Introduction
Hepatitis C virus (HCV) infection is a major cause
of chronic liver disease in individuals living with human
immunodeficiency virus (HIV) (PLWH) under anti-retroviral therapy
(ART) (1,2) due to shared common routes of
transmission. Worldwide, ~2.3 million individuals are estimated to
be coinfected with HIV and HCV (2).
A cohort study of the Asia HIV observational database reported
≤15.2% of HCV coinfection in HIV-infected individuals (3) and studies in Thai PLWH have also
suggested a high prevalence of 4-11% (4-7).
This coinfection causes a higher risk of progressive hepatitis and
accelerates the progression to liver fibrosis, cirrhosis and
hepatocellular carcinoma (HCC) (1,2,8). HIV
infection causes hepatitis C-mediated liver fibrosis by direct and
indirect mechanisms. HIV directly activates hepatocytes, hepatic
stellate cells and Kupffer cells, which cooperatively participate
in inflammation and fibrogenesis in the liver. HIV infection also
causes microbial translocation, indirectly inducing liver injury,
impaired HCV immune responses and fibrogenesis (2). While studies support the pathological
effects of coinfection in both HIV- and HCV-mediated disease
progression, advancements in the management of HIV-infected
patients with ART in the past decade (1,2) and
the use of direct-acting antivirals (DAAs) for chronic HCV
infection adds more complexity to the treatment and care of chronic
liver disease caused by HIV and HCV coinfection.
Previous studies have indicated significant liver
fibrosis and multiple risk factors for the severity and progression
of liver diseases in PLWH, including age, sex, CD4+ cell
count, CD4+/CD8+ ratio, HIV RNA levels,
alcohol consumption, diabetes mellitus and HCV coinfection
(4,9-11).
Although studies in HCV-infected patients have demonstrated various
clinical conditions associated with liver fibrosis and progression
to HCC either before or after the DAA era, such as age, duration of
infection, diabetes mellitus and alcohol consumption (12,13),
the progression of chronic hepatitis C in PLWH may be more advanced
and complicated (14). Moreover,
studies have indicated glucose and lipid abnormalities associated
with commonly used anti-retroviral (ARV) drug regimens in PLWH
(15,16). However, disordered glucose and lipid
metabolism in HCV-coinfected patients with HIV under ART remains to
be elucidated. The present study aimed to examine the prevalence
and associated clinical features of liver fibrosis in Thai patients
coinfected with HIV and HCV under suppressive ART. In particular,
metabolic markers in this patient group were determined. The
present study provided data supporting the development of
diagnosis, treatment and care for HCV-coinfected PLWH.
Materials and methods
Study population, clinical data and
laboratory investigation
A retrospective cross-sectional study was conducted
on 105 patients coinfected with HIV and HCV attending
Bamrasnaradura Infectious Diseases Institute (Thailand) between
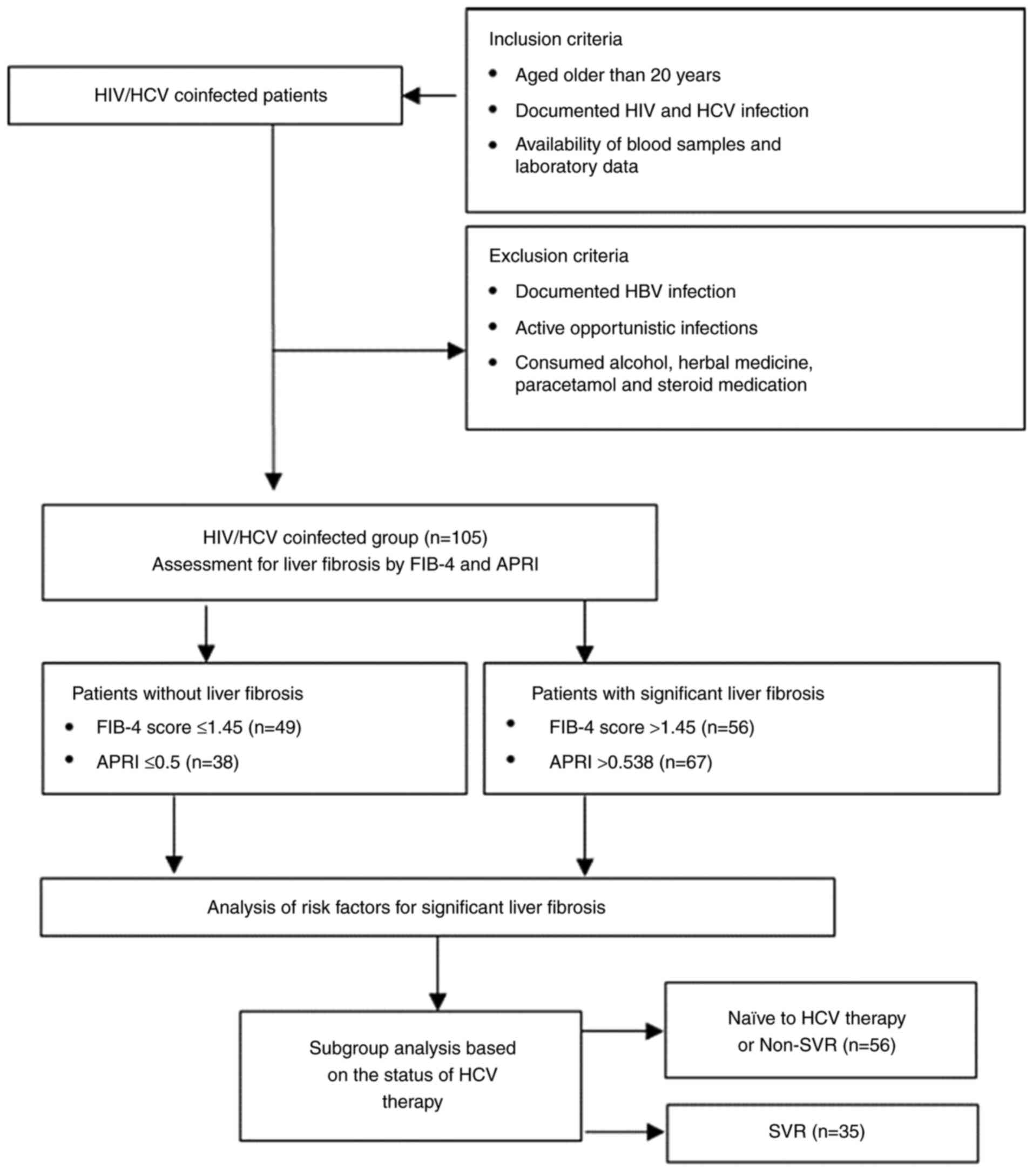
November 2016 and December 2017. As shown in Fig. 1, the patients were aged >20
years, with documented HIV and HCV infection and blood samples and
clinical data were available (Table
I). Patients with documented hepatitis B virus (HBV) infection,
active opportunistic infections (OIs) and who regularly consumed
alcohol, herbal medicine, paracetamol and steroid medications were
excluded from the study. All subjects provided written informed
consent. The study protocol was reviewed and approved by the Human
Ethics Committee No. 3, Thammasat University (approval no.
