Introduction
Mental disorders are considered a global public
health issue and continue to be a major burden worldwide (1). As stated by the National Institute of
Mental Health, these disorders mainly affect the mentality,
behavior or emotions of an individual, and can vary in terms of
impairment degree, significantly influencing their activities,
education, employment and social participation. Among the mental
disorders that can lead to psychosis is schizophrenia (SZ), which
is considered one of the most severe and debilitating psychiatric
disorders with a mean lifetime prevalence of ~1% of the population
(2). This percentage varies
according to ethnicity, culture and geographical area (3). In the Arab world, in particular,
several studies have reported that SZ affects 0.7-5.6% of the
population (4). Recurrent episodes
of psychosis characterized by hallucinations, delusions and
cognitive impairment represent the primary symptoms of the disease.
These symptoms can vary across patients and throughout the course
of the disease (5). Genetically,
there is considerable evidence suggesting that SZ has a
heritability rate of 66-85% (6);
the remaining influence may be attributed to environmental factors.
Nevertheless, the exact mechanism by which gene-environment
interactions influence the susceptibility to SZ remains
unclear.
Genome-wide association studies have traditionally
been used to investigate various complex disorders (7-11).
Previous association studies have aimed to identify genetic
variations, known as single nucleotide polymorphisms (SNPs), within
genes with known neurological functions and to investigate their
contribution to the development of SZ (7,10,11).
Various effects of these variants have been reported, and both
common and rare variants have been associated with either large or
small individual susceptibility to the disease (12-15).
These variants could influence the expression of genes involved in
brain development, providing researchers with valuable insights
into the brain dysfunction underlying the symptoms of SZ. The
neurodevelopmental hypothesis of SZ previously proposed that the
interaction between genetic and environmental factors, such as
vitamin D (VD) deficiency, can alter brain function during early
critical phases of brain development, causing brain impairment and
dysfunction (16-19).
VD is considered a neurosteroid regulator that exerts its action
through binding to the VD receptor (VDR) in numerous tissues,
including the brain (20,21). Therefore, VD is essential for proper
neurodevelopment, and cognitive and behavioral function.
Based on the previous literature, VD imbalance has
been associated with the development of numerous psychiatric
disorders, including SZ (20,22,23).
Accordingly, a number of studies have investigated the genetic
determinants of this hormone (24-27).
Research has aimed to determine whether specific genetic variations
in VD metabolism genes are associated with VD levels or VD-related
health outcomes. In the present study, eight SNPs were investigated
within VD metabolism-related genes to assess their potential
association with SZ susceptibility in Jordanian patients. These
SNPs included rs10741657, a 5' UTR A/G substitution associated with
altered enzyme activity of CYP2R1 and hypovitaminosis D,
where the GG genotype is linked to decreased [25(OH)D] levels
compared with the AA genotype (28,29).
Additionally, the CYP27B1 SNPs rs10877012 (G>T) and
rs4646536 (A>G) have been reported to influence circulating
calcitriol serum levels and to be associated with type 1 diabetes
(30). Furthermore, the rs6013897
SNP located at the 3' flanking region of the CYP24A1 gene
has been revealed to be positively associated with circulating
[25(OH)D] levels (31). Other SNPs
distributed across the VDR gene, such as rs2228570,
rs1544410, rs731236 and rs7975232 (32-34),
have been extensively studied and were revealed to affect gene
expression and VDR protein levels, with variable levels of
distribution across ethnicities and sexes. These SNPs have been
associated with neurodevelopmental and neuropsychiatric disorders,
including SZ, mental health disorders and autism, although the
findings are conflicting. In order to create a panel of key SNPs
linked to SZ, the present study intended to identify how these
studied SNPs, which were selected based on their known influence on
VD metabolism and their association with neurological disorders,
might be associated with SZ susceptibility in a group of Jordanian
patients with SZ.
Materials and methods
Study participants
A total of 400 subjects were enrolled in the present
study and divided into two groups: i) 200 patients diagnosed with
SZ attending a psychiatric clinic at King Abdullah University
Hospital and Princess Basma Teaching Hospital (Irbid, Jordan); ii)
200 healthy controls free of any psychosis-related symptoms
attending the National Center for Diabetes Endocrinology and
Genetics (Amman, Jordan) for routine health care. The present study
was approved by the Institutional Review Board Committee (approval
no. 2019/626) of Jordan University of Science and Technology
(Irbid, Jordan) and written informed consent was obtained from all
participants before enrollment in the present study.
Sample collection
After collecting the consent forms signed by all of
the enrolled patients with SZ and controls, two peripheral blood
samples were collected in plain (5 ml) and EDTA (4 ml) tubes. The
samples were collected between May 1, 2020 and September 30, 2021.
Serum was separated after centrifuging blood samples in plain tubes
at 10,000 x g for 10 min at 4˚C and was stored at -80˚C for later
use to measure VD concentration. Notably, hemolyzed samples were
excluded from the present study. Blood samples in EDTA tubes were
used for DNA extraction and further analysis.
Inclusion and exclusion criteria
Patients were diagnosed by a proficient psychiatrist
according to the diagnostic criteria for SZ based on the ICD-10
(DSM-V) (35). Experienced
psychiatrists conducted these diagnoses, following standard
guidelines to ensure accuracy. Patients were treated according to
the latest best practices and medical standards as outlined by the
guidelines issued by the American Psychiatric Association. The
participants who met the following criteria were included: i)
Either sex between 18-60 years old; ii) sporadic and familial
cases; iii) no history of other mental disorders; iv) no history of
blood transfusion within 1 month; v) no physical and nervous system
diseases, such as brain trauma; vi) no history of alcohol abuse and
drug abuse. The exclusion criteria were: i) Presence of other
medical conditions, which may produce psychotic SZ-related
symptoms, such as epilepsy, metabolic disturbance, brain lesions,
limbic encephalitis, stroke, multiple sclerosis and dementia; ii)
presence of other diseases or medications known to affect VD, such
as arthritis, osteoporosis, end-stage renal disease,
hypothyroidism, rickets, corticosteroid therapy and malabsorption
syndromes; and iii) individuals with drug-induced psychosis,
acquired brain injuries and intellectual disabilities.
Genomic DNA extraction
Genomic DNA was extracted using the QIAamp DNA Mini
Kit (cat. no. 51304; Qiagen GmbH) according to the manufacturer's
instructions. DNA concentration was measured using a Nanodrop
(Thermo Fisher Scientific, Inc.) and integrity was verified using
2% agarose gel electrophoresis.
SNP selection and genotyping
For SNP genotyping, two sets of allele-specific
primers for each SNP were designed using the PRIMER1 tool
(http://primer1.soton.ac.uk/primer1.html).
Tetra-amplification refractory mutation system-polymerase chain
reaction (T-ARMS-PCR) was carried out for rs10741657, rs10877012,
rs4646536, rs6013897, rs2228570, rs1544410, rs731236 and rs7975232
genotyping. The primer sequences are listed in Table SI. The annealing temperature for
each primer set was optimized through gradient PCR that was carried
out on a Veriti™ Dx 96-well Thermal Cycler (Thermo Fisher
Scientific, Inc.). Each PCR was carried out in a total volume of 20
µl, containing 4 µl HOT FIREPol® Blend Master Mix (cat.
no. 04-27-00115; Solis BioDyne), 0.5 µl each primer, 1 µl DNA
template and 13 µl nuclease-free water. The PCR cycling conditions
were as follows: Initial denaturation at 95˚C for 10 min, followed
by 35 cycles of denaturation at 95˚C for 15 sec, annealing at
61-64˚C for 30 sec, extension at 72˚C for 2 min, and a final
extension at 72˚C for 10 min. Subsequently, 10 µl PCR products were
loaded onto a 3% agarose gel, and a 100-bp ladder (cat. no. MBT049;
HiMedia Laboratories) was used for size comparison between DNA
fragments. For gel visualization, a UV transilluminator (Cleaver
Scientific Ltd.) was used.
