Introduction
Due to the progressive increase in the number of
patients with Alzheimer's disease (AD), which is currently
estimated to be more than 46 million individuals worldwide, AD has
become a significant concern for the elderly population (1). AD stands as the primary condition
associated with cognitive dysfunction, language difficulties and
alterations in personality (2,3). A key
neuropathological characteristic linked to AD symptoms is the
presence of senile plaques and neurofibrillary tangles, which are
significant indicators of neuronal loss. Additionally, various
mechanisms have been investigated to further explain the
pathogenesis of AD, including cholinergic dysfunction, oxidative
damage, deposits of amyloid beta (Aβ), hyperphosphorylation of tau
protein and the aggregation of senile plaques (4,5).
The central cholinergic system plays a vital role in
memory processing whereby the loss of the cholinergic neurons,
neurotransmitters and their receptors results in learning and
memory impairment (6,7). Cholinergic dysfunction is caused by an
elevation of acetylcholinesterase (AChE) activity and a reduction
in acetylcholine (ACh) concentration in the brain, which are the
main drivers of cognitive impairment (8). Scopolamine has been widely used in
in vivo models to evaluate potential agents for the
treatment of dementia (9).
Scopolamine, which acts as a blocker of the muscarinic cholinergic
receptor, leads to cognitive dysfunction by reducing cholinergic
neurotransmission and heightening oxidative stress in the brain
(10,11). Furthermore, scopolamine leads to the
shrinking of neuronal cells and initiates degenerative alterations
within the hippocampus (12).
Glutamate is the major fast-excitatory
neurotransmitter in the hippocampal network and is essential for
learning and memory. Dysfunctions in this system are considered to
be causally linked to neurodegenerative disorders (13). Glutamate receptor subunit 1 (GluR1)
is one of the four subunits of
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)
receptors, which are critical for synaptic plasticity and learning
processes (14). Notably, a
reduction in GluR1 is observed in the hippocampal formation of
patients with AD, resulting in impaired synaptic transmission and
potentially contributing to cognitive dysfunction (15). Additionally, several animal studies
have shown that decreased expression of GluR1 in the hippocampus
may be associated with learning and memory deficits (16,17).
Therefore, acetylcholine and glutamate are now recognised as key
contributors to memory processes.
Emerging evidence indicates that the interactions
between these neurotransmitters may be crucial for certain memory
types, with acetylcholine particularly facilitating glutamate
activity by coordinating the acquisition and recall phases in the
cortex and hippocampus (18).
Glutamatergic neurotransmission, through the AMPA receptor and the
N-methyl d-aspartate (NMDA) receptor, induces long-term
potentiation (LTP), which strengthens synapses with repeated use
and is essential for learning and memory. Besides glutamatergic
mechanisms, acetylcholine and its receptors markedly contribute to
both the induction and maintenance of LTP (19). Thus, medications capable of
enhancing cholinergic transmission while mitigating oxidative
stress may potentially reverse memory deficits induced by
scopolamine (20). Currently,
existing medications can only provide temporary alleviation from AD
symptoms, which is accompanied by adverse effects. Hence, there is
an imperative need to develop natural compounds for an effective
treatment against AD.
Pinostrobin (5-hydroxy-7-methoxy flavanone) is a
substituted flavonoid isolated from Boesenbergia rotunda
(L.), that has been identified as the main active ingredient
responsible for its pharmacological properties, including
antioxidant (21,22), anti-inflammatory (23,24)
and anti-apoptotic (25)
activities. In addition, our previous findings showed that
pinostrobin exerts protective effects against chronic restraint
stress and nerve crush injury (26,27).
Nevertheless, the potential of using pinostrobin to improve
scopolamine-induced cognitive impairment has not yet been examined.
Thus, the present study was conducted to explore whether
pinostrobin could mitigate memory impairment in this model. It also
investigated whether pinostrobin could enhance the function of the
cholinergic and glutamatergic systems.
Materials and methods
Chemicals
Scopolamine used in the present study was obtained
from (cat. no. S1875; MilliporeSigma). Donepezil
(Aricept® 10 mg tablet) was purchased from the Eisai
Co., Ltd.). Scopolamine was dissolved in 0.9% sodium chloride
(NaCl) and administered by i.p. injection to rats (3 mg/kg) as
described in a previous study (28). Donepezil was dissolved in water and
orally administered at a dose of 5 mg/kg in accordance with an
established report (29).
Isolation of pinostrobin
The preparation and structural characterisation of
pinostrobin (the purity of which was determined by NMR analysis)
was described in our previous study (26). Briefly, fresh rhizomes of B.
rotunda were purchased from a market in Phayao, Thailand. A
plant specimen was authenticated by the Walai Rukhavej Botanical
Research Institute, Mahasarakham University, MahaSarakham, Thailand
(No. S. Sedlak 19-1). The ethanolic crude extract (251 g) was
dissolved in methanol and then subjected to open column
chromatography (Arch. No. 7734, pore size Å, particle size 70-230
mesh, Merck), using silica gel as the absorbent. The column was
washed with a gradient of dichloromethane-hexane mixture for
separation. Fractions exhibiting similar patterns, as determined by
thin-layer chromatography analysis, were combined and
recrystallised with methanol to yield pinostrobin. Structural
analysis was conducted using 13C- and 1H-NMR
spectroscopy (Bruker Avance DRX 500 Spectrometer; Bruker
Corporation) and the NMR spectra were compared with the existing
literature for identification as follow: 1H NMR (500 MHz,
acetone-d6): 2.80 (dd, J=17.2, 3.0 Hz; 1H, H-3a), 3.06 (dd, J=17.2,
13.0 Hz; 1H, H-3b), 3.79 (s, 3H; -OCH3), 5.39 (dd, J=13.0, 3.0 Hz;
1H, H-2), 6.06 (m, 2H, H-6, H-8), 7.41 (m, 5H; H-2', H-3', H-3',
H-5', H-6'). 13C NMR (150 MHz, acetone-d6): 43.1 (C-3), 55.7
(C-7-OCH3), 79.2 (C-2), 94.3 (C-8), 95.1 (C-6), 103.1 (C-10), 126.1
(C-2', C-3', C-5', C-6'), 128.9 (C-4'), 138.4 (C-1'), 162.8 (C-5),
164.1 (C-9), 167.9 (C-7), 195.8 (C-4). (Central Science Laboratory,
Faculty of Science, Chiang Mai University, Thailand).
Experimental animals and
treatments
A total of 30 male Wistar rats (aged 8 weeks and
weighing 220-250 g) were obtained from Nomura Siam International.
The rats were allowed a week for acclimation prior to the
commencement of the experiments. All experimental procedures were
conducted under a 12-h light/dark cycle with standard chow and
water available ad libitum in a room with a constant
temperature (21±1˚C) and humidity (35-60%). All experiments were
approved by the Ethics Committee of the Laboratory Animal Research
Center, University of Phayao, Thailand (approval no. 640104029).
