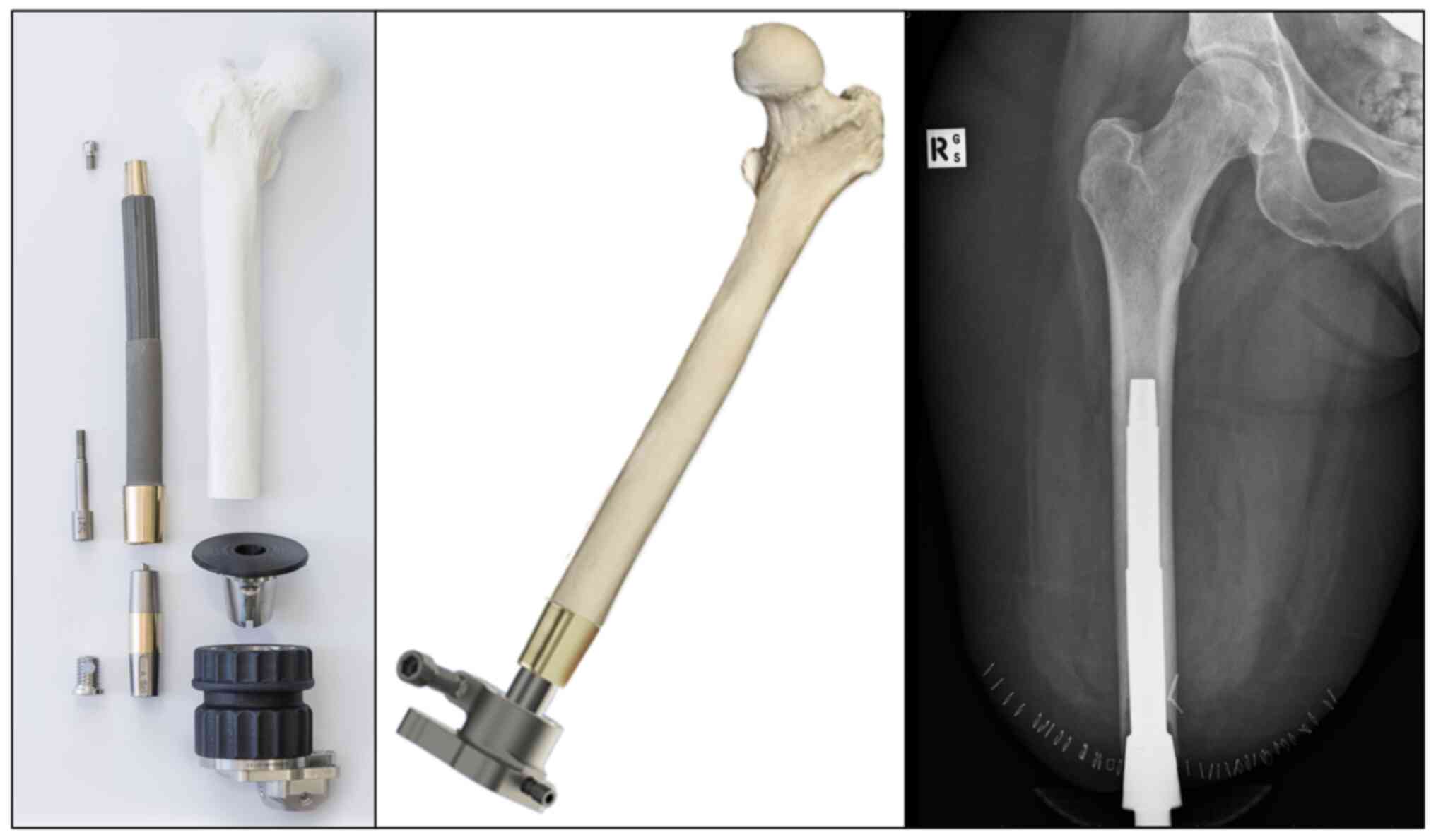
1. Introduction
Osseointegration implant (OI) technology is based on
the attachment and integration of bone to an artificial implant,
used in different orthopaedic applications to enable
musculoskeletal repair. The term ‘osseointegration’ was first
introduced in dentistry by Dr R. Branemark, who used titanium for
dental implants in a clinical study in 1965(1). Over the last two decades, OI has
evolved to provide a new method of rehabilitation following limb
amputation, which has been reported to increase patient
satisfaction and quality of life (QOL) compared to traditional
socket prostheses (2-4).
Since its initial application in dentistry, osseointegration
surgery has revolutionised orthopaedics and rehabilitation, and led
to improved quality of life and functional restoration for amputees
(5). In the context of this paper,
OI refers specifically to those implants that have been used for
limb rehabilitation in amputees.
Amputations significantly affect mobility and QOL,
occurring as a result of non-communicable diseases such as
diabetes, or due to trauma, tumours and congenital diseases
(6). In Australia, ~8,000
amputations occur annually, largely as a result of complications of
diabetes (7). In 2017, there were
57.7 million individuals worldwide with an amputation from
traumatic causes, such as falls and road injuries (6). Conventional amputation rehabilitation
methods utilise socket prostheses attached to the residual limb in
the form of a cup, but users experience functional and aesthetic
limitations that impact their QOL. Common issues with socket
prostheses include discomfort, sweating in the socket, phantom limb
pain and ill-fit of the socket due to the dynamic nature of the
residual limb (8-10).
These issues have prompted the development of new OI technology to
directly attach the prosthetic device to the bone of the residual
limb (Fig. 1). A range of implant
types have evolved, such as integral leg prosthesis (ILP),
osseointegration prosthetic limb (OPL), osseointegrated prostheses
for the rehabilitation of amputees (OPRA) and percutaneous
osseointegrated prosthesis (POP), most of which involve a titanium
implant for interfacing with bone together with certain design
variations, except for the ILP made from cobalt-chromium-molybdenum
(5,6).
