Introduction
Acute coronary syndrome (ACS) is a cardiovascular
disease with a multifactorial etiology, which often involves acute
myocardial infarction and unstable angina (1,2).
Several factors have been linked to the development of ACS,
including hypertension, diabetes, dyslipidemia, obesity, smoking,
age, sex and genetic background (3). This disease imposes a heavy burden,
particularly on developing countries, and is a leading cause of
mortality in Mexico (4). According
to the Instituto Nacional de Estadística y Geografia, in 2022 heart
disease was the second cause of mortality in Mexico. In
Southeastern Mexico the mortality rate is 15.9%, slightly below the
national rate of 17.7% (5).
The diagnostic process of ACS is often complicated
by its varied clinical features. Hence, some biomarkers, including
troponins, creatine kinase MB (CK-MB), B-type natriuretic peptides,
copeptin, C-reactive protein (CRP), IL-6, D-dimers and fibrinogen,
have evolved as crucial instruments for the diagnosis ACS (6). CRP is an acute-phase protein that
plays a key role in chronic and acute inflammation. It is used as a
general biomarker of systemic inflammation and participates in the
pathogenesis of cardiovascular diseases, such as endothelial
dysfunction and atherosclerosis (7,8). CRP
has been associated with the presence of coronary artery disease
(CAD) (9) and a higher risk of
future cardiovascular events in apparently healthy individual
(10). Genetic polymorphisms in the
interleukin genes as well as in the CRP gene have been associated
with minor elevations in CRP (11).
The single nucleotide polymorphisms (SNPs) in the promoter region,
exon 2 and the 3' untranslated region (UTR) of the CRP gene have
been reported to influence CRP serum levels (12-14);
similarly, other SNPs have been shown to induce changes in
transcription factor binding and gene promoter activity (15,16).
The association of SNPs in the CRP gene with plasma CRP levels and
cardiovascular disease has been reported in previous studies
(17-19).
A genome-wide association study found no association of variants in
the CRP locus and cardiovascular hearth disease, but five genetic
loci were strongly associated with CRP levels (20).
Another study in an American population found no
association in 1059G/C polymorphism in the human CRP gene however,
it suggested that genetic and environmental determinants each
importantly contribute to the vascular risk associated with
inflammation (21).
In the literature, the polymorphisms of the CRP gene
2667GG/GC (rs1800947), 3872CC/CT (rs1205), 5237GG/GA (rs2808630)
and 3006AA/AC (rs3093066) are known to be associated with changes
of the CRP production in vivo and cardiovascular diseases
(22,23). However, it remains to be elucidated
whether polymorphisms in the CRP gene are associated with
susceptibility to ACS in patients of Mexico. For this reason, the
current study analyzed the relationship between four SNPs of the
CRP gene, namely 2667 GG/GC, 3872CC/CT, 5237GG/GA and 3006AA/AC,
and ACS in a group of individuals from Southeastern Mexico.
Materials and methods
Subjects
The present retrospective and descriptive study
included patients who attended the cardiology department at the
Hospital Regional de Alta Especialidad-Ciudad Salud (HRAE-CS;
Chiapas, Mexico) between March 2010 and December 2012. It included
a cohort of 252 patients, 159 male (63.1%) and 93 female (36.9%),
with an age range of 22-81 years and a median age of 53.5. In
total, 114 patients were diagnosed with primary ACS according to
the American College of Cardiology (ACA) criteria (24) and were included in the ACS group
under the following criteria: i) Persistent chest pain for >20
min; ii) typical electrocardiographic changes including new
pathologic Q waves; iii) ST-segment elevation of >1 mm; and iv)
increased plasma levels of CK-MB >2-fold higher than the upper
reference limit and/or troponin-T >0.1 µg/ml.
In parallel, 138 healthy individuals were included
in the control group according to the ACA criteria (24): i) Individuals without a history of
cardiovascular disease; and ii) individuals without evidence of
other systemic diseases.