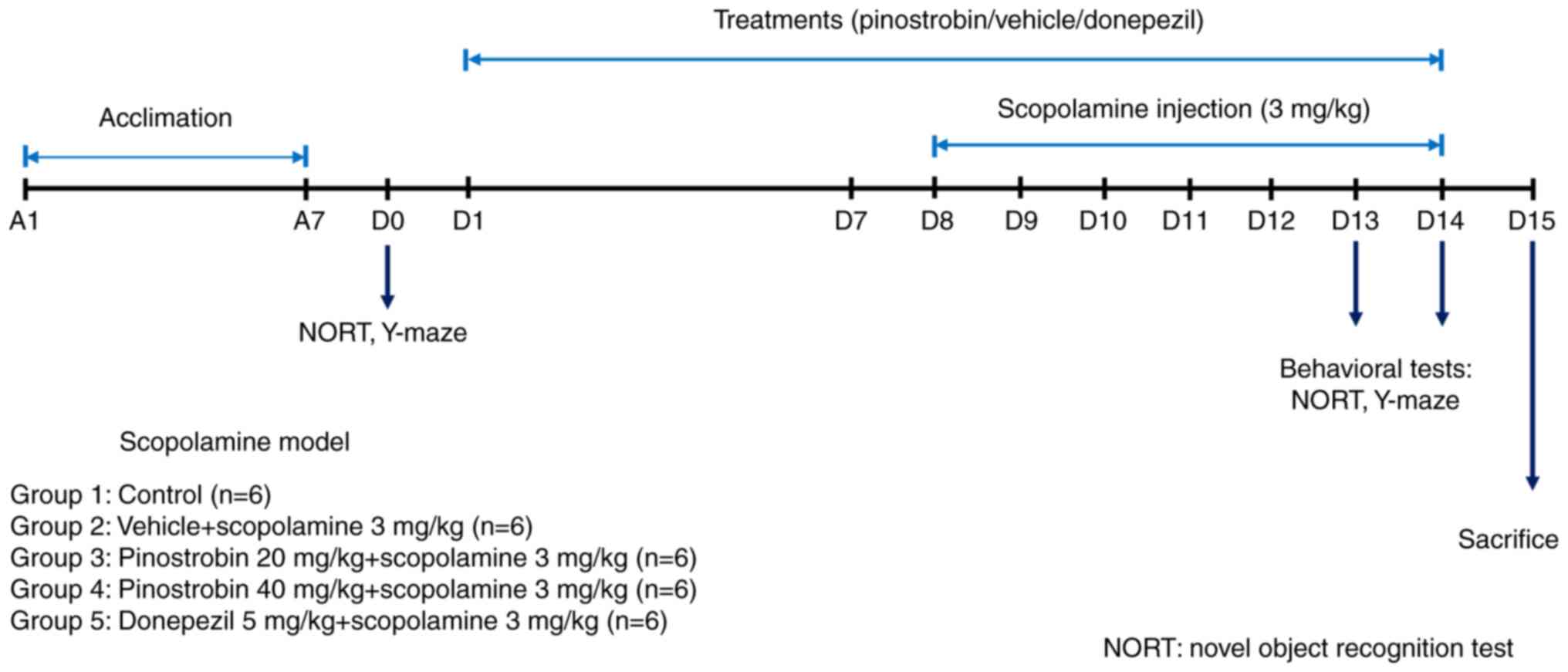
All rats were randomly divided into five groups (6 rats per group)
as follows: group 1 received vehicle as control, group 2 received
vehicle + scopolamine (3 mg/kg, i.p.), group 3 received pinostrobin
(20 mg/kg, p.o.) + scopolamine, group 4 received pinostrobin (40
mg/kg, p.o.) + scopolamine and group 5 received donepezil (5 mg/kg,
p.o.) + scopolamine. The animals were administered 1%
carboxymethylcellulose (cat. no. 21904; MilliporeSigma), which was
used as the vehicle or pinostrobin (at doses of 20 or 40 mg/kg) or
donepezil (5 mg/kg) via oral gavage daily for 14 days. During the
final 7 days of treatment, a daily intraperitoneal injection of
scopolamine (3 mg/kg) was administered. The cognitive performance
of all animals was assessed by using the novel object recognition
test (NORT) and the Y-maze test on day 0 for baseline data and on
day 13 and 14 to identify treatment effects (Fig. 1).
Tissue preparation
At the end of the experimental period, all animals
were anesthetised using thiopental sodium (70 mg/kg) and
transcardially perfused with 0.1 M PBS for the evaluation of
biochemical and immunohistochemical parameters. The brains (n=30)
were immediately collected and separated into two hemispheres. The
left hemisphere of the hippocampus was isolated and homogenised in
Tris-buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, protease
inhibitor, pH 7.4). After centrifugation at 9,279 x g at 4˚C for 15
min, the supernatant was stored at -80˚C for later biochemical
evaluation. The right hemisphere was fixed with ice-cold 4%
paraformaldehyde for 24 h and cryopreserved in 30% sucrose for
later immunohistochemistry analysis. The brains were cut into 30 µm
sections using a Leica CM1950 cryostat (Leica Microsystems GmbH)
and stored in an anti-freeze solution (4˚C).
Total protein concentration
The protein concentrations were determined using the
method of Lowry (30). Bovine serum
albumin (BSA; cat. no. 9048-46-8; MilliporeSigma) was used as a
standard and protein concentrations were expressed as mg/ml.
Biochemical analysis. Measurement of
the lipid peroxidation product
Estimation of the malondialdehyde (MDA)
concentration was performed according to the protocol previously
described (31,32) with some modifications. The
absorbance was measured at 532 nm using a microplate reader
(Synergy H1; BioTek; Agilent Technologies, Inc.). The results were
presented as µmol/mg protein.
Measurement of superoxide dismutase
(SOD) activity
The activity of SOD was examined by commercial assay
kit (cat. no. S19160; MilliporeSigma) according to the
manufacturer's instructions. The SOD activity was measured from the
curve of the standard solution and expressed as U/mg protein.
Measurement of reduced glutathione
(GSH)
A reduced GSH assay was investigated according to
the method described by Polycarp et al (33) and Srdjenovic et al (32). The absorbance of each sample mixture
was determined at 412 nm. The result was expressed as µmol/mg
protein.
Measurement of acetylcholinesterase
(AChE) activity
The activity of AChE, which is responsible for the
degradation of acetylcholine in tissue samples, was determined
using a commercial acetylcholinesterase colorimetric kit (cat. no.
ab138871; Abcam). The assay was performed according to the
manufacturer's instructions and the activity was measured from the
curve of the standard solution. The activity was expressed as U/mg
protein.
Immunohistochemical staining
To accomplish immunohistochemistry staining, the
free-floating sections were washed and treated in
H2O2 followed by blocking solution (5% normal
horse serum in 0.1 M PBS) (cat. no. 16050130; Gibco; Thermo Fisher
Scientific). Subsequently, sections were incubated with the primary
antibody, which was rabbit anti-choline acetyltransferase (ChAT;
1:100; cat. no. AB143; MilliporeSigma) or anti-glutamate receptor 1
(GluR1; 1:200; cat. no. AB1504; MilliporeSigma) at 4˚C overnight.
The sections were washed in 0.1 M PBS for 30 min and incubated for
2 h at room temperature with biotinylated donkey anti-rabbit
antibody (cat. no. 711-065-152; 1:500; Jackson ImmunoResearch
Europe, Ltd.). The sections were rinsed in 0.1 M PBS followed by 1
h of incubation in extravidin peroxidase (1:1,000) and then
visualized using the 3,3'-diaminobenzidine (DAB) reaction as
previously described (34). For
immunohistochemistry evaluation, hippocampal images (six images per
group) of subregion cornu ammonis 1 (CA1), cornu ammonis 2 (CA2),
cornu ammonis 3 (CA3) and dentate gyrus (DG) were captured at 40x
magnification using a Ni-U upright microscope (Nikon Corporation)
with NIS Element Imaging Software version 5 (Nikon Corporation).
The hippocampus is composed of various subregions: CA1, CA2, CA3
and the DG. Based on the basic anatomical organisation of the
hippocampal formation and on the morphological and connectivity
differences, ChAT and GluR1 immunostaining was analysed in the
pyramidal layer of CA1, CA2 and CA3 subregions and the granular
layer of DG.
Evaluation of ChAT and GluR1
immunostaining
The quantitative immunoreactivity of the ChAT and
GluR1 proteins within the hippocampus were determined using the
thresholding function of Image J software (v1.53; National
Institutes of Health). Image thresholding is a commonly utilised
method for quantitatively assessing changes in immunolabelled
materials, as previously documented (35). The data are presented as the
percentage of labelling area (% immunoreactivity).
Behavioural studies. NORT
The experimental process used to assess recognition
memory was the same as that described in our previous study
(26). One day prior to the
experiment, the rats were allowed to explore the empty open field
for 5 min. On the experiment day, during the training phase, two
identical objects (A1 and A2) were placed in two corners of the
open field. Each rat was placed in the middle of the open field and
allowed to freely explore these two identical objects for 5 min.
After 4 h of post-training phase, one old object was replaced by a
new object (B) and the rat was left to inspect the two objects for
5 min. The open field was cleaned with 70% ethanol before the next
rat was tested to avoid affecting subsequent test results. The
percentage of recognition index (RI) was calculated using a formula
as previously described (36).
Y-maze test
The Y-maze test was performed to measure working
memory related to the hippocampus in animals according to the
instructions in our previous work (26). The maze was Y-shaped with three arms
that were designated as regions A, B and C. A single rat was placed
at one of three enclosed arms for free exploration for a total of 8
min. A spontaneous alternation occurred when an animal entered all
three arms in a sequence of three consecutive entries. The maze was
cleaned with 70% ethanol to avoid affecting subsequent sessions.
The percentage of spontaneous alternation was considered to
indicate working memory and was computed according to the previous
study (37) as follows:
Statistical analysis
GraphPad Prism version 9 (Dotmatics) was used to
analyse the data. Statistical analysis was performed using the
one-way ANOVA test, followed by Tukey's post-hoc test for multiple
comparisons. Data are expressed as the mean ± SEM. P<0.05
was considered to indicate a statistically significant
difference.
