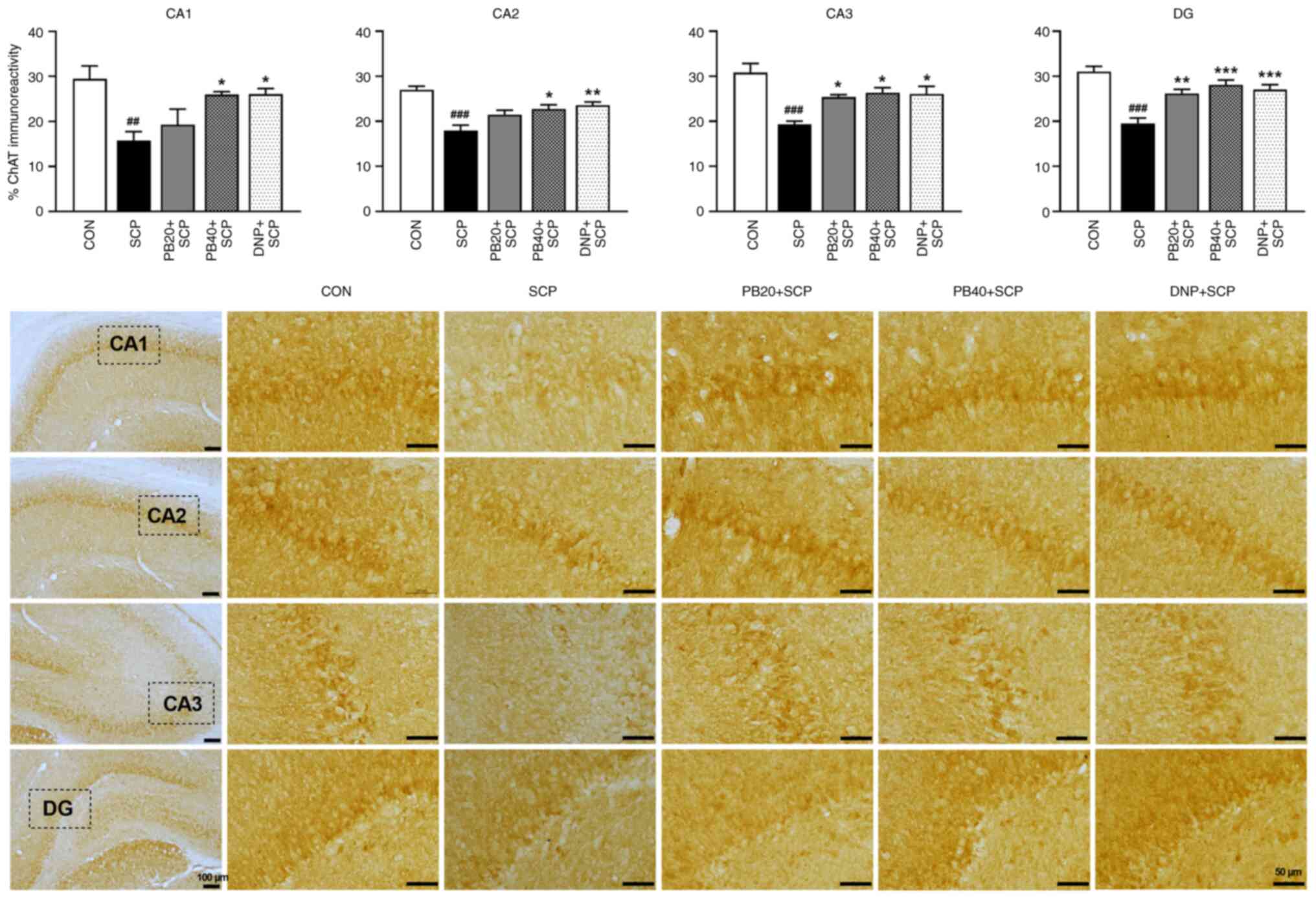
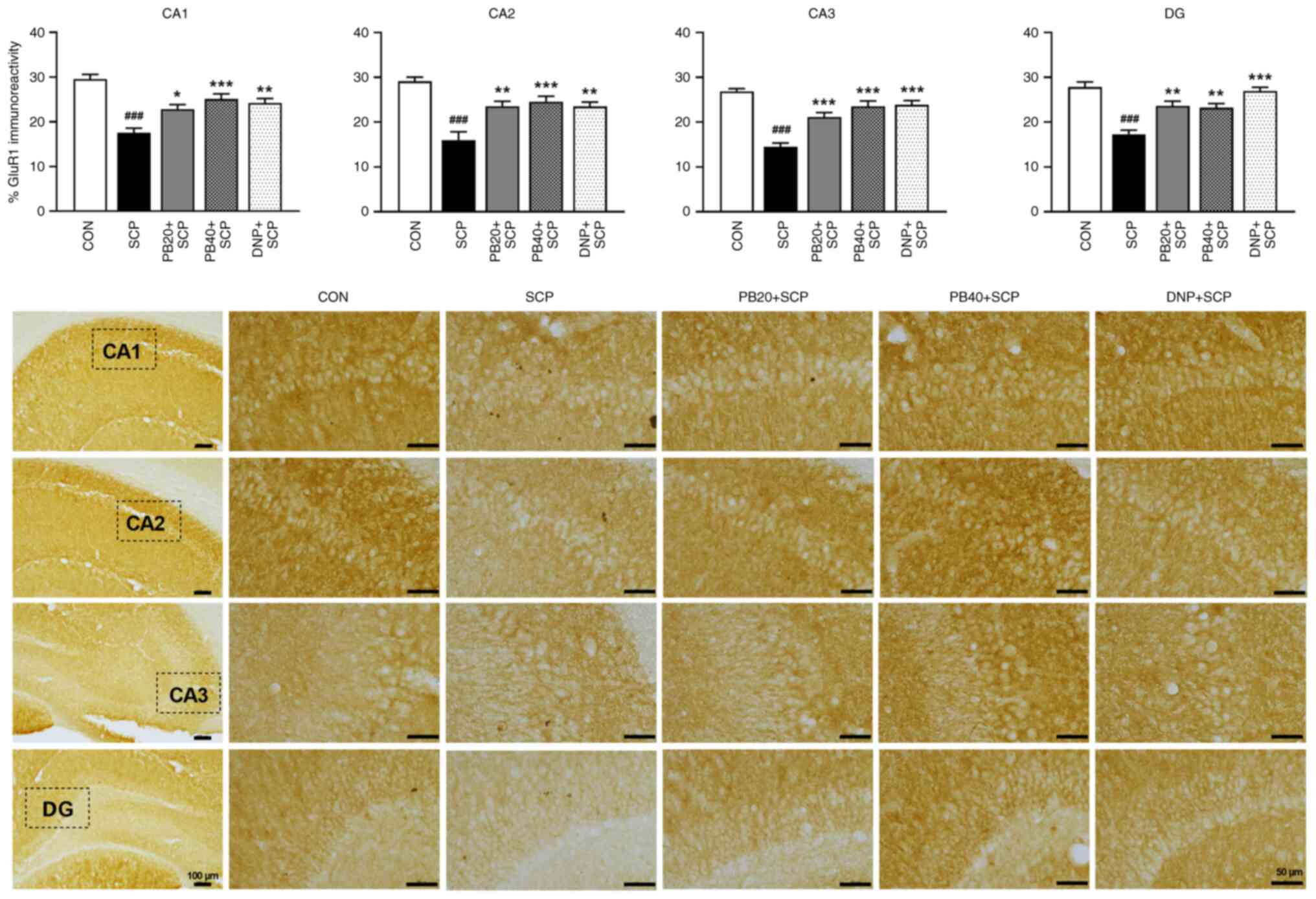
|
1
|
Mat Nuri TH, Hong YH, Ming LC, Mohd Joffry
S, Othman MF and Neoh CF: Knowledge on Alzheimer's disease among
public hospitals and health clinics pharmacists in the State of
Selangor, Malaysia. Front Pharmacol. 8(739)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Djeuzong E, Kandeda AK, Djiogue S,
Stéphanie L, Nguedia D, Ngueguim F, Djientcheu JP, Kouamouo J and
Dimo T: Antiamnesic and neuroprotective effects of an aqueous
extract of ziziphus jujuba mill. (rhamnaceae) on
scopolamine-induced cognitive impairments in rats. Evid Based
Complement Alternat Med. 2021(5577163)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shabani S and Mirshekar MA: Diosmin is
neuroprotective in a rat model of scopolamine-induced cognitive
impairment. Biomed Pharmacother. 108:1376–1383. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mahdy K, Shaker O, Wafay H, Nassar Y,
Hassan H and Hussein A: Effect of some medicinal plant extracts on
the oxidative stress status in Alzheimer's disease induced in rats.