Based on their medical history, no subjects in the
control group suffered from ACS or any related pathology. The age
and sex of the control and the ACS groups were matched. The
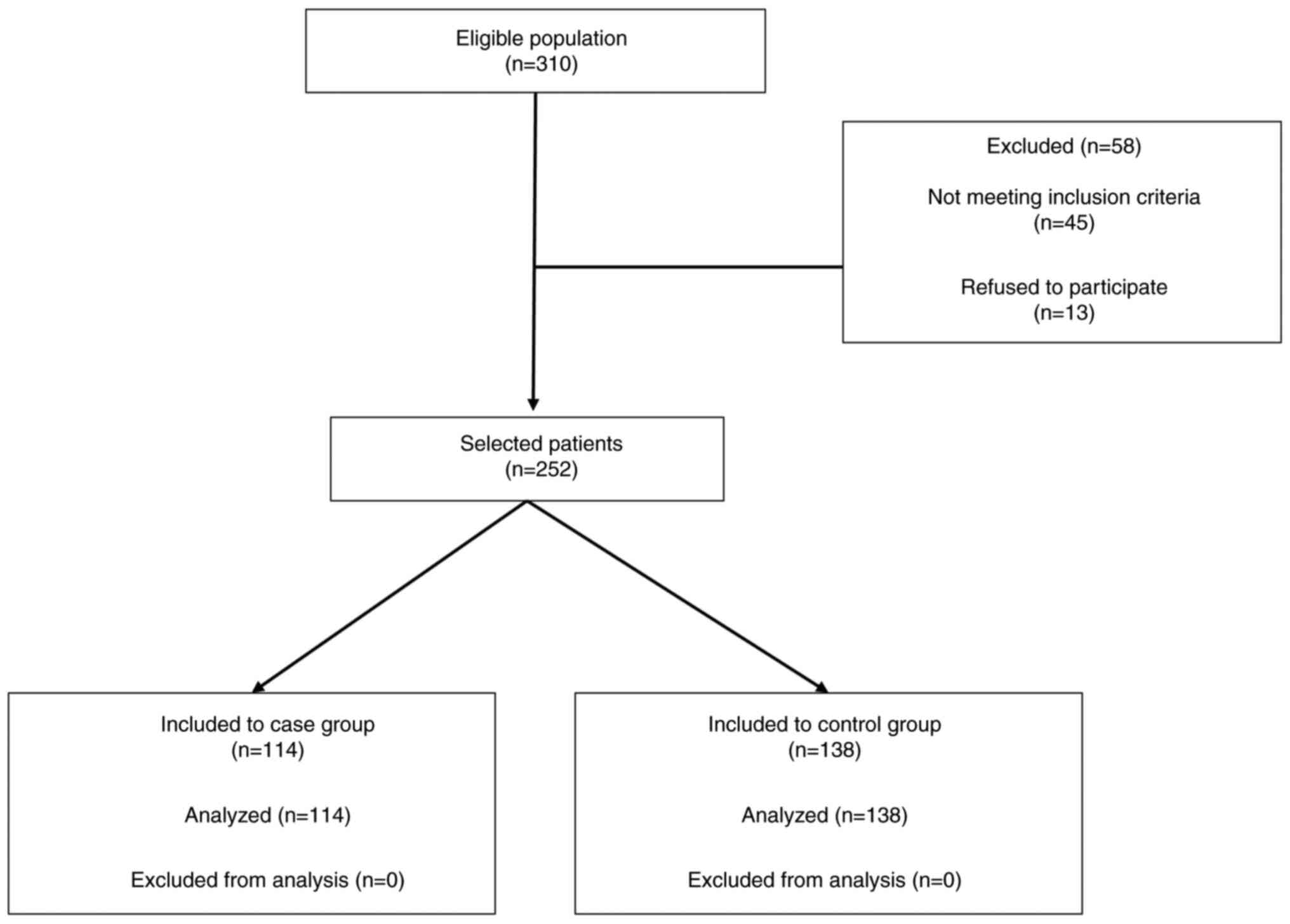
selection of patients is shown in the STROBE flow (Fig. 1). The current study was conducted in
accordance with The Declaration of Helsinki. All participants
provided written informed consent before taking their blood
samples. The study was approved by the HRAE-CS Ethics Committee
(approval no. 02/2010).
DNA extraction and genotyping
For each patient, 5 ml of blood was drawn into a
purple top EDTA tube and stored at 4˚C for a maximum of 1 week to
prevent degradation of nucleic acids. DNA was extracted from
EDTA-treated venous blood samples using the QIAamp DNA Blood Mini
kit (Qiagen GmbH) and stored at -20˚C until use. DNA was quantified
in an Eppendorf 6131 Biophotometer (Eppendorf SE) and sample
concentrations were adjusted to 50 ng/µl. Genotyping was performed
in a StepOne Real-Time PCR System with the TaqMan Genotyping Master
Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.). Specific
assays for the SNPs 3872C>T [rs1205; genotyping primer:
ACTTCCAGTTTGGCTTCTGTCCTCA(C/T)AGTCTCTCTCCATGTGGCAAACAAG],
5237G>A [rs2808630; genotyping primer:
AGGCCAGAGGCTGTCTACCAGACTA(G/A)GTATAGTAAGATGCAAGCAACTGAA],
2667C>G [rs1800947; genotyping primer:
AGATGGTGTTAATCTCATCTGGTGA(C/G)AGCACAAAGTCCCACATGTTCACAT] and
3006A>C [rs3093066; genotyping primer:
ATGTCACTGGCCTCATGCTTTGCAC(A/C)TTACAAAGTGAGTAATGTGTGCTGA] previously
associated with cardiovascular disease (CVD) in the CRP gene
(25) were purchased from Thermo
Fisher Scientific, Inc. with ID C_7479334_10, C_12035003_10,
C_11663840_10 and C_27452296_10, respectively. PCR protocol
included initial denaturation 1 cycle at 95˚C for 10 min, followed
by 40 cycles of denaturation at 95˚C for 15 seg and
annealing/extension at 60˚C for 1 min. The genotyping was performed
following the manufacturer's protocol. Genotype calls were made
upon visualization of the allelic discrimination charts in which
the clusters were identified.
