1. Introduction
Acute pancreatitis (AP) is a potentially fatal
inflammatory disease, which, in its severe form, is associated with
a mortality rate of 15-25% and often caused by pancreatic enzyme
activation from various etiologies and is characterized by local
inflammatory reactions in the pancreas (1-4).
The etiology of AP includes mainly gallstones and excessive alcohol
consumption (5-7).
Patients with more severe conditions can develop systemic
inflammatory response syndrome (SIRS), which may be accompanied by
organ dysfunction (6-8).
Clinically, it is characterized by acute upper abdominal pain and
elevated levels of blood amylase or lipase (6,7). AP
has a rapid onset and changes rapidly. When accompanied by multiple
organ dysfunction and local complications of the pancreas, the
mortality rate significantly increases (9,10). At
present, the pathogenesis of AP is not very clear.
In the early stages of AP, various stimulating
factors cause premature activation of trypsin in pancreatic acinar
cells (1,11). This process involves various
pathogenesis mechanisms, such as pathological calcium signaling in
pancreatic acinar cells, changes in pH, coexistence and autophagy,
the cleavage of trypsinogen by the lysosomal hydrolytic enzyme
tissue protease B to trypsin, and decreased activity of the
pancreatic acinar cell trypsin inhibitor (11-13).
Once trypsin is activated, various damaging pancreatic digestive
enzymes become active, and the pancreas and adjacent tissues
undergo self-digestion, causing local inflammation in the pancreas
and the secretion of a large amount of TNF-α. Inflammatory
cytokines can cause necrosis of pancreatic acinar cells and enter
the bloodstream, promoting the secretion of inflammatory cytokines
such as IL-1, IL-6 and IL-8, resulting in a waterfall effect
(9,14,15).
The activation of endoplasmic reticulum stress, the unfolded
protein response, autophagy, oxidative stress, lysosomal and
mitochondrial dysfunction, and signaling pathways are all
mechanisms underlying the pathogenesis of AP (10,16-19).
Oxidative stress is a stress state that causes an
imbalance between oxidative and antioxidative effects in the body,
leading to inflammatory infiltration of neutrophils, increased
protease secretion and the production of numerous oxidative
intermediates, causing oxidative damage and interfering with the
metabolic activities of normal organs (18,20,21).
There are two types of antioxidant systems in the body. One is the
enzyme antioxidant system, which includes superoxide dismutase,
catalase and glutathione peroxidase (22,23).
The other is the nonenzymatic antioxidant system, which includes
ertenionine, vitamin C, vitamin E, glutathione and melatonin
(24,25). When human and animal cells are
stimulated by nitrogen and nitrogen compounds, as well as calcium
and pathogens, the balance between oxidation and antioxidant
systems is disrupted, thus promoting the increased production and
accumulation of reactive oxygen species (ROS) in the cell and
eventually leading to an oxidative stress response (20,26).
The redox balance of body cells determines the longevity of cells.
In the past few decades, numerous studies have emphasized the role
of oxidative stress in the acute inflammatory response (27-29).
Currently, oxidative stress is considered not only a key mediator
associated with early local events of AP but also a key mediator
associated with systemic inflammatory response syndrome (20,30).
Oxidative stress is involved in the occurrence and development of
pancreatitis. The different stages and concentrations of ROS have
different effects. Relevant reports suggest that ROS are beneficial
for cell apoptosis during the acute phase, while reducing necrosis
and preventing severe pancreatic damage (18,20,24).
ROS appear to protect acinar cells, but high concentrations can
lead to pancreatic damage. The present review examines the role of
oxidative stress in the development of AP.
2. Reactive oxygen species
ROS are free radicals containing oxygen atoms,
including superoxide anions, hydroxyl radicals, hydrogen peroxide,
hypochlorous acid and singlet oxygen (18,31).
They have complex signaling functions and play important roles in
the development of inflammatory diseases. Oxidative stress occurs
when there is an imbalance between the antioxidant defense system
and the production of ROS (32).
ROS can quickly combine with nitric oxide (NO) to form reactive
nitrogen species (RNS), decreasing the endogenous antioxidant
protection ability of the body and participating in the occurrence
and development of AP (20,21).
Biofilms contain polyunsaturated fatty acids, which
are highly susceptible to ROS attack and undergo lipid
peroxidation. Lipid peroxidation is a chain reaction process of
unsaturated fatty acid oxidation degradation that has three stages:
Initiation, extension, and termination (33). The extension stage of lipid
peroxidation produces various free radicals, such as lipid
peroxidation free radicals, lipid oxygen free radicals and lipid
free radicals (34). The
termination stage involves the production of various small molecule
products, such as malondialdehyde (MDA), which can cause damage to
various cellular functions, which is closely related to the
occurrence and development of various diseases and is an important
marker of the early severity of disease (35,36).
It is well known that ROS serve numerous important biological
functions, including the regulation of redox-sensitive
transcription factors, redox-sensitive signal transduction pathways
and direct interactions with various molecules (18,20).
The large amount of ROS produced by oxidative stress can cause cell
necrosis or apoptosis through different pathways. At present, it
has been confirmed that the oxidative stress response is related to
various diseases, and there is notable research in cardiology,
neurology and endocrinology, as well as in other fields (37). Vasopressin can protect the
myocardium by antioxidation and inhibition of mitochondrial
permeability, reducing myocardial ischemia-reperfusion injury
(38). Taurine can prevent and
treat vascular dysfunction by reducing the vascular oxidative
stress response (39). Vitamin E
has been proven to reduce oxidative stress responses in the liver
and kidneys, thereby playing a protective role (40). Mangiferin can inhibit nuclear factor
κB (NF-κB) and increase catalase activity to protect cells and has
therapeutic significance in clinical practice (41,42).