VD concentration measurement
Serum 25(OH)D levels were determined using the
commercially available Roche Elecsys Vitamin D total II
electrochemiluminescence immunoassay and Cobas E801 auto analyzer
(Roche Diagnostics GmbH).
Statistical analysis
Statistical analysis was carried out using SPSS
software (Version 22; IBM Inc.). The genotype frequencies of all
SNPs were compared using a χ2 test or Fisher's exact
test when >20% of expected cell counts are <5. Logistic
regression analysis was performed to determine the odds ratio (OR)
and 95% confidence interval associated with SZ risk, with the
control considered the reference group. Each polymorphism was
tested for Hardy-Weinberg equilibrium (HWE) using χ2
test or Fisher's exact test. P<0.05 was considered to indicate a
statistically significant difference.
Moreover, a total of 370 cases were included for the
receiver operating characteristic (ROC) curve analysis after
excluding individuals with missing genotype data for any of the
investigated SNPs. For the generalized linear model, the analysis
was conducted on a subset of 251 cases due to a lack of VD
measurement data or genotype data for one of the studied SNPs. ROC
curve analysis was performed using the classify module in SPSS
software, encompassing all studied SNPs, to predict the presence of
SZ. Subsequently, the generalized linear model was employed,
utilizing binary logistic regression in the type of model menu. The
dependent variable in the response menu was set as the status of
the samples, referencing the control samples. The predictor menu
included the three studied SNPs as factors, while VD levels and age
served as covariates. The wild-type genotype for each SNP was
designated as the baseline category, coded as 1. Within the model
menu, the main effect for all SNPs and VD was specified. Finally,
the likelihood ratio test with profile likelihood was utilized for
model evaluation. These comprehensive methods were implemented to
investigate the predictive utility of the included SNPs through ROC
curve analysis and to explore the association between genetic
variants, VD levels, age and SZ status using generalized linear
modeling techniques.
Furthermore, SRplot (http://www.bioinformatics.com.cn/en) was used to
perform Mann-Whitney U test when comparing VD levels between
patients with SZ and controls, which included 272 cases in total.
The effect of sex variation between groups on the genotyping
frequency was assessed using Pearson's χ2 test or
Fisher's exact test. Linkage disequilibrium (LD) analysis was
conducted using the SNP linkage LD heatmap module available on
SRplot (http://www.bioinformatics.com.cn/srplot). This module
calculates pairwise LD statistics, measured by R2,
between SNPs. These statistical data are visually represented in a
triangular heatmap, where the extent of LD between SNP pairs is
indicated through a color-coded scale. The color key is used to
denote R2 values, enhancing the visual interpretation of
LD strength. Additionally, the heatmap integrates gene models with
SNP sites marked by colored asterisks, providing a clear genetic
landscape and facilitating the identification of regions with
strong LD. This graphical representation allows for an efficient
analysis and easy interpretation of the LD patterns across the
genomic regions studied.
Results
Demographic characteristics of the
sample population
A summary of the demographic data of the patients
with SZ and controls is presented in Table I. The average age of the control
group was ~57 years (range, 18-76 years), while it was 42 years in
the SZ group (range, 19-78 years). The case and control groups
exhibited a significant difference in their mean age. Among the SZ
group, there were 36 female and 164 male patients and in the
control group, there were 158 female and 42 male healthy
individuals.
 | Table IBaseline characteristics of patients
with schizophrenia and controls. |
Table I
Baseline characteristics of patients
with schizophrenia and controls.
| A, Characteristic
(sex) |
|---|
| Subjects | Male | Female |
|---|
| All subjects
(n=400) | 206 (51.5%) | 194 (48.5%) |
| Patients
(n=200) | 164 (82%) | 36 (18%) |
| Healthy controls
(n=200) | 42 (21%) | 158 (79%) |
| B, Characteristic
(mean age, years) |
| Patients | 42.09±58 | |
| Healthy
controls | 57.38±73 | |
Identification of VD metabolic pathway
gene SNPs
T-ARMS-PCR was carried out to amplify the DNA
fragments of the eight SNPs in VD metabolic pathway genes,
including CYP2R1 (rs10741657), CYP27B1 (rs10877012
and rs4646536), CYP24A1 (rs6013897) and VDR
(rs2228570, rs1544410, rs731236 and rs7975232). T-ARMS-PCR is a
genotyping method designed to detect SNPs by utilizing two pairs of
primers in a single PCR reaction: One pair flanking the SNP (outer
primers) and another pair that specifically anneals depending on
the allele presence (allele-specific or inner primers). This
technique generates two products per allele: One common product and
one allele-specific product, which allows for the determination of
the zygosity of the sample directly by gel electrophoresis
(36). While T-ARMS-PCR is
efficient for detecting known variants, Sanger sequencing is
essential for validation. Due to budget constraints, the cost of
sequencing and limited local sequencing facilities, Sanger
sequencing was not possible for utilization in the present study.
Therefore, the T-ARMS-PCR method was utilized as a practical
alternative for variant detection. Fig. S1, Fig.
S2, Fig. S3, Fig. S4, Fig.
S5, Fig. S6, Fig. S7 and Fig. S8 illustrate the T-ARMS-PCR
genotyping results for the studied SNPs.
Genotype and allele frequencies of
SNPs among patients and controls
CYP2R1 (rs10741657; A>G). The genotype and
allele frequencies of rs10741657 among all participants are
summarized in Tables II and
SII, respectively. Statistical
analysis of the results revealed a significant difference in
genotype and allele frequencies among patients and controls
(P<0.0001). The findings also suggested that the AA genotype and
the A allele were more prevalent among patients with SZ compared
with the controls.
 | Table IIGenotype frequencies of the studied
SNPs in the sample population. |
Table II
Genotype frequencies of the studied
SNPs in the sample population.