Results
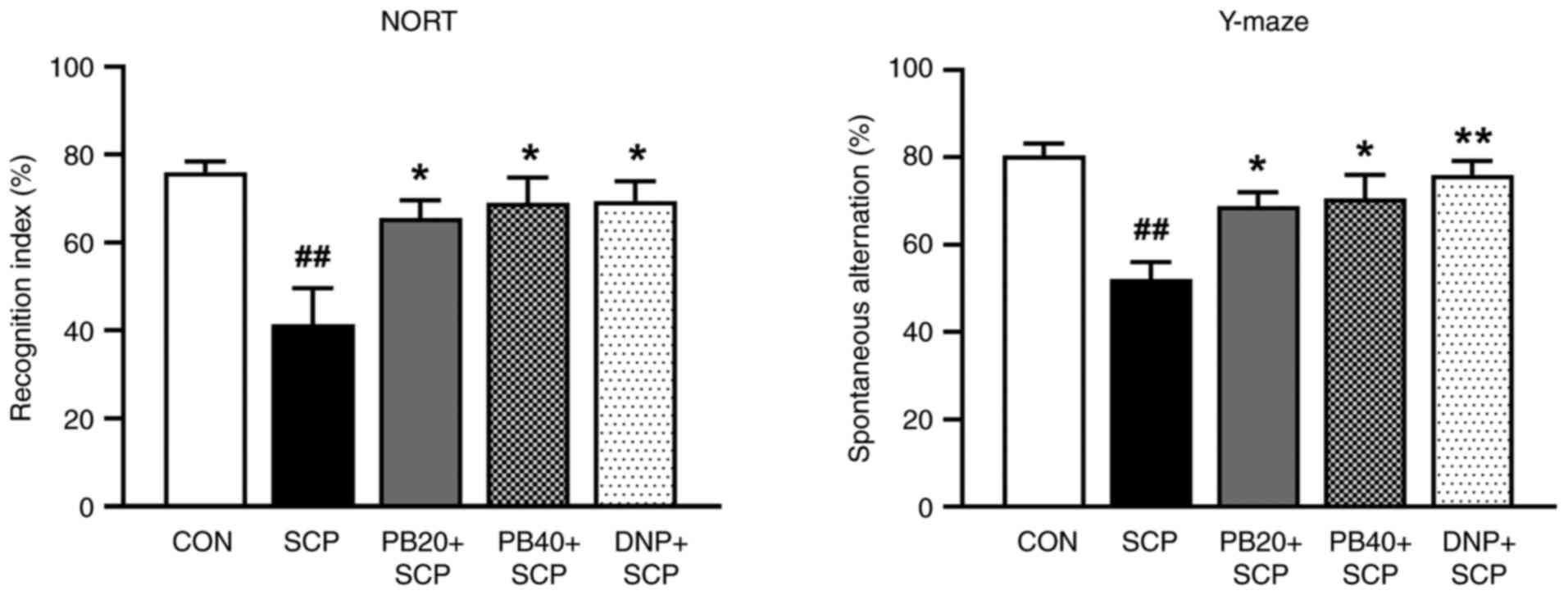
Pinostrobin reverses
scopolamine-induced memory impairment in the NORT and Y-maze
test
The NORT was used to measure whether pinostrobin
could restore scopolamine-induced recognition deficit. The
scopolamine-treated group demonstrated a reduction in RI by
decreased preference for the novel object compared with the control
group (P<0.01). However, the administration of
pinostrobin (20 or 40 mg/kg) or donepezil notably enhanced
recognition memory compared with the scopolamine-treated group
(P<0.05). Moreover, the scopolamine-treated group showed
a significant memory deficit by their decreased percentage of
spontaneous alternation in the Y-maze test compared with that of
the control group (P<0.01). However, oral administration
of pinostrobin (20 or 40 mg/kg) markedly improved the cognitive
performance as compared with the scopolamine-treated group
(P<0.05). Under the same conditions, the standard drug
donepezil also showed a reversal of the scopolamine-induced
cognitive impairment seen in the scopolamine-treated group
(P<0.01) as shown in Fig.
2.
Pinostrobin modulates
scopolamine-induced disruption of oxidative stress and antioxidant
defence in the hippocampus
Fig. 3 shows the
results for oxidative stress and antioxidant markers in the
hippocampus. In the scopolamine-induced group, the level of MDA,
which is a key product of lipid peroxidation, was markedly higher
than that in the control group (P<0.001). Furthermore,
both pinostrobin at a dose of 40 mg/kg and donepezil exhibited
significant reductions in the MDA levels when compared with the
scopolamine-treated group (P<0.05; Fig. 3A). In addition, the activities of
scavenging enzymes, including SOD and GSH, were examined. Notably,
there was a significant decrease in the activities of SOD in the
scopolamine-treated group compared with the control group
(P<0.001), whereas treatment with of pinostrobin or
donepezil resulted in a noticeable increase in SOD activity as
compared with the scopolamine-treated group (PB 20 mg/kg,
P<0.05; PB 40 mg/kg, P<0.01; DNP,
P<0.001; Fig. 3B).
Moreover, treatment with pinostrobin at a high dose (40 mg/kg) or
with donepezil markedly reversed the scopolamine-induced reduction
of GSH activity in the hippocampus as compared with the
scopolamine-treated group (PB 40 mg/kg, P<0.05; DNP,
P<0.01; Fig. 3C).
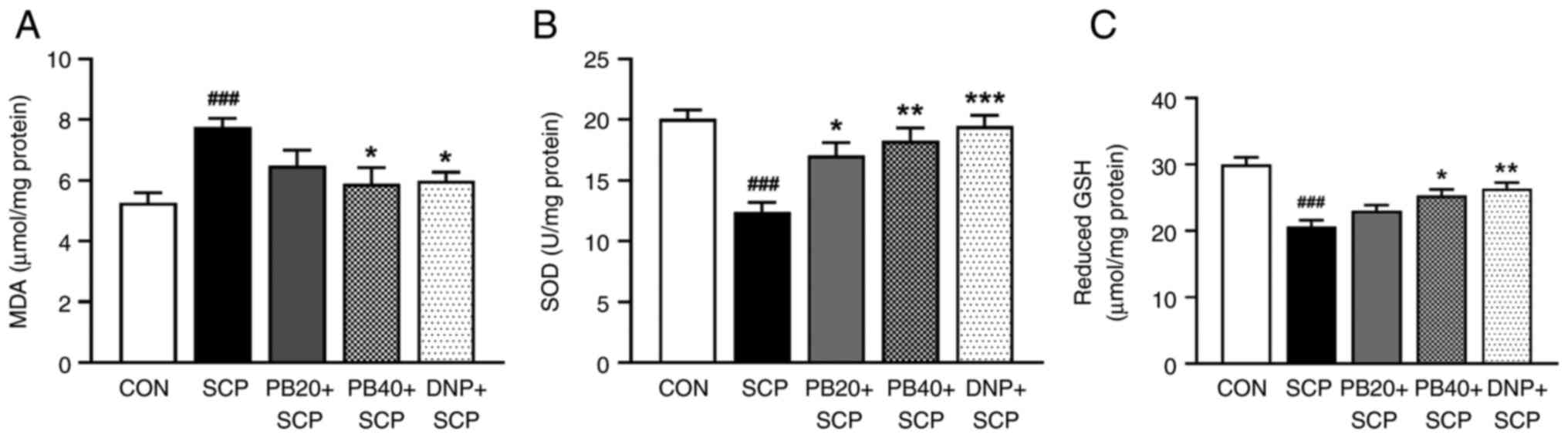 | Figure 3PB modulates SCP-induced oxidative
stress indicators in the hippocampus. Graphs exhibit the (A) MDA
level, (B) SOD activity and (C) reduced GSH activity. Data are
expressed as the mean ± SEM. ###P<0.001 vs. the
control group; *P<0.05, **P<0.01,
***P<0.001 vs. the SCP-treated group. PB,
pinostrobin; SCP, scopolamine; MDA, malondialdehyde; SOD,
superoxide dismutase; GSH, reduced glutathione; CON, control; DNP,
donepezil. |
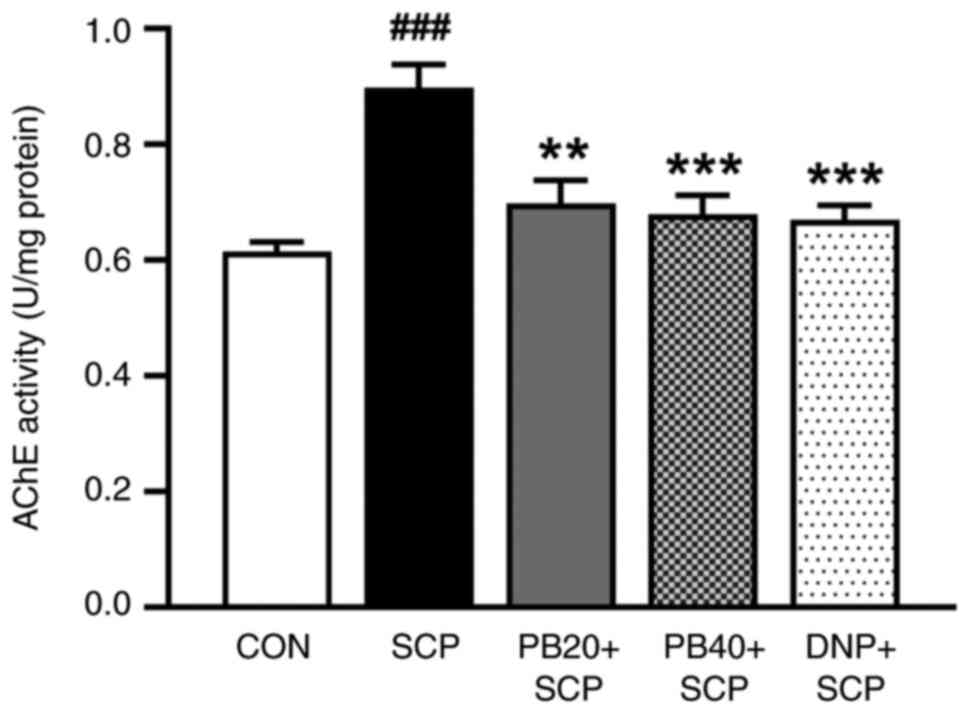
Pinostrobin attenuates
scopolamine-induced AChE hyperactivation in the hippocampus
To evaluate the effect of pinostrobin on cholinergic
function, the AChE enzymatic activity in the hippocampus was also
determined. As illustrated in Fig.