Statistical analysis
Data are presented as mean ± standard deviation
(age) or percentages. For inferential statistics, the normal
distribution of the data was first determined using the
Kolmogorov-Smirnov test and the equality of variances was confirmed
using the Levene test. The age of the groups was compared using the
Student's unpaired t-test. Fisher's exact test was used to compare
categorical values. The association between various cardiovascular
risk variables (age, male, diabetes, smoking, alcoholism, arterial
hypertension, dyslipidemia, sedentary lifestyle and obesity), or
CRP genotypes, with the presence of ACS was determined using odds
ratios (OR) and 95% confidence intervals (95% CI) using univariate
binary logistic regression analyses. Subsequently, significant
predictors were added to the multivariate model and with forward
stepwise logistic regression the most parsimonious model was
identified. The probability used for the stepwise regression was
set at 0.15 for variable input and 0.25 for removal. Analysis
involving a two-way interaction (A x B, where A and B are the
genotypes 2667CC/CG and 3872CC/CT) was made in a separate
multivariate model that did not include genotypes of other
polymorphisms. The multivariate analysis was considered pertinent
because various metabolic and vascular disorders have been
previously reported to be associated with both the exposure
variable of interest (CRP genotypes) and the outcome variable (ACS)
(26-29).
Multivariate logistic regression allowed for the necessary
statistical adjustments for probable confounding variables,
resulting in an adjusted OR (AdOR). Data were analyzed using SPSS
V.21 (IBM Corp.) apart from linkage disequilibrium (LD) and
haplotype analyses, which were performed using SNPStats software
(30). P<0.05 was considered to
indicate a statistically significant difference.
Results
Main clinical characteristics in
ACS
Demographic and clinical parameters of the patients
with ACS and control group are presented in Table I. In total, 252 participants were
included in the present study. Male individuals were the majority
in both groups, with 55.8% in the control and 71.9% in the ACS
group. Significant differences were found between the group control
and ACS in age, sex, T2DM, smoking, alcohol, HBP, dyslipidemia and
sedentary lifestyle (P<0.05). On the other hand, the obesity
incidence was not found significantly different (P=0.876). Except
for T2DM, the aforementioned variables are considered risk factors
for ACS.
 | Table IMain clinical characteristics of the
participating subjects according to the presence or absence of
acute coronary syndrome. |
Table I
Main clinical characteristics of the
participating subjects according to the presence or absence of
acute coronary syndrome.
| | Acute coronary
syndrome | |
|---|
| Variable | Total | No | Yes | P-value |
|---|
| Age (years) | 51.8±13.5 | 45.8±11.9 | 59.2±11.6 |
<0.001a |
| Sex | | | | 0.009b |
|
Malec | 63.1% | 55.8% | 71.9% | |
|
Female | 36.9% | 44.2% | 28.1% | |
| T2DM | | | |
<0.001b |
|
Yes | 30.6% | 13.0% | 51.8% | |
|
No | 69.4% | 87.0% | 48.2% | |
| Smoking | | | | 0.008b |
|
Yesc | 35.7% | 28.3% | 44.7% | |
|
No | 64.3% | 71.7% | 55.3% | |
| Alcohol | | | |
<0.001b |
|
Yesc | 52.0% | 38.4% | 74.7% | |
|
No | 48.0% | 61.6% | 25.3% | |
| HBP | | | |
<0.001b |
|
Yesc | 42.1% | 17.4% | 71.9% | |
|
No | 57.9% | 82.6% | 28.1% | |
| Dyslip | | | |
<0.001b |
|
Yesc | 27.4% | 10.1% | 48.2% | |
|
No | 72.6% | 89.9% | 51.8% | |
| Sedentarism | | | |
<0.001b |
|
Yesc | 38.7% | 23.9% | 63.1% | |
|
No | 61.3% | 76.1% | 36.9% | |
| Obesity | | | | 0.876b |
|
Yesc | 26.4% | 26.8% | 25.6% | |
|
No | 73.6% | 73.2% | 74.4% | |
Genotype of the C-reactive protein in
ACS
Allele and genotype frequencies of the four
polymorphisms in the patients with ACS and controls were in
Hardy-Weinberg equilibrium (P>0.05; data not shown). The
frequency of the genotypes 2667CC/CG, 3872CC/CT, and 3006AA/AC were
significantly higher in the group of patients with ACS, compared
with the control group (26.1 vs. 57.0%, P<0.001; 66.7 vs. 80.7%,
P=0.015; and 8.0 vs. 16.7%, P=0.049; respectively). The genotype
5237GG/GA did not show a statistical significance between groups
(P=0.467; Table II). Furthermore,
the analysis involved a two-way interaction between the
2667*3872 polymorphisms, showing that the combination of
genotypes 2667CC/CG*3872CC/CT was significantly higher
in the group with ASC (43.0%) compared with the control group
(18.1%) (P<0.001; Table
II).
 | Table IIDistribution of the analyzed
genotypes of CRP according to the presence or absence of acute
coronary syndrome. |
Table II
Distribution of the analyzed
genotypes of CRP according to the presence or absence of acute
coronary syndrome.