Unsaturated fatty acids can exert antioxidant effects on cell
membranes. Dietary flavonoids and their sources have a protective
effect on cardiovascular disease through antioxidant activity
(43). Some traditional Chinese
medicines have also been proven to have antioxidant stress response
effects. Studies have shown that Gegenqinlian decoction can
significantly increase the activity of superoxide dismutase and
reduce the activity of malondialdehyde, nitric oxide synthase
(NOS), tumor necrosis factor, interleukin, and inflammatory
cytokines in the colon, thereby regulating the balance between
oxidants and antioxidants and having a protective effect on
ulcerative colitis (44).
Schisandrin B has been revealed to activate the Nrf2/ARE pathway to
alleviate cisplatin-induced oxidative stress damage in renal cells
(31).
Research has shown that the large amount of ROS
produced by oxidative stress can cause cell necrosis or apoptosis
through different pathways: i) Mitochondrial dysfunction and
disruption of lipid and lysosomal membranes (10); ii) disruption of the intracellular
environment of the cytoplasm, causing intracellular Ca2+
overload (45); iii) increased
lipid peroxidation of the cell membrane caused by free radicals
which reduces membrane fluidity, and fluidity enhances
permeability, and increases extracellular calcium ion influx,
leading to cell death (46,47); iv) activation of the
apoptosis-related genes, Bax and p53, on the mitochondrial membrane
of pancreatic acinar cells, as well as the release of cytochrome
c (48); v) activation of
the oxidative stress-sensitive transcription nuclear factor factor
κB (NF-κB) (46); vi) activation of
the c-Jun amino terminal kinase (JNK) and p38 mitogen-activated
protein kinase (p38 MAPK) pathways (49); vii) activation of CD95 receptors and
in turn cell apoptosis; and viii) production of inflammatory
mediators (50). Changes in
signaling pathways are controlled by the level of the oxidative
stress response, and the degree of oxidation-reduction plays a more
important role in pathological and physiological processes than
does the accumulation of oxidative damage (30). In addition, changes in the oxidative
stress response and redox degree play different roles in
pathological and physiological processes involving different
cellular components, and their combined effect is stronger than any
individual effect. The oxidative stress response and redox
reactions can produce different physiological and pathological
outcomes in different organs, tissues and cells.
The antioxidant system includes in vivo
antioxidants such as vitamin C, selenium, and nicotinamide adenine
dinucleotide, as well as enzymes such as superoxide dismutase
(SOD), peroxidase, catalase, glutathione peroxidase (GPx) and
glutathione reductase, and antioxidant mechanisms such as cellular
autophagy (18). Research has shown
that GPx, glutathione and metallothionein play the first line of
defense against oxidative stress and antioxidant imbalance during
the AP process (51). Glutathione
and glutathione disulfide are in equilibrium, and the ratio between
the two is a reliable indicator of oxidative stress, as it reflects
the balance between antioxidant status and the pro-oxidant response
in cells. Mitochondria contain small molecule antioxidants such as
ascorbic acid, glutathione and vitamin E. Both ascorbic acid and
glutathione are actively transported to mitochondria, thereby
preventing oxidative damage to mitochondrial DNA (52). Melatonin is a product of the pineal
gland that is released from the intestinal mucosa in response to
food intake. Specific receptors for melatonin have been detected in
numerous gastrointestinal tissues, including the pancreas.
Melatonin and its precursor L-tryptophan can alleviate the severity
of AP and protect pancreatic gland tissue from damage caused by
acute inflammatory reactions (53).
Previous research has shown that nicotinamide adenine dinucleotide
phosphate oxidase inhibitors can significantly reduce AP-related
inflammation and oxidative stress parameters (54). Coenzyme Q10 is the only endogenous
lipid soluble antioxidant with favorable antioxidant and
anti-inflammatory effects (55). It
is the most common type of coenzyme Q in human tissues. Autophagy
is the main metabolic process in the body. Autophagy plays an
important role in clearing defective or harmful cytoplasm,
organelles, denatured proteins and lipids, and recycling their
components to meet energy and biological needs. Under stress,
autophagy plays a crucial role in clearing free radicals and
maintaining protective cell function (18,19).
3. Oxidative stress and acute
pancreatitis
AP is a sterile inflammation of the pancreas that
can initially progress from mild self-limiting local inflammation
to life-threatening severe AP (SAP) as the disease progresses
(22). A series of local and
systemic complications may occur, and in severe cases, multiple
organ dysfunction syndrome (MODS) may even occur (12). ROS act as both signaling molecules
and inflammatory mediators in the early stages of AP, playing
important roles in both local pancreatic injury and cellular damage
to extrapancreatic organs (16,39).
The acute inflammatory response begins with immune cells.
Neutrophils are the first batch of cells that adhere to endothelial
cells, and they begin to migrate through the vascular wall at the
site of infection to engulf invading pathogens. The ROS removal
system is impaired, and autophagy defects lead to an increase in
oxidative stress levels in mitochondria (10,25).
Oxidative stress directly affects biological molecules such as DNA,
proteins and lipids, regulating gene transcription and protein
expression (1). Once the stimulus
persists or overwhelms, it can lead to disease progression and
complications. Free radicals react with polyunsaturated fatty acids
in the cell membrane to produce intermediate products such as
malondialdehyde and 4-hydroxynonanal, leading to cell membrane
damage and cell death (56). ROS
are recognized as important causes of pancreatic cell death
(18). In pancreatitis-associated
MODS, ROS directly cause cellular damage and regulate
redox-sensitive transcription factors and redox-sensitive signaling
pathways (42). In 1995, Ward et
al proposed that disrupting the calcium balance of acinar cells
is the basis for the development of pancreatic injury. These
authors suggested that various pathogenic factors of AP exacerbate
the course of AP by causing excessive release of acinar calcium
ions or disrupting low resting levels of calcium ions. Continuous
calcium overload may activate degradable calpain, phospholipase or
other enzymes and destroy proenzyme particles, inducing autophagy
and/or lysosomal activation of digestive enzyme reactions (57). Cytoplasmic Ca2+ has been
described as a key regulator of the development of AP (45,47). A
large body of evidence suggests that Ca2+ signaling is
closely related to ROS (47). The
ROS produced by mitochondrial electron transport chains may
interact with targets involved in the dynamic balance of cellular
Ca2+, thereby altering their activity (45). For example, 2- and 3-inositol
1,4,5-triphosphate receptors and ryanodine receptors, which contain
multiple cysteine residues, are sensitive to ROS, indicating that
the regulatory effect of free radicals on Ca2+ release
channels may affect disease progression (45). There are numerous factors that
induce the formation of AP, such as caerulein over-stimulation,
bile salt, nonoxidative alcohol metabolites (FAEs) and fatty acids,
and these stimulating factors induce the formation of mitochondrial
membrane permeability transition pores (MPTPs), which are solute
channels regulated by cyclophilin D (CypD), and then lead to enzyme
activation, ATP depletion and cell necrosis. ROS play a crucial
role in the formation of the MPTP, which leads to cell necrosis by
altering the conformation and activity of CypD (10,45,58).