Eur Rev Med Pharmacol Sci. 16 (Suppl 3):S31–S42. 2012.PubMed/NCBI
|
|
5
|
von Bernhardi R, Eugenin-von Bernhardi L
and Eugenin J: Microglial cell dysregulation in brain aging and
neurodegeneration. Front Aging Neurosci. 7(124)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Blake MG, Krawczyk MC, Baratti CM and
Boccia MM: Neuropharmacology of memory consolidation and
reconsolidation: Insights on central cholinergic mechanisms. J
Physiol Paris. 108:286–291. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lee JS, Kim HG, Lee HW, Han JM, Lee SK,
Kim DW, Saravanakumar A and Son CG: Hippocampal memory enhancing
activity of pine needle extract against scopolamine-induced amnesia
in a mouse model. Sci Rep. 5(9651)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Choi JH, Lee EB, Jang HH, Cha YS, Park YS
and Lee SH: Allium hookeri extracts improve scopolamine-induced
cognitive impairment via activation of the cholinergic system and
anti-neuroinflammation in mice. Nutrients. 13(2890)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tang KS: The cellular and molecular
processes associated with scopolamine-induced memory deficit: A
model of Alzheimer's biomarkers. Life Sci.
233(116695)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hussain H, Ahmad S, Shah SWA, Ullah A, Ali
N, Almehmadi M, Ahmad M, Khalil AAK, Jamal SB, Ahmad H and Halawi
M: Attenuation of scopolamine-induced amnesia via cholinergic
modulation in mice by synthetic curcumin analogs. Molecules.
27(2468)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kim Y, Kim J, He M, Lee A and Cho E:
Apigenin ameliorates scopolamine-induced cognitive dysfunction and
neuronal damage in mice. Molecules. 26(5192)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Safar MM, Arab HH, Rizk SM and El-Maraghy
SA: Bone marrow-derived endothelial progenitor cells protect
against scopolamine-induced alzheimer-like pathological
aberrations. Mol Neurobiol. 53:1403–1418. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Danysz W and Parsons CG: Alzheimer's
disease, β-amyloid, glutamate, NMDA receptors and
memantine-searching for the connections. Br J Pharmacol.
167:324–352. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Eder P, Reinprecht I, Schreiner E,
Skofitsch G and Windisch M: Increased density of glutamate receptor
subunit 1 due to Cerebrolysin treatment: An immunohistochemical
study on aged rats. Histochem J. 33:605–612. 2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dewar D, Chalmers DT, Graham DI and
McCulloch J: Glutamate metabotropic and AMPA binding sites are
reduced in Alzheimer's disease: an autoradiographic study of the
hippocampus. Brain Res. 553:58–64. 1991.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ding R, Li Y, Du A, Yu H, He B, Shen R,
Zhou J, Li L, Cui W, Zhang G, et al: Changes in hippocampal AMPA
receptors and cognitive impairments in chronic ketamine addiction
models: Another understanding of ketamine CNS toxicity. Sci Rep.