Since its recent application in the last two
decades, OI has demonstrated significant promise as an alternative
treatment for amputees who have failed to mobilise with traditional
socket prostheses. Lower limb OI allows for direct skeletal
attachment, which has been shown to improve range of motion
(2) and mobility (3), and reduce the amount of energy used
when mobilising (11). The
mechanisms by which OI integrates with bone and induces
regenerative bone growth has been described in a number of studies
(12). As OI has only recently been
introduced into clinical practice, the understanding of their
efficacy and associated analysis techniques are limited to patient
questionnaires and functional outcome measurements. While these are
effective indicators of patient satisfaction and indirect
predictors of implant integration, an objective measurement of
periprosthetic bone health as a direct indication of implant
integration is rarely used.
Bone mineral density (BMD) is the gold standard for
measuring bone health, and is clinically used to diagnose
bone-related diseases or determine the risk of developing fractures
(13). The BMD can be measured by
dual-energy X-ray absorptiometry (DEXA), quantitative computed
tomography and quantitative magnetic resonance imaging (14). Most well-known for its use in the
diagnosis of osteoporosis and identifying high fracture risk
populations, the BMD is also used to assess the effectiveness of
treatment (15) and rehabilitation
(16). The BMD can be measured at
multiple anatomical locations; however, femoral neck measurements
are the best predictor of a future fracture near the hip (17). BMD measurements compare the density
of the patient's bone to that of healthy, young individuals,
referred to as the T-score (18). A
previous study described the effects of lower limb OI on BMD and
potential benefits in restoring more natural biomechanical loading
of the femoral neck (19). While
this is important in ensuring a return to baseline function in
lower limb amputees, the local periprosthetic effects of OI for
assessing risk of failure or fracture at the implant site have
remained to be properly characterised. Objective measurements
obtained through bone imaging may serve as a window for
understanding how OI integrates biologically with the skeleton or
whether the patient is at risk of developing future complications,
and guide amputees in their rehabilitation or direct engineers to
develop implants with greater efficacy. For numerous years, it has
been understood that bone loading through resistance exercises and
low-impact activities supports positive BMD changes in older adults
(20). It has also been shown that
OI leads to non-uniform load distribution that influences the
outcome of bone regeneration, with higher load areas having more
favourable outcomes (21).
The efficacy of OI technology for treating
amputations is currently measured by QOL and functional testing.
BMD as an objective measure to reflect post-implantation bone
quality has significant applicability in clinical practice for
assessing implant integration and hence predicting the risk of
periprosthetic fracture or implant failure. While previous reviews
have evaluated the efficacy of OI on amputee rehabilitation
(6,22-24),
none had a focused approach to analysing BMD measurements as an
objective indicator of bone quality post-implantation. The present
review captures all existing studies that reported BMD measurements
following OI surgery in amputees and reflects on the usefulness of
the BMD as a predictor of bone regeneration, implant failure and
post-operative complications, as well as its role in guiding
patients through rehabilitation and engineers in implant
development. The findings of relevant studies were summarised and
discussed in the form of a narrative review due to the limited
evidence currently available. Systematic reviews or meta-analyses
may be possible in the future following further build-up of
high-quality clinical evidence on this emerging topic area.
2. Selection of studies for analysis
PubMed, Medline, Scopus and Web of Science were
searched using combinations of the key words ‘amputation’,
‘osseointegration’ and ‘bone mineral density’ from inception to
March 1st, 2024. The reference lists of included studies were also
manually scanned for any additional studies missed by the
electronic search. Details of the search strategy are provided in
Table SI.
The eligibility criteria for selecting studies for
subsequent analysis were as follows: i) All types of clinical
studies involving human participants; ii) the study involved
trans-femoral or trans-tibial amputees rehabilitated using OI,
through either a single-stage or two-stage surgical procedure; and
iii) the study involved quantitative BMD measurement using one or
more bone imaging techniques. Studies were not used for analysis if
they were: i) Non-human studies or experimental studies performed
in a laboratory; ii) reviews or other non-original research
studies; iii) studies of which the full text was unavailable; and
iv) studies not written in English.
Due to limited research on OI retrieved using the
above criteria, additional studies involving BMD measurements
following total hip arthroplasty (THA) and total knee arthroplasty
(TKA) were included as supporting information (Table SII) for comparison and
discussion.
Relevant data from each selected study on OI were
extracted and summarised in Table
I, including: i) Basic study characteristics (first author,
country, year of publication); ii) study design (type, sample size,
implant type, follow-up duration); iii) analyses performed (BMD
measurements, other measurements); and iv) main BMD-related
findings and limitations stated. The quality of included studies
was evaluated based on the tool for assessing risk of bias in
non-randomised studies of interventions (25). The risk of bias was assessed
according to 9 domains, in which each study was assigned a high,
low or unclear risk. An overall risk of bias judgement was then
made based on the results in the 9 domains, as presented in the
last column of Table SIII, as low,
moderate, serious or critical risk of bias.
 | Table ISummary of included studies. |
Table I
Summary of included studies.