In addition, Armstrong et al reported that ROS alter
mitochondrial bioenergetics independently of CypD and alter the
mode of pancreatic cell death, leading to a transition from
apoptosis to necrosis (59). In
alcoholic AP, alcohol metabolism increases fatty acid ethyl esters
(FAEEs) through its nonoxidative metabolism and leads to
acetaldehyde, acetate and ROS through its oxidative metabolism.
FAEEs have various harmful effects, such as disrupting
Ca2+ homeostasis in acinar cells and activating
pathological proenzyme transcription factors. Acetaldehyde enhances
the function of the latter, leading to increased production of
proinflammatory cytokines (60).
Ethanol also increases inducible NOS (iNOS) production, which can
produce NO free radicals. The mechanism underlying the induction of
oxidative stress and ethanol-induced pancreatic injury is complex
and may involve oxidative stress (61). The interaction between endoplasmic
reticulum stress and inflammation leads to the harmful effect of
ethanol on the pancreas.
Increasing evidence suggests that the interaction
between oxidative stress and cytokines is associated with the
development of AP, leading to uncontrolled inflammatory cascade
amplification and MODS. Proinflammatory cytokines and oxidative
stress mainly trigger each other by activating mitogen-activated
protein kinases and NF-κB, leading to amplification of the
inflammatory cascade (42,44). In addition, proinflammatory
cytokines, especially TNF-α, interact with oxidative stress to form
a vicious cycle in AP. An increase in TNF-α accumulation promotes
the production of other inflammatory cytokines, including IL-1β and
IL-6. This leads to the activation of the inflammatory cascade,
resulting in damage to multiple tissues and organs. The levels of
IL-1β and IL-6 have been demonstarted to be correlated with the
severity of AP (27,30).
H2O2 promotes the apoptosis of
acinar cells at low concentrations, while higher concentrations
cause rapid necrosis of acinar cells (62). Booth et al reported
contradictory findings in clinical trials of antioxidants. The
production of ROS may constitute a protective mechanism for
handling stress in pancreatic acinar cells, as bile acid-induced
ROS increase cell apoptosis and reduce necrosis (63). Apoptosis can maintain the integrity
of the plasma membrane, while necrotic cells release their
components, thereby damaging neighboring cells and promoting
inflammation (62,63). ROS cannot only be considered as
playing a negative role, as they can also be beneficial.
Considering the role of ROS in pancreatic pathophysiology, it
should be recognized that there are multiple targets for ROS. A
more cautious approach should be undertaken when dealing with
ROS.
4. Antioxidant therapy for acute
pancreatitis
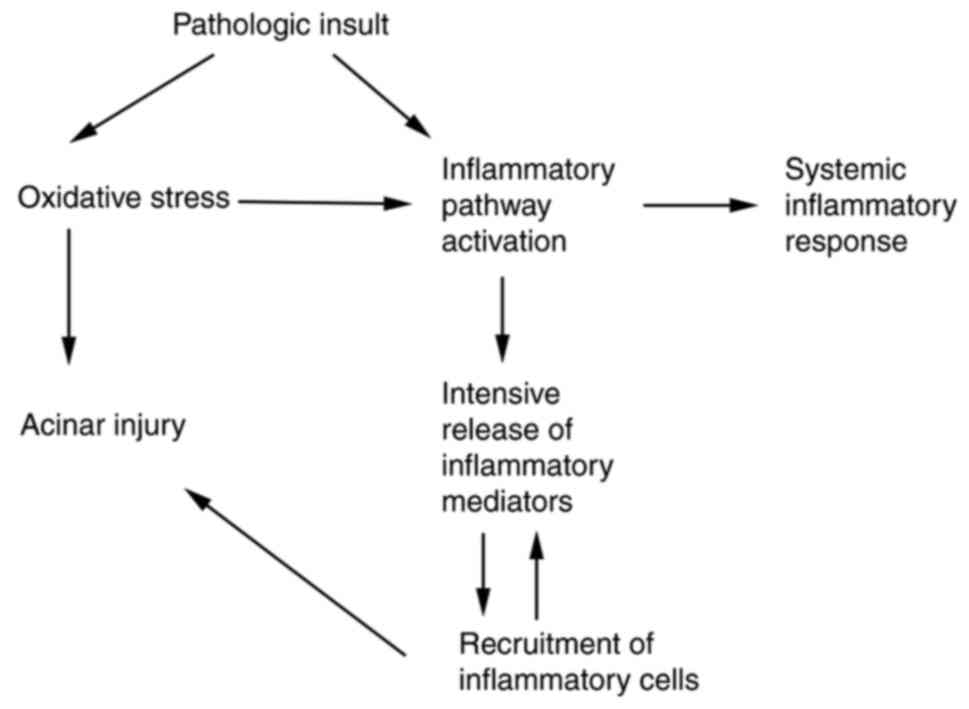
Due to the important role of oxidative stress in the
pathogenesis of AP (Fig. 1), a
number of studies in both humans and animal models have analyzed
the association between AP and oxidative metabolism (8,22). The
use of antioxidants in combination with conventional therapy may
improve organ and tissue damage caused by oxidative stress.