4, compared with the control group, the levels of AChE in the
hippocampus were significantly increased in the scopolamine-induced
group (P<0.01). Importantly, the administration of
pinostrobin (20 or 40 mg/kg) or donepezil significantly suppressed
the scopolamine-induced overactivation of AChE activity in the
hippocampus when compared with the scopolamine-treated group (PB 20
mg/kg, P<0.01; PB 40 mg/kg, P<0.001; DNP,
P<0.001).
Pinostrobin attenuates
scopolamine-induced alteration of ChAT expression in the
hippocampus
To investigate whether pinostrobin affected the
neural response in scopolamine-injected rats, ChAT expression was
determined in the hippocampus using immunohistochemistry as shown
in Fig. 5. The scopolamine-treated
group significantly decreased the expression of ChAT in all
subregions of the hippocampus (CA1, P<0.05; CA2, CA3 and
DG, P<0.001) compared with the control group. Markedly,
treatment with pinostrobin at a dose of 20 mg/kg significantly
suppressed the decrease of ChAT immunoreactivity in CA3 and DG of
the hippocampus compared with the scopolamine-treated group (CA3;
P<0.05, DG; P<0.01). More notably, pinostrobin
at a high dose (40 mg/kg) markedly inhibited the reduction of ChAT
expression in all subregions of the hippocampus (CA1, CA2 and CA3;
P<0.05, DG; P<0.001), which was similar to that
observed in the donepezil group (CA1; P<0.05, CA2;
P<0.01, CA3; P<0.05, DG; P<0.001)
compared with the scopolamine-treated group.
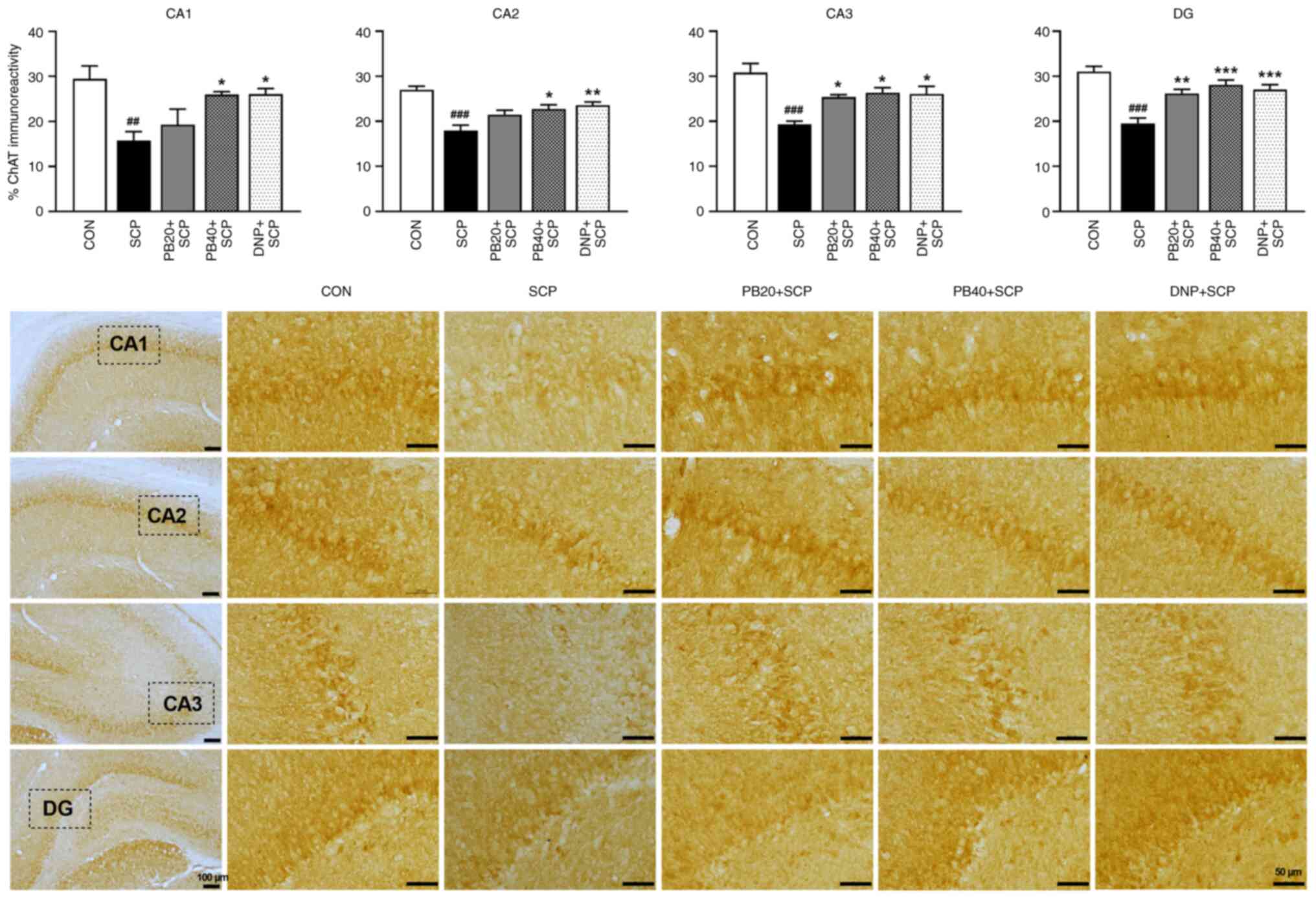 | Figure 5PB attenuates SCP-induced alteration
of ChAT expression in the hippocampus. Representative
photomicrographs showed ChAT expression measured by a percentage of
immunoreactivity in CA1, CA2, CA3 and DG of the hippocampus. Scale
bar, 50 µm; magnification, x40. Data are expressed as the mean ±
SEM. ##P<0.01, ###P<0.001 vs. the
control group; *P<0.05, **P<0.01,
***P<0.001 vs. the scopolamine-treated group. PB,
pinostrobin; SCP, scopolamine; ChAT, choline acetyltransferase;
CA1, cornu ammonis 1; CA2, cornu ammonis 2; CA3, cornu ammonis 3;
DG, dentate gyrus; CON, control; DNP, donepezil. |
Pinostrobin attenuates
scopolamine-induced alteration of GluR1 expression in the
hippocampus
With regard to the effect of pinostrobin on the
activity of glutamate receptors in scopolamine-treated rats, the
expression of GluR1 in the hippocampus was further determined using
immunohistochemistry analysis as shown in Fig. 6. A reduction in GluR1 expression in
the scopolamine-treated group was observed in all subregions of the
hippocampus compared with the control group (P<0.001).
Markedly, treatments with pinostrobin (20 or 40 mg/kg)
significantly enhanced the expression of GluR1 compared with the
scopolamine-treated group (PB 20 mg/kg, CA1, P<0.05; CA2,
P<0.01; CA3, P<0.001; DG, P<0.01; PB
40 mg/kg, CA1, CA2 and CA3, P<0.001; DG;
P<0.01). Moreover, these changes were potentially
reversed by treatment with the AChE inhibitor donepezil (CA1 and
CA2, P<0.01; CA3 and DG, P<0.001), compared
with the scopolamine-treated group.