070/2560) and the Bamrasnaradura Infectious Diseases Institute
(approval no. R005/60). The patients were previously characterized
and the distribution and clinical significance of HCV genotypes in
the present study group were reported in a previous study (17).
 | Table ICharacteristics of Thai patients
coinfected with HIV and HCV according to the presence of
significant liver fibrosis assessed by FIB-4 score and APRI
(n=105). |
Table I
Characteristics of Thai patients
coinfected with HIV and HCV according to the presence of
significant liver fibrosis assessed by FIB-4 score and APRI
(n=105).
| | FIB-4 | | APRI | |
|---|
| Characteristic | All n (%) | ≤1.45 | >1.45 | P-value | ≤0.5 | >0.5 | P-value |
|---|
| All patients | 105 | 49 (46.7) | 56 (53.3) | - | 38 (36.2) | 67 (63.8) | - |
| Sex | | | | | | | |
|
M | 95 (90.5) | 46 (48.4) | 49 (51.6) | | 35 (36.8) | 60 (63.2) | |
|
F | 10 (9.5) | 3 (30.0) | 7 (70.0) | 0.331a | 3 (30.0) | 7 (70.0) | 0.745a |
| Age, years | | | | | | | |
|
≤40 | 25 (23.8) | 16 (64.0) | 9 (36.0) | | 8 (32.0) | 17 (68.0) | |
|
41-50 | 48 (45.7) | 25 (52.1) | 23 (47.9) | | 18 (37.5) | 30 (62.5) | |
|
>50 | 32 (30.5) | 8 (25.0) | 24 (75.0) | 0.008b | 12 (37.5) | 20 (62.5) | 0.883 |
| CD4+
cell count, cells/µl (n=102) | | | | | | | |
|
≥350 | 87 (85.3) | 45 (51.7) | 42 (48.3) | | 34 (39.1) | 53 (60.9) | |
|
<350 | 15 (14.7) | 3 (20.0) | 12 (80.0) | 0.027a,b | 3 (20.0) | 12 (80.0) | 0.245 |
| Antiretroviral
therapy (n=100) | | | | | | | |
|
Others | 15 (15.0) | 6 (40.0) | 9 (60.0) | | 4 (26.7) | 11 (73.3) | |
|
2NRTIs and
NNRTI | 78 (78.0) | 40 (51.3) | 38 (48.7) | 0.144 | 30 (38.5) | 48 (61.5) | 0.650 |
|
2NRTIs and
PI | 7 (7.0) | 1 (14.3) | 6 (85.7) | | 3 (42.9) | 4 (57.1) | |
| HCV genotypes
(n=73) | | | | | | | |
|
G1 | 40 (54.8) | 20 (50.0) | 20 (50.0) | | 15 (37.5) | 25 (62.5) | |
|
G3 | 27 (37.0) | 10 (37.0) | 17 (63.0) | | 9 (33.3) | 18 (66.7) | |
|
G6 | 6 (8.2) | 2 (33.3) | 4 (66.7) | 0.498 | 3 (50.0) | 3 (50.0) | 0.743 |
| Pretreatment HCV
viral load, log IU/ml (n=71) | | | | | | | |
|
<5.00 | 7 (9.9) | 3 (42.9) | 4 (57.1) | | 3 (42.9) | 4 (57.1) | |
|
≥5.00 | 64 (90.1) | 25 (39.1) | 39 (60.9) | 1.000a | 19 (29.7) | 45 (70.3) | 0.669a |
| SVR to HCV therapy
(n=100) | | | | | | | |
|
SVR | 35 (35.0) | 21 (60.0) | 14 (40.0) | | 23 (65.7) | 12 (34.3) | |
|
Non-SVR | 7 (7.0) | 3 (42.9) | 4 (57.1) | | 2 (28.6) | 5 (71.4) | |
|
Naïve to
treatment | 49 (49.0) | 21 (42.9) | 28 (51.1) | 0.173 | 10 (20.4) | 39 (79.6) |
<0.001b |
|
On
treatment | 9 (9.0) | 2 (22.2) | 7 (77.8) | | 1 (11.1) | 8 (88.9) | |
| FBS, mg/dl
(n=104) | | | | | | | |
|
<110 | 80 (76.9) | 38 (47.5) | 42 (52.5) | | 29 (36.2) | 51 (63.8) | |
|
≥110 | 24 (23.1) | 11 (45.8) | 13 (54.2) | 1.000a | 9 (37.5) | 15 (62.5) | 1.000a |
| Cholesterol, mg/dl
(n=100) | | | | | | | |
|
<200 | 83 (83.0) | 37 (44.6) | 46 (55.4) | | 26 (31.3) | 57 (68.7) | |
|
≥200 | 17 (17.0) | 10 (58.8) | 7 (41.2) | 0.301a | 10 (58.8) | 7 (41.2) | 0.050a |
| HDL, mg/dl
(n=99) | | | | | | | |
|
M <40, F
<50 | 36 (36.4) | 15 (41.7) | 21 (58.3) | | 9 (25.0) | 27 (75.0) | |
|
M ≥40, F
≥50 | 63 (63.6) | 33 (52.4) | 30 (47.6) | 0.403a | 29 (46.0) | 34 (54.0) | 0.053a |
| LDL, mg/dl
(n=101) | | | | | | | |
|
<130 | 78 (77.2) | 35 (44.9) | 43 (55.1) | | 22 (28.2) | 56 (71.8) | |
|
≥130 | 23 (22.8) | 13 (56.5) | 10 (43.5) | 0.352a | 16 (69.6) | 7 (30.4) | 0.001a,b |
| TG, mg/dl
(n=102) | | | | | | | |
|
<150 | 68 (66.7) | 30 (44.1) | 38 (55.9) | | 21 (30.9) | 47 (69.1) | |
|
≥150 | 34 (33.3) | 17 (50.0) | 17 (50.0) | 0.674a | 15 (44.1) | 19 (55.9) | 0.196a |
| TG/HDL ratio
(n=96) | | | | | | | |
|
M <2.6, F
<1.7 | 45 (46.9)) | 20 (44.4) | 25 (55.6) | | 15 (33.3) | 30 (66.7) | |
|
M ≥2.6, F
≥1.7 | 51 (53.1) | 26 (51.0) | 25 (49.0) | 0.522 | 21 (41.2) | 30 (58.8) | 0.428 |
| TG-glucose index
(n=101) | | | | | | | |
|
<9.2 | 100(100) | 47 (46.5) | 54 (53.5) | | 36 (35.6) | 65 (64.4) | |
|
≥9.2 | 0 (0) | 0 (0) | 0 (0) | ND | 0 (0) | 0 (0) | ND |
Clinical data and laboratory
investigation
Data collected from the medical records of the
patients included age, sex, HIV infection diagnosis, duration of
HIV infection, currently used ARV drugs, duration of ART, duration
of HCV infection, HCV and HBV infection diagnosis, pretreatment HCV
viral load, HCV genotypes, current HCV treatment status and OIs.