Treatment with antioxidant agents has been shown to reduce acinar
cell damage and edema in several animal models. The antioxidants
commonly used by the investigators are N-acetylcysteine (NAC)
(64), α-tocopherol (65), β-carotin serlabo (66), selenium (67,68),
melatonin (69,70), resveratrol, mitochondrial-targeted
antioxidants (71), pyrrolidine
dithiocarbamate (72), carnitine,
traditional Chinese medicine [carvacrol (73), total saponin of Panax
notoginseng (74)], and vitamin
C (75).
NAC reduces oxidative stress parameters, serum
amylase levels, and serum calcium and lactate dehydrogenase levels
and reduces histopathological scores in combination with hyperbaric
oxygen. The protective effects of NAC are, at least, partly due to
a decrease in the production of TNF-α by acinar cells, which is
concomitant with the inhibition of NF-κB(p65) nuclear translocation
(64). α-Tocopherol is a phenolic
antioxidant that can inhibit the autoxidation of lipids by
scavenging free radicals and reacting with singlet oxygen (65). In patients with mild AP, the
concentration of β-carotenoids was revealed to be significantly
greater than that in patients with SAP, as β-carotenoids have
antioxidant activities and are effective in protecting the human
body against various oxidative stress-related diseases.
β-carotenoids can increase the level of antioxidants in the body,
trapping ROS and reducing oxidative damage to important
biomolecules such as membrane lipids, enzymatic proteins and DNA,
thereby ameliorating oxidative stress (66). Currently, the role of the
micronutrient selenium is receiving increasing attention. The
selenium content in the toenails of patients with SAP was revealed
to be lower, and the selenium concentration in red blood cells was
also revealed to be lower (67,68).
In a rain frog hormone-induced AP model melatonin was demonstrated
to reduce inflammation levels by regulating NF-κB, confirming its
effective antioxidant and anti-inflammatory functions (69,70).
Mitochondrion-targeted antioxidants have recently been shown to
have cytoprotective effects, while the recently reported
mitochondrion-targeted antioxidant SkQ1 scavenges ROS at nanoscale
concentrations (71,76). The antioxidant pyrrolidine
dithiocarbamate may inhibit NF-κB activation, thereby blocking
TNF-α synthesis and thus indirectly inhibiting high mobility group
protein generation and reducing pancreatic tissue damage in rats
with SAP (72). The antioxidant
effect of traditional Chinese medicine has improved treatment
efficacy. In recent years, natural extracts or artificially
synthesized antioxidants have been widely explored, and monocyclic
aromatic hydrocarbons from carvacrol, which can regulate oxidative
stress and reduce pancreatic cell damage, have been shown to have
favorable antioxidant activity in SAP rat models. Carvacrol
potentially alleviates hyperuricemia-induced oxidative stress and
inflammation by regulating the ROS/NRLP3/NF-κB pathway, thereby
exerting protective effects against joint degeneration (77). By upregulating the expression level
of miR-181b, it can be concluded that the total saponins in
Panax notoginseng can significantly reduce
taurocholide-induced pancreatic injury and increase cell apoptosis,
indicating its extensive potential in the treatment of
taurocholide-induced SAP (74).
In recent years, the clinical application of vitamin
C in various diseases of the body has become increasingly
widespread. As the most important water-soluble antioxidant in the
body, vitamin C has shown favorable clinical efficacy for burns
(78), acute and chronic pain
(79), severe treatment, sepsis
(80), ischemia-reperfusion injury
(81), blood pressure control
(82), emotional improvement in
hospitalized patients (83), and
acute kidney disease and for reducing the mortality rate of
critically ill patients (84). Du
et al observed the clinical efficacy of high-dose vitamin C
(10 g/d, daily intravenous infusion) in patients with AP. The
results showed that vitamin C improved cellular antioxidant and
immune abilities and exerted a favorable therapeutic effect on AP
(75). Currently, clinical research
on antioxidant therapy for AP mainly involves the combined
application of multiple antioxidants. However, there are relatively
few clinical and laboratory studies on the use of high-dose vitamin
C alone for antioxidant therapy. The clinical benefits of combining
vitamin C antioxidant therapy in patients with acute inflammation,
traumatic stress and critical illness, warrant further research on
the basic and clinical aspects of high-dose (>5 g/d) vitamin C
participation in AP antioxidant therapy.
Clinical studies have shown that the consumption of
serum antioxidants during the course of AP is positively correlated
with the severity of the condition (85). However, there is still controversy
over whether antioxidant therapy should be supplemented during SAP
treatment. In an L-arginine-induced rat AP model, Handharm et
al used a combination of intravenous infusion of NAC, selenium
and vitamin C for antioxidant therapy. Early active antioxidant
therapy can significantly reduce damage to the pancreas and
extrapancreatic organs (86).
However, the clinical study of Siriwardena et al, which
included 43 patients with SAP, and where antioxidant therapy was
administered within 7 days after patient admission (combined use of
NAC, selenium and vitamin C) indicated that patients did not have
any clinical benefits. Thus, it appears that the effectiveness and
rationality of antioxidant use as adjunctive therapy for SAP have
been called into question (87).
5. Conclusion
Oxidative stress disrupts the activation of cellular
Ca2+ and the excessive release of inflammatory
mediators. As the disease progresses, various factors accelerate
the production of ROS, further disrupting the balance between the
oxidative and antioxidant systems and forming a vicious cycle. This
promotes the occurrence of oxidative stress at various stages of
AP. In recent years, the role of antioxidant adjuvant therapy in
the comprehensive treatment of AP and other diseases has gradually
attracted the attention of clinical physicians, but related
research remains in its early stages. A large amount of clinical
and basic research still needs to be carried out. Future studies
are required to clarify the link between the different
concentrations of antioxidants and the severity of AP. In addition,
the dynamic changes of oxidative stress in the course of AP can be
eludicated by monitoring the changes in indicators such as free
radical content and antioxidant enzyme activity in the body of a
patient, thereby providing a basis for evaluating the condition and
adjusting treatment plans. In summary, the relationship between
oxidative stress and AP is a challenging and promising research
field. Through continuous in-depth research and exploration, more
precise and effective strategies for the treatment and prevention
of AP may be provided, bringing better quality of life and health
well-being to patients.
