Introduction
A clear intraoperative tumor border definition is
essential for complete tumor resection and strongly improves
patient outcomes (1). The
intraoperative evaluation of tumor borders is performed by visual
inspection followed by histological analysis. This procedure is
error-prone and time-consuming. Evolving imaging techniques allow
better visualization of the operation field in real-time. One such
technique is near-infrared (NIR) fluorescence-guided surgery
(2,3). Fluorophores are used as contrast
agents. Indocyanine green (ICG) has been approved by the Food and
Drug Administration and European Medicine Agency. It is widely used
in diagnostic medical imaging for perfusion and angiographic
applications (4,5). Additionally, due to its advantages
such as long retention time and higher signal-to-noise ratio
compared with other fluorophores, its potential use is still under
investigation in biomedical research (6,7).
Several studies have shown an association between
tumor tissue and ICG signals in vivo (8,9). This
has led to the introduction of ICG for tumor imaging in several
tumor entities (10) with a focus
on sentinel lymph nodes (11). One
potential use is the visualization of tumor areas in patients with
head and neck squamous cell carcinoma (HNSCC) (12), which has low 5-year survival rates
of ~50% and may benefit from technologies for better visualization
and tumor border definition. In HNSCC failing to fully remove the
tumor with adequate margins during surgery is the primary cause of
patient death (13). To the best of
our knowledge, there has not been any study showing evidence of
tumor-specific cellular uptake of ICG in patients, even though
results have shown preferential cellular uptake in tumor
xenografts; ICG labeling of tumor is indirect and seems to be
driven by higher endocytotic activity of tumor cells in combination
with the disruption of tight junctions (14). This leads to enhanced permeability
and retention (EPR) effect by which macromolecules accumulate in
tumor tissue (15). To the best of
our knowledge, however, little is known about the histological
distribution of ICG following injection in human cancer samples at
the microscopic level.
The present study aimed to evaluate the tumor and
non-tumor cellular uptake of ICG and its spatial distribution at a
microscopic level. A high-magnification imaging approach was used
for the detection of ICG in HNSCC cell lines and spatial
distribution of ICG within tissue samples from patients with
HNSCC.
Materials and methods
Cells and tissue samples
The human squamous cell carcinoma cell line SCC026
[cat. no. ACC-658, Research Resource Identifier (RRID):CVCL_2221]
and immortalized human skin cell line HaCaT (RRID:CVCL_0038; cat.
no. 300493; passage number of cryopreservation, 31) were obtained
from Leibniz Institute DSMZ Germany. Growth medium for SCC06
consisted of 80% Eagle's Minimal Essential Medium (Thermo Fisher
Scientific, Inc.), 20% fetal bovine serum (Thermo Fisher
Scientific, Inc.) and 2 mM L-glutamine. Growth medium for HaCaT
consisted of 90% Dulbecco's Modified Eagle's Medium (Thermo Fisher
Scientific, Inc.), 10% fetal bovine serum and 2 mM L-glutamine.
Tissue samples from five patients with HNSCC were
collected at Jena University Hospital, Jena, Germany between
September 2019 and November 2021. The study was approved by the
ethics committee of the Jena University Hospital (approval no.
4291-12/14) and written informed consent was obtained from all
patients. All patients were male, ranging in age from 61 to 70
years. Exclusion criteria were other malignancies or multimorbidity
that prevent surgery, such as advanced patient age, cardiovascular,
respiratory, neurological or cognitive impairment and coagulopathy
(16). No criterion was applied
with regards to sex but due to the sex ratio of head and neck
cancer being nearly 4:1 (78.9% male) (17) and the small patient cohort, only
male patients were recruited. One sample originated from the
oropharynx, two from the oral cavity and two from the larynx. An
overview of patients and their clinical data is provided in
Table I including TNM cancer
staging (18). The samples
originated from the tumor area as well as adjacent healthy tissue
(distance >5 mm, clear margins). All patients received ICG
intravenously during tumor surgery according to a standardized
protocol (3). ICG dissolved in
distilled water (5 ml; 25 mg/15 ml solution; Pulsion Medical
Systems SE, Getinge) was administered intravenously during surgery
after anesthesia induction and endoscopic exposure of the tumor. To
confirm successful ICG administration near-infrared endoscopy was
performed. At 30 min after ICG injection, specimens were collected.
