314:1818–1831. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Targownik LE, Fisher DA and Saini SD: AGA
clinical practice update on de-prescribing of proton pump
inhibitors: Expert review. Gastroenterology. 162:1334–1342.
2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ahrens D, Chenot JF, Behrens G, Grimmsmann
T and Kochen MM: Appropriateness of treatment recommendations for
PPI in hospital discharge letters. Eur J Clin Pharmacol.
66:1265–1271. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Al-Aly Z, Maddukuri G and Xie Y: Proton
pump inhibitors and the Kidney: Implications of current evidence
for clinical practice and when and how to deprescribe. Am J Kidney
Dis. 75:497–507. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Northuis CA, Bell EJ, Lutsey PL, George
KM, Gottesman RF, Mosley TH, Whitsel EA and Lakshminarayan K:
Cumulative use of proton pump inhibitors and risk of dementia: The
atherosclerosis risk in communities study. Neurology.
101:e1771–e1778. 2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yang YX, Lewis JD, Epstein S and Metz DC:
Long-term proton pump inhibitor therapy and risk of hip fracture.
JAMA. 296:2947–2953. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tseng HJ, Cheng CM, Tsai SJ, Lin WC, Bai
YM, Tsai CF, Su TP, Li CT, Chen TJ and Chen MH: Proton pump
inhibitor exposure and acute myocardial infarction risk: A nested
cohort study. Cardiovasc Toxicol. 21:444–450. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lo WK and Chan WW: Proton pump inhibitor
use and the risk of small intestinal bacterial overgrowth: A
meta-analysis. Clin Gastroenterol Hepatol. 11:483–490.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Choudhury A, Jena A, Jearth V, Dutta AK,
Makharia G, Dutta U, Goenka M, Kochhar R and Sharma V: Vitamin B12
deficiency and use of proton pump inhibitors: A systematic review
and meta-analysis. Expert Rev Gastroenterol Hepatol. 17:479–487.
2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Joo MK, Park JJ and Chun HJ: Proton pump
inhibitor: The dual role in gastric cancer. World J Gastroenterol.
25:2058–2070. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li DK, Yan P, Abou-Samra AB, Chung RT and
Butt AA: Proton pump inhibitors are associated with accelerated
development of cirrhosis, hepatic decompensation and hepatocellular
carcinoma in noncirrhotic patients with chronic hepatitis C
infection: Results from ERCHIVES. Aliment Pharmacol Ther.
47:246–258. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mahmud N, Serper M, Taddei TH and Kaplan
DE: The association between proton pump inhibitor exposure and key
liver-related outcomes in patients with cirrhosis: A veterans
affairs cohort study. Gastroenterology. 163:257–269.e6.
2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
De Roza MA, Kai L, Kam JW, Chan YH, Kwek
A, Ang TL and Hsiang JC: Proton pump inhibitor use increases
mortality and hepatic decompensation in liver cirrhosis. World J
Gastroenterol. 25:4933–4944. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Eusebi LH, Rabitti S, Artesiani ML, Gelli
D, Montagnani M, Zagari RM and Bazzoli F: Proton pump inhibitors:
Risks of long-term use. J Gastroenterol Hepatol. 32:1295–1302.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Freedberg DE, Kim LS and Yang YX: The
risks and benefits of long-term use of proton pump inhibitors:
Expert review and best practice advice from the American
gastroenterological association. Gastroenterology. 152:706–715.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Vilar-Gomez E, Vuppalanchi R, Gawrieh S,
Samala N and Chalasani N: CAP and LSM as determined by VCTE are
independent predictors of all-cause mortality in the US adult
population. Hepatology. 77:1241–1252. 2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rinella ME, Neuschwander-Tetri BA,
Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, Kleiner DE and
Loomba R: AASLD practice guidance on the clinical assessment and
management of nonalcoholic fatty liver disease. Hepatology.