A limitation in the present review, is that only the
relationship between oxidative stress and AP was explored.
Therefore, the relationship between chronic pancreatitis and
oxidative stress should be investigated in the future.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
YC, FY and XH contributed equally to the
acquisition, analysis and systematization of the data, manuscript
writing and critical revision of the manuscript for important
intellectual content. All the authors have read and approved the
final version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mayerle J, Sendler M, Hegyi E, Beyer G,
Lerch MM and Sahin-Toth M: Genetics, cell biology, and
pathophysiology of pancreatitis. Gastroenterology. 156:1951–1968
e1. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Patel HR, Diaz Almanzar VM, LaComb JF, Ju
J and Bialkowska AB: The role of MicroRNAs in pancreatitis
development and progression. Int J Mol Sci. 24(1057)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fu X, Xiu Z and Xu H: Interleukin-22 and
acute pancreatitis: A review. Medicine (Baltimore).
102(e35695)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cai Y, Shen Y, Gao L, Chen M, Xiao M,
Huang Z and Zhang D: Karyopherin Alpha 2 Promotes the Inflammatory
Response in Rat Pancreatic Acinar Cells Via Facilitating NF-ĸB
Activation. Dig Dis Sci. 61:747–757. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Qiu M, Zhou X, Zippi M, Goyal H, Basharat
Z, Jagielski M and Hong W: Comprehensive review on the pathogenesis
of hypertriglyceridaemia-associated acute pancreatitis. Ann Med.
55(2265939)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zerem E, Kurtcehajic A, Kunosic S, Zerem
Malkocevic D and Zerem O: Current trends in acute pancreatitis:
Diagnostic and therapeutic challenges. World J Gastroenterol.
29:2747–2763. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hong W, Pan J, Goyal H and Zippi M:
Editorial: Acute pancreatitis infection: Epidemiology, prevention,
clinical characteristics, treatment, and prediction. Front Cell
Infect Microbiol. 13(1175195)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Saluja A, Dudeja V, Dawra R and Sah RP:
Early intra-acinar events in pathogenesis of pancreatitis.
Gastroenterology. 156:1979–1993. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Qu C, Wei M and Li WQ: Extrapancreatic and
pancreatic infection in acute pancreatitis. Eur J Gastroenterol
Hepatol. 33:598–599. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hu Z, Wang D, Gong J, Li Y, Ma Z, Luo T,
Jia X, Shi Y and Song Z: MSCs Deliver hypoxia-treated mitochondria
reprogramming acinar metabolism to alleviate severe acute
pancreatitis injury. Adv Sci (Weinh). 10(e2207691)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wiley MB, Mehrotra K, Bauer J, Yazici C,
Bialkowska AB and Jung B: Acute Pancreatitis: Current clinical
approaches, molecular pathophysiology, and potential therapeutics.
Pancreas. 52:e335–e343. 2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ge P, Luo Y, Okoye CS and Chen H, Liu J,
Zhang G, Xu C and Chen H: Intestinal barrier damage, systemic
inflammatory response syndrome, and acute lung injury: A
troublesome trio for acute pancreatitis. Biomed Pharmacother.
132(110770)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Scurt FG, Bose K, Canbay A, Mertens PR and
Chatzikyrkou C: Acute kidney injury following acute pancreatitis
(AP-AKI): Definition, Pathophysiology, Diagnosis and Therapy. Z
Gastroenterol. 58:1241–1266. 2020.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
14
|
Rodriguez-Nicolas A, Jimenez P, Carmona
FD, Martín J, Matas Cobos AM, Ruiz-Cabello F and Redondo-Cerezo E:
Association between Genetic polymorphisms of inflammatory response
genes and acute pancreatitis. Immunol Invest. 48:585–596.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Komara NL, Paragomi P, Greer PJ, Wilson
AS, Breze C, Papachristou GI and Whitcomb DC: Severe acute
pancreatitis: Capillary permeability model linking systemic
inflammation to multiorgan failure. Am J Physiol Gastrointest Liver
Physiol. 319:G573–G583. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lee PJ and Papachristou GI: New insights
into acute pancreatitis. Nat Rev Gastroenterol Hepatol. 16:479–496.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Han X, Li B, Bao J, Wu Z, Chen C, Ni J,
Shen J, Song P, Peng Q, Wan R, et al: Endoplasmic reticulum stress
promoted acinar cell necroptosis in acute pancreatitis through
cathepsinB-mediated AP-1 activation. Front Immunol.
13(968639)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kong L, Deng J, Zhou X, Cai B, Zhang B,
Chen X, Chen Z and Wang W: Sitagliptin activates the p62-Keap1-Nrf2
signalling pathway to alleviate oxidative stress and excessive
autophagy in severe acute pancreatitis-related acute lung injury.
Cell Death Dis. 12(928)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang T, Gan Y and Zhu S: Association
between autophagy and acute pancreatitis. Front Genet.
14(998035)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Padureanu V, Florescu DN, Padureanu R,
Ghenea AE, Gheonea DI and Oancea CN: Role of antioxidants and
oxidative stress in the evolution of acute pancreatitis (Review).
Exp Ther Med. 23(197)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang J, Sha X, Wu D, Wu B, Pan X, Pan LL,
Gu Y and Dong X: Formononetin alleviates acute pancreatitis by
reducing oxidative stress and modulating intestinal barrier. Chin
Med. 18(78)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Burzynski J, Fichna J and Tarasiuk A:
Putative molecular targets for vitamin A in neutralizing oxidative
stress in acute and chronic pancreatitis-a systematic review.