| Gene | SNP | Genotype | Healthy controls
n=170 (%) | Patients n=200
(%) | OR (95% CI) | P-value |
|---|
| CYP2R1 | rs10741657 | GG | 134(79) | 112(56) | 1 | <0.0001 |
| | | AG | 30(18) | 72(36) | 2.87
(1.75-4.71) | |
| | | AA | 6(4) | 16(8) | 3.19
(1.21-8.43) | |
| CYP27B1 | rs10877012 | GG | 152(89) | 144(72) | 1 | <0.0001 |
| | | GT | 18(11) | 50(25) | 2.93
(1.63-5.26) | |
| | | TT | 0 (0) | 6(3) | - | |
| | rs4646536 | AA | 104(61) | 140(70) | 1 | 0.18 |
| | | AG | 58(34) | 51(26) | 0.65
(0.41-1.03) | |
| | | GG | 8(5) | 9(4) | 0.84
(0.31-2.24) | |
| CYP24A1 | rs6013897 | TT | 72(42) | 129(64) | 1 | <0.0001 |
| | | TA | 96(56) | 47(24) | 0.27
(0.17-0.43) | |
| | | AA | 2(1) | 24(12) | 6.70
(1.54-29.16) | |
| VDR | rs2228570 | AA | 7(4) | 10(5) | 1 | 0.81 |
| | | AG | 78(46) | 96(48) | 1.11
(0.73-1.69) | |
| | | GG | 85(50) | 94(47) | 1.29
(0.47-3.54) | |
| | rs1544410 | CC | 47(28) | 63(32) | 1 | 0.45 |
| | | CT | 86(51) | 88(44) | 0.76
(0.47-1.23) | |
| | | TT | 37(22) | 49(24) | 0.99
(0.56-1.75) | |
| | rs731236 | AA | 63(37) | 75(38) | 1 | 0.48 |
| | | AG | 82(48) | 87(44) | 0.89
(0.57-1.40) | |
| | | GG | 25(15) | 38(19) | 1.28
(0.70-2.34) | |
| | rs7975232 | CC | 27(16) | 19(10) | 1 | 0.15 |
| | | CA | 75(44) | 101(50) | 1.14
(0.74-1.78) | |
| | | AA | 68(40) | 80(40) | 0.60
(0.31-1.17) | |
CYP27B1 (rs10877012; G>T). The
distribution of rs10877012 genotypes and alleles among patients
with SZ and controls are shown in Tables II and SII. A significant difference was shown in
both genotype and allele frequencies between the two groups
(P<0.0001). The results also indicated a higher prevalence of
the TT genotype and the T allele in patients with SZ compared with
the controls.
CYP27B1 (rs4646536; A>G). The genotype and
allele frequencies among patients with SZ and controls are revealed
in Tables II and SII. Statistical analysis of the data
revealed no significant differences in both genotype and allele
frequencies between the two groups (P=0.18 and 0.165,
respectively).
CYP24A1 (rs6013897; T>A). The genotype and
allele distributions of the rs6013897 SNP in patients with SZ and
controls are shown in Tables II
and SII. The AA genotype was
significantly more frequent in the patient group (P<0.0001).
Regarding the allele frequency, there was no statistically
significant association identified; however, the A allele appeared
to be slightly more frequent in the patient group.
VDR (rs2228570; A>G). The frequencies of
the rs2228570 genotypes and alleles among patients with SZ and
controls are revealed in Tables II
and SII. The genotype and allele
distributions were not significantly different between patients and
controls (P=0.81 and 0.26, respectively).
VDR (rs1544410; C>T). The genotype and
allele frequencies for the rs1544410 SNP are presented in Tables II and SII. Data analysis revealed no
significant differences between patients and controls regarding
both genotype and allele distributions (P=0.45 and 0.88,
respectively).
VDR (rs731236; A>G). The frequencies of
genotypes and alleles for rs731236 among the two groups are
summarized in Tables II and
SII. Data analysis revealed no
significant differences between patients and controls in both
genotype and allele distributions (P=0.48 and 0.51,
respectively).
VDR (rs7975232; C>A). The genotype and
allele frequencies of the rs7975232 SNP are illustrated in Tables II and SII. Statistical analysis of genotype and
allele frequencies revealed no significant difference between
patients with SZ and controls (P=0.15 and 0.21, respectively).
Based on the findings obtained, three SNPs:
CYP2R1 (rs10741657; A>G), CYP27B1 (rs10877012;
G>T) and CYP24A1 (rs6013897; T>A) exhibited
statistically significant differences between the two groups. To
evaluate the impact of sex variation on the results, a Pearson's
χ2 test or Fisher's exact test was conducted, which
revealed no significant differences in the frequency of SNPs among
controls (male and female controls): χ2=0.685, P=0.953
for CYP24A1 (rs6013897; T>A); χ2=1.20, P=0.549
for CYP27B1 (rs10877012; G>T); and χ2=2.92,
P=0.571 for CYP2R1 (rs10741657; A>G). Similarly, there
were no significant differences in the frequency of these SNPs
among patients (male and female patients): χ2=0.449
(P=0.799) for CYP24A1 (rs6013897; T>A);
χ2=1.90 (P=0.387) for CYP27B1 (rs10877012;
G>T); and χ2=1.48 (P=0.477) for CYP2R1
(rs10741657; A>G) (Table III).
These results confirmed that male or female sex does not have a
significant impact on the genotype frequency between patients and
controls.
 | Table IIISignificance of differences in
frequency of assigned SNPs among males and females of both study
groups. |
Table III
Significance of differences in
frequency of assigned SNPs among males and females of both study
groups.
| | Control | Patients |
|---|
| Gene/SNP | χ2 | P-value | χ2 | P-value |
|---|
| CYP2R1
(rs10741657) | 2.92 | 0.57 | 1.48 | 0.48 |
| CYP27B1
rs10877012) | 1.20 | 0.55 | 1.90 | 0.39 |
| CYP24A1
(rs6013897) | 0.69 | 0.95 | 0.45 | 0.80 |
HWE
Differences between observed and expected genotype
frequencies for each SNP were determined by χ2 test or
Fisher's exact test to assess the deviation from HWE and to
identify any possible genotyping error that could exist. As
demonstrated in Table IV, the
genotype and allele frequency of five SNPs (rs10741657, rs10877012,
rs1544410, rs731236 and rs7975232) were in HWE. However, two SNPs
(rs6013897 and rs2228570) deviated significantly from HWE in both
cases and controls.
 | Table IVHardy-Weinberg equilibrium tests for
SNPs in the case and control groups. |
Table IV
Hardy-Weinberg equilibrium tests for
SNPs in the case and control groups.