| First author,
year | Study type | Country | Study
size/type | Analyses | BMD measurement
technique | Other
measurements | Initial BMD
measurement | Final BMD
measurement | Follow-up
duration | Change in BMD | Main BMD-related
outcomes | Limitations
stated | (Refs.) |
|---|
| Haket et al,
2017 | Consecutive
case-control, level 3 prognostic study | The
Netherlands | 27
patients/ILP | Femoral hip BMD,
periprosthetic cortical thickness | DEXA and
X-rays | Nil | Pre-operatively,
significant difference in BMD of hip neck between healthy and
amputated legs, with mean difference of 0.24 g/cm2 | On the amputated
side, BMD was 0.68, 0.67 and 0.69 g/cm2 pre-operatively
and 12 and 24 months post-operatively, respectively | 12 and 24
months | Insignificant
change in BMD, but initial and final values not reported | Bilateral hip BMD
and periprosthetic cortical thickness were improved with OI. BMD of
hip neck on amputated side was substantially lower than that of the
contralateral side, but did not show significant change at 24-month
follow-up | Limited control of
rotation during X-rays affecting accuracy of cortical thickness
measurements. Follow-up period was relatively short | (21) |
| Hansen et
al, 2018 | Precision study and
validation | Denmark | 2 cadaveric femoral
bones/OPRA | BMD | DEXA | Nil | Not reported | Not reported | Not reported | Neutral (0°) ROI:
1, 1.95 g/cm2; 2, 1.72 g/cm2; 3, 1.74
g/cm2; 4, 1.74 g/cm2; 5, 1.58
g/cm2; 6, 1.57 g/cm2; 7, 1.65
g/cm2. 0-10° flexion ROI: 1, 0.4 g/cm2; 2,
1.2 g/cm2; 3, 5.9 g/cm2; 4, 3.1
g/cm2; 5, 0.3 g/cm2; 6, 2.1 g/cm2;
7, 1.7 g/cm2. 0-20° flexion ROI: 1, 3.8
g/cm2; 2, 3.9 g/cm2; 3, 9.1 g/cm2;
4, 9.9 g/cm2; 5, 6.3 g/cm2; 6, 2.3
g/cm2; 7, 0.0 g/cm2. 0-10° external rotation
ROI: 1, 1.0 g/cm2; 2, 3.7 g/cm2; 3, 2.7
g/cm2; 4, 1.9 g/cm2; 5, 0.6 g/cm2;
6, 0.6 g/cm2; 7, 2.2 g/cm2. 0-20° external
rotation ROI: 1, 0.7 g/cm2; 2, 6.6 g/cm2; 3,
0.7 g/cm2; 4, 0.8 g/cm2; 5, 0.5
g/cm2; 6, 1.1 g/cm2; 7, 2.7
g/cm2 | In most positions,
the mean BMD showed a significant change compared to the neutral
position. 5% flexion or rotation had the most impact on average BMD
in the majority of ROIs | The femur was only
examined in the anterior-posterior position. Edge-detection errors
were present due to the software being designed for different
anatomical structures. Population represented a heterogeneous group
with a large variation in BMD values among patients | (26) |
| Hansen et
al, 2019 | Prospective cohort
study | Denmark | 19 patients-NRI vs.
later RI/integrum AB | Periprosthetic BMD
and bone marker turnover | DEXA | Blood samples:
Parathyroid hormone, vitamin D, calcium ion, N-terminal propeptide
of type-I procollagen, C-terminal telopeptide of type-I collagen,
osteocalcin and bone-specific alkaline phosphatase | Proximal femur: -
Intact side: 1.03 g/cm2; Amputated side: 0.66
g/cm2; Spine: L1-L4: 1.13 g/cm2 | RI group (n=8):
Proximal femur: - Intact side, 0.97 g/cm2; Amputated
side, 0.55 g/cm2; Spine L1-L4, 1.09 g/cm2.
NRI group (n = 11): Proximal femur: - Intact side, 1.03
g/cm2; Amputated side, 0.68 g/cm2; Spine
L1-L4, 1.15 g/cm2 | 30 months | Mean BMD in RI
group reduced between 27 and 38% at 30-month follow-up. Mean BMD in
NRI group returned to baseline values after 30 months | Mean proximal femur
BMD on amputated side decreased by 9% at 6 months after S1 surgery.
Mean proximal femur BMD on intact side did not change at all
time-points No difference in mean proximal femur BMD between RI and
NRI groups at any follow-up. Precision measurement of BMD was 1.2%
for spine (L1-L$), 2.1% for proximal femur (amputated), and 1.1%
for proximal femur (intact). | Matching criteria
were required to be extended for 27 control patients; control group
consisted of patients with hip or knee osteoarthritis. Lower
activity level compared to normal populations, hence control group
could have lower BMD compared to average population. Small sample
size and patient group heterogeneity could have resulted in
errors. | (28) |
| Thomson et
al, 2019a | Retrospective
cohort study | Australia | 28 patients-15 in
ILP group and 13 in OPL group | Periprosthetic BMD
and femoral BMD | X-rays | Nil | Not reported | Not reported | 36 months | Not reported | ILP group had
greater decrease in BMD than OPL group in most zones Periprosthetic
BMD decreased in all zones along the length of both implant
types | Femoral soft-tissue
distribution was assumed to be equal to the baseline measurement of
each patient, as their BMI had not changed significantly. There
were differences in contours in ROI during measurements | (29) |
| Thomson et
al, 2019b | Prospective cohort
study | Australia | 48 patients-15 TTA,
22 TFAL and 11 TFAS/ILP or OPL | Spine and femoral
BMD | DEXA | 6MWT, TUG | Spine (L2-L4):TTA,
1.3164 g/cm2; TFAL, 1.268 g/cm2; TFAS, 1.173
g/cm2. Femur neck operative: TTA, 0.9747
g/cm2; TFAL, 0.709 g/cm2; TFAS, 0.6725
g/cm2. Femur neck contralateral: TTA, 1.072
g/cm2; TFAL, 1.016 g/cm2; TFAS, 1.01
g/cm2 | Femur neck
operative: TFAL, 1.078 g/cm2 after 1 year and 0.364
g/cm2 after 3 years | 12 and 36
months | Operative femoral
neck Z-score of 0.719 average increase across all. groups No
significant change in spine BMD for any group | BMD was restored in
patients with OI. Statistically significant increase in Z-scores on
operative side for TFAL and TFAS groups from pre-operative to
follow-up. No significant change in TTA group on operative
side | Mean femoral neck
Z-scores were lower than expected, which could be due to the lower
mobility of patients within this study compared to the literature.
In the TFAS group, a lag screw was passing through the cancellous
bone in the operative femoral neck, as this region was excluded
from the study; the results showed a higher BMD and Z-score on the
operative femur neck | (19) |
| Sinclair et
al, 2022 | Prospective cohort
study | USA | 10
patients/POP | Adverse events,
periprosthetic BMD and radiographs | DEXA and
X-rays | Prosthetic put on
and take off time, 6MWT and Q-TFA | Not reported | Pre-loading:
Medial, 1.44 g/cm2; Proximal, 1.66 g/cm2;
Lateral, 1.39 g/cm2; Ipsilateral, hip, 0.84
g/cm2; Lumbar spine, 1.31 g/cm2. Post-loading
at 26 months: Medial, 1.97 g/cm2; Proximal, 1.62
g/cm2; Lateral, 2.19 g/cm2; Ipsilateral hip,
0.88 g/cm2; Lumbar spine, 1.35 g/cm2 | 3, 6, 13 and 26
months | Significant
increase in average BMD in lumbar spine, medial and lateral areas.