Naunyn Schmiedebergs Arch Pharmacol. 396:1361–1370. 2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
He J, Ma M, Li D, Wang K, Wang Q, Li Q, He
H, Zhou Y, Li Q, Hou X and Yang L: Sulfiredoxin-1 attenuates injury
and inflammation in acute pancreatitis through the ROS/ER
stress/Cathepsin B axis. Cell Death Dis. 12(626)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang D, Li L, Li J, Wei Y, Tang J, Man X
and Liu F: Colchicine improves severe acute pancreatitis-induced
acute lung injury by suppressing inflammation, apoptosis and
oxidative stress in rats. Biomed Pharmacother.
153(113461)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yuan J, Wei Z, Xin G, Liu X, Zhou Z, Zhang
Y, Yu X, Wan C, Chen Q, Zhao W, et al: Vitamin B(12) attenuates
acute pancreatitis by suppressing oxidative stress and improving
mitochondria dysfunction via CBS/SIRT1 pathway. Oxid Med Cell
Longev. 2021(7936316)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jin H, Zhao K, Li J, Xu Z, Liao S and Sun
S: Matrine alleviates oxidative stress and ferroptosis in severe
acute pancreatitis-induced acute lung injury by activating the
UCP2/SIRT3/PGC1α pathway. Int Immunopharmacol.
117(109981)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bansod S, Chilvery S, Saifi MA, Das TJ,
Tag H and Godugu C: Borneol protects against cerulein-induced
oxidative stress and inflammation in acute pancreatitis mice model.
Environ Toxicol. 36:530–539. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Neri M, Fineschi V, Di Paolo M, Pomara C,
Riezzo I, Turillazzi E and Cerretani D: Cardiac oxidative stress
and inflammatory cytokines response after myocardial infarction.
Curr Vasc Pharmacol. 13:26–36. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pu X, Li F, Lin X, Wang R and Chen Z:
Oxidative stress and expression of inflammatory factors in lung
tissue of acute mountain sickness rats. Mol Med Rep.
25(49)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
El Morsy EM and Ahmed MAE: Carvedilol
attenuates l-arginine induced acute pancreatitis in rats through
modulation of oxidative stress and inflammatory mediators. Chem
Biol Interact. 327(109181)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kojayan GG, Alizadeh RF, Li S and Ichii H:
Reducing pancreatic fibrosis using antioxidant therapy targeting
Nrf2 Antioxidant Pathway: A possible treatment for chronic
pancreatitis. Pancreas. 48:1259–1262. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Choi J, Oh TG, Jung HW, Park KY, Shin H,
Jo T, Kang DS, Chanda D, Hong S, Kim J, et al: Estrogen-Related
Receptor ү maintains pancreatic acinar cell function and identity
by regulating cellular metabolism. Gastroenterology. 163:239–256.
2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ma D, Jiang P, Jiang Y, Li H and Zhang D:
Effects of Lipid Peroxidation-mediated ferroptosis on severe acute
pancreatitis-induced intestinal barrier injury and bacterial
translocation. Oxid Med Cell Longev. 2021(6644576)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu K, Liu J, Zou B, Li C, Zeh HJ, Kang R,
Kroemer G, Huang J and Tang D: Trypsin-Mediated sensitization to
ferroptosis increases the severity of pancreatitis in mice. Cell
Mol Gastroenterol Hepatol. 13:483–500. 2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Abdelzaher WY, Ahmed SM, Welson NN,
Marraiki N, Batiha GE and Kamel MY: Vinpocetine ameliorates
L-arginine induced acute pancreatitis via Sirt1/Nrf2/TNF pathway
and inhibition of oxidative stress, inflammation, and apoptosis.
Biomed Pharmacother 2021: 133: 110976.
|
|
36
|
Liang X, Hu C, Liu C, Yu K, Zhang J and
Jia Y: Dihydrokaempferol (DHK) ameliorates severe acute
pancreatitis (SAP) via Keap1/Nrf2 pathway. Life Sci.
261(118340)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Forman HJ and Zhang H: Targeting oxidative
stress in disease: Promise and limitations of antioxidant therapy.
Nat Rev Drug Discov. 20:689–709. 2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Nazari A, Mohamadi A, Imani AR, Faghihi M,
Tarahi MJ, Moghimian M and Cheraghi M: Effect of vasopressin on
electrocardiographic changes produced by ischemia-reperfusion in
rats. Pak J Pharm Sci. 34:1409–1414. 2021.PubMed/NCBI
|
|
39
|
Baliou S, Adamaki M, Ioannou P, Pappa A,
Panayiotidis MI, Spandidos DA, Christodoulou I, Kyriakopoulos AM
and Zoumpourlis V: Protective role of taurine against oxidative
stress (Review). Mol Med Rep. 24(605)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Amini L, Chekini R, Nateghi MR, Haghani H,
Jamialahmadi T, Sathyapalan T and Sahebkar A: The effect of
combined vitamin C and vitamin E supplementation on oxidative
stress markers in women with endometriosis: A Randomized,
triple-blind placebo-controlled clinical trial. Pain Res Manag.
2021(5529741)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Li W, Wang K, Liu Y, Wu H, He Y, Li C,
Wang Q, Su X, Yan S, Su W, et al: A novel drug combination of
mangiferin and cinnamic acid alleviates rheumatoid arthritis by
inhibiting TLR4/NFĸB/NLRP3 activation-induced pyroptosis. Front
Immunol. 13(912933)2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Feng M, Wei S, Zhang S and Yang Y:
Anti-Inflammation and anti-pyroptosis activities of mangiferin via
suppressing NF-ĸB/NLRP3/GSDMD signaling cascades. Int J Mol Sci.
23(10124)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Khan J, Deb PK, Priya S, Medina KD, Devi
R, Walode SG and Rudrapal M: Dietary Flavonoids: Cardioprotective
potential with antioxidant effects and their pharmacokinetic,
toxicological and therapeutic concerns. Molecules.
26(4021)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xu BL, Zhang GJ and Ji YB: Active
components alignment of Gegenqinlian decoction protects ulcerative
colitis by attenuating inflammatory and oxidative stress. J
Ethnopharmacol. 162:253–260. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Maleth J and Hegyi P: Ca2+ toxicity and
mitochondrial damage in acute pancreatitis: Translational overview.