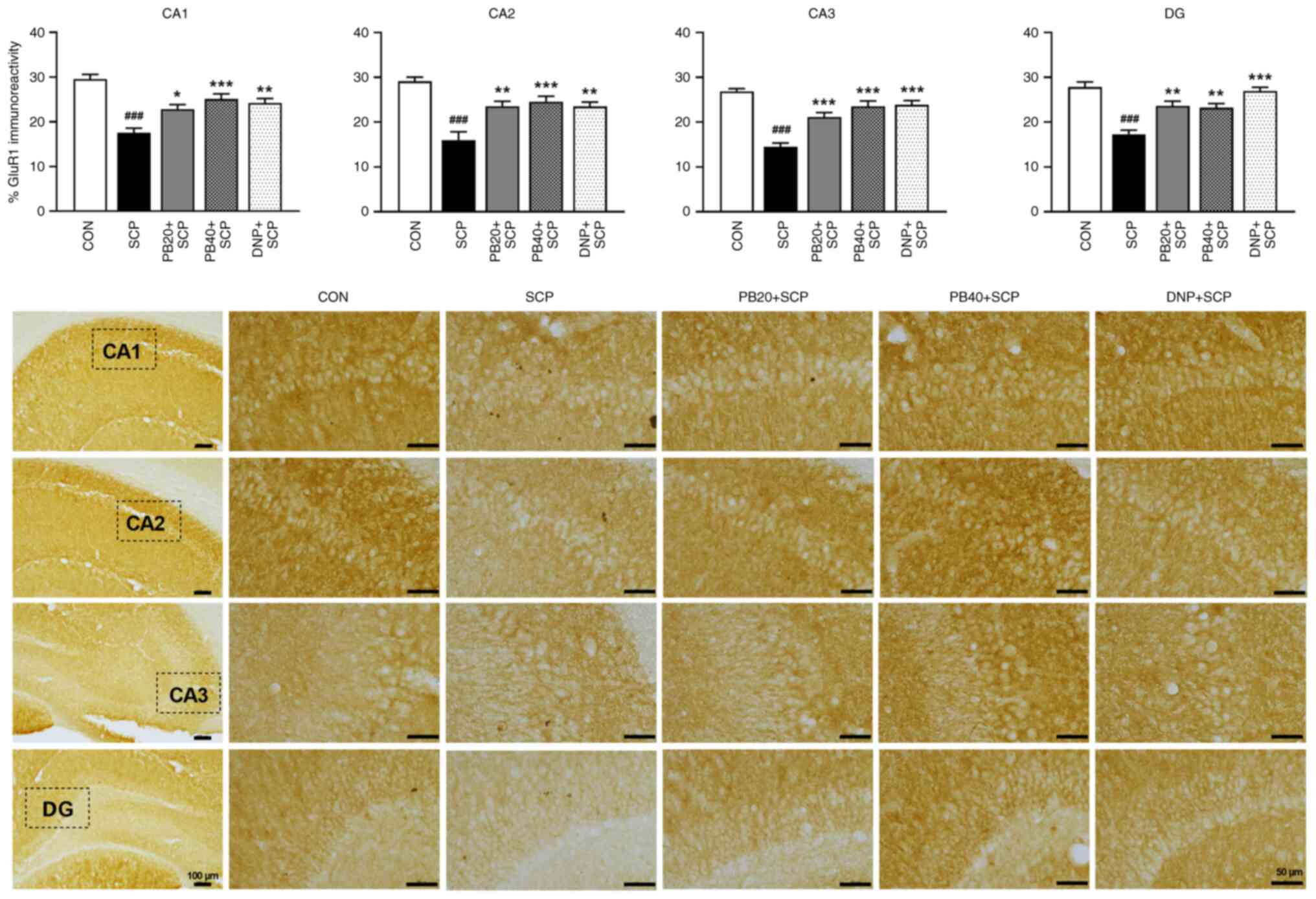 | Figure 6PB attenuates SCP-induced alteration
of GluR1 expression in the hippocampus. Representative
photomicrographs showed GluR1 expression represented by a
percentage of immunoreactivity in CA1, CA2, CA3 and DG of the
hippocampus. Scale bar, 50 µm; magnification, x40. Data are
expressed as the mean ± SEM. ###P<0.001 vs. the
control group; *P<0.05, **P<0.01,
***P<0.001 vs. the scopolamine-treated group. PB,
pinostrobin; SCP, scopolamine; GluR1, glutamate receptor 1; CA1,
cornu ammonis 1; CA2, cornu ammonis 2; CA3, cornu ammonis 3; DG,
dentate gyrus; CON, control; DNP, donepezil. |
Discussion
The present study demonstrated for the first time,
to the best of the authors' knowledge, that pinostrobin, one of the
core active compounds of Boesenbergia rotunda, probably
attenuates memory impairment by the regulation of the oxidative
stress and cholinergic system in an animal model of AD induced by
scopolamine. Scopolamine is a muscarinic acetylcholine receptor
antagonist, which induces cognitive impairment (38). Scopolamine has been shown to cause
surges of oxidative stress including increased reactive oxygen
species (ROS) production in the cortex and the hippocampus
(39). Several behavioural tasks
have been tentatively used to examine learning and memory
performance in scopolamine models, including the Morris water maze
test, novel objective recognition test, passive avoidance test and
Y-maze test (8,10,29).
In the current study, the NORT and Y-maze test were selected to
probe the effects of pinostrobin on scopolamine-induced memory
impairment. From a pragmatic standpoint, both the NORT and Y-maze
test proved highly effective, demanding minimal, brief training.
The NORT is considered to be a consistent behavioural model based
on the time spent exploring new objects that appraises recognition
memory (36). Recognition memory is
a form of sporadic memory that is regularly observed to be
declining during aging in humans and in patients with AD (40). The Y-maze test is a foundation model
for evaluating working memory impairment, which is based on the
willingness of animals to explore new environments (41). Therefore, controlled animals desire
to explore a new arm of the maze rather than the one they have
previously visited. The results of the present study demonstrated
that scopolamine-treated rats illustrated a significant memory
deficit by decreasing the percentages of RI in the NORT and
spontaneous alternation in the Y-maze test. These observations
align with several previous findings (10,42,43).
Unexpectedly, treatment of rats with pinostrobin notably enhanced
the cognitive performance that had been impaired by the injection
of scopolamine. Additionally, to assess the neuroprotective
mechanisms of pinostrobin against scopolamine-induced cognitive
impairment, the effects of pinostrobin on the related changes in
oxidative stress markers and cholinergic activity were
investigated. Oxidative stress can occur when there is excessive
production of ROS under various conditions and it contributes to
the pathogenesis of several neurodegenerative disorders such as AD
(32,44). Studies have demonstrated that
scopolamine-induced memory impairment is associated with increased
oxidative stress within the brain (45,46).
Scopolamine administration decreases the activity of SOD, catalase,
glutathione s-transferase and glutathione peroxidase (GPx)
(47) and it also increases the
level of MDA, a marker of lipid peroxidation (48). Thus, oxidative stress appears to be
a pivotal factor in cognitive decline induced by scopolamine.
Furthermore, the present study showed a significant increase in the
hippocampal MDA concentration that was observed after scopolamine
injection. Similarly, it has been reported that the administration
of scopolamine for 7 days increased the levels of MDA in the brains
of mice (11). Moreover,
scopolamine has been associated with elevated Aβ accumulation and
heightened oxidative stress within the hippocampus of rats
(49). Aβ-mediated oxidative stress
can cause metabolic alterations in the brain, such as lipid
peroxidation and ROS production (50) leading to synaptic loss and cognitive
dysfunction (51). Moreover,
previous studies have indicated that injection of scopolamine
causes oxidative stress by decreasing the activities of antioxidant
enzymes (SOD or GPx) and increasing lipid peroxidation in the brain
(52,53). These findings are consistent with
the present results; the activities of SOD and GSH were markedly
decreased in the scopolamine-treated group. Alternatively,
treatment with pinostrobin or donepezil markedly reversed the
scopolamine-induced increase in MDA concentration. Furthermore, the
reduced activities of SOD and GSH in the hippocampus were markedly
increased by pinostrobin. Therefore, the present results indicated
that pinostrobin might help to alleviate scopolamine-induced memory
deficits by reducing oxidative stress.
Cognitive function is linked to the cholinergic
system and acetylcholine, a neurotransmitter that is widely spread
across the nervous system and plays a vital role in memory and
learning processes (54).
Acetylcholine is degraded in the synaptic cleft by AChE, which
converts it to acetic acid and choline. Excessive activation of
AChE activity can lead to a shortage of ACh and cognitive
dysfunction (10). Studies have
demonstrated that scopolamine injection enhances the level of AChE
(55,56). An elevated level of AChE metabolises
more acetylcholine and decreases its level in the synapses and then
disturbs cholinergic neurotransmission, which results in memory
impairment (8). A previous study
suggested that inhibitors of AChE can potentially reverse memory
deficit induced by scopolamine (57). However, the precise mechanism of
increased AChE activity by scopolamine remains to be elucidated.
Some evidence suggests that scopolamine-induced oxidative stress
may act as a potential mechanism for increasing AChE activity that
results in cognitive impairment (42,58).