6(38771)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang YJ, Chen HB, Wei B, Wang W, Zhou PL,
Zhan JQ, Hu MR, Yan K, Hu B and Yu B: Cognitive decline is
associated with reduced surface GluR1 expression in the hippocampus
of aged rats. Neurosci Lett. 591:176–181. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Aigner TG: Pharmacology of memory:
Cholinergic-glutamatergic interactions. Curr Opin Neurobiol.
5:155–160. 1995.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Francis PT, Parsons CG and Jones RW:
Rationale for combining glutamatergic and cholinergic approaches in
the symptomatic treatment of Alzheimer's disease. Expert Rev
Neurother. 12:1351–1365. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wu Y, Luo X, Liu X, Liu D, Wang X, Guo Z,
Zhu L, Tian Q, Yang X and Wang JZ: Intraperitoneal administration
of a novel TAT-BDNF peptide ameliorates cognitive impairments via
modulating multiple pathways in two Alzheimer's rodent models. Sci
Rep. 5(15032)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fahey JW and Stephenson KK: Pinostrobin
from honey and Thai ginger (Boesenbergia pandurata): A potent
flavonoid inducer of mammalian phase 2 chemoprotective and
antioxidant enzymes. J Agric Food Chem. 50:7472–7476.
2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ijaz MU, Shahzadi S, Hamza A, Azmat R,
Anwar H, Afsar T, Shafique H, Bhat MA, Naglah AM, Al-Omar MA and
Razak S: Alleviative effects of pinostrobin against cadmium-induced
renal toxicity in rats by reducing oxidative stress, apoptosis,
inflammation, and mitochondrial dysfunction. Front Nutr.
10(1175008)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wu D, Nair MG and DeWitt DL: Novel
compounds from Piper methysticum Forst (Kava Kava) roots and their
effect on cyclooxygenase enzyme. J Agric Food Chem. 50:701–705.
2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Patel NK and Bhutani KK: Pinostrobin and
Cajanus lactone isolated from Cajanus cajan (L.) leaves inhibits
TNF-α and IL-1β production: In vitro and in vivo experimentation.
Phytomedicine. 21:946–953. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhao LL, Jayeoye TJ, Ashaolu TJ and
Olatunji OJ: Pinostrobin, a dietary bioflavonoid exerts
antioxidant, anti-inflammatory, and anti-apoptotic protective
effects against methotrexate-induced ovarian toxicity in rats.
Tissue Cell. 85(102254)2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Thongrong S, Surapinit S, Promsrisuk T,
Jittiwat J and Kongsui R: Pinostrobin alleviates chronic restraint
stress-induced cognitive impairment by modulating oxidative stress
and the function of astrocytes in the hippocampus of rats. Biomed
Rep. 18(20)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kongsui R, Surapinit S, Promsrisuk T and
Thongrong S: Pinostrobin from Boesenbergia rotunda
attenuates oxidative stress and promotes functional recovery in rat
model of sciatic nerve crush injury. Braz J Med Biol Res.
56(e12578)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lin J, Huang L, Yu J, Xiang S, Wang J,
Zhang J, Yan X, Cui W, He S and Wang Q: Fucoxanthin, a marine
carotenoid, reverses scopolamine-induced cognitive impairments in
mice and inhibits acetylcholinesterase in vitro. Mar Drugs.
14(67)2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Woo Y, Lim JS, Oh J, Lee JS and Kim JS:
Neuroprotective effects of euonymus alatus extract on
scopolamine-induced memory deficits in mice. Antioxidants (Basel).
9(449)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
31
|
Nakmareong S, Kukongviriyapan U,
Pakdeechote P, Donpunha W, Kukongviriyapan V, Kongyingyoes B,
Sompamit K and Phisalaphong C: Antioxidant and vascular protective
effects of curcumin and tetrahydrocurcumin in rats with
L-NAME-induced hypertension. Naunyn Schmiedebergs Arch Pharmacol.