Philos Trans R Soc Lond B Biol Sci. 371(20150425)2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Du W, Liu G, Shi N, Tang D, Ferdek PE,
Jakubowska MA, Liu S, Zhu X, Zhang J, Yao L, et al: A microRNA
checkpoint for Ca(2+) signaling and overload in acute pancreatitis.
Mol Ther. 30:1754–1774. 2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Gerasimenko JV, Gerasimenko OV and
Petersen OH: The role of Ca2+ in the pathophysiology of
pancreatitis. J Physiol. 592:269–280. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Nakamura Y, Do JH, Yuan J, Odinokova IV,
Mareninova O, Gukovskaya AS and Pandol SJ: Inflammatory cells
regulate p53 and caspases in acute pancreatitis. Am J Physiol
Gastrointest Liver Physiol. 298:G92–G100. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Cao MH, Xu J, Cai HD, Lv ZW, Feng YJ, Li
K, Chen CQ and Li YY: p38 MAPK inhibition alleviates experimental
acute pancreatitis in mice. Hepatobiliary Pancreat Dis Int.
14:101–106. 2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Pardalis V, Palli E, Lambropoulou M,
Tsigalou C, Anagnostoulis S, Garoufalis G, Bolanaki H, Simopoulos C
and Karayiannakis AJ: Expression of Fas (CD95/APO-1) and Fas ligand
(FasL) in experimentally-induced acute pancreatitis. J Invest Surg.
27:65–72. 2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Milnerowicz H, Bukowski R, Jablonowska M,
Sciskalska M and Milnerowicz U: The antioxidant profiles, lysosomal
and membrane enzymes activity in patients with acute pancreatitis.
Mediators Inflamm. 2014(376518)2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Gao L, Chong E, Pendharkar S, Phillips A,
Ke L, Li W and Windsor JA: The Challenges and effects of ascorbic
acid treatment of acute pancreatitis: A systematic review and
meta-analysis of preclinical and clinical studies. Front Nutr.
8(734558)2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zhao Q, Tang X, Huang J, Li J, Chen Q, Sun
Y and Wu J: Melatonin attenuates endoplasmic reticulum stress in
acute pancreatitis. Pancreas. 47:884–891. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Cao WL, Xiang XH, Chen K, Xu W and Xia SH:
Potential role of NADPH oxidase in pathogenesis of pancreatitis.
World J Gastrointest Pathophysiol. 5:169–177. 2014.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Shin JY, Choi JW, Kim DG, Zhou ZQ, Shin
YK, Seo JH, Song HJ, Choi BM, Bae GS and Park SJ: Protective
effects of Coenzyme Q10 against acute pancreatitis. Int
Immunopharmacol. 88(106900)2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Gukovsky I, Pandol SJ and Gukovskaya AS:
Organellar dysfunction in the pathogenesis of pancreatitis.
Antioxid Redox Signal. 15:2699–2710. 2011.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Ward JB, Petersen OH, Jenkins SA and
Sutton R: Is an elevated concentration of acinar cytosolic free
ionised calcium the trigger for acute pancreatitis? Lancet.
346:1016–1019. 1995.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Mukherjee R, Mareninova OA, Odinokova IV,
Huang W, Murphy J, Chvanov M, Javed MA, Wen L, Booth DM, Cane MC,
et al: Mechanism of mitochondrial permeability transition pore
induction and damage in the pancreas: inhibition prevents acute
pancreatitis by protecting production of ATP. Gut. 65:1333–1346.
2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Armstrong JA, Cash NJ, Morton JC, Tepikin
AV, Sutton R and Criddle DN: Mitochondrial targeting of
antioxidants alters pancreatic acinar cell bioenergetics and
determines cell fate. Int J Mol Sci. 20(1700)2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Vela S, Guerra A, Farrell G, Trivedi S,
Chaffin H, Rood C, Singh R, Kostenko S, Chang YH, Snozek C, et al:
Pathophysiology and biomarker potential of fatty acid ethyl ester
elevation during alcoholic pancreatitis. Gastroenterology.
161:1513–1525. 2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Lee J, Lim JW and Kim H: Lycopene inhibits
oxidative stress-mediated inflammatory responses in
ethanol/palmitoleic acid-stimulated pancreatic acinar AR42J cells.
Int J Mol Sci. 22(2101)2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Armstrong JA, Cash NJ, Ouyang Y, Morton
JC, Chvanov M, Latawiec D, Awais M, Tepikin AV, Sutton R and
Criddle DN: Oxidative stress alters mitochondrial bioenergetics and
modifies pancreatic cell death independently of cyclophilin D,
resulting in an apoptosis-to-necrosis shift. J Biol Chem.
293:8032–8047. 2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Booth DM, Murphy JA, Mukherjee R, Awais M,
Neoptolemos JP, Gerasimenko OV, Tepikin AV, Petersen OH, Sutton R
and Criddle DN: Reactive oxygen species induced by bile acid induce
apoptosis and protect against necrosis in pancreatic acinar cells.
Gastroenterology. 140:2116–2125. 2011.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Minati MA, Libert M, Dahou H, Jacquemin P
and Assi M: N-Acetylcysteine reduces the pro-oxidant and
inflammatory responses during pancreatitis and pancreas
tumorigenesis. Antioxidants (Basel). 10(1107)2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Xie C, Wang P, Gu Z and Yang R: Spermidine
alleviates oxidative damage and enhances phenolic compounds
accumulation in barley seedlings under UV-B stress. J Sci Food
Agric. 103:648–656. 2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Ali MY, Sina AA, Khandker SS, Neesa L,
Tanvir EM, Kabir A, Khalil MI and Gan SH: Nutritional composition
and bioactive compounds in tomatoes and their impact on human
health and disease: A review. Foods. 10(45)2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Shi Y, Han L, Zhang X, Xie L, Pan P and
Chen F: Selenium alleviates cerebral ischemia/reperfusion injury by
regulating oxidative stress, mitochondrial fusion and ferroptosis.