| | Controls | | Patients | |
|---|
| Gene/SNP | Genotype | Observed
genotype | Expected
genotype | P-value | Observed
genotype | Expected
genotype | P-value |
|---|
| CYP2R1
(rs10741657) | A/A | 6 | 3.2 | 0.08 | 16 | 13.5 | 0.36 |
| | A/G | 37 | 42.5 | | 72 | 77 | |
| | G/G | 143 | 140.2 | | 112 | 109.5 | |
| CYP27B1
(rs10877012) | G/G | 175 | 175.4 | 0.50 | 144 | 142.8 | 0.52 |
| | G/T | 18 | 17.2 | | 50 | 52.4 | |
| | T/T | 0 | 0.4 | | 6 | 4.8 | |
| CYP27B1
(rs4646536) | A/A | 114 | 114.3 | 0.91 | 140 | 137 | 0.13 |
| | A/G | 62 | 61.5 | | 51 | 57.1 | |
| | G/G | 8 | 8.3 | | 9 | 5.95 | |
| CYP24A1
(rs6013897) | T/T | 76 | 90.4 | <0.001 | 129 | 116.3 | <0.001 |
| | T/A | 106 | 77.1 | | 47 | 72.4 | |
| | A/A | 2 | 16.4 | | 24 | 11.3 | |
| VDR
(rs2228570) | A/A | 7 | 13 | 0.03 | 10 | 16.8 | 0.02 |
| | A/G | 88 | 76 | | 96 | 82.4 | |
| | G/G | 105 | 111 | | 94 | 100.8 | |
| VDR
(rs1544410) | C/C | 57 | 56.2 | 0.82 | 63 | 57.2 | 0.10 |
| | C/T | 98 | 99.6 | | 88 | 99.5 | |
| | T/T | 45 | 44.2 | | 49 | 43.2 | |
| VDR
(rs731236) | A/A | 75 | 75.6 | 0.85 | 75 | 70.2 | 0.16 |
| | A/G | 96 | 94.7 | | 87 | 96.6 | |
| | G/G | 29 | 29.6 | | 38 | 33.2 | |
| VDR
(rs7975232) | C/C | 33 | 30.4 | 0.43 | 19 | 24.2 | 0.11 |
| | C/A | 90 | 95.2 | | 101 | 90.7 | |
| | A/A | 77 | 74.4 | | 80 | 85.2 | |
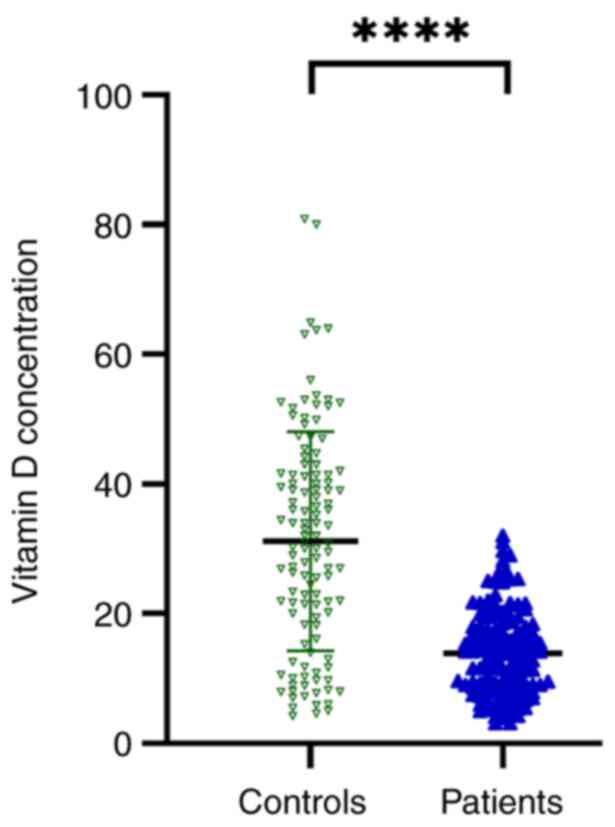
VD is decreased in patients with
SZ
The Mann-Whitney U test was utilized to compare VD
levels between individuals with SZ and controls, as shown in
Fig. 1. The results demonstrated
that VD levels were significantly lower (P<0.0001) in patients
with SZ (13.8 ng/ml) compared with those in the control group (31.3
ng/ml), indicating that the levels of VD varied significantly
between the two groups.
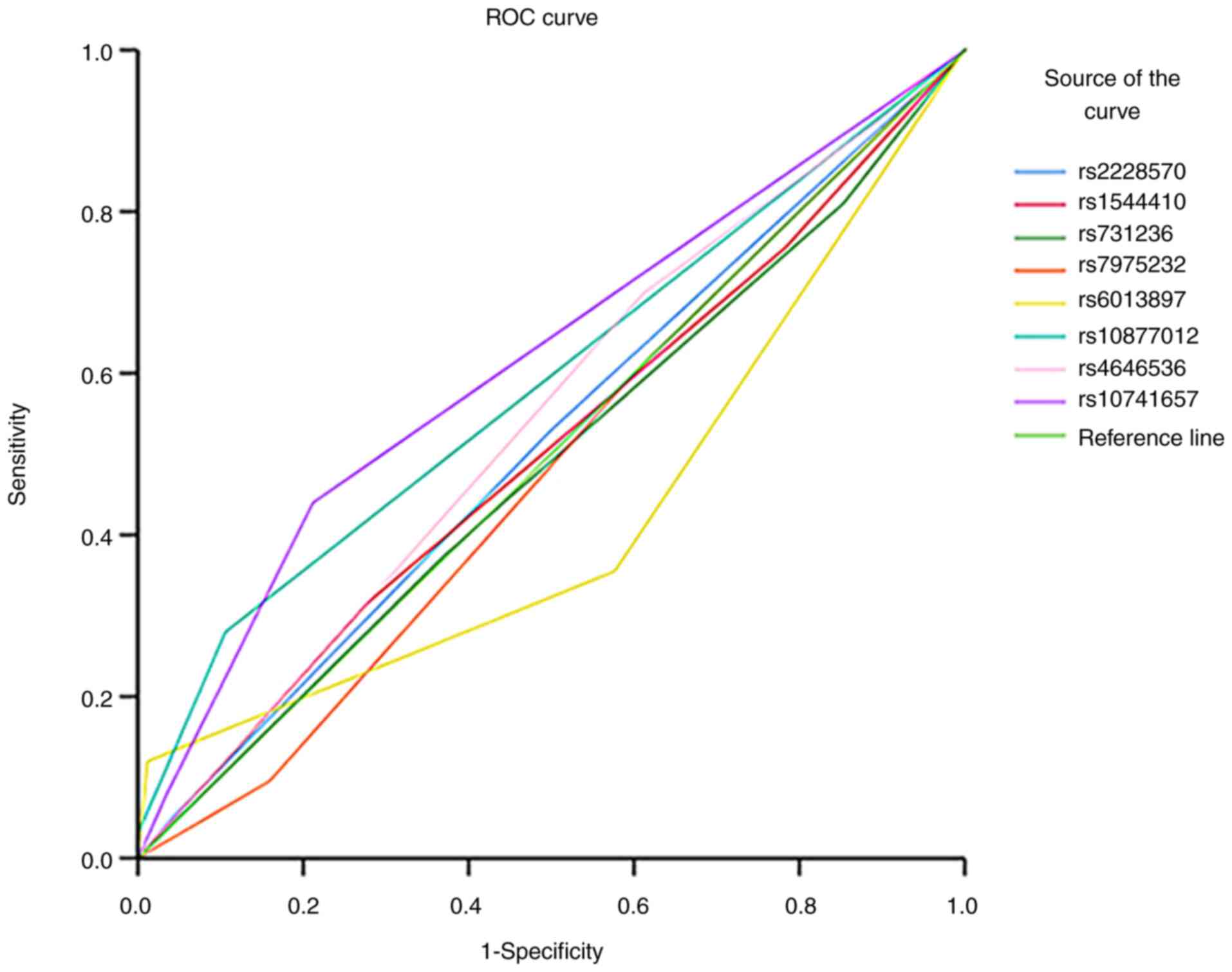
rs10741657 has the highest
discriminative capacity
The area under the curve (AUC) analysis was
conducted to assess the predictive performance of genetic variants
in distinguishing between patients with SZ and controls (Fig. 2). The AUC values for each test
result variable (rs2228570, rs1544410, rs731236, rs7975232,
rs6013897, rs10877012, rs4646536 and rs10741657) ranged from 0.422
to 0.615. Notably, rs10741657 exhibited the highest AUC value of
0.615, indicating improved discriminative ability compared with the
other variants, whereas rs6013897 showed the lowest AUC value of
0.422. The statistical significance of the AUC values varied across
the tested variants, with rs10877012 and rs10741657 demonstrating
significant discriminative abilities (P=0.003 and P<0.001,
respectively). These findings suggested varying levels of
predictive power among the tested genetic variants in
distinguishing schizophrenic states, with rs10741657 revealing the
most promising discriminatory performance.
SZ predictor SNPs
The binary logistic model revealed significant
associations between genetic variants, VD levels and the likelihood
of having SZ, as shown in Table V.