383:519–529. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Srdjenovic B, Mrdjanovic J, Galovic AJ,
Kladar N, Bozin B, Jurisic V and Bogdanovic G: Effect of ELF-EMF on
antioxidant status and micronuclei in K562 cells and normal
lymphocytes. Open Life Sci. 9:931–940. 2014.
|
|
33
|
Polycarp TN, Obukowho EB and Yusoff SM:
Changes in haematological parameters and oxidative stress response
of goats subjected to road transport stress in a hot humid tropical
environment. Comp Clin Pathol. 25:285–293. 2016.
|
|
34
|
Hinwood M, Tynan RJ, Charnley JL, Beynon
SB, Day TA and Walker FR: Chronic stress induced remodeling of the
prefrontal cortex: Structural re-organization of microglia and the
inhibitory effect of minocycline. Cereb Cortex. 23:1784–1797.
2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Johnson SJ and Walker FR: Strategies to
improve quantitative assessment of immunohistochemical and
immunofluorescent labelling. Sci Rep. 5(10607)2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Batool Z, Sadir S, Liaquat L, Tabassum S,
Madiha S, Rafiq S, Tariq S, Batool TS, Saleem S, Naqvi F, et al:
Repeated administration of almonds increases brain acetylcholine
levels and enhances memory function in healthy rats while
attenuates memory deficits in animal model of amnesia. Brain Res
Bull. 120:63–74. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mooshekhian A, Sandini T, Wei Z, Van
Bruggen R, Li H, Li XM and Zhang Y: Low-field magnetic stimulation
improved cuprizone-induced depression-like symptoms and
demyelination in female mice. Exp Ther Med. 23(210)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Klinkenberg I and Blokland A: The validity
of scopolamine as a pharmacological model for cognitive impairment:
A review of animal behavioral studies. Neurosci Biobehav Rev.
34:1307–1350. 2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Rahimzadegan M and Soodi M: Comparison of
memory impairment and oxidative stress following single or repeated
doses administration of scopolamine in rat hippocampus. Basic Clin
Neurosci. 9:5–14. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Terry AV and Callahan PM: Nicotinic
acetylcholine receptor ligands, cognitive function, and preclinical
approaches to drug discovery. Nicotine Tob Res. 21:383–394.
2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kraeuter AK, Guest PC and Sarnyai Z: The
Y-maze for assessment of spatial working and reference memory in
mice. Methods Mol Biol. 1916:105–111. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Afzal M, Alzarea SI, Alharbi KS, Alzarea
AI, Alenezi SK, Alshammari MS, Alquraini AH and Kazmi I: Rosiridin
attenuates scopolamine-induced cognitive impairments in rats via
inhibition of oxidative and nitrative stress leaded caspase-3/9 and
TNF-α signaling pathways. Molecules. 27(5888)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Bhuvanendran S, Kumari Y, Othman I and
Shaikh MF: Amelioration of cognitive deficit by embelin in a
scopolamine-induced Alzheimer's disease-like condition in a rat
model. Front Pharmacol. 9(665)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Li J, O W, Li W, Jiang ZG and Ghanbari HA:
Oxidative stress and neurodegenerative disorders. Int J Mol Sci.
14:24438–24475. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Fan Y, Hu J, Li J, Yang Z, Xin X, Wang J,
Ding J and Geng M: Effect of acidic oligosaccharide sugar chain on
scopolamine-induced memory impairment in rats and its related
mechanisms. Neurosci Lett. 374:222–226. 2005.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Budzynska B, Boguszewska-Czubara A,
Kruk-Slomka M, Skalicka-Wozniak K, Michalak A, Musik I and Biala G:
Effects of imperatorin on scopolamine-induced cognitive impairment
and oxidative stress in mice. Psychopharmacology (Berl).