| | Acute coronary
syndrome | |
|---|
| CRP genotypes | Total (%) | No (%) | Yes (%) |
P-valuea |
|---|
| 2667 | | | | |
|
2667CC/CG | 40.1 | 26.1 | 57.0 | <0.001 |
| 2667GG | 59.9 | 73.9 | 43.0 | |
| 3872 | | | | |
|
3872CC/CT | 73.0 | 66.7 | 80.7 | 0.015 |
|
3872TT | 27.0 | 33.3 | 19.3 | |
| 5237 | | | | |
|
5237GG/GA | 29.4 | 29.0 | 29.8 | 0.467 |
|
5237AA | 70.6 | 71.0 | 70.2 | |
| 3006 | | | | |
|
3006AA/AC | 11.9 | 8.0 | 16.7 | 0.049 |
|
3006CC | 88.1 | 92.0 | 83.3 | |
|
2667*3872b | | | | |
|
CC/CG*CC/CT* | 29.4 | 18.1 | 43.0 | <0.001 |
|
CC/CG*TT | 10.7 | 8.0 | 14.0 | |
|
GG*CC/CT | 43.7 | 48.6 | 37.7 | |
|
GG*TT | 16.3 | 25.4 | 5.3 | |
Association of C-reactive protein
polymorphisms with acute coronary syndrome
The univariate logistic regression analysis showed
that age, maleness, T2DM, smoking, alcohol, HBP, dyslipidemia and
sedentary lifestyle were factors associated with ACS (Table III). The multivariate analysis
reveals that only age, T2DM, alcohol, HBP and sedentary lifestyle
had an association with ACS (Table
III). The univariate model reveals that genotypes 2667CC/CG,
3872CC/CT and 3006AA/AC were ACS-associated (OR=3.75, 95% CI:
2.21-6.39; OR=2.09, 95% CI: 1.16-3.75; and OR=2.3, 95% CI:
1.04-5.08, respectively; Table
III). The multivariate model showed that genotypes 2667CC/CG
(AdOR=4.82, 95% CI: 1.69-13.72) and 3872CCGG/CTGA (AdOR=3.78, 95%
CI: 1.1-12.92) remain associated to ACS independently of other
clinical variables or genotypes analyzed; while the 3006AA/AC
genotype lost relevance and was not included in the multivariate
model. The interaction of the 2667CC/CG and 3872CC/CT genotypes
increased the risk for ACS in the univariate and multivariate model
(OR=3.40, 95% CI: 1.92-6.02; and AdOR=4.14, 95% CI: 1.49-11.52,
respectively). However, the probability of developing ACS generated
by this combination of genotypes was not higher than that generated
by the 2667CC/CG genotype alone (Table III).
 | Table IIIUnivariate and multivariate logistic
regression analyses of factors associated with ACS. |
Table III
Univariate and multivariate logistic
regression analyses of factors associated with ACS.
| | Univariate
model | Multivariable
model |
|---|
| | cOR | 95% CI | P-value | aOR | 95% CI | P-value |
|---|
| Age | 1.09 | 1.06 | 1.12 | <0.001 | 1.15 | 1.08 | 1.21 | <0.001 |
| Male | 2.03 | 1.19 | 3.44 | 0.009 | | | | |
| T2DM | 7.15 | 3.86 | 13.25 | <0.001 | 4.76 | 1.56 | 14.49 | 0.006 |
| Smoking | 2.05 | 1.21 | 3.46 | 0.007 | | | | |
|
Alcohola | 4.74 | 2.59 | 8.64 | <0.001 | 9.15 | 3.14 | 26.63 | <0.001 |
| HBP | 12.17 | 6.67 | 22.1 | <0.001 | 5.40 | 1.89 | 15.30 | 0.002 |
| Dyslip | 8.25 | 4.25 | 16.0 | <0.001 | 2.87 | 0.96 | 8.99 | 0.060 |
| Sedentarism | 5.44 | 3.01 | 9.82 | <0.001 | 16.36 | 4.74 | 56.42 | <0.001 |
| Obesity | 0.94 | 0.50 | 1.75 | 0.845 | | | | |
| CRP genotypes |
|
2667CC/CG | 3.75 | 2.21 | 6.39 | <0.001 | 4.82 | 1.69 | 13.72 | 0.003 |
|
3872CC/CT | 2.09 | 1.16 | 3.75 | 0.013 | 3.78 | 1.11 | 12.92 | 0.034 |
|
5237GG/GA | 1.04 | 1.60 | 1.79 | 0.884 | | | | |
|
3006AA/AC | 2.30 | 1.04 | 5.08 | 0.038 | | | | |
|
2667*3872b | 3.40 | 1.92 | 6.02 | <0.001 | 4.14 | 1.49 | 11.52 | 0.006 |
Haplotype analysis and LD
The most frequent haplotype in the population was H1
T/A/G/C, with 29.15% of occurrence, followed by H2 C/A/G/C with
28.46%, H3 C/A/C/C with 11.92% and H4 T/A/C/C with 10.68%;
together, they represent 80.21% of the total haplotypes. Due to the
frequency of the remaining haplotypes in the population, only four
were analyzed. Haplotypes numbers H2, H3, and H4 showed an
association with risk of ACS (OR=14.86, 95% CI: 2.62-84.15,
P=0.0026; OR=29.98, 95% CI: 4.38-205.34, P=7x10-4 and
OR=100.56, 95% CI: 4.64-2177.88, P=0.0037 respectively) after
adjusting for age, sex and other clinical variables (T2DM, smoking,
alcohol, hypertension, dyslipidemia, sedentary lifestyle and
obesity), as shown in Table IV.