Neurochem Res. 47:2992–3002. 2022.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Silvestrini A, Mordente A, Martino G,
Bruno C, Vergani E, Meucci E and Mancini A: The role of selenium in
oxidative stress and in nonthyroidal Illness Syndrome (NTIS): An
overview. Curr Med Chem. 27:423–449. 2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Kruk J, Aboul-Enein BH and Duchnik E:
Exercise-induced oxidative stress and melatonin supplementation:
Current evidence. J Physiol Sci. 71(27)2021.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Chitimus DM, Popescu MR, Voiculescu SE,
Panaitescu AM, Pavel B, Zagrean L and Zagrean AM: Melatonin's
impact on antioxidative and anti-inflammatory reprogramming in
homeostasis and disease. Biomolecules. 10(1211)2020.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Dvoretskaya Y, Glanz V, Gryaznova M,
Syromyatnikov M and Popov V: Mitochondrial Antioxidant SkQ1 has a
beneficial effect in experimental diabetes as based on the analysis
of expression of microRNAs and mRNAs for the oxidative metabolism
regulators. Antioxidants (Basel). 10(1749)2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Zhang ZW, Zhang QY, Zhou MT, Liu NX, Chen
TK, Zhu YF and Wu L: Antioxidant inhibits HMGB1 expression and
reduces pancreas injury in rats with severe acute pancreatitis. Dig
Dis Sci. 55:2529–2536. 2010.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Kilic Y, Geyikoglu F, Colak S, Turkez H,
Bakir M and Hsseinigouzdagani M: Carvacrol modulates oxidative
stress and decreases cell injury in pancreas of rats with acute
pancreatitis. Cytotechnology. 68:1243–1256. 2016.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Liu MW, Wei R, Su MX, Li H, Fang TW and
Zhang W: Retraction Note: Effects of Panax notoginseng saponins on
severe acute pancreatitis through the regulation of mTOR/Akt and
caspase-3 signaling pathway by upregulating miR-181b expression in
rats. BMC Complement Med Ther. 22(306)2022.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Du WD, Yuan ZR, Sun J, Tang JX, Cheng AQ,
Shen DM, Huang CJ, Song XH, Yu XF and Zheng SB: Therapeutic
efficacy of high-dose vitamin C on acute pancreatitis and its
potential mechanisms. World J Gastroenterol. 9:2565–2569.
2003.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Weniger M, Reinelt L, Neumann J, Holdt L,
Ilmer M, Renz B, Hartwig W, Werner J, Bazhin AV and D'Haese JG: The
analgesic effect of the mitochondria-targeted antioxidant SkQ1 in
pancreatic inflammation. Oxid Med Cell Longev.
2016(4650489)2016.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Riaz M, Al Kury LT, Atzaz N, Alattar A,
Alshaman R, Shah FA and Li S: Carvacrol alleviates
hyperuricemia-induced oxidative stress and inflammation by
modulating the NLRP3/NF-ĸB pathwayt. Drug Des Devel Ther.
16:1159–1170. 2022.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Nakajima M, Kojiro M, Aso S, Matsui H,
Fushimi K, Kaita Y, Goto H, Yamaguchi Y and Yasunaga H: Effect of
high-dose vitamin C therapy on severe burn patients: A nationwide
cohort study. Crit Care. 23(407)2019.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Xia J, Li D, Yu G, Xu B, Gao X, Wang H, Ma
Y, Li X and Xiong Y: Effects of hypovitaminosis D on preoperative
pain threshold and perioperative opioid use in colorectal cancer
surgery: A cohort study. Pain Physician. 25:E1009–E1019.
2022.PubMed/NCBI
|
|
80
|
Lamontagne F, Masse MH, Menard J, Sprague
S, Pinto R, Heyland DK, Cook DJ, Battista MC, Day AG, Guyatt GH, et
al: Intravenous vitamin C in adults with sepsis in the intensive
care unit. N Engl J Med. 386:2387–2398. 2022.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Ko SH, Jun JH, Oh JE, Shin E, Kwak YL and
Shim JK: Effect of high-dose vitamin C on renal
ischemia-reperfusion injury. Biomed Pharmacother.
173(116407)2024.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Yuan X, Li X, Ji Z, Xiao J, Zhang L, Zhang
W, Su H, Kaliannan K, Long Y and Shao Z: Effects of vitamin C
supplementation on blood pressure and hypertension control in
response to ambient temperature changes in patients with essential
hypertension. Clin Exp Hypertens. 41:414–421. 2019.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Sim M, Hong S, Jung S, Kim JS, Goo YT,
Chun WY and Shin DM: Vitamin C supplementation promotes mental
vitality in healthy young adults: Results from a cross-sectional
analysis and a randomized, double-blind, placebo-controlled trial.
Eur J Nutr. 61:447–459. 2022.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Xu F, Wen Y, Hu X, Wang T and Chen G: The
potential use of vitamin C to prevent kidney injury in patients
with COVID-19. Diseases. 9(46)2021.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Bonham MJ, Abu-Zidan FM, Simovic MO, Sluis
KB, Wilkinson A, Winterbourn CC and Windsor JA: Early ascorbic acid
depletion is related to the severity of acute pancreatitis. Br J
Surg. 86:1296–1301. 1999.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Hardman J, Shields C, Schofield D, McMahon
R, Redmond HP and Siriwardena AK: Intravenous antioxidant
modulation of end-organ damage in L-arginine-induced experimental
acute pancreatitis. Pancreatology. 5:380–386. 2005.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Siriwardena AK, Mason JM, Balachandra S,
Bagul A, Galloway S, Formela L, Hardman JG and Jamdar S:
Randomised, double blind, placebo controlled trial of intravenous
antioxidant (n-acetylcysteine, selenium, vitamin C) therapy in
severe acute pancreatitis. Gut. 56:1439–1444. 2007.PubMed/NCBI View Article : Google Scholar
|