The samples were snap frozen in liquid nitrogen and stored at -80˚C
until measurement. Sections (12 µm thickness) were cut with a
cryotome (Leica Biosystems). Fresh frozen sections were prepared
and transported under low light to prevent photo-bleaching.
 | Table IOverview of patients and their
clinical data. |
Table I
Overview of patients and their
clinical data.
| Patient | Age, years | Cancer | Tumor
localization | TNM (stage) |
|---|
| 1 | 61 | Floor of the
mouth | Floor of the
mouth | pT1(2) cN0c M0 L0 V0
Pn0 R0 (I) |
| 2 | 54 | Floor of the
mouth | Floor of the
mouth | pT3 pN0(0/24) cM0 L0
V0 Pn0 R0; (II) |
| 3 | 67 | Oropharyngeal | Oropharynx | cT2 cN3b cM0
(III) |
| 4 | 70 | Laryngeal | Vocal fold | cT1a cN0 cM0
(I) |
| 5 | 61 | Laryngeal | Larynx | cT1b cN0 cM0
(I) |
Fluorescence microscopy
All fluorescence images were captured using an
inverted light microscope (Axio Observer Z1/7; Carl Zeiss AG) with
a universal LED illumination system (pE-4000; CoolLED Ltd.) and
sCMOS camera (ORCA-Fusion BT; Hamamatsu Photonics K.K.) with an 1x
camera adapter. Images of cell cultures were captured using a 63x
lens (LD Plan-Neofluar 63x/0.75 Korr M27; Carl Zeiss AG). Images of
tissue sections were captured using a 40x lens (LD Plan-Neofluar
40x/0.6 Korr M27; Carl Zeiss AG). ICG was detected using the ICG HC
filter set (AHF Analysentechnik AG). Ethidium homodimer-1 was
detected using a 45 Texas Red filter set (Carl Zeiss AG).
Calcein-AM was detected using a 44 FITC filter set (Carl Zeiss AG).
Hoechst 33342 was detected using a 96 HE BFP filter set (Carl Zeiss
AG). All fluorescence images were shown in pseudo-colors. ZEN 3.5
blue edition imaging software (Carl Zeiss AG) was used for all
experiments. For fluorescence intensity in tissue sections, the
mean pixel intensity of the corresponding tissue area was
calculated and compared using Python software (Version 3.7, Python
Software Foundation). Tissue areas including artifacts were
identified and removed for the calculation.
SCC026 and HaCaT cells were seeded at a density of
0.1x106 cells/ml on a surface area of 1.9 cm²/dish. The
cells were incubated at 37˚C and 5% CO2 for 24 h until
they reached 70-90% confluence. To investigate ICG uptake, growth
medium was removed and fresh medium containing 0.6 µg/ml ICG
(Verdye™, Diagnostic Green GmbH) was added. All steps involving ICG
or fluorescent agents were performed under low light conditions to
prevent photobleaching. Cells were incubated with ICG medium at
37˚C and 5% CO2 for different time lengths (1, 45 and 90
min, 2.5 and 24.0 h). ICG medium was removed, cells were washed
with Dulbecco's Phosphate-Buffered Saline (DPBS; Sigma-Aldrich
Chemie GmbH) and fresh growth medium was added. The cells were
subjected to ICG fluorescence imaging. To distinguish live from
dead cells, cells were stained with calcein AM and ethidium
homodimer-1 (both Thermo Fisher Scientific, Inc.). Staining
solution consisted of DPBS with 2x10-3 mM/l calcein AM
and 4x10-3 mM/l ethidium homodimer-1. The medium was
removed, the cells were washed with DPBS and 150 µl staining
solution was added to the cells. Cells were incubated with the
fluorescence staining solution at room temperature for 30 min
before fluorescence imaging. The exposure time was automatically
determined by the imaging software (ZEN 3.5 blue edition imaging
software; Carl Zeiss AG, micro-shop.zeiss.com/de/de/softwarefinder/software-categories/zen-blue).
Additionally, a control group of cells underwent the same
incubation and staining conditions without treatment with ICG. The
influence on the cell survival was judged visually.
SCC026 and HaCaT cells were seeded with a density of
0.075x106 cells/ml on a surface area of 1.9 cm²/dish.
The cells were incubated at 37˚Cand 5% CO2 for 24 h
until they reached 70-90% confluence. To investigate the ICG
retention, the cells were incubated at 37˚C with 0.6 µg/ml ICG for
2.5 h. ICG medium was then removed, and fresh growth medium added.
The cells were incubated in ICG-free growth medium at 37˚C and 5%
CO2 (0.5, 2.5, 4.0, 4.5, 5.0, 5.5, 6.0 and 24.0 h). To
differentiate between cell nucleus and cytoplasm, cells were
stained with Hoechst 33342 (Thermo Fisher Scientific, Inc.) and
calcein AM. Staining solution consisted of DPBS with
8.115x10-3 mM/l Hoechst 33342 and 2x10-3 mM/l
calcein AM. The medium was removed, the cells were washed with DPBS
and 150 µl staining solution was added to the cells. Cells were
incubated with the fluorescence staining solution at room
temperature for 30 min before ICG/calcein/Hoechst fluorescence
imaging. The exposure time was automatically determined by the
imaging software.