232:931–942. 2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Uma G and Maheswari SU: Neuroprotective
effects of polyherbal formulation (Indian NONI) on
scopolamine-induced memory impairment in mice. Int J Pharm Pharm
Sci. 6:354–357. 2014.
|
|
48
|
Abd-El-Fattah MA, Abdelakader NF and Zaki
HF: Pyrrolidine dithiocarbamate protects against
scopolamine-induced cognitive impairment in rats. Eur J Pharmacol.
723:330–338. 2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Hernández-Rodríguez M, Arciniega-Martínez
IM, García-Marín ID, Correa-Basurto J and Rosales-Hernández MC:
Chronic administration of scopolamine increased GSK3βP9, beta
secretase, amyloid beta, and oxidative stress in the hippocampus of
wistar rats. Mol Neurobiol. 57:3979–3988. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Butterfield DA, Castegna A, Lauderback CM
and Drake J: Evidence that amyloid beta-peptide-induced lipid
peroxidation and its sequelae in Alzheimer's disease brain
contribute to neuronal death. Neurobiol Aging. 23:655–664.
2002.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Gul S, Attaullah S, Alsugoor MH, Bawazeer
S, Shah SA, Khan S, Salahuddin HS and Ullah M: Folicitin abrogates
scopolamine induced oxidative stress, hyperlipidemia mediated
neuronal synapse and memory dysfunction in mice. Heliyon.
9(e16930)2023.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Guo C, Shen J, Meng Z, Yang X and Li F:
Neuroprotective effects of polygalacic acid on scopolamine-induced
memory deficits in mice. Phytomedicine. 23:149–155. 2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Liao J, Nai Y, Feng L, Chen Y, Li M and Xu
H: Walnut oil prevents scopolamine-induced memory dysfunction in a
mouse model. Molecules. 25(1630)2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ghias M, Shoaib M, Ali Shah SW, Umar MN
and Ullah S, Ali N, Shah I and Ullah S: Nootropic effects of
synthetic flavonoid derivatives on scopolamine induced memory
impairment in mice via cholinesterase inhibition and antioxidant
system. Pak J Pharm Sci. 32 (5 Suppl):S2325–S2332. 2019.PubMed/NCBI
|
|
55
|
Soodi M, Naghdi N, Hajimehdipoor H,
Choopani S and Sahraei E: Memory-improving activity of Melissa
officinalis extract in naive and scopolamine-treated rats. Res
Pharm Sci. 9:107–114. 2014.PubMed/NCBI
|
|
56
|
Yadang FSA, Nguezeye Y, Kom CW, Betote
PHD, Mamat A, Tchokouaha LRY, Taiwé GS, Agbor GA and Bum EN:
Scopolamine-induced memory impairment in mice: Neuroprotective
effects of carissa edulis (forssk.) valh (apocynaceae) aqueous
extract. Int J Alzheimers Dis. 2020(6372059)2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Chaudhaery SS, Roy KK, Shakya N, Saxena G,
Sammi SR, Nazir A, Nath C and Saxena AK: Novel carbamates as orally
active acetylcholinesterase inhibitors found to improve
scopolamine-induced cognition impairment: Pharmacophore-based
virtual screening, synthesis, and pharmacology. J Med Chem.
53:6490–6505. 2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Härtl R, Gleinich A and Zimmermann M:
Dramatic increase in readthrough acetylcholinesterase in a cellular
model of oxidative stress. J Neurochem. 116:1088–1096.