Data collected from a screening questionnaire included weight,
height, alcohol consumption, steroid and paracetamol drug intake
and herbal medicine use. All patients underwent clinical
examination and blood samples were subjected to testing for HIV
viral load (Roche Molecular Diagnostics); CD4+ cell
count (BD Biosciences); platelet count (Beckman Coulter, Inc.);
liver function tests, including aspartate aminotransferase (AST)
and alanine aminotransferase (ALT) (Roche Diagnostics); fasting
blood sugar (FBS); and serum lipid levels, including cholesterol,
low-density lipoprotein (LDL), high-density lipoprotein (HDL) and
triglyceride (TG) (Roche Diagnostics).
Sustained virologic response (SVR) was defined as
negative HCV RNA at 6 months after completing HCV treatment
(18). Liver fibrosis was evaluated
by fibrosis score-4 (FIB-4), which was classified into class 1
(≤1.45), class 2 (1.46-3.25) and class 3 (>3.25) (19,20)
and AST-to-platelet ratio index (APRI), which was classified into
class 1 (≤0.5), class 2 (0.51-1.5) and class 3 (>1.5) (21). Abnormal levels of FBS were defined
as ≤110 mg/dl (22-24).
According to the National Cholesterol Education Program, Adult
Treatment Panel III guidelines (24), lipid profile abnormalities included
hypercholesterolemia, defined as cholesterol levels ≥200 mg/dl;
hypertriglyceridemia, defined as TG levels ≥150 mg/dl; high LDL
cholesterol, defined as ≥130 mg/dl; and low HDL cholesterol,
defined as <40 mg/dl in men and <50 mg/dl in women (24). Metabolic markers, including TG/HDL
ratio and TG-glucose index, were also determined to predict
cardiometabolic disease. In the present study, the cut-off values
of TG/HDL ratio were 2.6 for men and 1.7 for women, while those of
TG-glucose index were 9.2 (25,26).
Statistical analysis
Data are presented as the mean ± standard
derivation, median (range) and percentages. The χ2 and
Fisher's exact tests were used to determine the association between
categorical variables and liver fibrosis, as assessed by FIB-4
score and APRI, whereas the Mann-Whitney U test was used to analyze
the association between liver fibrosis or HCV therapy and
continuous variables. The level of statistical significance was set
at P<0.05. Univariate and multivariate logistic regression was
performed to determine the risks for liver fibrosis. Odds ratio
(OR) with 95% confidence interval (CI) values and P-values were
calculated. P<0.05 was considered to indicate a statistically
significant difference. The statistical analysis was performed
using SPSS version 15.0 (SPSS Inc.).
Results
The present study was conducted on 105 patients with
HIV and HCV coinfection according to the flow diagram in Fig. 1. The study consisted of 95 (90.5%)
male and 10 (9.5%) female patients, with median ages of 46 (27-67)
years and 47 (34-61) years, respectively. The mean CD4+
cell count was 566±2.66 cells/ml and 91% (93/102) of patients had
undetectable levels of HIV viral load (<20 copies/ml). The
majority of patients (99/100) were on ART, 78% (78/100) on 2
nucleoside reverse transcriptase inhibitors (2NRTIs) and
non-nucleoside reverse transcriptase inhibitors (NNRTIs), 7%
(7/100) on 2NRTIs and protease inhibitors (PIs). and 15% (15/100)
on other antiretroviral drugs. Only 1% (1/100) of the patients were
naïve to ART. The median duration of ART was 9 (2-20) years. In
this patient group (Table I), the
prevalence of patients coinfected with HCV genotypes 1, 3 and 6
were 54.8% (40/73), 37% (27/73) and 8.2% (6/73), respectively and
90.1% (64/71) of the patients had pretreatment HCV viral load
>5.0 log IU/ml (17). A total of
51% (51/100) of the patients were on HCV therapy with 35% (35/100)
achieving SVR, 7% (7/100) not achieving SVR and 9% (9/100)
currently receiving treatment. The prevalence of patients having
significant liver fibrosis assessed by FIB-4 scores >1.45 was
53.3% and by APRI >0.5 was 63.8%. The rates of patients with
abnormal levels of FBS were 23.1% (24/104), whereas those with
hypercholesterolemia, hypertriglyceridemia, high LDL and low HDL
cholesterol were 17% (17/100), 33.3% (34/102), 22.8% (23/101) and
36.4% (36/99), respectively. In the present study group, the median
values of metabolic markers, TG/HDL ratio and TG-glucose index,
were 2.62 (0.48-23.94) and 4.69 (4.3-5.66) respectively. The rates
of patients at risk for cardiometabolic diseases with a TG/HDL
ratio >2.6 for men and 1.7 for women were 53.1% (51/96).