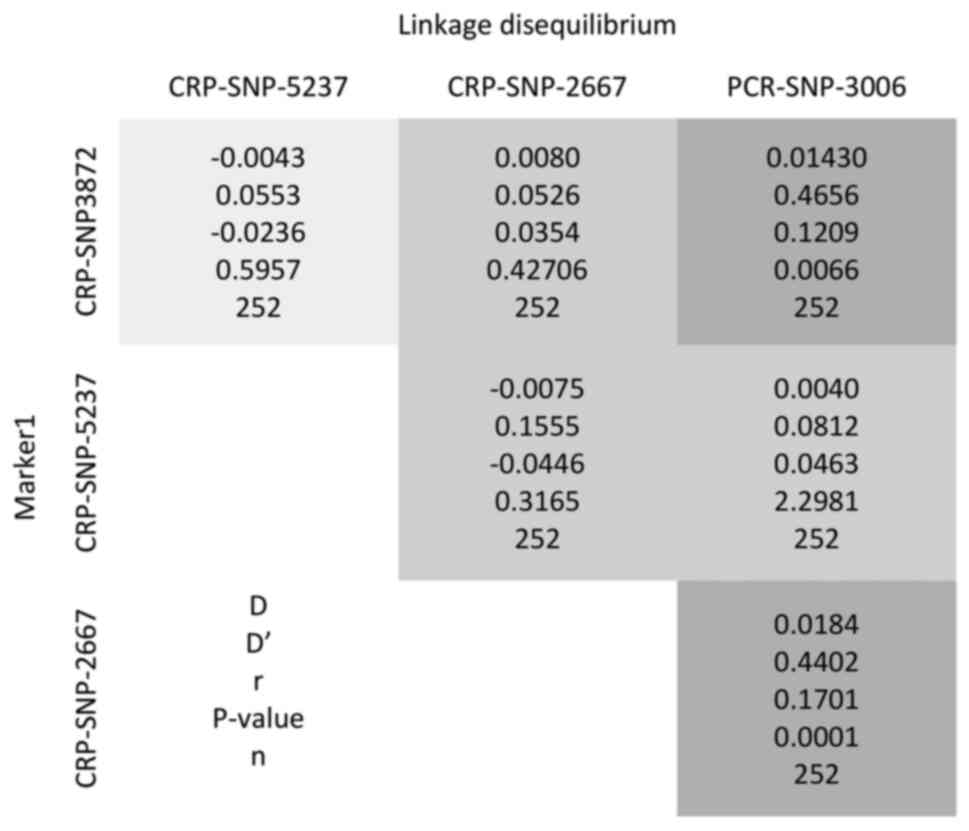
The polymorphism 3006 exhibited a strong LD with 3872 and 2667
(with D 0.0143, D'=0.4556, P=0.006; and
D=0.0184, D'=0.4402, P<0.001, respectively), as
shown in Fig. 2. The genotype
3006AA/AC rs3093066 was associated with ACS in the univariate
model. This polymorphism was previously associated with CRP levels
in Caucasian American and African American young adults (25). However, this polymorphism does not
seem to be an independent risk factor for the genesis of ACS in the
population in the present study, since other clinical or genetic
factors studied here make it lose relevance in a multivariate
analysis. A probable explanation for this fact is that polymorphism
3006 exhibited a strong LD with 3872 and 2667 (with D=0.0143,
D'=0.4556, P<0.05; and D=0.0184, D'=0.4402, P<0.05,
respectively). This means that the alleles of the 3006
polymorphisms are nonrandomly associated with the alleles at nearby
polymorphic sites 3872 and 2667. Therefore, it is likely that the
3006AA/AC genotype is not an independent risk factor, but rather a
‘linked or predicted’ factor by the genotypes 2667CC/CG and
3872CC/CT, an aspect that was detected in the multivariate
model.
 | Table IVHaplotype association with ACS
(n=252, adjusted by sex, age, T2DM, smoking, alcohol, hypertension,
dyslipidemia, sedentarism and obesity). |
Table IV
Haplotype association with ACS
(n=252, adjusted by sex, age, T2DM, smoking, alcohol, hypertension,
dyslipidemia, sedentarism and obesity).
| Haplotype | CRP3872 | CRP5237 | CRP2667 | CRP3006 | Freq | OR (95% CI) | P-value |
|---|
| H1 | T | A | G | C | 0.2915 | 1.00 | - |
| H2 | C | A | G | C | 0.2846 | 14.86
(2.62-84.15) | 0.0026 |
| H3 | C | A | C | C | 0.1192 | 29.98
(4.38-205.34) |
7x10-4 |
| H4 | T | A | C | C | 0.1068 | 100.56
(4.64-2177.88) | 0.0037 |
Discussion
Clinical parameters
ACS is a spectrum of myocardial ischemic disorders,
including myocardial infarction with ST-segment elevation,
myocardial infarction with non-ST segment elevation and unstable
angina, with a multifactorial origin. In addition, previous works
have linked genetic polymorphisms with the development of ACS
(13,14,31).