2011.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Eshaghi Ghalibaf MH, Rajabian A, Parviz M,
Akbarian M, Amirahmadi S, Vafaee F and Hosseini M: Minocycline
alleviated scopolamine-induced amnesia by regulating antioxidant
and cholinergic function. Heliyon. 9(e13452)2023.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Oda Y: Choline acetyltransferase: The
structure, distribution and pathologic changes in the central
nervous system. Pathol Int. 49:921–937. 1999.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Sohn E, Lim HS, Kim YJ, Kim BY and Jeong
SJ: Annona atemoya leaf extract improves scopolamine-induced memory
impairment by preventing hippocampal cholinergic dysfunction and
neuronal cell death. Int J Mol Sci. 20(3538)2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Lim DW, Son HJ, Um MY, Kim IH, Han D, Cho
S and Lee CH: Enhanced cognitive effects of demethoxycurcumin, a
natural derivative of curcumin on scopolamine-induced memory
impairment in mice. Molecules. 21(1022)2016.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Olayinka JN, Eduviere A, Adeoluwa O,
Akinluyi E, Obisesan A, Akawa O and Adebanjo A: Quercetin mitigates
scopolamine-induced memory dysfunction: Impact on oxidative stress
and cholinergic mechanisms. Metab Brain Dis. 37:265–277.
2022.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Cheng YJ, Lin CH and Lane HY: Involvement
of cholinergic, adrenergic, and glutamatergic network modulation
with cognitive dysfunction in Alzheimer's disease. Int J Mol Sci.
22(2283)2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Hascup KN, Findley CA, Sime LN and Hascup
ER: Hippocampal alterations in glutamatergic signaling during
amyloid progression in AβPP/PS1 mice. Sci Rep.
10(14503)2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Kwon O, Feng L, Druckmann S and Kim J:
Schaffer collateral inputs to CA1 excitatory and inhibitory neurons
follow different connectivity rules. J Neurosci. 38:5140–5152.
2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Royo M, Escolano BA, Madrigal MP and
Jurado S: AMPA receptor function in hypothalamic synapses. Front
Synaptic Neurosci. 14(833449)2022.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Hayashi Y, Shi SH, Esteban JA, Piccini A,
Poncer JC and Malinow R: Driving AMPA receptors into synapses by
LTP and CaMKII: requirement for GluR1 and PDZ domain interaction.
Science. 287:2262–2267. 2000.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Henley JM and Wilkinson KA: AMPA receptor
trafficking and the mechanisms underlying synaptic plasticity and
cognitive aging. Dialogues Clin Neurosci. 15:11–27. 2013.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Bye CM and McDonald RJ: A specific role of
hippocampal NMDA receptors and arc protein in rapid encoding of
novel environmental representations and a more general long-term
consolidation function. Front Behav Neurosci. 13(8)2019.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Yamada K, Arai M, Suenaga T and Ichitani
Y: Involvement of hippocampal NMDA receptors in encoding and
consolidation, but not retrieval, processes of spontaneous object
location memory in rats. Behav Brain Res. 331:14–19.
2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Li C, Tang B, Feng Y, Tang F, Pui-Man Hoi
M, Su Z and Ming-Yuen Lee S: Pinostrobin exerts neuroprotective
actions in neurotoxin-induced Parkinson's disease models through
Nrf2 induction. J Agric Food Chem. 66:8307–8318. 2018.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Gao WY, Chen PY, Chen SF, Wu MJ, Chang HY
and Yen JH: Pinostrobin inhibits proprotein convertase
subtilisin/kexin-type 9 (PCSK9) gene expression through the
modulation of FoxO3a protein in HepG2 cells. J Agric Food Chem.
66:6083–6093. 2018.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Kicuntod J, Khuntawee W, Wolschann P,
Pongsawasdi P, Chavasiri W, Kungwan N and Rungrotmongkol T:
Inclusion complexation of pinostrobin with various cyclodextrin
derivatives. J Mol Graph Model. 63:91–98. 2016.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Yang ZH, Sun X, Qi Y, Mei C, Sun XB and Du
GH: Uptake characteristics of pinocembrin and its effect on
p-glycoprotein at the blood-brain barrier in in vitro cell
experiments. J Asian Nat Prod Res. 14:14–21. 2012.PubMed/NCBI View Article : Google Scholar
|