Ipsilateral hip and proximal regions also showed an increase in
BMD, but were not significant | OI showed
improvements in BMD | Small sample size,
single-centre study, no female participants, and small number of
surgeons conducted the OI surgery | (27) |
3. Characteristics of selected studies
A total of 1,634 articles were retrieved from
database searches, of which 576 were considered potentially
eligible based on title and abstract. Following in-depth screening
and selection of articles based on eligibility criteria, 6 articles
(19,21,26-29)
were used for analysis in the present study (Table I).
The selected studies were from Europe, Australia and
the USA. Study types included observational cohort studies
(prospective or retrospective), consecutive case control studies
and a precision study. All studies had a sample size of <50
patients and used a variety of OI types, including ILP, OPL, OPRA
and POP. All studies assessed OI for trans-femoral amputations,
while only one study by Thomson et al (19) included both trans-femoral and
trans-tibial amputees. Follow-up durations varied, ranging between
12 and 36 months in most studies. BMD measurements had been
performed using DEXA in 3 studies, DEXA and X-rays in 2 studies and
X-rays only in 1 study.
4. Quality of selected studies
Assessment of risk of bias for each study selected
for analysis is shown in Table
SIII. Although there was variation among studies, the majority
of selected studies had a low risk of bias across the assessed
domains. In the ‘conflict of interest’ and ‘incomplete outcome
data’ domains, all studies showed unclear risk of bias. In the
other 7 domains, high risk of bias was seen in 7/7 domains in one
study (26), 6/7 domains in another
study (21) and 2/7 domains in a
third study (27), while the
remaining studies showed low risk of bias in all 7 domains. Of the
two studies rated to have overall serious risk of bias, one
included 27 patients who underwent OI at a single institution and
were followed up for various time periods that were grouped into
averages of 12 and 24 months. DEXA measurements were used to
determine the BMD at the hip neck of healthy and amputated sides
using a software and values of changes in BMD were reported
(21). However, specific methods
for DEXA measurements as well as the initial and final BMD values
were not stated. The other study used 2 cadaveric femoral bones
from patients who received OI and reported BMD changes measured by
DEXA as a result of altering leg position during scanning (26). The study focused on investigating
the precision of BMD measurements and did not report the absolute
or change in BMD values before and after OI surgery, or the time
after OI when the BMD measurements were made. There was no control
limb and statistical methods were not described. Significant
variations in study design can potentially introduce risk of bias
in the studies selected for analysis in this review and the quality
of studies should be considered when drawing conclusions from the
findings.
5. BMD measurements for OI in amputees
Of the six selected studies, two reported
insignificant changes in BMD following OI surgery in amputees,
while the remaining studies showed varying results. Haket et
al (21) found an insignificant
change in BMD from baseline to 24 months, which was similarly
reflected in the study by Hansen et al (28), indicating that the BMD returned to
baseline values at 30 months following a reduction. Thomson et
al (29) found a reduction in
the BMD at >24 months follow-up compared to baseline
measurements, which was speculated to be caused by stress shielding
of the implant. In a different study, Thomson et al
(19) reported Z-score results
implying that OI could effectively support bone remodelling and
increase BMD in the femoral neck of the amputated limb. This was
supported by the study by Sinclair et al (27), which noted an increase in BMD at the
ipsilateral hip and proximal to the implant stem, although the
change was not significant. One study did not report initial and
final BMD measurements, as this was a validation study performed
using cadaver limbs (26).
Due to the limited evidence retrieved on BMD
measurements in lower limb amputees rehabilitated using OI, a
supplementary table was generated to summarise studies reporting
BMD measurements in patients who received an osseointegrated
prosthesis for hip or knee joint replacement procedures (THA and
TKA; Table SII). Many of these
supplementary studies on both THA and TKA reported an overall
decrease in BMD for all measured regions of interest (ROIs)
(30-35).
In addition, others found variations in BMD changes from no change
to decreased BMD post-surgery in different ROIs (36,37).
Certain studies noted that the BMD tended to decrease at proximal
ROIs (38,39), with an eventual approach back to
baseline levels at the 6-month follow-up (38).
A number of studies on THA and TKA found an increase
in BMD at the tip of the implant but a notable decrease in the
middle ROIs (40,41), and one found insignificant changes
in BMD post-implantation (42).
Interestingly, one study also found that a short stem implant
resulted in significantly less BMD loss compared to a standard
straight stem (43).
To combat the apparent reduction in BMD
post-implantation, studies have investigated factors that may help
maintain BMD. One study found that the use of zoledronic acid led
to a minor increase in BMD by 2.2% at 12 months, while no change
was noted in the placebo group (44). Another study found that cementless
implants led to an increase in average BMD at the 3- and 6-month
follow-ups, while cemented implants led to decreased BMD (45).
6. Discussion of current findings from BMD
measurements following OI surgery
Of the >500 articles considered potentially
eligible for inclusion in the present study, only 6 met the
inclusion and exclusion criteria, emphasising the limited evidence
on the use of BMD measurements for evaluating the outcomes of OI
surgery in rehabilitating trans-femoral and trans-tibial amputees.
Many of the excluded articles focused on osseointegration in dental
implants, or animal or computer models of osseointegration in
amputated limbs (46,47). Other articles focused on BMD
measurements in amputees without implants (48), or only reported subjective
measurements such as quality of life surveys following OI surgery
(4,49). Although excluded from this review,
these studies can be used to gain a better understanding of the
research space.
BMD can be measured by different methods, including
DEXA, X-rays and radiostereometric analysis. The included studies
used DEXA and/or X-rays to gather BMD data, while the supplementary
studies on THA or TKA used all three methods individually or in
combination. The BMD of the implant group can be compared to the
average BMD of a young, healthy adult to give a T-score. The
included studies generally pointed to the eventual restoration of
baseline BMD in lower limb amputees rehabilitated with OI.
In a study by Haket et al (21) in 2017, changes in bone remodelling
were observed at 12 and 24 months following femoral OI surgery
using the ILP implant, which were measured through DEXA and
anteroposterior (AP) radiographs. While AP radiographs showed an
increase in cortical thickness, reflecting bone regeneration and
restoration of natural biomechanics, there was no significant
change in BMD values after 2 years. The authors speculated that the
lack of change in BMD could be because the newly formed bone around
the implant had not reached a normal density, yet. All participants
continued to use their OI after this study, with 0 of 27 patients
listed as a failed case. However, in this study, the lack of
correlation between change in BMD and success of the OI surgery
raised doubts about the ability of the BMD to be used as an
objective measure of OI outcome. It should be noted that this study
presented a high risk of bias across numerous items; hence, further
investigation with longer follow-up and a larger sample size is
warranted.