77:1797–1835. 2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kim D, Konyn P, Cholankeril G and Ahmed A:
Physical activity is associated with nonalcoholic fatty liver
disease and significant fibrosis measured by FibroScan. Clin
Gastroenterol Hepatol. 20:e1438–e1455. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Eddowes PJ, Sasso M, Allison M, Tsochatzis
E, Anstee QM, Sheridan D, Guha IN, Cobbold JF, Deeks JJ, Paradis V,
et al: Accuracy of fibroscan controlled attenuation parameter and
liver stiffness measurement in assessing steatosis and fibrosis in
patients with nonalcoholic fatty liver disease. Gastroenterology.
156:1717–1730. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ciardullo S and Perseghin G: Statin use is
associated with lower prevalence of advanced liver fibrosis in
patients with type 2 diabetes. Metabolism.
121(154752)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bedossa P and Poynard T: An algorithm for
the grading of activity in chronic hepatitis C. The METAVIR
cooperative study group. Hepatology. 24:289–293. 1996.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Leung CW and Tapper EB: Sugar-sweetened
beverages are associated with increased liver stiffness and
steatosis among apparently healthy adults in the United States.
Clin Gastroenterol Hepatol. 20:959–961.e1. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Vilar-Gomez E, Nephew LD, Vuppalanchi R,
Gawrieh S, Mladenovic A, Pike F, Samala N and Chalasani N:
High-quality diet, physical activity, and college education are
associated with low risk of NAFLD among the US population.
Hepatology. 75:1491–1506. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Phan H, Richard A, Lazo M, Nelson WG,
Denmeade SR, Groopman J, Kanarek N, Platz EA and Rohrmann S: The
association of sex steroid hormone concentrations with
non-alcoholic fatty liver disease and liver enzymes in US men.
Liver Int. 41:300–310. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yang HH, Chen GC, Zhou MG, Xie LF, Jin YY,
Chen HT, Chen ZK, Kong YH, Yuan CZ and Li ZH: Association of age at
first birth and risk of non-alcoholic fatty liver disease in women:
Evidence from the NHANES. Hepatol Int. 17:303–312. 2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Siddiqui MS, Vuppalanchi R, Van Natta ML,
Hallinan E, Kowdley KV, Abdelmalek M, Neuschwander-Tetri BA, Loomba
R, Dasarathy S, Brandman D, et al: Vibration-controlled transient
elastography to assess fibrosis and steatosis in patients with
nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol.
17:156–163.e2. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chalasani N, Younossi Z, Lavine JE,
Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM and Sanyal AJ:
The diagnosis and management of nonalcoholic fatty liver disease:
Practice guidance from the American association for the study of
liver diseases. Hepatology. 67:328–357. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rinella ME, Lazarus JV, Ratziu V, Francque
SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab
JP, et al: A multisociety Delphi consensus statement on new fatty
liver disease nomenclature. Ann Hepatol. 29(101133)2024.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Younossi ZM, Koenig AB, Abdelatif D, Fazel
Y, Henry L and Wymer M: Global epidemiology of nonalcoholic fatty
liver disease-meta-analytic assessment of prevalence, incidence,
and outcomes. Hepatology. 64:73–84. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Buzzetti E, Pinzani M and Tsochatzis EA:
The multiple-hit pathogenesis of non-alcoholic fatty liver disease
(NAFLD). Metabolism. 65:1038–1048. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Magee N, Ahamed F, Eppler N, Jones E,
Ghosh P, He L and Zhang Y: Hepatic transcriptome profiling reveals
early signatures associated with disease transition from
non-alcoholic steatosis to steatohepatitis. Liver Res. 6:238–250.
2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shen H and Liangpunsakul S: Histamine
H2-receptor antagonist use is associated with lower prevalence of
nonalcoholic fatty liver disease: A population-based study from the
national health and nutrition examination survey, 2001-2006. J Clin
Gastroenterol. 50:596–601. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Pyo JH, Kim TJ, Lee H, Choi SC, Cho SJ,
Choi YH, Min YW, Min BH, Lee JH, Kang M, et al: Proton pump
inhibitors use and the risk of fatty liver disease: A nationwide
cohort study. J Gastroenterol Hepatol. 36:1235–1243.