However, no patients with a TG-glucose index >9.2 were
observed.
The characteristics of the patients according to the
presence of significant liver fibrosis are shown in Table I. The majority of characteristics of
the patients with and without significant liver fibrosis, as
evaluated by FIB-4 score and APRI, were similar. However, age and
CD4+ cell count were significantly associated with liver
fibrosis, as assessed by FIB-4 scores (P=0.008 and P=0.027,
respectively); and HCV treatment status, cholesterol and LDL levels
were associated with liver fibrosis, as assessed by APRI
(P<0.001, P=0.050 and P=0.001, respectively). The data were
consistent with the univariate and multivariate analyses of risk
factors for significant liver fibrosis in the present study group
(Table II). Multivariate analysis
indicated that patients aged >50 years had an 11.4 times higher
risk (P=0.001) and those with a CD4+ cell count <350
cells/µl had a 5.7 times higher risk (P=0.017) for liver fibrosis,
as assessed by FIB-4 scores, than those aged ≤40 years and with a
CD4+ cell count ≥350 cells/µl respectively. Univariate
and multivariate analyses indicated that patients naïve to HCV
treatment had 7.5 times (P=0.001) and 5.4 times (P=0.003) higher
risks than those with SVR, respectively. Similarly, patients who
were on HCV treatment had 15.3 times (P=0.015) and 12.7 times
(P=0.033) higher risks than patients with SVR, respectively.
Notably, the univariate analysis indicated that patients developing
hypercholesterolemia, high HDL and high LDL cholesterol had lower
risks than those with normal levels (OR 0.3, 95% CI 0.1-0.9,
P=0.037; OR 0.4, 95% CI 0.2-0.9, P=0.041; OR 0.2, 95% CI 0.1-0.5,
P=0.001, respectively). However, only patients with high LDL
cholesterol remained at a lower risk (OR 0.1, 95% CI 0.3-0.8,
P=0.029) according to multivariate analysis adjusted for sex, age,
CD4+ cell count, ARV treatment, HCV genotypes,
pretreatment HCV viral load, HCV treatment, FBS and a lipid
panel.
 | Table IIUnivariate and multivariate analyses
of risks for liver fibrosis in Thai patients coinfected with HIV
and HCV (n=105). |
Table II
Univariate and multivariate analyses
of risks for liver fibrosis in Thai patients coinfected with HIV
and HCV (n=105).
| Characteristic | Enrolled patients N
(%) | Patients with FIB-4
>1.45 | Crude OR (95%
CI) | P-value | Adjusted OR (95%
CI) | P-value | Patients with APRI
>0.5 | Crude OR (95%
CI) | P-value | Adjusted OR (95%
CI) | P-value |
|---|
| Sex | | | | | | | | | | | |
|
M | 95 (90.5) | 49 (87.5) | 1 | | | | 60 (89.6) | 1 | | | |
|
F | 10 (9.5) | 7 (12.5) | 2.2 (0.5-9.0) | 0.276 | | | 7 (10.4) | 1.4 (0.3-5.6) | 0.669 | | |
| Age, years | | | | | | | | | | | |
|
≤40 | 25 (23.8) | 9 (16.0) | 1 | | 1 | | 17 (25.4) | 1 | | | |
|
41-50 | 48 (45.7) | 23 (41.1) | 1.6 (0.6-4.4) | 0.332 | 2.5 (0.7-8.0) | 0.123 | 30 (44.7) | 0.8 (0.3-2.2) | 0.642 | | |
|
>50 | 32 (30.5) | 24 (42.9) | 5.3 (1.7-16.7) | 0.004a | 11.4
(2.8-47.0) | 0.001a | 20 (29.9) | 0.8 (0.3-2.4) | 0.666 | | |
| CD4+
cell count, cells/µl (n=102) | | | | | | | | | | | |
|
≥350 | 87 (85.3) | 42 (77.8) | 1 | | 1 | | 53 (81.5) | 1 | | | |
|
<350 | 15 (14.7) | 12 (22.2) | 4.3 (1.1-16.3) | 0.032a | 5.7 (1.4-24.1) | 0.017a | 12 (18.5) | 2.6 (0.7-9.8) | 0.167 | | |
| ARV drugs
(n=100) | | | | | | | | | | | |
|
Others | 15 (15.0) | 9 (17.0) | 1 | | | | 11 (17.5) | 1 | | | |
|
2NRTIs and
NNRTI | 78 (78.0) | 38 (71.7) | 0.6 (0.2-2.0) | 0.426 | | | 48 (76.2) | 0.6 (0.2-2.0) | 0.389 | | |
|
2NRTIs and
PI | 7 (7.0) | 6 (11.3) | 4 (0.4-42.2) | 0.249 | | | 4 (6.3) | 0.5 (0.1-3.2) | 0.451 | | |
| HCV genotypes
(n=73) | | | | | | | | | | | |
|
G1 | 40 (54.8) | 20 (48.8) | 1 | | | | 25 (54.4) | 1 | | | |
|
G3 | 27 (37.0) | 17 (41.4) | 1.7 (0.6-4.6) | 0.297 | | | 18 (39.1) | 1.2 (0.4-3.3) | 0.727 | | |
|
G6 | 6 (8.2) | 4 (9.8) | 2.0 (0.3-12.2) | 0.452 | | | 3 (6.5) | 0.6 (0.1-3.4) | 0.561 | | |
| Pretreatment HCV
viral load, log IU/ml (n=71) | | | | | | | | | | | |
|
<5.00 | 7 (9.9) | 4 (9.3) | 1 | | | | 4 (8.2) | 1 | | | |
|
≥5.00 | 64 (90.1) | 39 (90.7) | 1.2 (0.2-5.7) | 0.845 | | | 45 (91.8) | 1.8 (0.4-8.7) | 0.479 | | |
| SVR to HCV therapy
(n=100) | | | | | | | | | | | |
|
SVR | 35 (35.0) | 14 (26.4) | 1 | | | | 12 (18.8) | 1 | | 1 | |
|
Non-SVR | 7 (7.0) | 4 (7.6) | 2.0 (0.4-10.3) | | | | 5 (7.8) | 4.8 (0.8-28.5) | 0.085 | 3.2 (0.4-28.2) | 0.297 |
|
Naïve to
treatment | 49 (49.0) | 28 (52.8) | 2.0 (0.8-4.8) | | | | 39 (60.9) | 7.5 (2.8-20.0) | 0.001a | 5.4 (1.7-16.7) | 0.003a |
|
On
treatment | 9 (9.0) | 7 (13.2) | 5.3 (0.9-29.1) | 0.057 | | | 8 (12.5) | 15.3
(1.7-137.4) | 0.015a | 12.7
(1.2-131.9) | 0.033a |
| FBS, mg/dl
(n=104) | | | | | | | | | | | |
|
<110 | 80 (76.9) | 42 (76.4) | 1 | | | | 51 (77.3) | 1 | | | |
|
≥110 | 24 (23.1) | 13 (23.6) | 1.1 (0.4-2.7) | 0.886 | | | 15 (22.7) | 0.9 (0.4-2.4) | 0.911 | | |
| Cholesterol, mg/dl
(n=100) | | | | | | | | | | | |