There were certain similarities in the observations
made by Thomson et al (29)
in 2019. This study monitored 28 patients with trans-femoral OI for
2 years, of which 15 received ILP and 13 received OPL. X-rays were
used to measure changes in BMD, indicating that the OPL implant led
to increased bone thickness, while in contrast to Haket et
al (21), the ILP implant led
to bone resorption at the distal end, which was suspected to be
caused by stress shielding. The study also showed statistically
significant decreases in BMD for both implant types at 2 years
after surgery. Lower BMD values are typically associated with a
higher risk of fracture, although none of the participants
sustained a fracture or had implant failure during the study
period. The findings of the present study again suggest that BMD
may not be an accurate indicator of OI success.
Another study by Thomson et al (19) from 2019 observed changes in BMD of
the femoral neck and spine for 3 years after OI implantation using
ILP or OPL. The focus of this study is noteworthy, since the other
included studies all assessed BMD around the implant site. The
femoral neck and spine are both sites frequently employed when
evaluating osteoporosis and fracture risk in patients without
amputation, and their relevance in this study arises from the
understanding that OI may help to restore native biomechanics
through the femur and hence potentially stimulate bone
regeneration. The results showed a statistically significant
increase in BMD at 1 year compared to baseline for all
trans-femoral participants receiving OI, while the increased BMD
observed in trans-tibial participants was not significant. This was
thought to be due to the retention of the full femur and knee joint
in trans-tibial amputees, allowing the femoral neck and spine to
maintain a more natural biomechanical state. In this study, BMD
measurements at relevant anatomical sites other than those around
the implant had a good correlation with the performance of OI in
the rehabilitation of lower limb amputation.
Hansen et al (26) published a validation study in 2018
with the aim of evaluating the precision and feasibility of a scan
protocol for conducting BMD measurements. This study used the
proximal part of two cadaveric human femoral bones with the OPRA
implant, mounted on a positioning jig to position them from neutral
(0°) to 20° flexion and rotation. DEXA scans were used to evaluate
variations in BMD as a result of changes in femur position angle.
Furthermore, 20 patient examinations were conducted to evaluate the
precision error for each of the elected ROIs. Importantly, this
study found significant changes in average BMD values depending on
the degree of flexion and rotation. The authors stressed the
importance of creating a reproducible scan protocol for conducting
BMD measurements in clinical studies to facilitate valid
cross-comparisons of study group results following OI surgery.
Hansen et al (28) published another study in 2019 using
the same scanning protocol established in the 2018 study to observe
changes in BMD and bone turnover markers over 30 months in 20
patients with OI. They noted a decrease in the average
periprosthetic BMD between the initial measurement and the 6-month
follow-up. During the study, 8 of the 19 participants required
their implant to be removed due to infection, pain or inability to
use the implant. These participants were moved into a separate
group (RI group) with their BMD measurements taken alongside
participants who did not require implant removal (NRI group). The
NRI group showed an increase in BMD that realigned to pre-operative
readings at 30 months, while the RI group showed a significant
reduction in BMD. In contrast to the studies discussed above, this
study indicated the possibility of using the BMD as a predictor for
OI failure in trans-femoral amputees.
Sinclair et al (27) published a prospective cohort study
in 2022 involving 10 patients implanted with POP to evaluate the
safety and efficacy of this press-fit OI. All participants were
male and underwent unilateral trans-femoral amputation at least 6
months prior to study commencement. BMD measurements were made
using DEXA scans taken at 2 years following OI surgery and
qualitative radiograph assessment. The study noted significant
increases in BMD of the lumbar spine, as well as in ROIs medial and
lateral to the distal porous coated region of the implant compared
to baseline. Furthermore, the BMD in the ipsilateral hip and region
proximal to the implant stem increased from baseline, although the
changes were not significant. Qualitative radiograph assessment
indicated an increase in bone density of the distal femur
throughout the length of the bone from baseline to 1 year
post-implantation. The perceived increase in BMD following OI
surgery may have a role in preventing degenerative musculoskeletal
changes after lower limb amputation.
Among the studies analysed in the present review, it
is clear that varied conclusions can be drawn when examining the
usefulness of the BMD as an objective tool for measuring the
success of OI surgery. Certain studies have shown an increase in
periprosthetic bone thickness following OI implantation but no
significant increase in periprosthetic BMD (21,29).
Other studies have observed no implant failure accompanied by a
trend of increasing BMD values post-implantation (19,27,28),
suggesting a potential association between BMD measurements and the
success of rehabilitation using OI. Regardless, the lack of a
standardised approach to the scanning and measurement procedure for
BMD readings in amputee patients receiving OI makes it difficult to
compare studies conducted by different research groups, as
identified by Hansen et al (26).
Although not directly comparable to the included
studies, the supplementary studies reporting BMD measurements for
OI surgery in THA and TKA patients provide valuable insight into
related clinical results over larger sample sizes and more varied
patient demographics. The experimental procedures and outcomes of
these studies may be beneficial in guiding the future
standardisation of BMD measurement protocols for patients receiving
OI for various indications. Overall, the findings of the
supplementary studies on THA and TKA were highly varied, reflecting
a lack of standardised methods for conducting BMD measurements as
well as heterogeneous study outcomes. The range of follow-up
periods was similar to the included studies, which was mostly
within 36 months, while patient demographics were mostly limited to
Western countries with the vast majority of studies being conducted
in Europe. The available evidence on BMD measurements in patients
with OI, both for amputation rehabilitation and other indications,
calls for higher-quality studies with larger sample sizes, longer
follow-up and wider demographics of populations in various
different continents.