2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Llorente C, Jepsen P, Inamine T, Wang L,
Bluemel S, Wang HJ, Loomba R, Bajaj JS, Schubert ML, Sikaroodi M,
et al: Gastric acid suppression promotes alcoholic liver disease by
inducing overgrowth of intestinal Enterococcus. Nat Commun.
8(837)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Huang H, Liu Z, Guo Y, Zeng Y, Shen S and
Xu C: Long-term use of proton pump inhibitors is associated with an
increased risk of nonalcoholic fatty liver disease. J Clin
Gastroenterol. 58:289–296. 2024.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Corleto VD, Festa S, Di Giulio E and
Annibale B: Proton pump inhibitor therapy and potential long-term
harm. Curr Opin Endocrinol Diabetes Obes. 21:3–8. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tsai MC, Liu YY, Lin CC, Wang CC, Wu YJ,
Yong CC, Chen KD, Chuah SK, Yao CC, Huang PY, et al: Gut microbiota
dysbiosis in patients with biopsy-proven nonalcoholic fatty liver
disease: A cross-sectional study in Taiwan. Nutrients.
12(820)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Demir M, Lang S, Hartmann P, Duan Y,
Martin A, Miyamoto Y, Bondareva M, Zhang X, Wang Y, Kasper P, et
al: The fecal mycobiome in non-alcoholic fatty liver disease. J
Hepatol. 76:788–799. 2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Schwimmer JB, Johnson JS, Angeles JE,
Behling C, Belt PH, Borecki I, Bross C, Durelle J, Goyal NP,
Hamilton G, et al: Microbiome signatures associated with
steatohepatitis and moderate to severe fibrosis in children with
nonalcoholic fatty liver disease. Gastroenterology. 157:1109–1122.
2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lee G, You HJ, Bajaj JS, Joo SK, Yu J,
Park S, Kang H, Park JH, Kim JH, Lee DH, et al: Distinct signatures
of gut microbiome and metabolites associated with significant
fibrosis in non-obese NAFLD. Nat Commun. 11(4982)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Garcia-Martinez I, Francés R, Zapater P,
Giménez P, Gómez-Hurtado I, Moratalla A, Lozano-Ruiz B, Bellot P,
González-Navajas JM and Such J: Use of proton pump inhibitors
decrease cellular oxidative burst in patients with decompensated
cirrhosis. J Gastroenterol Hepatol. 30:147–154. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ciardullo S, Rea F, Savaré L, Morabito G,
Perseghin G and Corrao G: Prolonged use of proton pump inhibitors
and risk of type 2 diabetes: Results from a large population-based
nested case-control study. J Clin Endocrinol Metab.
107:e2671–e2679. 2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Czarniak P, Ahmadizar F, Hughes J, Parsons
R, Kavousi M, Ikram M and Stricker BH: Proton pump inhibitors are
associated with incident type 2 diabetes mellitus in a prospective
population-based cohort study. Br J Clin Pharmacol. 88:2718–2726.
2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Czwornog JL and Austin GL: Association of
proton pump inhibitor (PPI) use with energy intake, physical
activity, and weight gain. Nutrients. 7:8592–8601. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Helgadóttir H, Metz DC, Lund SH,
Gizurarson S, Jacobsen EI, Asgeirsdóttir GA, Yngadóttir Y and
Björnsson ES: Study of gender differences in proton pump inhibitor
dose requirements for GERD: A double-blind randomized trial. J Clin
Gastroenterol. 51:486–493. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Nguyen TH, Thrift AP, Ramsey D, Green L,
Shaib YH, Graham DY and El-Serag HB: Risk factors for Barrett's
esophagus compared between African Americans and non-Hispanic
Whites. Am J Gastroenterol. 109:1870–1880. 2014.PubMed/NCBI View Article : Google Scholar
|