|
<200 | 83 (83.0) | 46 (86.8) | 1 | | | | 57 (89.1) | 1 | | 1 | |
|
≥200 | 17 (17.0) | 7 (13.2) | 0.6 (0.2-1.6) | 0.287 | | | 7 (10.9) | 0.3 (0.1-0.9) | 0.037a | 2.0 (0.3-13.4) | 0.489 |
| HDL, mg/dl
(n=99) | | | | | | | | | | | |
|
M <40, F
<50 | 36 (36.4) | 21 (41.2) | 1 | | | | 27 (44.3) | 1 | | 1 | |
|
M ≥40, F
≥50 | 63 (63.6) | 30 (58.8) | 0.6 (0.3-1.5) | 0.306 | | | 34 (55.7) | 0.4 (0.2-0.9) | 0.041a | 0.5 (0.1-1.7) | 0.269 |
| LDL, mg/dl
(n=101) | | | | | | | | | | | |
|
<130 | 78 (77.2) | 43 (81.1) | 1 | | | | 56 (88.9) | 1 | | 1 | |
|
≥130 | 23 (22.8) | 10 (18.9) | 0.6 (0.2-1.6) | 0.328 | | | 7 (11.1) | 0.2 (0.1-0.5) | 0.001a | 0.1 (0.3-0.8) | 0.029a |
| TG, mg/dl
(n=102) | | | | | | | | | | | |
|
<150 | 68 (66.7) | 38 (69.1) | 1 | | | | 47 (71.2) | 1 | | 1 | |
|
≥150 | 34 (33.3) | 17 (30.9) | 0.8 (0.3-1.8) | 0.575 | | | 19 (28.8) | 0.6 (0.2-1.3) | 0.189 | 0.3 (0.1-1.1) | 0.082 |
Analysis by the Mann-Whitney U test, shown in
Table III, indicated
significantly higher levels of AST in patients with moderate and
advanced liver fibrosis, as assessed by FIB-4 scores (P=0.004 and
P<0.001, respectively) and APRI (both P<0.001) and higher
levels of ALT were detected in patients with moderate and advanced
liver fibrosis, as assessed by APRI (both P<0.001), than those
without fibrosis. Patients with moderate and advanced fibrosis
evaluated by FIB-4 scores were significantly older than those
without liver fibrosis (P<0.031 and P<0.001, respectively).
Consistent with the univariate and multivariate analyses,
considerably lower levels of cholesterol, HDL and LDL were observed
in the patients with advanced liver fibrosis, with a FIB-4 score
>3.25 and APRI >1.5 (all P<0.05).
 | Table IIIMedian levels of lipid profiles, age,
CD4+ cell count and liver enzymes stratified by FIB-4
scores and APRI in Thai patients coinfected with HIV and HCV. |
Table III
Median levels of lipid profiles, age,
CD4+ cell count and liver enzymes stratified by FIB-4
scores and APRI in Thai patients coinfected with HIV and HCV.
| | FIB-4 score | APRI |
|---|
| Laboratory
parameter | ≤1.45 (n=49) | 1.46-3.25
(n=35) | P-value | >3.25
(n=21) | P-value | ≤0.5 (n=38) | 0.51-1.5
(n=41) | P-value | >1.5 (n=26) | P-value |
|---|
| Age, years
(n=105) | 43.0 (27-65) | 47.0 (36-67) | 0.031a | 55.0 (39-63) |
<0.001b | 45.0 (29-65) | 44.0 (32-67) | 0.423 | 48.0 (27-63) | 0.345 |
| CD4+
cell count, cells/µl (n=102) | 534.5
(218-1,748) | 507.5 (92-991) | 0.477 | 470.0 (5-828) | 0.066 | 529.0
(218-1,748) | 559.5
(92-1,114) | 0.717 | 477.0 (5-824) | 0.147 |
| AST, U/l
(n=105) | 37.0 (17-189) | 57.0 (27-189) | 0.004a | 82.0 (48-420) |
<0.001b | 27.5 (17-64) | 57.0 (31-115) |
<0.001b | 98.5 (48-420) |
<0.001b |
| ALT, U/l
(n=105) | 42.0 (14-436) | 52.0 (11-264) | 0.583 | 62.0 (21-235) | 0.139 | 28.0 (11-138) | 65.0 (12-188) |
<0.001b | 97.5 (21-436) |
<0.001b |
| FBS, mg/dl
(n=104) | 96.0 (67-128) | 101.0 (84-160) | 0.140 | 102.5 (81-146) | 0.056 | 96.5 (84-126) | 99.0 (82-160) | 0.599 | 99 (67-157) | 0.339 |
| Cholesterol, mg/dl
(n=100) | 180.0
(102-242) | 173.0
(113-267) | 0.182 | 140.5 (82-205) |
<0.001b | 183.0
(102-267) | 173.5
(113-235) | 0.121 | 151.0 (82-205) |
<0.001b |
| HDL, mg/dl
(n=99) | 45.0 (27-99) | 51.0 (22-114) | 0.419 | 35.0 (11-79) | 0.009a | 46.5 (28-99) | 51.0 (22-114) | 0.742 | 40.0 (11-79) | 0.017a |
| LDL, mg/dl
(n=101) | 120.5 (67-174) | 106.0 (69-188) | 0.257 | 94.0 (37-153) | 0.006a | 123.5 (79-188) | 117.0 (67-182) | 0.570 | 91.0 (37-134) |
<0.001b |
| TG, mg/dl
(n=102) | 126.0 (67-447) | 108.0 (54-419) | 0.138 | 136.0 (56-383) | 0.433 | 127.5 (64-349) | 112.5 (54-447) | 0.310 | 130.0 (56-419) | 0.285 |
As univariate and multivariate analyses indicated a
significant association of HCV treatment status with liver fibrosis
evaluated by APRI, laboratory parameters, including lipid profiles,
age, CD4+ cell count and liver enzymes stratified by HCV
treatment status were analyzed by the Mann-Whitney U test (Table IV). The data showed that there was
no significant difference between the median ages and
CD4+ cell counts in the SVR and with naïve to HCV
treatment or non-SVR patient groups. The SVR group had
significantly higher median levels of liver enzymes, AST and ALT
and liver fibrosis, as determined by FIB-4 and APRI, than those in
the naïve to HCV treatment or non-SVR patient groups (P<0.001,
P<0.001, P=0.047 and P<0.001, respectively), indicating that
the therapy achieving SVR had improved liver pathology. Consistent
with the lower levels of lipids, cholesterol, HDL and LDL observed
in the patients with advanced liver fibrosis, median levels of
cholesterol and LDL in patients in the naïve to treatment or
non-SVR groups were significantly lower than those observed in the
SVR group (P=0.031 and P=0.039, respectively). These data may
suggest an involvement of lipid metabolism in liver fibrosis in
patients with HIV and HCV coinfection.