7. Towards the standardisation of BMD
measurement protocols
The studies analysed in the present review utilised
DEXA and X-ray analysis as the primary techniques for measuring
BMD. DEXA scans can be taken in various positions with different
ROI placement. This introduces potential variations in BMD
measurements and resulting measurement values, driving the need to
develop a standardized measurement protocol that can be applied to
all OI types to evaluate implant survival. Hansen et al
(26) highlighted the importance of
having a reproducible set-up for DEXA scans to reduce BMD
measurement errors. The Lunar Prodigy Scanner (GE Healthcare) was
used in this study as an acceptable device for precise BMD
measurement in proximity to the OI. With a common protocol in place
that introduces minimal variations in limb positioning when
measuring BMD, scans from different research groups and clinical
settings will be comparable. Given that the majority of included
studies used DEXA scans to assess BMD and presented useful data
from their investigations, it is proposed that DEXA should be used
as the universal technique for measuring BMD considering its
accessibility.
All of the selected studies reported BMD
measurements after OI surgery in trans-femoral amputees, with all
but one study investigating periprosthetic bone remodelling. There
was considerable variation in the observed changes in BMD across
different ROIs and across different time-points, both among the
included studies and at times within the same study. In a standard
protocol, it is advisable to conduct BMD measurements both in the
periprosthetic region and at other relevant anatomical locations,
such as the femoral neck and spine. At least 7 ROIs should be used
in each region and follow-up should be conducted for at least 36
months.
8. Limitations
The current review provides the first summary of the
current evidence on the quantitative assessment of BMD in
trans-femoral and trans-tibial amputees rehabilitated using OI, to
the best of our knowledge. A number of limitations should be
considered when drawing conclusions from the findings given the
small number of studies available in this topic area. First and
foremost, amputee rehabilitation using OI surgery is a small but
evolving field, with a limited amount of clinical evidence that has
only built up in the last two decades. The lack of standardisation
in this field regarding patient characteristics, surgical
protocols, implant selection, outcome measures, follow-up period
and other study characteristics introduces significant
heterogeneity among available studies, making it difficult to
perform meaningful comprehensive and comparative analyses or draw
conclusions on current outcomes without overinterpreting the data.
In the present review, the very restricted number of six studies
that fit the selection criteria meant that it was not possible to
perform a comprehensive analysis in the form of a systematic review
or meta-analysis, or to further categorise study characteristics or
outcomes, e.g. by anatomical location of the implant surgery, type
of surgical protocol or implant type used. However, this first
review on assessing BMD in OI surgery may serve as a starting point
and a call for future in-depth studies, e.g. using a retrospective
case-control design, larger sample sizes, longer follow-up and
incorporation of standardised BMD measurements. Furthermore, the
present study did not analyse the supplementary results presented
on BMD measurements for implant surgery in patients with THA and
TKA in detail. It was outside the intention of this review to
discuss findings from THA and TKA studies, as the implants used
were to replace joints rather than to reconstruct lower limb
amputations. The implant materials, design, surgical placement and
outcome measures were completely different from OI in amputees and
hence not directly comparable to the six studies selected for the
present review. In addition, the vast majority of patients with THA
and TKA received the indication for osteoarthritis or other
degenerative joint disorders, while amputations are mostly
indicated due to traumatic injuries. These diverging patient
characteristics also make meaningful comparisons challenging.
Hence, only the THA and TKA studies were used to supplement our
discussion on measuring BMD for amputees rehabilitated with OI and
to strengthen the call for a more standardised BMD measurement
protocol. Secondly, the majority of the included studies were
conducted in Europe involving a predominantly Caucasian population,
which may not reflect the outcomes in other demographic populations
and ethnic groups. Furthermore, the studies mostly reported
outcomes in trans-femoral amputees despite the increasing
prevalence of OI in trans-tibial amputees. From the results of a
single available study (19), the
BMD-related outcomes are not generalisable across these two types
of patients. Finally, the present review did not separately analyse
patients who underwent OI surgery through the single or two-stage
surgical procedure, since the majority of existing studies used the
conventional two-stage procedure. Among various two-stage protocols
for limb reconstruction and rehabilitation using OI, a period of
4-18 months is required from the time of the initial surgery
(50). A surgery is first performed
to insert the implant, and a second surgery is then performed
several months later to create a percutaneous skin opening, which
then allows prosthesis fitting. The single-stage OI procedure has
evolved since 2014, predominantly being performed in Australia and
combining the two-stage procedure into a single surgery, thereby
shortening the recovery time to 3-6 weeks (50). Due to the greatly reduced recovery
time, as well as savings on cost and healthcare resources by
removing multiple surgeries, single-stage OI has the potential to
gain greater popularity in the coming years. It should be noted,
however, that although the single-stage study protocol (50) produced pilot study data in 10
patients indicating improvements in quality of life and functional
outcomes compared to pre-operative values, the study findings for
single-stage OI have not yet been published and outcome comparisons
with two-stage surgery are not available. Whether the difference in
OI surgical protocol may impact post-operative changes in BMD
remains to be investigated in future studies.
9. Conclusion
The development of OI technology has demonstrated
great potential in improving patient quality of life and functional
outcomes, which is becoming a more preferred choice in the
rehabilitation of lower limb amputees for returning to normal
activities. BMD changes are currently not commonly measured in this
population, but have potential to provide additional information on
the progress of bone remodelling following OI placement. The
limited available evidence suggests that OI may help restore a
healthy BMD in lower limb amputees, although post-operative BMD
changes were not strongly correlated with the success of OI surgery
or periprosthetic fracture risk. Significant variability was
observed among the results of studies analysed in this review,
calling for future investigations with larger sample sizes and
longer follow-up times, as well as the development of a
standardised protocol for measuring BMD in patients with OI.
Supplementary Material
Search strategies used to retrieve
relevant studies.
Summary of studies reporting
post-implantation BMD measurements in THA and TKA.
Risk of bias assessment of included
studies.
Acknowledgements
Not applicable.
Funding
Funding: The authors acknowledge funding from the National
Health and Medical Research Council (grant no. GNT1120249).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author
Authors' contributions
Study design: JJL and DX; acquisition, analysis and
interpretation of data: JCC, SPP, AG, CS, AMK, EMC, DX and JJL;
manuscript drafting/critical revision: JCC, SPP, AG, CS, AMK, EMC,
XL, AO, WL, MAM, JJL and DX. All authors have read and approved the
final manuscript for submission. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Buser D, Sennerby L and De Bruyn H: Modern
implant dentistry based on osseointegration: 50 years of progress,
current trends and open questions. Periodontol. 73:7–21.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hagberg K, Häggström E, Uden M and
Brånemark R: Socket versus bone-anchored trans-femoral prostheses:
Hip range of motion and sitting comfort. Prosthet Orthot Int.