The present study analyzed the association between four
polymorphisms in the promoter region of the CRP gene and ACS in a
group of individuals from Southern Mexico. In the present study
variables such as age, maleness, T2DM, alcohol consumption,
smoking, HBP, dyslipidemia and sedentary lifestyle showed
significant differences between the groups (Table I), a result which is consistent with
previous reports (16,32-34).
Hypertension, dyslipidemia and smoking are the three modifiable
risk factors most strongly and independently associated with
coronary heart disease (CHD) (35).
Recently, a study showed that factors such as age 70 years old,
femaleness sex, HBP, T2DM and hypertension are more frequent in
patients with combined endpoint (cardiovascular mortality,
mortality from stroke, myocardial infarction and stroke/transient
ischemic attack in 10-year follow-up) in comparison with the
control group in German patients (P<0.05) (36). DM is linked with comorbidities such
as CAD (37) and represents 25-30%
of the patients with ACS. Patients with ACS and DM, present poorer
outcomes regarding CV morbidity and mortality, compared with
individuals with ACS but not with DM. In addition, T2DM prolongs
hospitalization time in patients with ACS and disruption of the
normal glucose homeostasis has been found in ~70-75% of patients
with CAD (38).
CRP polymorphisms and ACS
Few studies have been conducted in the Hispanic
population to establish an association between gene polymorphisms
and ACS. In the present study, the genotype 3872CC/CT (rs1205) was
found to be a risk factor for ACS (AdOR=3.78; 95% CI: 1.11-12.92,
P=0.034). The only study conducted in Mexico with this SNP did not
show any association with ACS in individuals from Western Mexico;
however, the allele T is associated with lower CRP concentration
(38). 3872CT has been associated
with low levels of CRP (33,39-42)
myocardial infarction (33) and CHD
(12), but other studies have not
shown an association with CHD (40)
or aortic stenosis (30). The
current results reported that the 5237GG/AA genotype did not show
an association with ACS (OR=1.04; 95% CI: 1.60-1.79, P=0.884);
which agrees with a previous report (12).
The 2667CC/CG genotypes (rs1800947) were found to be
a risk factor for ACS in the present study population (AdOR=4.82,
95%CI: 1.69-13.72, P=0.003). These polymorphisms have been
associated with CHD (43), coronary
artery disease (44), myocardial
infarction (45), cerebrovascular
ischemia-related disease (46),
cardiovascular disease mortality (23) and other diseases such as diabetes
(47), prostatic (48) and colorectal cancer (49) and microangiopathic stroke (50). In addition, other investigations
have not found an association (33,15,51,52).
Allele G from rs1800947 has been associated with low CRP levels in
coronary artery disease (44),
myocardial infarction (53) and
adverse cardiovascular (54).
The genotype 3006AA/AC rs3093066 was associated with
ACS in the univariate model. This polymorphism was previously
associated with CRP levels in European American and African
American young adults (25).
However, this polymorphism does not seem to be an independent risk
factor for the genesis of ACS in the present population, since
other clinical or genetic factors studied here make it lose
relevance in a multivariate analysis. A probable explanation for
this is that polymorphism 3006 exhibited a strong LD with 3872 and
2667 (with D=0.0143, D'=0.4556, P<0.05; and D=0.0184, D'=0.4402,
P<0.05, respectively). This meant that the alleles of the 3006
polymorphism were non-randomly associated with the alleles at
nearby polymorphic sites 3872 and 2667. Therefore, it is likely
that the 3006AA/AC genotype is not an independent risk factor, but
rather a ‘linked or predicted’ factor by the genotypes 2667CC/CG
and 3872CC/CT, an aspect that was detected in the multivariate
model. The LD between polymorphism 3006 with polymorphisms 3872 and
2667 may reflect the natural selection history, gene conversion and
mutation history of the analyzed population (from Chiapas,
southeast of Mexico) (55), which
although it can be classified as mestizo (individuals of mixed
ancestry with a European and an indigenous background), has a
strong Mayan ethnological and linguistic affiliations (56). Future analyses will be necessary in
this respect. On the other hand, the present results showed that
haplotype numbers H2 (C/A/G/C), H3 (C/A/C/C) and H4 (T/A/C/C)
showed an association with risk ACS. Similar results have been
shown in the US population (12).
Polymorphisms in the CRP promoter region would
increase the risk of ACS by favoring an elevation of this protein,
which is a clear sign of systemic inflammation (57,58).
The expression of the CRP gene is modulated by several SNPs in the
promoter region and these SNPs affects changes in gene promoter
activity, RNA structure or turnover, transcription factor binding
and transcriptional activity (25,59).
The mechanisms by which SNP 2667 affects the expression of CRP are
unclear, so it remains possible that 2667 is in strong LD with a
polymorphism in a distal regulatory element not within the region
scanned for polymorphisms (25).