ICG fluorescence imaging were performed on fresh
frozen sections. Individual images were combined with an overlap of
15% to obtain ICG fluorescence images of the entire tissue section.
The exposure time for all images was set at 10 sec. Tissue sections
were stained with hematoxylin and eosin (HE; hematoxylin: 5 min,
Eosin: 20 sec, at room temperature) after ICG fluorescence imaging
and submitted to an experienced pathologist for analysis (including
the search for necrotic tissue or cells) and annotation. The
annotated HE images were co-registered with the ICG fluorescence
images to investigate the distribution of ICG inside the
tissue.
Results
ICG imaging of cell cultures
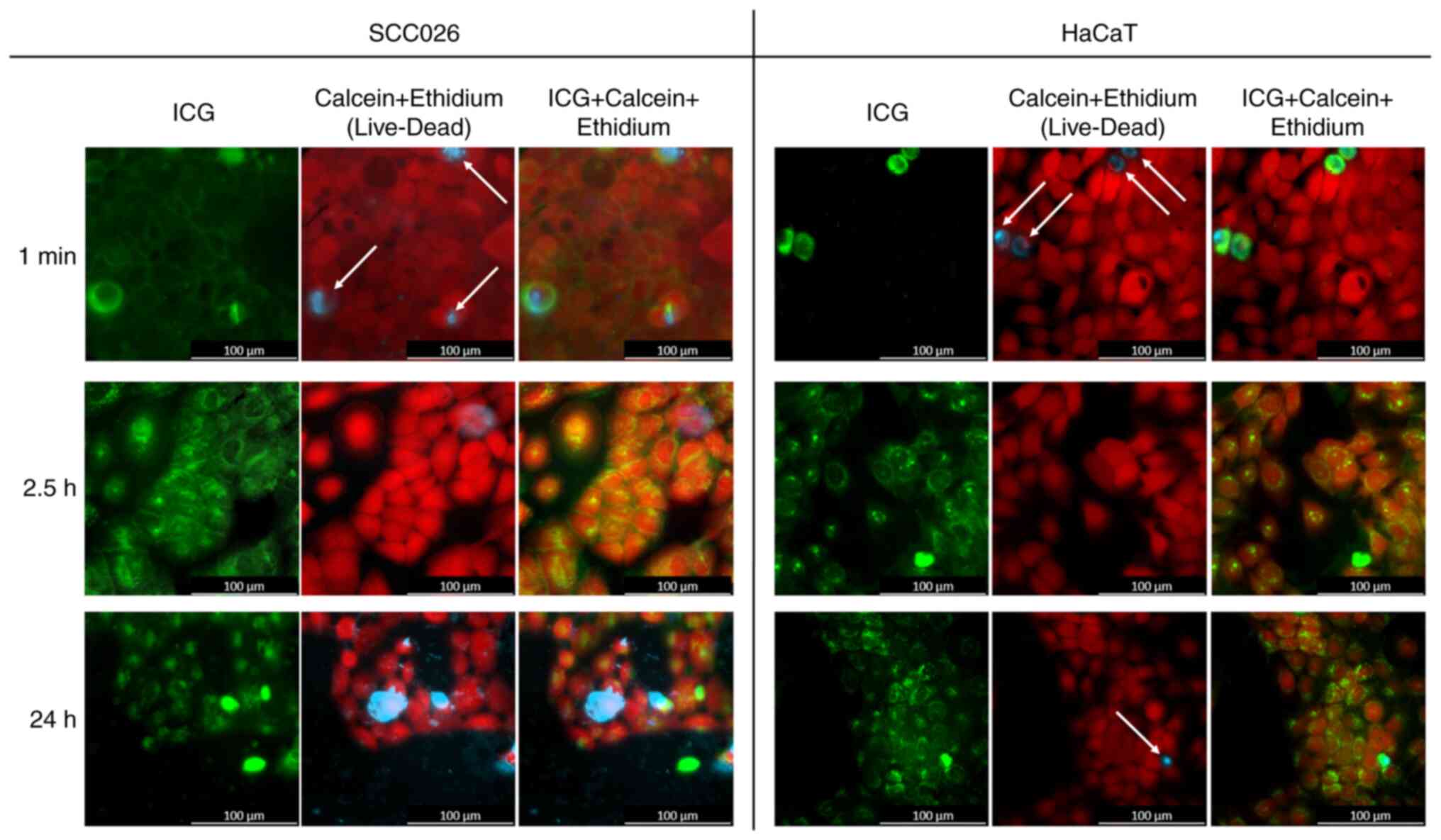
No notable effect of ICG on cell survival was
observed. The number of dead cells was similar in the ICG and the
control group (data not shown). Uptake of ICG by dead cells was
observed after 1 min of incubation in ICG medium. Overall, dead
cells showed earlier and higher ICG uptake than living cells
(Fig. 1A).