The present results demonstrated that the significant dysfunction
of the cholinergic system was observed in scopolamine-treated rats
was accompanied by an increase in AChE activity in the hippocampus
along with memory impairment, which is in accordance with previous
studies (28,59). However, treatment with pinostrobin
and donepezil significantly decreased AChE activity in the
hippocampus. Furthermore, ChAT, the enzyme responsible for
acetylcholine biosynthesis, is one of the most explicit markers for
examining the functional state of cholinergic neurons in the
central nervous system (60).
Studies have indicated that scopolamine leads to reduced ChAT
activity, which is associated with dementia (8,61).
Hence, the present study aimed to determine cholinergic function by
evaluating the expression of ChAT in the hippocampal CA1, CA2, CA3
and DG regions. The immunohistochemistry results revealed that the
scopolamine-treated group showed a significant reduction in ChAT
expression in all subregions of the hippocampus. These results are
consistent with previous research that demonstrate a reduction in
ChAT expression in the CA3 region of the hippocampus following
scopolamine administration (62).
Similarly, a 7-day regimen of scopolamine injections (3 mg/kg)
markedly decreased ChAT expression in the CA1, CA3 and DG regions
of the hippocampus (63). However,
supplementation with pinostrobin at a low dose (20 mg/kg) markedly
enhanced the function of ChAT in the hippocampal CA3 and DG. In
addition, a high dose of pinostrobin (40 mg/kg) or of donepezil
markedly restored the downregulation of hippocampal ChAT expression
in all subregions. Accordingly, the modulation of the cholinergic
activity was counteracted by pinostrobin, suggesting that it could
play a significant role in restoring cognitive function.
Furthermore, the glutamatergic system also plays a
crucial role in cognitive function and working memory in various
brain areas such as the prefrontal cortex and the hippocampus
(64,65). The Schaffer collateral pathway is a
key neural route in the hippocampus, particularly in the CA1 area.
It consists of axons from pyramidal neurons in the CA3 region
projecting to pyramidal neurons in the CA1 region. Glutamate is
essential for the normal functioning of the Schaffer collateral
pathway in the hippocampus, mediating fast synaptic transmission
through AMPA receptors and synaptic plasticity through NMDA
receptors. This relationship is fundamental for processes such as
learning and memory (66). The
function of AMPA receptors (AMPARs) is important for synaptic
plasticity and fundamental circuit of memory and learning (67). The cellular trafficking, synaptic
anchoring and overall function of AMPARs are influenced by the
specific subunits that make up the core ion channel, which is a
tetramer composed of GluA1-GluA4 subunits (also known as
GluR1-GluR4). The activity of AMPARs is dependent on neuronal
activity. Consequently, a model that specifies subunit-dependent
trafficking of AMPARs has been suggested and LTP is considered to
require sequences or domains of the GluA1 subunit (68). Alterations in the number and
function of AMPARs are crucial for synaptic plasticity and the
cognitive decline associated with ageing. AMPARs are highly dynamic
proteins, which are precisely regulated through processes of
trafficking, recycling, degradation and replacement (69).
Studies have reported the involvement of hippocampal
NMDA receptors in the learning and memory process (70,71).
However, the critical role of AMPA receptors in cognitive function
within the hippocampus, particularly in neurodegenerative animal
models, has not been well evaluated. Therefore, the purpose of the
present study was to investigate the function of glutamatergic
AMPARs following scopolamine administration. The present study
revealed that scopolamine injection markedly reduced GluR1
expression within the hippocampus. A previous study indicated that
a decrease in GluA1 expression may contribute to cognitive deficits
associated with ketamine-induced neurotoxicity (16). However, the present findings
demonstrated that pinostrobin and donepezil treatment substantially
increased GluR1 expression in the hippocampus of rats treated with
scopolamine. Although the alleviating effect of pinostrobin has
been determined against oxidative stress, cholinergic and
glutamatergic dysfunction related to memory impairment induced by
scopolamine in adult male rats, further investigations are
recommended to verify the molecular mechanisms of pinostrobin
against scopolamine-induced memory deficit involved in cholinergic,
glutamatergic and inflammatory signalling pathways.
Pinostrobin demonstrates neuroprotective effects by
mitigating cognitive impairment, decreasing oxidative stress and
enhancing neuronal density and astrocyte function in the
hippocampus (26,72). Although previous studies have not
detailed the exact mechanism by which pinostrobin crosses the
blood-brain barrier, its pharmacological properties, such as
antioxidant and anti-inflammatory effects, suggest that it may
interact with transporters or mechanisms that facilitate this
process (73). Moreover, molecular
dynamics simulations have shown that pinostrobin can form inclusion
complexes with beta-cyclodextrin derivatives, potentially improving
its stability and solubility for enhanced bioavailability and
transport across biological barriers, including the blood-brain
barrier (74). Although not
explicitly studied for pinostrobin, other flavonoids such as
pinocembrin are known to interact with transporters such as
P-glycoprotein (P-gp) and multidrug resistance proteins, which
could potentially play a role in the transport of pinostrobin
(75). However, further research is
needed to elucidate the specific transport mechanisms or
interactions that enable pinostrobin's penetration of the
blood-brain barrier, which would deepen our understanding of its
neuroprotective effects.
A limitation of the present study was the lack of
hippocampal NMDA receptor measurement, for an improved
understanding of the involvement of hippocampal glutamate receptors
in cognitive function associated with neurological disorders;
defining the underlying molecular mechanisms of NMDA and AMPA
glutamate is essential for future research. Furthermore, to provide
more robust data on the nootropic effects of pinostrobin or
donepezil alone on cognitive function and oxidative stress markers
without the interference of scopolamine, future studies should
include groups of animals that do not receive scopolamine
treatment. The present study also provided recommendations for
assessing locomotor activity by using the open field that may
influence cognitive function. This is important for distinguishing
between cognitive enhancements and changes in general activity
levels. Lastly, it is further advised to use scoring methods for
histopathological examination that are effective and validate for
quantifying histological changes.
The current study revealed that oral administration
of pinostrobin could improve the cognitive deficit induced in an AD
model by scopolamine. Administration of scopolamine resulted in an
increase in oxidative stress and AChE activity was potentially
alleviated by the treatment with pinostrobin. Additionally,
pinostrobin also enhanced the expression of ChAT and GluR1 in the
hippocampus. Based on these results, the present study proposes
that pinostrobin may serve as a potential therapeutic agent for
restoring memory function by affecting oxidative stress and
cholinergic activity in the hippocampus.
Acknowledgements
Not applicable.
Funding
Funding: The current study was financially supported by the
Thailand Science Research and Innovation fund and the University of
Phayao, Phayao, Thailand (grant no. FF65-RIM121).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ST was responsible for conceptualization,
investigation, methodology, writing, reviewing and editing. TP was
responsible for formal analysis, reviewing and editing the
manuscript. NS was responsible for methodology, reviewing and
editing the manuscript. SS was responsible for methodology and
formal analysis. JJ was responsible for formal analysis, reviewing
and editing the manuscript. RK was responsible for
conceptualization, data curation, formal analysis, funding
acquisition, investigation, methodology, project administration,
supervision, validation, writing the original draft and writing,
reviewing and editing the manuscript. RK and ST confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethical approval and consent to
participate
All experiments were approved by the Ethics
Committee of the Laboratory Animal Research Center, University of
Phayao, Thailand (approval no. 640104029).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mat Nuri TH, Hong YH, Ming LC, Mohd Joffry
S, Othman MF and Neoh CF: Knowledge on Alzheimer's disease among
public hospitals and health clinics pharmacists in the State of
Selangor, Malaysia. Front Pharmacol. 8(739)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Djeuzong E, Kandeda AK, Djiogue S,
Stéphanie L, Nguedia D, Ngueguim F, Djientcheu JP, Kouamouo J and
Dimo T: Antiamnesic and neuroprotective effects of an aqueous
extract of ziziphus jujuba mill. (rhamnaceae) on
scopolamine-induced cognitive impairments in rats. Evid Based
Complement Alternat Med. 2021(5577163)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shabani S and Mirshekar MA: Diosmin is
neuroprotective in a rat model of scopolamine-induced cognitive
impairment. Biomed Pharmacother. 108:1376–1383. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mahdy K, Shaker O, Wafay H, Nassar Y,
Hassan H and Hussein A: Effect of some medicinal plant extracts on
the oxidative stress status in Alzheimer's disease induced in rats.