 | Table IVMedian levels of lipid profiles, age,
CD4+ cell count, liver enzymes and liver fibrosis
markers stratified by status of HCV therapy in patients coinfected
with HIV and HCV. |
Table IV
Median levels of lipid profiles, age,
CD4+ cell count, liver enzymes and liver fibrosis
markers stratified by status of HCV therapy in patients coinfected
with HIV and HCV.
| Laboratory
parameter | SVR (n=35) | Naïve to therapy or
non-SVR (n=56) | P-value |
|---|
| Age, years
(n=91) | 48.0
(37.0-65.0) | 45.0
(27.0-67.0) | 0.062 |
| CD4+
cell count, cells/µl (n=91) | 530.0
(190.0-1,288.0) | 551.0
(5.0-1,748.0) | 0.365 |
| AST, U/l
(n=91) | 31.0
(17.0-109.0) | 67.0
(20.0-189.0) |
<0.001b |
| ALT, U/l
(n=91) | 33.0
(11.0-138.0) | 75.0
(12.0-436.0) |
<0.001b |
| FIB-4 score
(n=91) | 1.2 (0.5-4.8) | 1.6 (0.0-13.9) | 0.047a |
| APRI (n=91) | 0.4 (0.1-2.4) | 1.0 (0.1-4.3) |
<0.001b |
| Cholesterol, mg/dl
(n=86) | 179.0
(131.0-242.0) | 171.0
(82.0-235.0) | 0.031a |
| HDL, mg/dl
(n=85) | 52.0
(27.0-79.0) | 45.0
(11.0-114.0) | 0.177 |
| LDL, mg/dl
(n=87) | 118.0
(75.0-188.0) | 106.0
(37.0-182.0) | 0.039a |
| TG, mg/dl
(n=88) | 120.0
(64.0-447.0) | 118.0
(54.0-442.0) | 0.985 |
Discussion
HCV coinfection is well-established as a major risk
for the progression of chronic liver disease in PLWH (1,2,10,27).
The present study was conducted on Thai patients coinfected with
HIV and HCV mostly on long-term suppressive ART. A total of 51% of
the present study group underwent HCV therapy with an SVR rate of
35%. The data indicated the high prevalence of patients developing
significant liver fibrosis and the relatively high rates of those
with abnormally high levels of FBS, lipid profiles, which were
total cholesterol, TG, LDL and HDL and TG/HDL in the present study
group. Previous studies have indicated that, in the era of ART,
HCV/HIV coinfection accelerates the progression of hepatitis to
advanced chronic liver disease and HCC and can cause higher
liver-related mortality (8,14,28,29).
Moreover, the commonly used ARV drugs in PLWH are associated with
impaired FBS and abnormal lipid profiles, contributing to
increasing risks for diabetes and cardiovascular disease (15,16).
Thus, the present study group has the potential to develop advanced
liver disease and metabolic disorders. However, metabolic
alterations in PLWH who are coinfected with HCV appear to be more
complicated. In the present study, clinical features associated
with significant liver fibrosis, particularly metabolic profiles,
in patients coinfected with HIV and HCV mostly on suppressive ART
were investigated.
Similar to previous studies on HCV- or HIV-infected
patients (4,10,12,13),
clinical features associated with significant liver fibrosis in
this HCV and HIV-coinfected patient group were identified. While
patients aged >50 years and with a CD4+ cell count
<350 cells/µl exhibited a high risk for significant liver
fibrosis, as assessed by FIB-4 scores, patients naïve to or
receiving HCV treatment carried a high risk for liver fibrosis, as
determined by APRI. Notably, the analysis also indicated a
significant association of lipid levels with liver fibrosis
evaluated by APRI. Patients who developed hypercholesterolemia,
high HDL and high LDL levels had lower risks for liver fibrosis
than those with normal levels, which was consistent with the
significant reduction of lipid levels in more advanced liver
fibrosis observed in the patients with FIB-4 scores >3.25 and
APRI >1.5 compared with those without liver fibrosis. In
addition, the data indicated that HCV treatment achieving SVR
significantly increased the levels of lipids, cholesterol and LDL,
in this HIV/HCV patient group. This was similar to the findings of
previous studies indicating lower serum levels of total
cholesterol, LDL, HDL and TG in HCV-monoinfected patients compared
with those in negative subjects (30,31).
The previous epidemiological study indicated that HCV infection
might cause hypolipidemia regardless of host factors, including
age, nutrition and hepatic damage (31). The involvement of hypolipidemia with
the outcome of liver transplantation has also been demonstrated in
a patient group with high rates of HCV infection (32). Therefore, the association of lipid
levels with liver fibrosis in HCV-coinfected PLWH observed in the
present study was possibly due to HCV coinfection and the status of
HCV treatment.