No notable ICG uptake by living cells could be
observed for the first 90 min (data not shown). ICG was only
detected in the intercellular space and inside dead cells. After
2.5 h, ICG was taken up and internalized by living cells in both
cell lines (Fig. 1B). At 24 h,
transport to the nucleus of living cells was observed in both cell
lines (Fig. 1C).
Imaging of tissue sections
For investigation of the in vivo distribution
of ICG, samples from patients following ICG injection and surgery
were used.
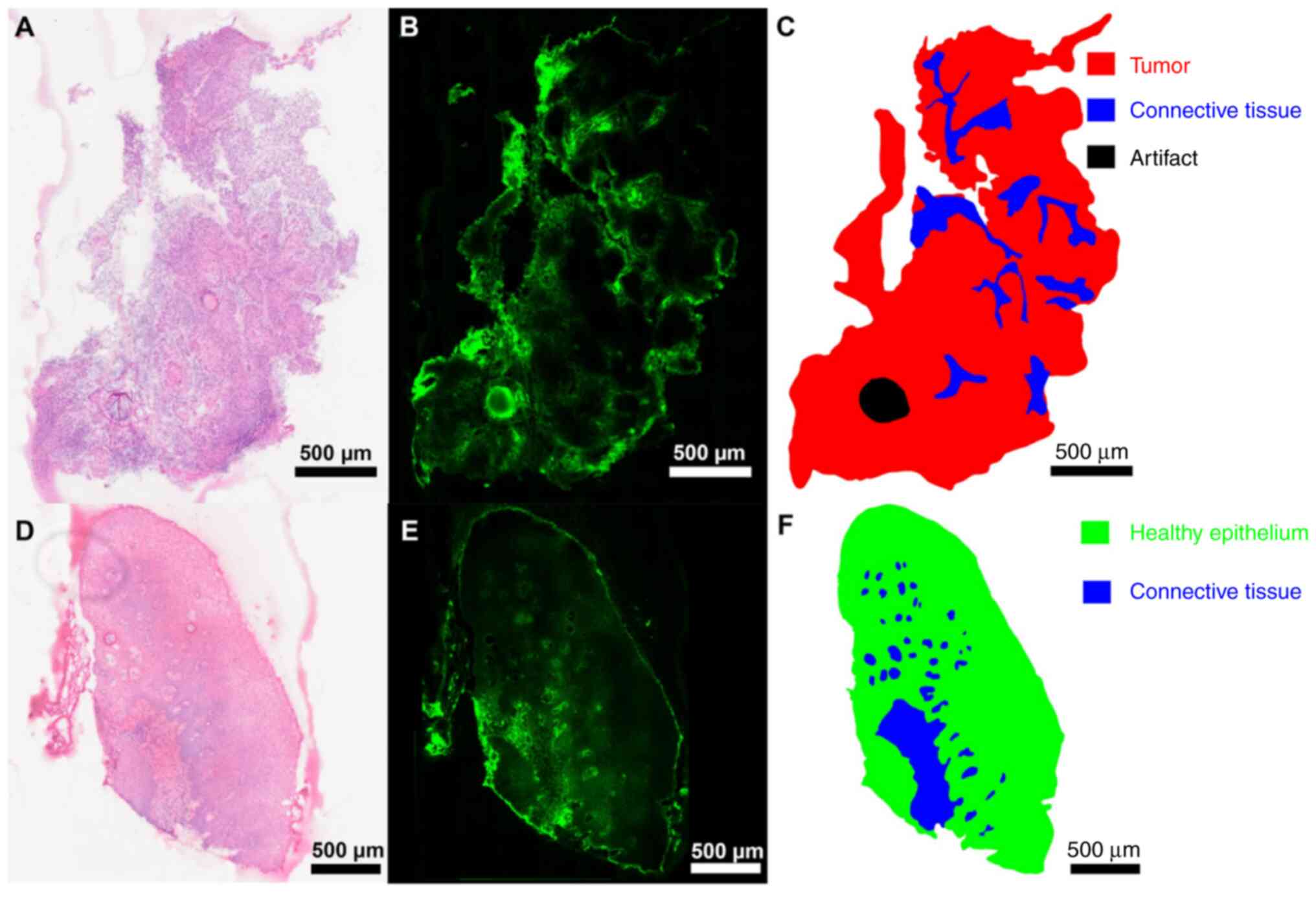
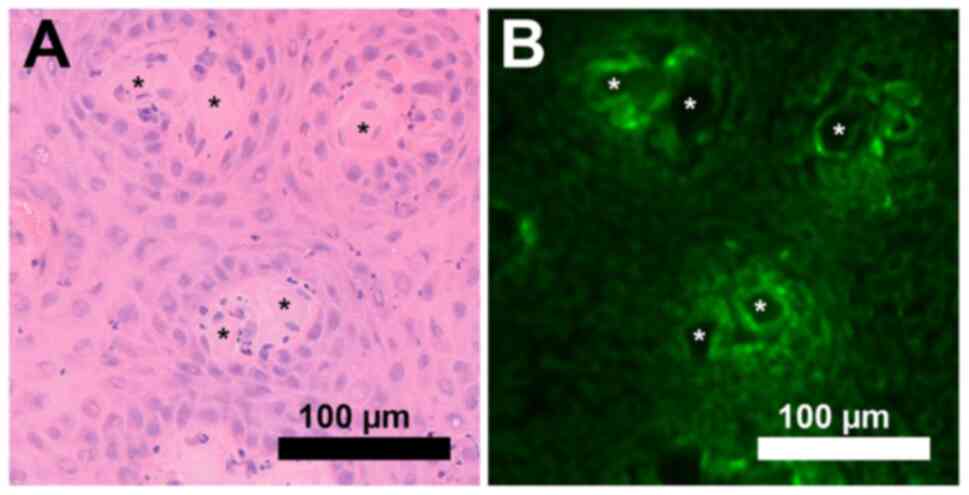
In all tissue sections, ICG distribution was
distinctive and not diffuse. Higher ICG signal intensity was
detected in connective tissue and surrounding cell clusters
(Fig. 2) and cell clusters
surrounding blood vessels (Fig. 3).
ICG signal intensity was studied in representative samples of
connective tissue and healthy epithelium as well as tumor tissue.
The signal intensity was higher in connective compared with tumor
tissue (Fig. 2A-C) and healthy
epithelium (Fig. 2D-F). ICG signal
intensity decreased with increasing distance from connective
tissue. Higher ICG signal intensity was also detected in the
connective tissue of healthy epithelial tissue located in close
proximity to tumor tissue (Fig.
2D-F). The higher ICG signals at the section edges were
associated with diffusion artifacts from thawing of the tissue
during the ICG measurement. This edge-zone was therefore excluded
from the analysis. Another artifact resulting from tissue
preparation (Fig. 2B and C) was also excluded. Fully resolved
microscopic images are provided as a publicly available dataset
(19).
Discussion
ICG angiography is used to examine choroidal blood
flow and associated pathologies (20). In tumor surgery, ICG has been
introduced in clinical practice to detect sentinel lymph nodes and
identify regional metastasis (11,21).
Many studies with ICG have been conducted to test its suitability
for determining tumor boundaries in vivo (22). Accurate labeling of tumor margins