Notably, rs10741657, rs10877012 and rs6013897 exhibited an
increased likelihood of developing SZ. For rs10741657, the AA
genotype had an OR of 4.911, although this was not significant
(P=0.093), while the TA genotype had an OR of 2.497 (P=0.022).
Regarding rs10877012, the GT genotype had an OR of 2.369 (P=0.087),
however this was not statistically significant. For rs6013897, the
AA genotype had an OR of 13.087 (P=0.096) without statistical
power, while the TA genotype had an OR of 0.267 (P<0.001)
indicating a protective effect and suggesting that having the
variant decreased the probability of having SZ. Additionally,
increased VD levels were revealed to be associated with a lower
probability of developing SZ, with an OR of 0.888 (P<0.001).
Finally, the overall model exhibited a strong statistical
significance (χ2=77.209, P<0.001) and good fit
(Hosmer and Lemeshow goodness-of-fit test: χ2=4.451,
P=0.814), underscoring the robustness of the associations
identified (data not shown).
 | Table VLogistic regression analysis of
significant single nucleotide polymorphisms and vitamin D
levels. |
Table V
Logistic regression analysis of
significant single nucleotide polymorphisms and vitamin D
levels.
| Variables | B | S.E. | Sig. | Exp (B) | 95% CI for Exp
(B) |
|---|
| CYP2R1
(rs10741657) A/A | 1.591 | 0.9478 | 0.093 | 4.911 | 0.968-42.925 |
| CYP2R1
(rs10741657) A/G | 0.915 | 0.4008 | 0.022 | 2.497 | 1.155-5.599 |
| CYP27B1
(rs10877012) G/T | 0.863 | 0.5044 | 0.087 | 2.369 | 0.910-6.673 |
| CYP24A1
(rs6013897) A/A | 2.572 | 1.5457 | 0.096 | 13.087 | 1.147-457.792 |
| CYP24A1
(rs6013897) T/A | -1.319 | 0.3586 | <0.001 | 0.267 | 0.130-0.534 |
| Vitamin D | -0.118 | 0.0174 | <0.001 | 0.888 | 0.856-0.917 |
LD analysis
LD analysis between all eight SNPs is shown in
Fig. S9. The LD heatmap revealed
that these SNPs do not exhibit significant associations, suggesting
an independent inheritance within the study population. Notably, no
SNP pairs demonstrated high LD (R² values close to 1.0), which
would indicate a tendency to be co-inherited.
Given the lack of significant LD, the haplotype
construction from these SNPs would likely result in arbitrary
combinations of alleles, as these do not represent true biological
interactions. This undermines the utility of haplotype analysis in
this context, as all SNPs do not appear to influence the phenotype
collectively. Instead, each SNP contributes independently, which
aligns with the observed distribution of allele frequencies and LD
patterns among the studied SNPs.
Discussion
SZ is considered one of the most severe and complex
psychiatric disorders with a strong hereditary tendency.
Accumulating studies have suggested that SZ is potentially linked
to disruptions in brain development that are induced by the
gene-environment interplay (12,37-39).
However, investigations into the factors and the underlying
pathophysiological mechanisms of this disease remain a concern for
researchers. SNPs have increasingly become the most popular
genotyping approach in association studies due to their genetic
stability and high abundance in the genome (9,40,41).
Several studies have revealed a strong relationship between VD and
the pathological mechanisms of SZ (22,42-44).
Some common SNPs of the VD metabolic pathway genes have been
revealed to be associated with the levels of circulating VD in
several diseases. The present study was conducted to investigate
the association of selected SNPs in VD metabolic genes with SZ in
the Jordanian population. Notably, three of these genes are
involved in VD synthesis (CYP2R1, CYP27B1 and
CYP24A1), while the fourth gene contributes to its
VDR (25,32,45,46).
The studied SNPs were selected due to their potential role as key
regulators in the VD pathway and their possible influence on the
gene/enzyme expression and function, which are associated with
altered VD serum levels. The genotype and allele frequency of the
SNPs, and their distribution between the SZ and control groups were
examined. Although to the best of the authors' knowledge, the
associations of CYP2R1 (rs10741657), CYP27B1
(rs10877012 and rs4646536), CYP24A1 (rs6013897) and
VDR (rs2228570, rs1544410, rs731236 and rs7975232) with SZ
have not been reported in case-control studies before, the present
study demonstrated associations of CYP2R1 (rs10741657),
CYP27B1 (rs10877012) and CYP24A1 (rs6013897) SNPs
with SZ among Jordanians. Such associations have not been
established before in any other population.
CYP2R1 is one of the key genes that is
involved in VD metabolism. It encodes a 25-hydroxylase, an enzyme
responsible for converting inactive pre-VD to 25(OH)D in the liver
(47). In the present study, no
deviation from the HWE for CYP2R1 (rs10741657; A>G) was
observed. It was revealed that the A allele and AA genotype of this
SNP were more frequent in SZ cases (P<0.0001) compared with
controls. Based on this result, the A allele may be significantly
associated with SZ susceptibility, while the G allele could confer
protection. Thus, the findings of the present study reveal a novel
association of this SNP with SZ. Consistent with these findings,
Wang et al (48) detected a
higher frequency of the A allele and AA genotype in the Chinese Han
population, and their association with an increased risk of
coronary heart disease. Conversely, a study in the German
population revealed a significant association between GG and GA
genotypes and type 1 diabetes mellitus, suggesting the G allele as
a risk allele (49). Differences
between these findings are primarily attributed to the ethnic
backgrounds of the studied populations and differences in sample
sizes. To date, to the best of the authors' knowledge, there is no
study that has evaluated the effect of CYP2R1 (rs10741657)
on the progression of SZ or any other mental disorder. As for
CYP27B1, it is another key gene in VD metabolism required
for the hydroxylation of 25(OH)D in the kidney to produce
[1,25(OH)2D] (50). In the present
study, no significant difference was detected between patients with
SZ and controls for CYP27B1 (rs4646536). However, a
statistically significant difference was revealed in genotype and
allele frequencies for CYP27B1 (rs10877012) between patient
and controls groups (P<0.0001). It was revealed that both the TT
genotype and T allele of rs10877012 were more frequent in patients
with SZ compared with the controls (P<0.0001), suggesting that
the T allele may confer a risk role in SZ susceptibility. To the
best of the authors' knowledge, no studies have examined the
association between these two SNPs and SZ or any other mental
disorder in any population. The CYP24A1 gene is another gene
in the VD metabolic pathway, which encodes a degradative enzyme
that regulates circulating VD (51). For this gene SNP (rs6013897),
a significant difference was revealed in genotype distribution
between SZ cases and controls (P<0.0001). The results revealed
that the TT genotype was more frequent by 8-fold in patients with
SZ compared with the controls (P<0.0001). Regarding the allele
frequency, the T allele frequency was slightly higher in patients
compared with the controls, however it was not statistically
significant. This finding requires more samples to be analyzed.
This SNP has not previously been reported to be associated with any
psychiatric diseases. Notably, the genotype frequencies of
CYP24A1 (rs6013897) deviated from the HWE. This deviation
could be due to several reasons including: i) The small sample
size, ii) the high consanguinity rate (non-random mating) in the
Jordanian population, iii) genotyping error, iv) copy number
variation, v) population substructure and vii) migration of
individuals (52-54).
Therefore, the analysis should be repeated on a larger sample size
and genotyping should be carried out using different techniques
such as direct sequencing or TaqMan probe assay.