Initially, the present study observed the relatively
high prevalence of patients at risk for cardiometabolic diseases,
as indicated by lipid profile abnormalities and TG/HDL ratio, which
was consistent with a previous study in PLWH (16). However, the present data indicated
no association between the abnormally high TG/HDL ratio and
significant liver fibrosis as evaluated by FIB-4 and APRI. In
addition, the logistic regression analysis indicated a negative
association of lipid levels, cholesterol, HDL and LDL, with liver
fibrosis as assessed by APRI. Indeed, HCV infection is closely
associated with host lipid metabolism (33). Serum lipids serve roles in HCV
virion circulation and entry into hepatocytes (33). The virus also employs different
types of lipids to facilitate virion production and assembly
(33). The inverse relationship
between lipid levels and liver fibrosis observed in the present
study may reflect the disruption of lipid metabolism caused by
hepatic damage during HCV infection. While it has been reported
that commonly used ARV drug regimens cause an increase in serum
lipid levels in PLWH (16), the
mechanisms underlying HCV infection-mediated host lipid metabolism
in PLWH on suppressive ART require further clarification.
The limitations of the present study include that it
contained a limited number of subjects, which may affect the
statistical significance of the variables tested. Clinical data
were obtained from retrospective medical reviews and the drug
regimens used for hepatitis C therapy were unavailable. However, as
the data collection was performed at a time when HCV therapy in
Thailand was performed based on the clinical practice guidelines
for the management of chronic hepatitis C 2015, all treated
HCV-infected patients were likely to have received a combination of
pegylated interferon and ribavirin (34). Thus, the present study provides data
before the use of DAA therapy in Thailand.
In conclusion, the present cross-sectional study
detected the high prevalence of liver fibrosis in HCV-coinfected
PLWH, evaluated by non-invasive markers, FIB-4 scores and APRI. The
analysis indicated that age, CD4+ cell count, HCV
treatment and lipid profiles, including cholesterol, HDL and LDL,
were significantly associated with liver fibrosis. Notably, the
reduction of serum lipid levels was significantly associated with
the severity of chronic liver disease. Long-term monitoring for
clinical outcomes related to lipid metabolism, particularly in
patients starting DAA therapy, is warranted.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Thailand Science
Research and Innovation Fundamental Fund fiscal year 2022 (grant
no. 2493097) and the Thammasat University Research Unit in
Diagnostic Molecular Biology of Chronic Diseases related to Cancer
(DMB-CDC). Somkid Srisopa is a recipient of the Research Grant for
Graduate Studies from the National Research Council of Thailand
(fiscal year 2018, contract no. 7/2561).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CA contributed to funding acquisition, resources,
supervision, study design, data analysis, manuscript preparation,
review and editing. SS performed experimental work, data collection
and analysis and manuscript preparation. DP contributed to the
statistical analysis. SS and CA confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The protocol of the present study was reviewed and
approved by the Human Ethics Committee no. 3, Thammasat University
(approval no. 070/2560) and Bamrasnaradura Infectious Diseases
Institute (approval no. R005/60), Thailand.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ganesan M, Poluektova LY, Kharbanda KK and
Osna NA: Human immunodeficiency virus and hepatotropic viruses
co-morbidities as the inducers of liver injury progression. World J
Gastroenterol. 25:398–410. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gobran ST, Ancuta P and Shoukry NH: A Tale
of Two Viruses: Immunological Insights Into HCV/HIV Coinfection.
Front Immunol. 12(726419)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen M, Wong WW, Law MG, Kiertiburanakul
S, Yunihastuti E, Merati TP, Lim PL, Chaiwarith R, Phanuphak P, Lee
MP, et al: Hepatitis B and C Co-Infection in HIV Patients from the
TREAT Asia HIV observational database: Analysis of risk factors and
survival. PLoS One. 11(e0150512)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Akekawatchai C, Sretapunya W,
Pipatsatitpong D and Chuenchit T: Hepatitis B or C virus
coinfection in and risks for transaminitis in human
immunodeficiency virus-infected Thais on combined antiretroviral
therapy. Asian Biomedicine. 9:353–361. 2015.
|
|
5
|
Law WP, Dore GJ, Duncombe CJ,
Mahanontharit A, Boyd MA, Ruxrungtham K, Lange JM, Phanuphak P and
Cooper DA: Risk of severe hepatotoxicity associated with
antiretroviral therapy in the HIV-NAT Cohort, Thailand, 1996-2001.
AIDS. 17:2191–2199. 2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sungkanuparph S, Wongprasit P, Manosuthi W
and Atamasirikul K: Compliance with hepatitis B and hepatitis C
virus infection screening among HIV-1 infected patients in a
resource-limited setting. Southeast Asian J Trop Med Public Health.
39:863–866. 2008.PubMed/NCBI
|
|
7
|
Thitipatarakorn S, Chinbunchorn T, Peelay
J, Seekaew P, Amatavete S, Sangsai M, Pankam T, Avihingsanon A,
Avery M, Phanuphak P, et al: Prevalence and the associated factors
of hepatitis B and hepatitis C viral infections among HIV-positive
individuals in same-day antiretroviral therapy initiation program
in Bangkok, Thailand. BMC Public Health. 22(144)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sun J, Althoff KN, Jing Y, Horberg MA,
Buchacz K, Gill MJ, Justice AC, Rabkin CS, Goedert JJ, Sigel K, et
al: Trends in hepatocellular carcinoma incidence and risk among
persons with HIV in the US and Canada, 1996-2015. JAMA Netw Open.
4(e2037512)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
DallaPiazza M, Amorosa VK, Localio R,
Kostman JR and Lo Re V III: Prevalence and risk factors for
significant liver fibrosis among HIV-monoinfected patients. BMC
Infect Dis. 10(116)2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chiraunyanann T, Changsri K, Sretapunya W,
Yuenyongchaiwat K and Akekawatchai C: CXCL12 G801A polymorphism is
associated with significant liver fibrosis in HIV-infected Thais: A
cross-sectional study. Asian Pac J Allergy Immunol. 37:162–170.