would assist during surgery; however studies have not yet been able
to clearly show whether this is possible (22,23).
Since it is difficult to study ICG distribution during surgery, the
aim of the present study was to investigate the application of ICG
using cell lines and tissue as model.
Earlier and higher uptake of ICG by dead cells was
observed. This was expected, since necrotic cells no longer have a
functioning cell barrier, allowing ICG to enter the cell more
easily (24). Egloff-Juras et
al (25) studied the uptake of
ICG by living cells in FaDu spheroids with similar results
regarding ICGpenetration after 24 h. While measured at slightly
different time intervals compared to this study, Egloff-Juras et
al also observed low ICG uptake in the first hour with
increasing uptake after 3 h. However, neither spheroids nor cell
cultures possess blood vessels, which limits the relevance of both
the present and aforementioned study. Chan et al (26) analyzed ICG uptake and retention in
sarcoma and breast cancer cell lines; ICG uptake but not retention
was associated with proliferation rate of the sarcoma cell lines.
Therefore, that for tumor detection in sarcoma surgery, ICG may
demonstrate higher utility in high-grade tumors, which display a
higher proliferation rate.
To understand the in vivo distribution of
ICG, studies on tissue from patients who received ICG intravenously
were performed. High ICG signals were primarily detected in
connective tissue. This indicated a leaking of ICG from blood
vessels, which supports the hypothesis of the EPR effect being the
cause of ICG accumulation in tumor tissue due to stronger
vascularization (27). However, the
strongly heterogenous distribution of ICG and the small patient
cohort limits the present findings. Following injection, ICG
rapidly binds non-covalently and reversibly to macromolecules such
as albumin in the bloodstream (28). Immature tumor vessels with
fenestrated endothelia and deficient basement membranes allow
extravasation of these macromolecules, while inefficient lymphatic
drainage leads to their retention (EPR effect) (10,15).