The last gene assessed in the present study was
VDR, which encodes a nuclear hormone receptor that is
expressed in several tissues and cells (55). The action of VD is mediated through
its binding with VDR. Polymorphisms of the VDR gene have
been reported and evaluated as genetic risk factors in various
disorders, such as issues with cognitive functioning and depressive
symptoms in old age (56),
Alzheimer's disease (57), mild
cognitive impairments and autism (58). In the present study, four common
SNPs of the VDR gene (rs2228570, rs1544410, rs731236 and
rs7975232) were examined. The genotype and allele frequencies
revealed no significant difference between the SZ and control
groups. The findings of the present study align with a previous
study conducted by Yan et al (32), who investigated the VDR gene
variant frequencies among 100 individuals with SZ and 189 control
subjects. This study also revealed a lack of associations between
these SNPs and SZ. Moreover, the results of Handoko et al
(33) support these findings,
confirming the absence of an association between VDR gene
variants and SZ.
Finally, the results of the present study
demonstrated significant differences in VD levels across the two
groups. The VD serum levels were revealed to be significantly lower
in the SZ group compared with those in the control group with mean
serum VD levels of 13.8 and 31.3 ng/ml for patients with SZ and
controls, respectively (Fig. 1).
Similarly, previous studies have supported the results of the
present study, indicating the high prevalence of VD deficiency
among patients with mental disorders, specifically SZ (42,59-62).
One of the primary limitations of the present study
is the insufficient sample size, which may have prevented the
detection of certain associations. In addition, the potential
clinical subtypes of SZ based on symptom profiles were unable to be
investigated, even though it is widely recognized that SZ has a
broad spectrum of symptoms. The present study did not focus on the
clinical description of the patients, since a number of them had
received medication for several years, while others started taking
medication at the time of sample collection and others were not on
regular medication. Therefore, the present study focused on a
broader diagnosis of SZ, rather than subclassifying it into
specific clinical subtypes to establish foundational SNP
associations. Furthermore, the genotyping and VD measurement were
not performed in the same samples. Hence, it was not possible to
further analyze the association between gene polymorphisms and VD
status. Moreover, a potential bias was introduced by the unequal
sex ratios in the sample population, which could influence the
generalizability of the findings of the present study. Ensuring
sex-matched samples in future studies is important to avoid
discrepancies that could affect the results, particularly in
genomic studies where biological sex may influence genetic
expression, methylation pattern and disease outcomes. The omission
of sex from the logistic regression analysis was justified by the
lack of association between sex and SNPs; however, more balanced
sex representation remains an important consideration for future
research.
In conclusion, the present study is an association
study that highlights the possible association between SNPs and SZ.
Moreover, known polymorphisms with high allele frequencies in the
general population are presented including rs10741657 (AF, 0.7344)
and rs10877012 (AF, 0.6501), similarly to other SNPs mentioned in
the present study. Finally, the importance of experimental
validation through functional studies is recognized. While the
study primarily focuses on genetic association analysis, the
authors are committed to exploring opportunities for future
investigations to directly address the limitations of the present
study.
Supplementary Material
Gel electrophoresis result of
tetra-amplification refractory mutation system-PCR products for the
three banding patterns of the rs10741657 polymorphism in the
CYP2R1 gene. Lane L: 100-bp ladder; the asterisk indicates
the band size of 500 bp. The size of the outer fragment is 416 bp.
Lanes 4, 6, 9, 11, and 12 represent the homozygous GG genotype (270
bp); lanes 1-3, 5, 8, 10, 13 and 14 represent the heterozygous AG
genotype (270 and 201 bp), and lanes 7 and 15 represent the AA
genotype (201 bp).
Gel electrophoresis result of
tetra-amplification refractory mutation system-PCR products for the
three banding patterns of the rs10877012 polymorphism in the
CYP27B1 gene. Lane L: 100-bp ladder; the asterisk indicates
the band size of 500 bp. The size of the outer fragment is 420 bp.
Lanes 1-3, 5, 6, 8, 10-13 and 15 represent the homozygous GG
genotype (275 bp), lanes 4 and 9 represent the heterozygous GT
genotype (275 and 205 bp), and lanes 7 and 14 represent the TT
genotype (205 bp).
Gel electrophoresis result of
tetra-amplification refractory mutation system-PCR products for the
three banding patterns of the rs4646536 polymorphism in the
CYP27B1 gene. Lane L: 100-bp ladder; the asterisk indicates
the band size of 500 bp. The size of the outer fragment is 394 bp.
Lanes 1-3, 5, 9, 11, 13 and 14 represent the homozygous AA genotype
(253 bp), lanes 4, 6-8, 10 and 12 represent the heterozygous AG
genotype (253 and 200 bp), and lane 15 indicates the GG genotype
(200 bp).
Gel electrophoresis result of
tetra-amplification refractory mutation system-PCR products for the
three banding patterns of the rs6013897 polymorphism in the
CYP24A1 gene. Lane L: 100-bp ladder; the asterisk indicates
the band size of 500 bp. The size of the outer fragment is 477 bp.
Lanes 2-6, 10, 13 and 15 represent the homozygous TT genotype (300
bp), lanes 1, 8, 11 and 12 represent the heterozygous TA genotype
(300 and 229 bp), and lanes 7, 9 and 14 represent the AA genotype
(229 bp).
Gel electrophoresis result of
tetra-amplification refractory mutation system-PCR products for the
three banding patterns of the FokI polymorphism (rs2228570)
in the VDR gene. Lane L: 100-bp ladder; the asterisk
indicates the band size of 500 bp. The size of the outer fragment
is 388 bp. Lanes 1-3 represent the homozygous GG genotype (187 bp),
and lanes 4-6 and 7-9 represent the heterozygous AG genotype (258
and 187 bp). The A allele band size is 258 bp.
Gel electrophoresis result of
tetra-amplification refractory mutation system-PCR products of the
BsmI polymorphism (rs1544410) in the VDR gene. The
size of the outer fragment is 403 bp. Lane L: 100-bp ladder; the
asterisk indicates the band size of 500 bp. Lanes 9 and 13
represent the homozygous CC genotype (260 bp), lanes 1, 5, 6, 11,
12, and 14, represent the heterozygous genotype CT (260 and 201
bp), and lanes 2-4, 7, 8 and 10 represent the TT genotype (201
bp).
Gel electrophoresis result of
tetra-amplification refractory mutation system-PCR products of the
TaqI polymorphism (rs731236) in the VDR gene. The
size of the outer fragment is 448 bp. Lane L: 100-bp ladder; the
asterisk indicates the band size of 500 bp. Lanes 2, 3, 5-8, 11 and
12 represent the AA genotype (284 bp), lanes 1, 9 and 15 represent
the homozygous GG genotype (216 bp), and lanes 4, 10, 13, 14 and 16
represent the heterozygous AG genotype (284 and 216 bp).
Gel electrophoresis result of
tetra-amplification refractory mutation system-PCR products of the
ApaI polymorphism (rs7975232) in the VDR gene. The
size of the outer fragment is 356 bp. Lane L: 100-bp ladder; the
asterisk indicates the band size of 500 bp. Lanes 3, 10, 11 and 13
represent the CC genotype (192 bp), lanes 2, 4, 6 and 12 represent
the homozygous AA genotype (216 bp), and lanes 1, 5, 7-9 and 14
represent the heterozygous CA genotype (216 and 192 bp).