2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Androutsakos T, Schina M, Pouliakis A,
Kontos A, Sipsas N and Hatzis G: Causative factors of liver
fibrosis in HIV-infected patients. A single center study. BMC
Gastroenterol. 20(91)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
de Torres M and Poynard T: Risk factors
for liver fibrosis progression in patients with chronic hepatitis
C. Ann Hepatol. 2:5–11. 2003.PubMed/NCBI
|
|
13
|
Luna-Cuadros MA, Chen HW, Hanif H, Ali MJ,
Khan MM and Lau DT: Risk of hepatocellular carcinoma after
hepatitis C virus cure. World J Gastroenterol. 28:96–107.
2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nguyen Truong T, Laureillard D, Lacombe K,
Duong Thi H, Pham Thi Hanh P, Truong Thi Xuan L, Chu Thi N, Luong
Que A, Vu Hai V, Nagot N, et al: High Proportion of HIV-HCV
coinfected patients with advanced liver fibrosis requiring
hepatitis C Treatment in Haiphong, Northern Vietnam (ANRS 12262).
PLoS One. 11(e0153744)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tadesse WT, Adankie BT, Shibeshi W, Amogne
W, Aklillu E and Engidawork E: Prevalence and predictors of glucose
metabolism disorders among People Living with HIV on combination
antiretroviral therapy. PLoS One. 17(e0262604)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Waters DD and Hsue PY: Lipid abnormalities
in persons living with HIV infection. Can J Cardiol. 35:249–259.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Somkid Srisopa DP and Chaereeporn
Akekawatchai: Genotypic distribution of hepatitis C virus in human
immunodeficiency virus-infected patients in Bamrasnaradura
Infectious Diseases Institute. Journal of the Medical Technologist
Association of Thailand. 48:7215–7226. 2020.
|
|
18
|
Cloherty G, Talal A, Coller K, Steinhart
C, Hackett J Jr, Dawson G, Rockstroh J and Feld J: Role of
serologic and molecular diagnostic assays in identification and
management of hepatitis C virus infection. J Clin Microbiol.
54:265–273. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sterling RK, Lissen E, Clumeck N, Sola R,
Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT,
Thomas DL, et al: Development of a simple noninvasive index to
predict significant fibrosis in patients with HIV/HCV coinfection.
Hepatology. 43:1317–1325. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Foca E, Fabbiani M, Prosperi M, Quiros
Roldan E, Castelli F, Maggiolo F, Di Filippo E, Di Giambenedetto S,
Gagliardini R, Saracino A, et al: Liver fibrosis progression and
clinical outcomes are intertwined: Role of CD4+ T-cell count and
NRTI exposure from a large cohort of HIV/HCV-coinfected patients
with detectable HCV-RNA: A MASTER cohort study. Medicine
(Baltimore). 95(e4091)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wai CT, Greenson JK, Fontana RJ,
Kalbfleisch JD, Marrero JA, Conjeevaram HS and Lok AS: A simple
noninvasive index can predict both significant fibrosis and
cirrhosis in patients with chronic hepatitis C. Hepatology.
38:518–526. 2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Federation WaID, World Health Organization
(WHO): Definition and diagnosis of diabetes mellitus and
intermediate hyperglycarmia. WHO, Geneva, 2006.
|
|
23
|
American Diabetes Association.
Classification and diagnosis of diabetes: Standards of medical care
in diabetesd-2021. Diabetes Care. 44 (Suppl 1):S15–S33.
2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Expert Panel on Detection, Evaluation and
Treatment of High Blood Cholesterol in Adults. Executive summary of
the third report of The National cholesterol education program
(NCEP) expert panel on detection, evaluation, and treatment of high
blood cholesterol in adults (Adult Treatment Panel III). JAMA.
285:2486–2497. 2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lelis DF, Calzavara JVS, Santos RD,
Sposito AC, Griep RH, Barreto SM, Molina MDCB, Schmidt MI, Duncan
BB, Bensenor I, et al: Reference values for the triglyceride to
high-density lipoprotein ratio and its association with
cardiometabolic diseases in a mixed adult population: The
ELSA-Brasil study. J Clin Lipidol. 15:699–711. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lertsakulbunlue S, Mungthin M, Rangsin R,
Kantiwong A and Sakboonyarat B: Trends in baseline
triglyceride-glucose index and association with predicted 10-year
cardiovascular disease risk among type 2 diabetes patients in
Thailand. Sci Rep. 13(12960)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zeremski M, Dimova RB, Pillardy J, de Jong
YP, Jacobson IM and Talal AH: Fibrosis progression in patients with
chronic hepatitis C virus infection. J Infect Dis. 214:1164–1170.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Medrano LM, Garcia-Broncano P, Berenguer
J, González-García J, Jiménez-Sousa MÁ, Guardiola JM, Crespo M,
Quereda C, Sanz J, Canorea I, et al: Elevated liver stiffness is
linked to increased biomarkers of inflammation and immune
activation in HIV/hepatitis C virus-coinfected patients. AIDS.
32:1095–1105. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Portocarrero Nunez JA, Gonzalez-Garcia J,
Berenguer J, Gallego MJV, Loyarte JAI, Metola L, Bernal E, Navarro
G, Del Amo J and Jarrín I: and the Cohort of the Spanish HIV
Research Network (CoRIS). Impact of co-infection by hepatitis C
virus on immunological and virological response to antiretroviral
therapy in HIV-positive patients. Medicine (Baltimore).
97(e12238)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Corey KE, Kane E, Munroe C, Barlow LL,
Zheng H and Chung RT: Hepatitis C virus infection and its clearance
alter circulating lipids: Implications for long-term follow-up.
Hepatology. 50:1030–1037. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Miyazaki T, Honda A, Ikegami T, Saitoh Y,
Hirayama T, Hara T, Doy M and Matsuzaki Y: Hepatitis C virus
infection causes hypolipidemia regardless of hepatic damage or
nutritional state: An epidemiological survey of a large Japanese
cohort. Hepatol Res. 41:530–541. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Boemeke L, Bassani L, Marroni CA and
Gottschall CB: Lipid profile in cirrhotic patients and its relation
to clinical outcome. Arq Bras Cir Dig. 28:132–135. 2015.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
33
|
Del Campo JA and Romero-Gomez M:
Modulation of host lipid metabolism by hepatitis C virus: Role of
new therapies. World J Gastroenterol. 21:10776–10782.
2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liver TAftSot: Thailand Practice Guideline
for Management of Chronic Hepatitis B and C 2015. https://thasl.org/en/thasl-guideline-2/.
|