29:153–163. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Frossard L, Hagberg K, Häggström E, Gow
DL, Brånemark R and Pearcy M: Functional outcome of transfemoral
amputees fitted with an osseointegrated fixation: Temporal gait
characteristics. J Prosthet Orthot. 22:11–20. 2010.
|
|
4
|
Hagberg K, Hansson E and Brånemark R:
Outcome of percutaneous osseointegrated prostheses for patients
with unilateral transfemoral amputation at two-year follow-up. Arch
Phys Med Rehabil. 95:2120–2127. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hoellwarth JS, Tetsworth K, Rozbruch SR,
Handal MB, Coughlan A and Al Muderis M: Osseointegration for
amputees: Current implants, techniques, and future directions. JBJS
Rev. 8(e0043)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hebert JS, Rehani M and Stiegelmar R:
Osseointegration for lower-limb amputation: A systematic review of
clinical outcomes. JBJS Rev. 5(e10)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dillon MP, Kohler F and Peeva V: Incidence
of lower limb amputation in Australian hospitals from 2000 to 2010.
Prosthet Orthot Int. 38:122–132. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dudek NL, Marks MB, Marshall SC and
Chardon JP: Dermatologic conditions associated with use of a
lower-extremity prosthesis. Arch Phys Med Rehabil. 86:659–663.
2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hagberg K and Brånemark R: Consequences of
non-vascular trans-femoral amputation: A survey of quality of life,
prosthetic use and problems. Prosthet Orthot Int. 25:186–194.
2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Legro MW, Reiber G, del Aguila M, Ajax MJ,
Boone DA, Larsen JA, Smith DG and Sangeorzan B: Issues of
importance reported by persons with lower limb amputations and
prostheses. J Rehabil Res Dev. 36:155–163. 1999.PubMed/NCBI
|
|
11
|
Van de Meent H, Hopman MT and Frölke JP:
Walking ability and quality of life in subjects with transfemoral
amputation: A comparison of osseointegration with socket
prostheses. Arch Phys Med Rehabil. 94:2174–2178. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Thesleff A, Brånemark R, Håkansson B and
Ortiz-Catalan M: Biomechanical characterisation of bone-anchored
implant systems for amputation limb prostheses: A systematic
review. Ann Biomed Eng. 46:377–391. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Marshall D, Johnell O and Wedel H:
Meta-analysis of how well measures of bone mineral density predict
occurrence of osteoporotic fractures. BMJ. 312:1254–1259.
1996.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu XS, Cohen A, Shane E, Yin PT, Stein
EM, Rogers H, Kokolus SL, McMahon DJ, Lappe JM, Recker RR, et al:
Bone density, geometry, microstructure, and stiffness:
Relationships between peripheral and central skeletal sites
assessed by DXA, HR-pQCT, and cQCT in premenopausal women. J Bone
Miner Res. 25:2229–2238. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Cummings SR, Palermo L, Browner W, Marcus
R, Wallace R, Pearson J, Blackwell T, Eckert S and Black D:
Monitoring osteoporosis therapy with bone densitometry: Misleading
changes and regression to the mean. JAMA. 283:1318–1321.
2000.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kelley GA, Kelley KS and Tran ZV: Exercise
and bone mineral density in men: A meta-analysis. J Appl Physiol
(1985). 88:1730–1736. 2000.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cummings SR, Black DM, Nevitt MC, Browner
W, Genant H, Cauley J, Ensrud K, Palermo L, Scott J and Vogt TM:
Bone density at various sites for prediction of hip fractures. The
study of osteoporotic fractures research group. Lancet. 341:72–75.
1993.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Faulkner KG: The tale of the T-score:
Review and perspective. Osteoporos Int. 16:347–352. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Thomson S, Lu W, Zreiqat H, Li JJ,
Tetsworth K and Al Muderis M: Proximal bone remodeling in lower
limb amputees reconstructed with an osseointegrated prosthesis. J
Orthop Res. 37:2524–2530. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Marques EA, Mota J and Carvalho J:
Exercise effects on bone mineral density in older adults: A
meta-analysis of randomized controlled trials. Age (Dordr).
34:1493–1515. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Haket LM, Frölke JPM, Verdonschot N,
Tomaszewski PK and van de Meent H: Periprosthetic cortical bone
remodeling in patients with an osseointegrated leg prosthesis. J
Orthop Res. 35:1237–1241. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Al Muderis MM, Lu WY, Li JJ, Kaufman K,
Orendurff M, Highsmith MJ, Lunseth PA and Kahle JT: Clinically
relevant outcome measures following limb osseointegration;
systematic review of the literature. J Orthop Trauma. 32:e64–e75.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gerzina C, Potter E, Haleem AM and Dabash
S: The future of the amputees with osseointegration: A systematic
review of literature. J Clin Orthop Trauma. 11:S142–S148.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kunutsor SK, Gillatt D and Blom AW:
Systematic review of the safety and efficacy of osseointegration
prosthesis after limb amputation. Br J Surg. 105:1731–1741.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sterne JAC, Hernán MA, Reeves BC, Savović
J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT,
Boutron I, et al: ROBINS-I: A tool for assessing risk of bias in
non-randomised studies of interventions. BMJ.
355(i4919)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hansen RL, Langdahl BL, Jørgensen PH,
Petersen KK, Søballe K and Stilling M: Bone mineral density
measurements around osseointegrated implants: A precision study and
validation of scan protocol for transfemoral amputees. J Clin
Densitom. 21:244–251. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sinclair S, Beck JP, Webster J, Agarwal J,
Gillespie B, Stevens P, Gililland J and Kubiak E: The first FDA
approved early feasibility study of a novel percutaneous bone
anchored prosthesis for transfemoral amputees: A prospective 1-year
follow-up cohort study. Arch Phys Med Rehabil. 103:2092–2104.