LD heatmap plot for all investigated
SNPs in the present study. The color key denoting r2
values ranges from blue to orange, with orange indicating tight LD.
Numbers 1-8 represent the studied SNPs. SNPs, single nucleotide
polymorphisms; LD, linkage disequilibrium.
List of primers used for
tetra-amplification refractory mutation system-PCR, including the
product size of each fragment.
Allele frequency for each genotype of
the studied SNPs in patients with schizophrenia and controls.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Jordan University of
Science and Technology (JUST), research (grant no. 20200068).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MS conceptualized, supervised and provided
administrative support for the present study, in addition to
writing, editing and reviewing the original draft, designing the
methodology, and analyzing and validating the raw data. RD
performed the experiments, wrote the original draft and collected
the samples. AAZ analyzed and curated the raw data and wrote the
original draft. AGK performed clinical assessment. ABD performed
the experiments and collected the samples. MS and AAZ confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participates
Ethical approval was obtained from the Institutional
Review Board Committee (approval no. 2019/626) of the Jordan
University of Science and Technology. Written informed consent was
obtained from all participants or next of kin before enrollment in
the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Whiteford HA, Ferrari AJ, Degenhardt L,
Feigin V and Vos T: The global burden of mental, neurological and
substance use disorders: An analysis from the global burden of
disease study 2010. PLoS One. 10(e0116820)2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Velligan DI and Rao S: The epidemiology
and global burden of schizophrenia. J Clin Psychiatry.
84(MS21078COM5)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rayan A and Obiedate K: The correlates of
quality of life among jordanian patients with schizophrenia. J Am
Psychiatr Nurses Assoc. 23:404–413. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Saab R, Moussaoui D, Tabet CC, El Hamaoui
Y, Salamoun MM, Mneimneh ZN and Karam EG: Epidemiology of
schizophrenia and related disorders in the Arab world. Arab J
Psychiatry. 22:1–9. 2011.
|
|
5
|
Williams U, Jones DJ and Reddon JR (eds):
Police response to mental health in Canada. Canadian Scholars'
Press, pp357, 2019.
|
|
6
|
Cardno AG, Marshall EJ, Coid B, Macdonald
AM, Ribchester TR, Davies NJ, Venturi P, Jones LA, Lewis SW, Sham
PC, et al: Heritability estimates for psychotic disorders: The
Maudsley Twin Psychosis Series. Arch Gen Psychiatry. 56:162–168.
1999.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wu Y, Cao H, Baranova A, Huang H, Li S,
Cai L, Rao S, Dai M, Xie M, Dou Y, et al: Multi-trait analysis for
genome-wide association study of five psychiatric disorders. Transl
Psychiatry. 10(209)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Momozawa Y and Mizukami K: Unique roles of
rare variants in the genetics of complex diseases in humans. J Hum
Genet. 66:11–23. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Visscher PM, Wray NR, Zhang Q, Sklar P,
McCarthy MI, Brown MA and Yang J: 10 Years of GWAS discovery:
Biology, function, and translation. Am J Hum Genet. 101:5–22.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mullins N, Forstner AJ, O'Connell KS,
Coombes B, Coleman JRI, Qiao Z, Als TD, Bigdeli TB, Børte S, Bryois
J, et al: Genome-wide association study of more than 40,000 bipolar
disorder cases provides new insights into the underlying biology.
Nat Genet. 53:817–829. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cano-Gamez E and Trynka G: From GWAS to
function: Using functional genomics to identify the mechanisms
underlying complex diseases. Front Genet. 11(424)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lv Y, Wen L, Hu WJ, Deng C, Ren HW, Bao
YN, Su BW, Gao P, Man ZY, Luo YY, et al: Schizophrenia in the
genetic era: A review from development history, clinical features
and genomic research approaches to insights of susceptibility
genes. Metab Brain Dis. 39:147–171. 2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Heinzer L and Curtis D: What have genetic
studies of rare sequence variants taught us about the aetiology of
schizophrenia? J Transl Genet Genom. 8:1–12. 2024.
|
|
14
|
Owen MJ, Legge SE, Rees E, Walters JTR and
O'Donovan MC: Genomic findings in schizophrenia and their
implications. Mol Psychiatry. 28:3638–3647. 2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Legge SE, Santoro ML, Periyasamy S,
Okewole A, Arsalan A and Kowalec K: Genetic architecture of
schizophrenia: A review of major advancements. Psychol Med.
51:2168–2177. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rodgers MD, Mead MJ, McWhorter CA, Ebeling
MD, Shary JR, Newton DA, Baatz JE, Gregoski MJ, Hollis BW and
Wagner CL: Vitamin D and child neurodevelopment-a post hoc
analysis. Nutrients. 15(4250)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bivona G, Gambino CM, Iacolino G and
Ciaccio M: Vitamin D and the nervous system. Neurol Res.
41:827–835. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Eyles DW: Vitamin D: Brain and behavior.
JBMR Plus. 5(e10419)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ye X, Zhou Q, Ren P, Xiang W and Xiao L:
The synaptic and circuit functions of vitamin D in neurodevelopment
disorders. Neuropsychiatr Dis Treat. 19:1515–1530. 2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Di Somma C, Scarano E, Barrea L,
Zhukouskaya VV, Savastano S, Mele C, Scacchi M, Aimaretti G, Colao
A and Marzullo P: Vitamin D and neurological diseases: An endocrine
view. Int J Mol Sci. 18(2482)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cui X, Gooch H, Petty A, McGrath JJ and
Eyles D: Vitamin D and the brain: Genomic and non-genomic actions.
Mol Cell Endocrinol. 453:131–143. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lally J and Gaughran F: Vitamin D in
schizophrenia and depression: A clinical review. BJPsych Adv.
25:240–248. 2019.
|
|
23
|
Cui X and Eyles DW: Vitamin D and the
central nervous system: Causative and preventative mechanisms in
brain disorders. Nutrients. 14(4353)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Voltan G, Cannito M, Ferrarese M, Ceccato
F and Camozzi V: Vitamin D: An overview of gene regulation, ranging
from metabolism to genomic effects. Genes (Basel).
14(1691)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bahrami A, Sadeghnia HR, Tabatabaeizadeh
SA, Bahrami-Taghanaki H, Behboodi N, Esmaeili H, Ferns GA, Mobarhan
MG and Avan A: Genetic and epigenetic factors influencing vitamin D
status. J Cell Physiol. 233:4033–4043. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sepulveda-Villegas M, Elizondo-Montemayor
L and Trevino V: Identification and analysis of 35 genes associated
with vitamin D deficiency: A systematic review to identify genetic
variants. J Steroid Biochem Mol Biol. 196(105516)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hyppönen E, Vimaleswaran KS and Zhou A:
Genetic determinants of 25-hydroxyvitamin D concentrations and
their relevance to public health. Nutrients.
14(4408)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Slater NA, Rager ML, Havrda DE and
Harralson AF: Genetic variation in CYP2R1 and GC genes associated
with vitamin D deficiency status. J Pharm Pract. 30:31–36.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhu JG, Ochalek JT, Kaufmann M, Jones G
and DeLuca HF: CYP2R1 is a major, but not exclusive, contributor to
25-hydroxyvitamin D production in vivo. Proc Natl Acad Sci USA.