However, a study showed that the minor alleles of the 2667 and 3872
polymorphisms linked with CRP haplotypes are associated with
decreased promoter activity (23).
As aforementioned, several SNPs have been associated with CHD and
with CRP levels. CRP is an inflammatory marker associated with
cardiovascular disease, T2DM and other pathology. It is probable
that CRP genotypes affect CRP synthesis and influence the onset of
clinical CVD or ACS (23).
Due to the aforementioned factors, it is
hypothesized that the number of subjects included in the present
study may be a limitation. However, the results shown can be
considered reliable, considering that it has previously been
postulated that it is necessary to have ≤10 individuals with the
event of interest for each predictor variable included in logistic
regression models (60) to improve
the performance of the model and reduce the risk of false positive
results. In the multivariate logistic regression model performed in
the present study (Table III),
eight predictor variables were included in the multivariate model,
analyzing 114 individuals with ACS and 138 subjects in the control
group. Therefore, the present study complied with the rule of
having ≤10 events per variable. However, some reports have
suggested that this rule is controversial (61,62).
Another limitation was that the serum levels of CRP were not
determined in the participants, so it would be relevant for future
research to quantify this protein to evaluate whether the risk
conferred by certain genotypes is directly related to the elevation
of CRP.
In conclusion, four polymorphisms in the promoter
region of the CRP gene in association with ACS were analyzed in the
present study. Variables such as age, T2DM, alcohol, HBP and
sedentary lifestyle were associated with ACS. To the best of the
authors' knowledge, this work provided the first evidence of the
association of CRP genotypes 2667CC/CG and 3872CC/CT with ACS in
Mexico. These genotypes were evidenced as risk factors independent
of other clinical risk factors in the multivariate analysis. It is
possible that a set of polymorphisms, along with environmental
factors, contribute to the development of chronic degenerative
diseases, modulating the increased risk for heart disease. However,
further studies are required to ascertain whether these
polymorphisms participate in the development of ACS.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data presented in this study are available on
request from the corresponding author.
Authors' contributions
ALR and LMCA were responsible for conceptualization.
IDE, ESG, ALR, BHO and ICQC were responsible for methodology. JAF,
ALR, IDE and VOG were responsible for software. ALR, LMCA, IDE and
ESG were responsible for formal analysis. ALR, RSGB, SGM and NLL
were responsible for investigation. JAF, IDE and VOG were
responsible for data curation. ALR, RSGB and NLL were responsible
for writing and preparation of the original draft. ALR, LMCA, IDE,
ICQC, SGM and NLL were responsible for writing, reviewing and
editing. LMCA, IDE and BHO were responsible for supervision. LMCA
was responsible for project administration. ALR and LMCA confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was conducted according to the
guidelines of the Declaration of Helsinki and was approved by the
HRAE-CS Ethics Committee, with a permit number 02/2010. Informed
consent was obtained from all subjects involved in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
Not applicable.
References
|
1
|
Birnbaum Y, Wilson JM, Fiol M, de Luna AB
A, Eskola M and Nikus K: ECG diagnosis and classification of acute
coronary syndromes. Ann Noninvasive Electrocardiol. 19:4–14.
2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Crea F and Liuzzo G: Pathogenesis of acute
coronary syndromes. J Am Coll Cardiol. 61:1–11. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hamm CW, Bassand JP, Agewall S, Bax J,
Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, et al:
Israrci ST-segment yükselmesi belirtileri göstermeyen hastalarda
akut koronersendromlarin (AKS) tedavi kilavuzlari. Turk Kardiyol
Dern Ars. 39:73–128. 2011.
|
|
4
|
Sanchis-Gomar F, Perez-Quilis C, Leischik
R and Lucia A: Epidemiology of coronary heart disease and acute
coronary syndrome. Ann Transl Med. 4(256)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
INEGI: Techninal note on statistics of
registered deaths. 2021. Mexico, 2022. Available at: https://www.inegi.org.mx/contenidos/programas/mortalidad/doc/defunciones_registradas_2021_nota_tecnica.pdf.
|
|
6
|
Katsioupa M, Kourampi I, Oikonomou E,
Tsigkou V, Theofilis P, Charalambous G, Marinos G, Gialamas I,
Zisimos K, Anastasiou A, et al: Novel biomarkers and their role in
the diagnosis and prognosis of acute coronary syndrome. Life
(Basel). 13(1992)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Momiyama Y, Ohmori R, Fayad ZA, Kihara T,
Tanaka N, Kato R, Taniguchi H, Nagata M, Nakamura H and Ohsuzu F:
Associations between plasma C-reactive protein levels and the
severities of coronary and aortic atherosclerosis. J Atheroscler
Thromb. 17:460–467. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Szmitko PE, Wang CH, Weisel RD, de Almeida
JR, Anderson TJ and Verma S: New markers of inflammation and
endothelial cell activation: Part I. Circulation. 108:1917–1923.