2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hansen RL, Langdahl BL, Jørgensen PH,
Petersen KK, Søballe K and Stilling M: Changes in periprosthetic
bone mineral density and bone turnover markers after
osseointegrated implant surgery: A cohort study of 20 transfemoral
amputees with 30-month follow-up. Prosthet Orthot Int. 43:508–518.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Thomson S, Thomson A, Tetsworth K, Lu W,
Zreiqat H and Al Muderis M: Radiographic evaluation of bone
remodeling around osseointegration implants among transfemoral
amputees. J Orthop Trauma. 33:e303–e308. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Boller S, Jahnke A, Augustin L, Ahmed G,
Rickert M and Ishaque BA: Age-related osseointegration of a short
hip stem: A clinical and radiological 24 months follow-up. Arch
Orthop Trauma Surg. 139:405–410. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Brinkmann V, Radetzki F, Delank KS,
Wohlrab D and Zeh A: A prospective randomized radiographic and
dual-energy X-ray absorptiometric study of migration and bone
remodeling after implantation of two modern short-stemmed femoral
prostheses. J Orthop Traumatol. 16:237–243. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gasbarra E, Iundusi R, Perrone FL,
Saturnino L and Tarantino U: Densitometric evaluation of bone
remodelling around Trabecular metal primary stem: A 24-month
follow-up. Aging Clin Exp Res. 27:S69–S75. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Leichtle UG, Leichtle CI, Schmidt B and
Martini F: Peri-prosthetic bone density after implantation of a
custom-made femoral component: A five-year follow-up. J Bone Joint
Surg Br. 88:467–471. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Massari L, Bistolfi A, Grillo PP, Borré A,
Gigliofiorito G, Pari C, Francescotto A, Tosco P, Deledda D, Ravera
L and Causero A: Periacetabular bone densitometry after total hip
arthroplasty with highly porous titanium cups: A 2-year follow-up
prospective study. Hip Int. 27:551–557. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Samy AM and El-Tantawy A: Stem length in
primary cementless total hip arthroplasty: Does it make a
difference in bone remodeling? Eur J Orthop Surg Traumatol.
29:1235–1242. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bedigrew KM, Ruh EL, Zhang Q, Clohisy JC,
Barrack RL and Nunley RM: 2011 Marshall Urist young investigator
award: When to release patients to high-impact activities after hip
resurfacing. Clin Orthop Relat Res. 470:299–306. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Stukenborg-Colsman CM, von der Haar-Tran
A, Windhagen H, Bouguecha A, Wefstaedt P and Lerch M: Bone
remodelling around a cementless straight THA stem: A prospective
dual-energy X-ray absorptiometry study. Hip Int. 22:166–171.
2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Augustin L, Boller S, Bobach C, Jahnke A,
Ahmed GA, Rickert M and Ishaque BA: Development of periprosthetic
bone mass density around the cementless Metha® short hip
stem during three year follow up-a prospective radiological and
clinical study. Int Orthop. 43:2031–2037. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hayashi S, Kuroda Y, Nakano N, Matsumoto
T, Kamenaga T, Maeda T, Niikura T and Kuroda R: Peri-prosthetic
bone remodeling of hydroxyapatite-coated compaction short stem was
not affected by stem alignment. J Orthop Surg Res.
17(131)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Gasbarra E, Celi M, Perrone FL, Iundusi R,
Di Primio L, Guglielmi G and Tarantino U: Osseointegration of
Fitmore stem in total hip arthroplasty. J Clin Densitom.
17:307–313. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lerch M, Kurtz A, Windhagen H, Bouguecha
A, Behrens BA, Wefstaedt P and Stukenborg-Colsman CM: The
cementless Bicontact® stem in a prospective dual-energy
X-ray absorptiometry study. Int Orthop. 36:2211–2217.
2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Cucchi D, Menon A, Galliera E, Messina C,
Zanini B, Marazzi MG, Massaccesi L, Compagnoni R, Romanelli MMC and
Randelli P: A prospective assessment of periprosthetic bone mineral
density and osteoimmunological biomarkers variations after total
knee replacement surgery. J Clin Densitom. 22:86–95.
2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lacko M, Filip V, Gharaibeh A, Lackova A,
Folvarsky M and Zamborsky R: Comparison of bone remodelling around
short stem and conventional straight stem in total hip replacement:
A prospective randomized radiographic and dual-energy X-ray
absorptiometric study. Bratisl Lek Listy. 122:548–554.
2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Aro E, Moritz N, Mattila K and Aro HT: A
long-lasting bisphosphonate partially protects periprosthetic bone,
but does not enhance initial stability of uncemented femoral stems:
A randomized placebo-controlled trial of women undergoing total hip
arthroplasty. J Biomech. 75:35–45. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Linde KN, Rytter S, Søballe K, Madsen F,
Langdahl B and Stilling M: Component migration, bone mineral
density changes, and bone turnover markers in cementless and
cemented total knee arthroplasty: A prospective randomized RSA
study in 53 patients with 2-year follow-up. Knee Surg Sports
Traumatol Arthrosc. 30:3100–3113. 2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Prochor P and Sajewicz E: The influence of
geometry of implants for direct skeletal attachment of limb
prosthesis on rehabilitation program and stress-shielding
intensity. Biomed Res Int. 2019(6067952)2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Tomaszewski PK, Verdonschot N, Bulstra SK,
Rietman JS and Verkerke GJ: Simulated bone remodeling around two
types of osseointegrated implants for direct fixation of upper-leg
prostheses. J Mech Behav Biomed Mater. 15:167–175. 2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sherk VD, Bemben MG and Bemben DA: BMD and
bone geometry in transtibial and transfemoral amputees. J Bone
Miner Res. 23:1449–1457. 2008.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Al Muderis M, Lu W and Li JJ:
Osseointegrated prosthetic limb for the treatment of lower limb
amputations: Experience and outcomes. Unfallchirurg. 120:306–311.
2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Al Muderis M, Lu W, Tetsworth K, Bosley B
and Li JJ: Single-stage osseointegrated reconstruction and
rehabilitation of lower limb amputees: The Osseointegration Group
of Australia accelerated protocol-2 (OGAAP-2) for a prospective
cohort study. BMJ Open. 7(e013508)2017.PubMed/NCBI View Article : Google Scholar
|