Eur Rev Med Pharmacol Sci. 16 (Suppl 3):S31–S42. 2012.PubMed/NCBI
|
|
5
|
von Bernhardi R, Eugenin-von Bernhardi L
and Eugenin J: Microglial cell dysregulation in brain aging and
neurodegeneration. Front Aging Neurosci. 7(124)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Blake MG, Krawczyk MC, Baratti CM and
Boccia MM: Neuropharmacology of memory consolidation and
reconsolidation: Insights on central cholinergic mechanisms. J
Physiol Paris. 108:286–291. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lee JS, Kim HG, Lee HW, Han JM, Lee SK,
Kim DW, Saravanakumar A and Son CG: Hippocampal memory enhancing
activity of pine needle extract against scopolamine-induced amnesia
in a mouse model. Sci Rep. 5(9651)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Choi JH, Lee EB, Jang HH, Cha YS, Park YS
and Lee SH: Allium hookeri extracts improve scopolamine-induced
cognitive impairment via activation of the cholinergic system and
anti-neuroinflammation in mice. Nutrients. 13(2890)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tang KS: The cellular and molecular
processes associated with scopolamine-induced memory deficit: A
model of Alzheimer's biomarkers. Life Sci.
233(116695)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hussain H, Ahmad S, Shah SWA, Ullah A, Ali
N, Almehmadi M, Ahmad M, Khalil AAK, Jamal SB, Ahmad H and Halawi
M: Attenuation of scopolamine-induced amnesia via cholinergic
modulation in mice by synthetic curcumin analogs. Molecules.
27(2468)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kim Y, Kim J, He M, Lee A and Cho E:
Apigenin ameliorates scopolamine-induced cognitive dysfunction and
neuronal damage in mice. Molecules. 26(5192)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Safar MM, Arab HH, Rizk SM and El-Maraghy
SA: Bone marrow-derived endothelial progenitor cells protect
against scopolamine-induced alzheimer-like pathological
aberrations. Mol Neurobiol. 53:1403–1418. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Danysz W and Parsons CG: Alzheimer's
disease, β-amyloid, glutamate, NMDA receptors and
memantine-searching for the connections. Br J Pharmacol.
167:324–352. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Eder P, Reinprecht I, Schreiner E,
Skofitsch G and Windisch M: Increased density of glutamate receptor
subunit 1 due to Cerebrolysin treatment: An immunohistochemical
study on aged rats. Histochem J. 33:605–612. 2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dewar D, Chalmers DT, Graham DI and
McCulloch J: Glutamate metabotropic and AMPA binding sites are
reduced in Alzheimer's disease: an autoradiographic study of the
hippocampus. Brain Res. 553:58–64. 1991.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ding R, Li Y, Du A, Yu H, He B, Shen R,
Zhou J, Li L, Cui W, Zhang G, et al: Changes in hippocampal AMPA
receptors and cognitive impairments in chronic ketamine addiction
models: Another understanding of ketamine CNS toxicity. Sci Rep.
6(38771)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang YJ, Chen HB, Wei B, Wang W, Zhou PL,
Zhan JQ, Hu MR, Yan K, Hu B and Yu B: Cognitive decline is
associated with reduced surface GluR1 expression in the hippocampus
of aged rats. Neurosci Lett. 591:176–181. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Aigner TG: Pharmacology of memory:
Cholinergic-glutamatergic interactions. Curr Opin Neurobiol.
5:155–160. 1995.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Francis PT, Parsons CG and Jones RW:
Rationale for combining glutamatergic and cholinergic approaches in
the symptomatic treatment of Alzheimer's disease. Expert Rev
Neurother. 12:1351–1365. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wu Y, Luo X, Liu X, Liu D, Wang X, Guo Z,
Zhu L, Tian Q, Yang X and Wang JZ: Intraperitoneal administration
of a novel TAT-BDNF peptide ameliorates cognitive impairments via
modulating multiple pathways in two Alzheimer's rodent models. Sci
Rep. 5(15032)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fahey JW and Stephenson KK: Pinostrobin
from honey and Thai ginger (Boesenbergia pandurata): A potent
flavonoid inducer of mammalian phase 2 chemoprotective and
antioxidant enzymes. J Agric Food Chem. 50:7472–7476.
2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ijaz MU, Shahzadi S, Hamza A, Azmat R,
Anwar H, Afsar T, Shafique H, Bhat MA, Naglah AM, Al-Omar MA and
Razak S: Alleviative effects of pinostrobin against cadmium-induced
renal toxicity in rats by reducing oxidative stress, apoptosis,
inflammation, and mitochondrial dysfunction. Front Nutr.
10(1175008)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wu D, Nair MG and DeWitt DL: Novel
compounds from Piper methysticum Forst (Kava Kava) roots and their
effect on cyclooxygenase enzyme. J Agric Food Chem. 50:701–705.
2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Patel NK and Bhutani KK: Pinostrobin and
Cajanus lactone isolated from Cajanus cajan (L.) leaves inhibits
TNF-α and IL-1β production: In vitro and in vivo experimentation.
Phytomedicine. 21:946–953. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhao LL, Jayeoye TJ, Ashaolu TJ and
Olatunji OJ: Pinostrobin, a dietary bioflavonoid exerts
antioxidant, anti-inflammatory, and anti-apoptotic protective
effects against methotrexate-induced ovarian toxicity in rats.
Tissue Cell. 85(102254)2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Thongrong S, Surapinit S, Promsrisuk T,
Jittiwat J and Kongsui R: Pinostrobin alleviates chronic restraint
stress-induced cognitive impairment by modulating oxidative stress
and the function of astrocytes in the hippocampus of rats. Biomed
Rep. 18(20)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kongsui R, Surapinit S, Promsrisuk T and
Thongrong S: Pinostrobin from Boesenbergia rotunda
attenuates oxidative stress and promotes functional recovery in rat
model of sciatic nerve crush injury. Braz J Med Biol Res.
56(e12578)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lin J, Huang L, Yu J, Xiang S, Wang J,
Zhang J, Yan X, Cui W, He S and Wang Q: Fucoxanthin, a marine
carotenoid, reverses scopolamine-induced cognitive impairments in
mice and inhibits acetylcholinesterase in vitro. Mar Drugs.
14(67)2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Woo Y, Lim JS, Oh J, Lee JS and Kim JS:
Neuroprotective effects of euonymus alatus extract on
scopolamine-induced memory deficits in mice. Antioxidants (Basel).
9(449)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
31
|
Nakmareong S, Kukongviriyapan U,
Pakdeechote P, Donpunha W, Kukongviriyapan V, Kongyingyoes B,
Sompamit K and Phisalaphong C: Antioxidant and vascular protective
effects of curcumin and tetrahydrocurcumin in rats with
L-NAME-induced hypertension. Naunyn Schmiedebergs Arch Pharmacol.
383:519–529. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Srdjenovic B, Mrdjanovic J, Galovic AJ,
Kladar N, Bozin B, Jurisic V and Bogdanovic G: Effect of ELF-EMF on
antioxidant status and micronuclei in K562 cells and normal
lymphocytes. Open Life Sci. 9:931–940. 2014.
|
|
33
|
Polycarp TN, Obukowho EB and Yusoff SM:
Changes in haematological parameters and oxidative stress response
of goats subjected to road transport stress in a hot humid tropical
environment. Comp Clin Pathol. 25:285–293. 2016.
|
|
34
|
Hinwood M, Tynan RJ, Charnley JL, Beynon
SB, Day TA and Walker FR: Chronic stress induced remodeling of the
prefrontal cortex: Structural re-organization of microglia and the
inhibitory effect of minocycline. Cereb Cortex. 23:1784–1797.
2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Johnson SJ and Walker FR: Strategies to
improve quantitative assessment of immunohistochemical and
immunofluorescent labelling. Sci Rep. 5(10607)2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Batool Z, Sadir S, Liaquat L, Tabassum S,
Madiha S, Rafiq S, Tariq S, Batool TS, Saleem S, Naqvi F, et al:
Repeated administration of almonds increases brain acetylcholine
levels and enhances memory function in healthy rats while
attenuates memory deficits in animal model of amnesia. Brain Res
Bull. 120:63–74. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mooshekhian A, Sandini T, Wei Z, Van
Bruggen R, Li H, Li XM and Zhang Y: Low-field magnetic stimulation
improved cuprizone-induced depression-like symptoms and
demyelination in female mice. Exp Ther Med. 23(210)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Klinkenberg I and Blokland A: The validity
of scopolamine as a pharmacological model for cognitive impairment:
A review of animal behavioral studies. Neurosci Biobehav Rev.
34:1307–1350. 2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Rahimzadegan M and Soodi M: Comparison of
memory impairment and oxidative stress following single or repeated
doses administration of scopolamine in rat hippocampus. Basic Clin
Neurosci. 9:5–14. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Terry AV and Callahan PM: Nicotinic
acetylcholine receptor ligands, cognitive function, and preclinical
approaches to drug discovery. Nicotine Tob Res. 21:383–394.