Chan et al (26) concluded
that the exact mechanisms underlying ICG uptake and retention
require further investigation.
Adjacent connective tissue also showed higher ICG
signals. The extent of ICG accumulation in adjacent connective
tissue requires further investigation. Patients differ in blood
volume and concentration of plasma proteins to which ICG binds,
resulting in different ICG concentrations in tissue samples. The
ICG dosage injected before surgery was applied based on a
standardized procedure (3,29). Furthermore, improved ICG injection
protocols for distinct clinical application considering time and
location of the injections in addition to dose are under
development (30). More objective
measurements are needed for large-scale multicenter studies. The
detection of higher ICG signals in dead cells in vitro
experiments led to a search for necrotic cells within the tissue.
No necrotic cells were detected in the analyzed tissue samples.
ICG signal intensity decreased with increasing
distance from the connective tissue, which indicates a
concentration gradient towards the periphery. This pattern was
consistent across all samples, regardless of the tumor
localization. Therefore, it was hypothesized that this pattern may
be universal in HNSCC. Using more samples from more patients and
comparing the ICG distribution using immunohistochemical staining
for more detailed tumor assessment, is required. As in the cell
culture experiments, uptake and internalization of ICG into the
cell, but not the nucleus, was detected. All samples were collected
30 min after intravenous administration of ICG. ICG thus had a
maximum contact time of 30 min with the tissue. By contrast with
the cell culture, transport to the cell nucleus was only observed
at 24 h. Further studies with later sampling times after ICG
administration cohort should be performed in the future to
investigate whether transport to the nucleus occurs after a longer
time. This is of particular relevance for a better understanding of
the mechanism underlying the second window ICG technique, in which
high dose ICG is administered 24 h before surgery (31). While this approach has led to
promising findings for laparoscopic fluorescence cholangiography,
it is less feasible in clinical applications due to practicability
concerns (32). These include
additional administrative challenges due to the need of treating
patients twice, following a strict timeline that is not well
aligned with daily clinical routine, as well as additional
costs.
ICG should be used as a tag for other markers with
tumor-specific labeling to allow a reliable and effective
intraoperative detection of tumor boundaries. A future application
of ICG may involve coupling to nanoparticles that enable
tumor-specific labeling, which is a topic currently under
investigation in a multitude of settings (33,34).
Another area of investigation is use of ICG to detect colorectal
liver metastasis; specific ICG uptake and retention of cholestatic
hepatocytes may increase negative tumor margins during surgery
(35,36). A recent study showed the potential
of ICG as a universal solid tumor marker if the fluorescence
lifetime is measured instead of the signal intensity (37) but questioned the validity of ICG
fluorescence intensity as a tumor marker.
In conclusion, no distinct uptake of ICG by tumor
cells in vitro could be observed. Highlighting of tumor
tissue when ICG is administered during surgery appears to be a
result of the EPR effect, rather than selective uptake by tumor
cells and therefore is not reliable enough to be used as a feasible
method of tumor border recognition in the clinic.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Thüringer
Aufbaubank/European Regional Development Fund (grant no. 2020 FGI
0029), Carl Zeiss Foundation, Virtual Workshop for Digitization in
Sciences (grant no. 0563-2.8/738/2) and Coherent Raman Imaging for
the molecular study of origin of diseases (EU Horizon 2020-ICT;
grant no. 101016923).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
OGL, FvE and FH conceived the study. Methodology and
experiments were performed by DP, HNN, RZ and GE. DP, FvE and FH
wrote the manuscript. Review and editing was performed by OGL and
DP. The work was supervised by FvE.DP and FvE confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the ethics committee of
the Jena University Hospital (approval no. 4291-12/14) and written
informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Iseli TA, Lin MJ, Tsui A, Guiney A,
Wiesenfeld D and Iseli CE: Are wider surgical margins needed for
early oral tongue cancer? J Laryngol Otol. 126:289–294.
2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dittberner A, Ziadat R, Hoffmann F,
Pertzborn D, Gassler N and Guntinas-Lichius O: Fluorescein-Guided
panendoscopy for head and neck cancer using handheld probe-based
confocal laser endomicroscopy: A pilot study. Front Oncol.
11(671880)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Schmidt F, Dittberner A, Koscielny S,
Petersen I and Guntinas-Lichius O: Feasibility of real-time
near-infrared indocyanine green fluorescence endoscopy for the
evaluation of mucosal head and neck lesions. Head Neck. 39:234–240.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Schaafsma BE, Mieog JS, Hutteman M, van
der Vorst JR, Kuppen PJ, Löwik CW, Frangioni JV, van de Velde CJ
and Vahrmeijer AL: The clinical use of indocyanine green as a
near-infrared fluorescent contrast agent for image-guided oncologic
surgery. J Surg Oncol. 104:323–332. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Atallah I, Milet C, Quatre R, Henry M,
Reyt E, Coll JL, Hurbin A and Righini CA: Role of near-infrared
fluorescence imaging in the resection of metastatic lymph nodes in
an optimized orthotopic animal model of HNSCC. Eur Ann
Otorhinolaryngol Head Neck Dis. 132:337–342. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lu CH and Hsiao JK: Indocyanine green: An
old drug with novel applications. Tzu Chi Med J. 33:317–322.