110:15650–15655. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bailey R, Cooper JD, Zeitels L, Smyth DJ,
Yang JH, Walker NM, Hyppönen E, Dunger DB, Ramos-Lopez E, Badenhoop
K, et al: Association of the vitamin D metabolism gene CYP27B1 with
type 1 diabetes. Diabetes. 56:2616–2621. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Carvalho IS, Gonçalves CI, Almeida JT,
Azevedo T, Martins T, Rodrigues FJ and Lemos MC: Association of
vitamin D pathway genetic variation and thyroid cancer. Genes
(Basel). 10(572)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yan J, Feng J, Craddock N, Jones IR, Cook
EH Jr, Goldman D, Heston LL, Chen J, Burkhart P, Li W, et al:
Vitamin D receptor variants in 192 patients with schizophrenia and
other psychiatric diseases. Neurosci Lett. 380:37–41.
2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Handoko HY, Nancarrow DJ, Mowry BJ and
McGrath JJ: Polymorphisms in the vitamin D receptor and their
associations with risk of schizophrenia and selected anthropometric
measures. Am J Hum Biol. 18:415–417. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lins TC, Vieira RG, Grattapaglia D and
Pereira RW: Population analysis of vitamin D receptor polymorphisms
and the role of genetic ancestry in an admixed population. Genet
Mol Biol. 34:377–385. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
First MB, Rebello TJ, Keeley JW, Bhargava
R, Dai Y, Kulygina M, Matsumoto C, Robles R, Stona AC and Reed GM:
Do mental health professionals use diagnostic classifications the
way we think they do? A global survey. World Psychiatry.
17:187–195. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Medrano RFV and De Oliveira CA: Guidelines
for the tetra-primer ARMS-PCR technique development. Mol
Biotechnol. 56:599–608. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Luvsannyam E, Jain MS, Pormento MKL,
Siddiqui H, Balagtas ARA, Emuze BO and Poprawski T: Neurobiology of
schizophrenia: A comprehensive review. Cureus.
14(e23959)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wahbeh MH and Avramopoulos D:
Gene-environment interactions in schizophrenia: A literature
review. Genes (Basel). 12(1850)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Schmitt A, Falkai P and Papiol S:
Neurodevelopmental disturbances in schizophrenia: Evidence from
genetic and environmental factors. J Neural Transm (Vienna).
130:195–205. 2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Watanabe K, Stringer S, Frei O, Umićević
Mirkov M, de Leeuw C, Polderman TJC, van der Sluis S, Andreassen
OA, Neale BM and Posthuma D: A global overview of pleiotropy and
genetic architecture in complex traits. Nat Genet. 51:1339–1348.
2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tam V, Patel N, Turcotte M, Bossé Y, Paré
G and Meyre D: Benefits and limitations of genome-wide association
studies. Nat Rev Genet. 20:467–484. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Cui X, McGrath JJ, Burne THJ and Eyles DW:
Vitamin D and schizophrenia: 20 Years on. Mol Psychiatry.
26:2708–2720. 2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Roy NM, Al-Harthi L, Sampat N, Al-Mujaini
R, Mahadevan S, Al Adawi S, Essa MM, Al Subhi L, Al-Balushi B and
Qoronfleh MW: Impact of vitamin D on neurocognitive function in
dementia, depression, schizophrenia and ADHD. Front Biosci
(Landmark Ed). 26:566–611. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
44
|
Zhu JL, Luo WW, Cheng X, Li Y, Zhang QZ
and Peng WX: Vitamin D deficiency and schizophrenia in adults: A
systematic review and meta-analysis of observational studies.
Psychiatry Res. 288(112959)2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Asadzadeh Manjili F, Kalantar SM,
Arsang-Jang S, Ghafouri-Fard S, Taheri M and Sayad A: Upregulation
of vitamin D-related genes in schizophrenic patients.
Neuropsychiatr Dis Treat. 14:2583–2591. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Dastani Z, Li R and Richards B: Genetic
regulation of vitamin D levels. Calcif Tissue Int. 92:106–117.
2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Cheng JB, Motola DL, Mangelsdorf DJ and
Russell DW: De-orphanization of cytochrome P450 2R1: A microsomal
vitamin D 25-hydroxilase. J Biol Chem. 278:38084–38093.
2003.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wang Q, Lin Z, Chen H, Ma T and Pan B:
Effect of cytochrome p450 family 2 subfamily R member 1 variants on
the predisposition of coronary heart disease in the Chinese Han
population. Front Cardiovasc Med. 8(652729)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ramos-Lopez E, Brück P, Jansen T, Herwig J
and Badenhoop K: CYP2R1 (vitamin D 25-hydroxylase) gene is
associated with susceptibility to type 1 diabetes and vitamin D
levels in Germans. Diabetes Metab Res Rev. 23:631–636.
2007.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Christakos S, Dhawan P, Verstuyf A,
Verlinden L and Carmeliet G: Vitamin D: Metabolism, molecular
mechanism of action, and pleiotropic effects. Physiol Rev.
96:365–408. 2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Bikle DD: Vitamin D metabolism, mechanism
of action, and clinical applications. Chem Biol. 21:319–329.
2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Lee S, Kasif S, Weng Z and Cantor CR:
Quantitative analysis of single nucleotide polymorphisms within
copy number variation. PLoS One. 3(e3906)2008.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Graffelman J, Jain D and Weir B: A
genome-wide study of Hardy-Weinberg equilibrium with next
generation sequence data. Hum Genet. 136:727–741. 2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wang J and Shete S: Testing departure from
Hardy-Weinberg proportions. Methods Mol Biol. 850:77–102.
2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Valdivielso JM and Fernandez E: Vitamin D
receptor polymorphisms and diseases. Clin Chim Acta. 371:1–12.
2006.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Kuningas M, Mooijaart SP, Jolles J,
Slagboom PE, Westendorp RGJ and Heemst D Van: VDR gene variants
associate with cognitive function and depressive symptoms in old
age. Neurobiol Aging. 30:466–473. 2009.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Liu N, Zhang T, Ma L, Wei W, Li Z, Jiang
X, Sun J, Pei H and Li H: Vitamin D receptor gene polymorphisms and
risk of Alzheimer disease and mild cognitive impairment: A
systematic review and meta-analysis. Adv Nutr. 12:2255–2264.
2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Zhang Z, Li S, Yu L and Liu J:
Polymorphisms in vitamin D receptor genes in association with
childhood autism spectrum disorder. Dis Markers.
2018(7862892)2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Eyles DW, Trzaskowski M, Vinkhuyzen AAE,
Mattheisen M, Meier S, Gooch H, Anggono V, Cui X, Tan MC, Burne
THJ, et al: The association between neonatal vitamin D status and
risk of schizophrenia. Sci Rep. 8(17692)2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Eyles DW, Burne THJ and McGrath JJ:
Vitamin D, effects on brain development, adult brain function and
the links between low levels of vitamin D and neuropsychiatric
disease. Front Neuroendocrinol. 34:47–64. 2013.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Crews M, Lally J, Gardner-Sood P, Howes O,
Bonaccorso S, Smith S, Murray RM, Di Forti M and Gaughran F:
Vitamin D deficiency in first episode psychosis: A case-control
study. Schizophr Res. 150:533–537. 2013.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Shahini N, Jazayeri SMMZ, Jahanshahi R and
Charkazi A: Relationship of serum homocysteine and vitamin D with
positive, negative, and extrapyramidal symptoms in schizophrenia: A
case-control study in Iran. BMC Psychiatry. 22(681)2022.PubMed/NCBI View Article : Google Scholar
|