2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kraeuter AK, Guest PC and Sarnyai Z: The
Y-maze for assessment of spatial working and reference memory in
mice. Methods Mol Biol. 1916:105–111. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Afzal M, Alzarea SI, Alharbi KS, Alzarea
AI, Alenezi SK, Alshammari MS, Alquraini AH and Kazmi I: Rosiridin
attenuates scopolamine-induced cognitive impairments in rats via
inhibition of oxidative and nitrative stress leaded caspase-3/9 and
TNF-α signaling pathways. Molecules. 27(5888)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Bhuvanendran S, Kumari Y, Othman I and
Shaikh MF: Amelioration of cognitive deficit by embelin in a
scopolamine-induced Alzheimer's disease-like condition in a rat
model. Front Pharmacol. 9(665)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Li J, O W, Li W, Jiang ZG and Ghanbari HA:
Oxidative stress and neurodegenerative disorders. Int J Mol Sci.
14:24438–24475. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Fan Y, Hu J, Li J, Yang Z, Xin X, Wang J,
Ding J and Geng M: Effect of acidic oligosaccharide sugar chain on
scopolamine-induced memory impairment in rats and its related
mechanisms. Neurosci Lett. 374:222–226. 2005.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Budzynska B, Boguszewska-Czubara A,
Kruk-Slomka M, Skalicka-Wozniak K, Michalak A, Musik I and Biala G:
Effects of imperatorin on scopolamine-induced cognitive impairment
and oxidative stress in mice. Psychopharmacology (Berl).
232:931–942. 2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Uma G and Maheswari SU: Neuroprotective
effects of polyherbal formulation (Indian NONI) on
scopolamine-induced memory impairment in mice. Int J Pharm Pharm
Sci. 6:354–357. 2014.
|
|
48
|
Abd-El-Fattah MA, Abdelakader NF and Zaki
HF: Pyrrolidine dithiocarbamate protects against
scopolamine-induced cognitive impairment in rats. Eur J Pharmacol.
723:330–338. 2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Hernández-Rodríguez M, Arciniega-Martínez
IM, García-Marín ID, Correa-Basurto J and Rosales-Hernández MC:
Chronic administration of scopolamine increased GSK3βP9, beta
secretase, amyloid beta, and oxidative stress in the hippocampus of
wistar rats. Mol Neurobiol. 57:3979–3988. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Butterfield DA, Castegna A, Lauderback CM
and Drake J: Evidence that amyloid beta-peptide-induced lipid
peroxidation and its sequelae in Alzheimer's disease brain
contribute to neuronal death. Neurobiol Aging. 23:655–664.
2002.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Gul S, Attaullah S, Alsugoor MH, Bawazeer
S, Shah SA, Khan S, Salahuddin HS and Ullah M: Folicitin abrogates
scopolamine induced oxidative stress, hyperlipidemia mediated
neuronal synapse and memory dysfunction in mice. Heliyon.
9(e16930)2023.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Guo C, Shen J, Meng Z, Yang X and Li F:
Neuroprotective effects of polygalacic acid on scopolamine-induced
memory deficits in mice. Phytomedicine. 23:149–155. 2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Liao J, Nai Y, Feng L, Chen Y, Li M and Xu
H: Walnut oil prevents scopolamine-induced memory dysfunction in a
mouse model. Molecules. 25(1630)2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ghias M, Shoaib M, Ali Shah SW, Umar MN
and Ullah S, Ali N, Shah I and Ullah S: Nootropic effects of
synthetic flavonoid derivatives on scopolamine induced memory
impairment in mice via cholinesterase inhibition and antioxidant
system. Pak J Pharm Sci. 32 (5 Suppl):S2325–S2332. 2019.PubMed/NCBI
|
|
55
|
Soodi M, Naghdi N, Hajimehdipoor H,
Choopani S and Sahraei E: Memory-improving activity of Melissa
officinalis extract in naive and scopolamine-treated rats. Res
Pharm Sci. 9:107–114. 2014.PubMed/NCBI
|
|
56
|
Yadang FSA, Nguezeye Y, Kom CW, Betote
PHD, Mamat A, Tchokouaha LRY, Taiwé GS, Agbor GA and Bum EN:
Scopolamine-induced memory impairment in mice: Neuroprotective
effects of carissa edulis (forssk.) valh (apocynaceae) aqueous
extract. Int J Alzheimers Dis. 2020(6372059)2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Chaudhaery SS, Roy KK, Shakya N, Saxena G,
Sammi SR, Nazir A, Nath C and Saxena AK: Novel carbamates as orally
active acetylcholinesterase inhibitors found to improve
scopolamine-induced cognition impairment: Pharmacophore-based
virtual screening, synthesis, and pharmacology. J Med Chem.
53:6490–6505. 2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Härtl R, Gleinich A and Zimmermann M:
Dramatic increase in readthrough acetylcholinesterase in a cellular
model of oxidative stress. J Neurochem. 116:1088–1096.
2011.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Eshaghi Ghalibaf MH, Rajabian A, Parviz M,
Akbarian M, Amirahmadi S, Vafaee F and Hosseini M: Minocycline
alleviated scopolamine-induced amnesia by regulating antioxidant
and cholinergic function. Heliyon. 9(e13452)2023.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Oda Y: Choline acetyltransferase: The
structure, distribution and pathologic changes in the central
nervous system. Pathol Int. 49:921–937. 1999.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Sohn E, Lim HS, Kim YJ, Kim BY and Jeong
SJ: Annona atemoya leaf extract improves scopolamine-induced memory
impairment by preventing hippocampal cholinergic dysfunction and
neuronal cell death. Int J Mol Sci. 20(3538)2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Lim DW, Son HJ, Um MY, Kim IH, Han D, Cho
S and Lee CH: Enhanced cognitive effects of demethoxycurcumin, a
natural derivative of curcumin on scopolamine-induced memory
impairment in mice. Molecules. 21(1022)2016.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Olayinka JN, Eduviere A, Adeoluwa O,
Akinluyi E, Obisesan A, Akawa O and Adebanjo A: Quercetin mitigates
scopolamine-induced memory dysfunction: Impact on oxidative stress
and cholinergic mechanisms. Metab Brain Dis. 37:265–277.
2022.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Cheng YJ, Lin CH and Lane HY: Involvement
of cholinergic, adrenergic, and glutamatergic network modulation
with cognitive dysfunction in Alzheimer's disease. Int J Mol Sci.
22(2283)2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Hascup KN, Findley CA, Sime LN and Hascup
ER: Hippocampal alterations in glutamatergic signaling during
amyloid progression in AβPP/PS1 mice. Sci Rep.
10(14503)2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Kwon O, Feng L, Druckmann S and Kim J:
Schaffer collateral inputs to CA1 excitatory and inhibitory neurons
follow different connectivity rules. J Neurosci. 38:5140–5152.
2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Royo M, Escolano BA, Madrigal MP and
Jurado S: AMPA receptor function in hypothalamic synapses. Front
Synaptic Neurosci. 14(833449)2022.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Hayashi Y, Shi SH, Esteban JA, Piccini A,
Poncer JC and Malinow R: Driving AMPA receptors into synapses by
LTP and CaMKII: requirement for GluR1 and PDZ domain interaction.
Science. 287:2262–2267. 2000.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Henley JM and Wilkinson KA: AMPA receptor
trafficking and the mechanisms underlying synaptic plasticity and
cognitive aging. Dialogues Clin Neurosci. 15:11–27. 2013.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Bye CM and McDonald RJ: A specific role of
hippocampal NMDA receptors and arc protein in rapid encoding of
novel environmental representations and a more general long-term
consolidation function. Front Behav Neurosci. 13(8)2019.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Yamada K, Arai M, Suenaga T and Ichitani
Y: Involvement of hippocampal NMDA receptors in encoding and
consolidation, but not retrieval, processes of spontaneous object
location memory in rats. Behav Brain Res. 331:14–19.
2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Li C, Tang B, Feng Y, Tang F, Pui-Man Hoi
M, Su Z and Ming-Yuen Lee S: Pinostrobin exerts neuroprotective
actions in neurotoxin-induced Parkinson's disease models through
Nrf2 induction. J Agric Food Chem. 66:8307–8318. 2018.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Gao WY, Chen PY, Chen SF, Wu MJ, Chang HY
and Yen JH: Pinostrobin inhibits proprotein convertase
subtilisin/kexin-type 9 (PCSK9) gene expression through the
modulation of FoxO3a protein in HepG2 cells. J Agric Food Chem.
66:6083–6093. 2018.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Kicuntod J, Khuntawee W, Wolschann P,
Pongsawasdi P, Chavasiri W, Kungwan N and Rungrotmongkol T:
Inclusion complexation of pinostrobin with various cyclodextrin
derivatives. J Mol Graph Model. 63:91–98. 2016.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Yang ZH, Sun X, Qi Y, Mei C, Sun XB and Du
GH: Uptake characteristics of pinocembrin and its effect on
p-glycoprotein at the blood-brain barrier in in vitro cell
experiments. J Asian Nat Prod Res. 14:14–21. 2012.PubMed/NCBI View Article : Google Scholar
|