2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kitai T, Inomoto T, Miwa M and Shikayama
T: Fluorescence navigation with indocyanine green for detecting
sentinel lymph nodes in breast cancer. Breast Cancer. 12:211–215.
2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yokoyama J, Fujimaki M, Ohba S, Anzai T,
Yoshii R, Ito S, Kojima M and Ikeda K: A feasibility study of NIR
fluorescent image-guided surgery in head and neck cancer based on
the assessment of optimum surgical time as revealed through dynamic
imaging. Onco Targets Ther. 6:325–330. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kedrzycki MS, Leiloglou M, Chalau V,
Chiarini N, Thiruchelvam PTR, Hadjiminas DJ, Hogben KR, Rashid F,
Ramakrishnan R, Darzi AW, et al: The impact of temporal variation
in indocyanine green administration on tumor identification during
fluorescence guided breast surgery. Ann Surg Oncol. 28:5617–5625.
2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vahrmeijer AL, Hutteman M, van der Vorst
JR, van de Velde CJ and Frangioni JV: Image-guided cancer surgery
using near-infrared fluorescence. Nat Rev Clin Oncol. 10:507–518.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Akrida I, Michalopoulos NV, Lagadinou M,
Papadoliopoulou M, Maroulis I and Mulita F: An updated review on
the emerging role of indocyanine green (ICG) as a sentinel lymph
node tracer in breast cancer. Cancers (Basel).
15(5755)2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cortese S, Kerrien E, Yakavets I,
Meilender R, Mastronicola R, Renard S, Leroux A, Bezdetnaya L and
Dolivet G: ICG-induced NIR fluorescence mapping in patients with
head & neck tumors after the previous radiotherapy.
Photodiagnosis Photodyn Ther. 31(101838)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hinni ML, Ferlito A, Brandwein-Gensler MS,
Takes RP, Silver CE, Westra WH, Seethala RR, Rodrigo JP, Corry J,
Bradford CR, et al: Surgical margins in head and neck cancer: A
contemporary review. Head Neck. 35:1362–1370. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Onda N, Kimura M, Yoshida T and Shibutani
M: Preferential tumor cellular uptake and retention of indocyanine
green for in vivo tumor imaging. Int J Cancer. 139:673–682.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu J: The enhanced permeability and
retention (EPR) Effect: The significance of the concept and methods
to enhance its application. J Pers Med. 11(771)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lim L, Chao M, Shapiro J, Millar JL, Kipp
D, Rezo A, Fong A, Jones IT, McLaughlin S and Gibbs P: Long-term
outcomes of patients with localized rectal cancer treated with
chemoradiation or radiotherapy alone because of medical
inoperability or patient refusal. Dis Colon Rectum. 50:2032–2039.
2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dittberner A, Friedl B, Wittig A, Buentzel
J, Kaftan H, Boeger D, Mueller AH, Schultze-Mosgau S, Schlattmann
P, Ernst T and Guntinas-Lichius O: Gender disparities in
epidemiology, treatment, and outcome for head and neck cancer in
germany: A population-based long-term analysis from 1996 to 2016 of
the thuringian cancer registry. Cancers (Basel).
12(3418)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Huang SH and O'Sullivan B: Overview of the
8th edition TNM classification for head and neck cancer. Curr Treat
Options Oncol. 18(40)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hoffmann F: S3_dataset_high resolution
microscopy images.zip., 2023. https://doi.org/10.6084/m9.figshare.22331380.v1.
|
|
20
|
Stanga PE, Lim JI and Hamilton P:
Indocyanine green angiography in chorioretinal diseases:
Indications and interpretation: An evidence-based update.
Ophthalmology. 110:15–21; quiz 22-3. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sethi HK, Sina EM, Mady LJ and Fundakowski
CE: Sentinel lymph node biopsy for head and neck malignancies
utilizing simultaneous radioisotope gamma probe and indocyanine
green fluorescence navigation. Head Neck. 46:212–217.
2024.PubMed/NCBI View Article : Google Scholar
|
|
22
|
De Ravin E, Venkatesh S, Harmsen S,
Delikatny EJ, Husson MA, Lee JYK, Newman JG and Rajasekaran K:
Indocyanine green fluorescence-guided surgery in head and neck
cancer: A systematic review. Am J Otolaryngol.
43(103570)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Belia F, Biondi A, Agnes A, Santocchi P,
Laurino A, Lorenzon L, Pezzuto R, Tirelli F, Ferri L, D'Ugo D and
Persiani R: The use of indocyanine green (ICG) and near-infrared
(NIR) fluorescence-guided imaging in gastric cancer surgery: A
narrative review. Front Surg. 9(880773)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang Y, Chen X, Gueydan C and Han J:
Plasma membrane changes during programmed cell deaths. Cell Res.
28:9–21. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Egloff-Juras C, Yakavets I, Scherrer V,
Francois A, Bezdetnaya L, Lassalle HP and Dolivet G: Validation of
a three-dimensional head and neck spheroid model to evaluate
cameras for NIR fluorescence-guided cancer surgery. International
Journal of Molecular Sciences. 22(1966)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chan CD, Brookes MJ, Tanwani R, Hope C,
Pringle TA, Knight JC and Rankin KS: Investigating the mechanisms
of indocyanine green (ICG) cellular uptake in sarcoma. BioRxiv
2021.2004. 2005.438013, 2021.
|
|
27
|
Jiang JX, Keating JJ, De Jesus EM, Judy
RP, Madajewski B, Venegas O, Okusanya OT and Singhal S:
Optimization of the enhanced permeability and retention effect for
near-infrared imaging of solid tumors with indocyanine green. Am J
Nucl Med Mol Imaging. 5:390–400. 2015.PubMed/NCBI
|
|
28
|
Cherrick GR, Stein SW, Leevy CM and
Davidson CS: Indocyanine green: Observations on its physical
properties, plasma decay, and hepatic extraction. J Clin Invest.
39:592–600. 1960.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Digonnet A, Van Kerckhove S, Moreau M,
Willemse E, Quiriny M, Ahmed B, de Saint Aubain N, Andry G and
Bourgeois P: Near infrared fluorescent imaging after intravenous
injection of indocyanine green during neck dissection in patients
with head and neck cancer: A feasibility study. Head Neck 38 Suppl.
1:E1833–E1837. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ahn HM, Son GM, Lee IY, Shin DH, Kim TK,
Park SB and Kim HW: Optimal ICG dosage of preoperative colonoscopic
tattooing for fluorescence-guided laparoscopic colorectal surgery.
Surg Endosc. 36:1152–1163. 2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Teng CW, Huang V, Arguelles GR, Zhou C,
Cho SS, Harmsen S and Lee JYK: Applications of indocyanine green in
brain tumor surgery: Review of clinical evidence and emerging
technologies. Neurosurg Focus. 50(E4)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Boogerd LSF, Handgraaf HJM, Huurman VAL,
Lam HD, Mieog JSD, van der Made WJ, van de Velde CJH and Vahrmeijer
AL: The best approach for laparoscopic fluorescence
cholangiography: Overview of the literature and optimization of
dose and dosing time. Surg Innov. 24:386–396. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Egloff-Juras C, Bezdetnaya L, Dolivet G
and Lassalle HP: NIR fluorescence-guided tumor surgery: New
strategies for the use of indocyanine green. Int J Nanomedicine.
14:7823–7838. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Borlan R, Focsan M, Maniu D and Astilean
S: Interventional NIR fluorescence imaging of cancer: Review on
next generation of dye-loaded protein-based nanoparticles for
real-time feedback during cancer surgery. Int J Nanomedicine.
16:2147–2171. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Achterberg FB, Sibinga Mulder BG, Meijer
RP, Bonsing BA, Hartgrink HH, Mieog JSD, Zlitni A, Park SM, Farina
Sarasqueta A, Vahrmeijer AL and Swijnenburg RJ: Real-time surgical
margin assessment using ICG-fluorescence during laparoscopic and
robot-assisted resections of colorectal liver metastases. Ann
Transl Med. 8(1448)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Achterberg FB, Bijlstra OD, Slooter MD,
Sibinga Mulder BG, Boonstra MC, Bouwense SA, Bosscha K, Coolsen
MME, Derksen WJM, Gerhards MF, et al: ICG-Fluorescence imaging for
margin assessment during minimally invasive colorectal liver
metastasis resection. JAMA Netw Open. 7(e246548)2024.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pal R, Lwin TM, Krishnamoorthy M, Collins
HR, Chan CD, Prilutskiy A, Nasrallah MP, Dijkhuis TH, Shukla S,
Kendall AL, et al: Fluorescence lifetime of injected indocyanine
green as a universal marker of solid tumours in patients. Nat
Biomed Eng. 7:1649–1666. 2023.PubMed/NCBI View Article : Google Scholar
|