2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gupta S, Gupta VK, Gupta R, Arora S and
Gupta V: Relationship of high-sensitive C-reactive protein with
cardiovascular risk factors, clinical presentation and angiographic
profile in patients with acute coronary syndrome: An Indian
perspective. Indian Heart J. 65:359–365. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Casas JP, Shah T, Hingorani AD, Danesh J
and Pepys MB: C-reactive protein and coronary heart disease: A
critical review. J Intern Med. 264:295–314. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Eklund CM: Proinflammatory cytokines in
CRP baseline regulation. Adv Clin Chem. 48:111–136. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pai JK, Mukamal KJ, Rexrode KM and Rimm
EB: C-reactive protein (CRP) gene polymorphisms, CRP levels, and
risk of incident coronary heart disease in two nested case-control
studies. PLoS One. 3(e1395)2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rios DL, Cerqueira CC, Bonfim-Silva R,
Araújo LJ, Pereira JF, Gadelha SR and Barbosa AA: Interleukin-1
beta and interleukin-6 gene polymorphism associations with
angiographically assessed coronary artery disease in Brazilians.
Cytokine. 50:292–296. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tonet AC, Karnikowski M, Moraes CF, Gomez
L, Karnikowski MGO, Córdova C and Nóbrega OT: Association between
the-174 G/C promoter polymorphism of the interleukin-6 gene and
cardiovascular disease risk factors in Brazilian older women. Braz
J Med Biol Res. 41:47–53. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Brull DJ, Serrano N, Zito F, Jones L,
Montgomery HE, Rumley A, Sharma P, Lowe GD, World MJ, Humphries SE
and Hingorani AD: Human CRP gene polymorphism influences CRP
levels: Implications for the prediction and pathogenesis of
coronary heart disease. Arterioscler Thromb Vasc Biol.
23:2063–2069. 2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kovacs A, Green F, Hansson LO, Lundman P,
Samnegård A, Boquist S, Ericsson CG, Watkins H, Hamsten A and
Tornvall P: A novel common single nucleotide polymorphism in the
promoter region of the C-reactive protein gene associated with the
plasma concentration of C-reactive protein. Atherosclerosis.
178:193–198. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Volanakis JE: Human C-reactive protein:
Expression, structure, and function. Mol Immunol. 38:189–197.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
NACB LMPG Committee Members. Myers GL,
Christenson RH, Cushman M, Ballantyne CM, Cooper GR, Pfeiffer CM,
Grundy SM, Labarthe DR, Levy D, et al: National academy of clinical
biochemistry laboratory medicine practice guidelines: Emerging
biomarkers for primary prevention of cardiovascular disease. Clin
Chem. 55:378–384. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang XH, Liu SQ, Wang YL and Jin Y:
Correlation of serum high-sensitivity C-reactive protein and
interleukin-6 in patients with acute coronary syndrome. Genet Mol
Res. 13:4260–4266. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Elliott P, Chambers JC, Zhang W, Clarke R,
Hopewell JC, Peden JF, Erdmann J, Braund P, Engert JC, Bennett D,
et al: Genetic Loci associated with C-reactive protein levels and
risk of coronary heart disease. JAMA. 302:37–48. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zee RYL and Ridker PM: Polymorphism in the
human C-reactive protein (CRP) gene, plasma concentrations of CRP,
and the risk of future arterial thrombosis. Atherosclerosis.
162:217–219. 2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu C, Jin P, Luo Y, Xu J, Kong C, Chen J,
Xie H and Zhou G: Association of single-nucleotide polymorphisms of
C-reactive protein gene with susceptibility to infantile sepsis in
Southern China. Med Sci Monit. 24:590–595. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lange LA, Carlson CS, Hindorff LA, Lange
EM, Walston J, Durda JP, Cushman M, Bis JC, Zeng D, Lin D, et al:
Association of polymorphisms in the CRP gene with circulating
C-reactive protein levels and cardiovascular events. JAMA.
296:2703–2711. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cannon CP, Brindis RG, Chaitman BR, Cohen
DJ, Cross JT Jr, Drozda JP Jr, Fesmire FM, Fintel DJ, Fonarow GC,
Fox KA, et al: 2013 ACCF/AHA key data elements and definitions for
measuring the clinical management and outcomes of patients with
acute coronary syndromes and coronary artery disease: A report of
the American college of cardiology foundation/American heart
association task force on clinical data standards (writing
committee to develop acute coronary syndromes and coronary artery
disease clinical data standards). Circulation. 127:1052–1089.
2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Carlson CS, Aldred SF, Lee PK, Tracy RP,
Schwartz SM, Rieder M, Liu K, Williams OD, Iribarren C, Lewis EC,
et al: Polymorphisms within the C-reactive protein (CRP) promoter
region are associated with plasma CRP levels. Am J Hum Genet.
77:64–77. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Zhao Y, Wang H, Liu S, Zhao X, Chen Y,
Yang Y, Wang W, Wu Y, Chen A, Tang J, et al: Association study of
CRP gene polymorphism and hypertension in Han Chinese population.
Gene. 512:41–46. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jebur HB, Masroor M, Ahmad H, Khan NA,
Akther J, Bharali D, Singh VK, Verma A, Khan S, Khan V, et al: CRP
gene polymorphism and their risk association with type 2 diabetes
mellitus. Open Access Maced J Med Sci. 7:33–37. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wei W, Yang S, Qiu Y, Wang H, Zhao X, Zhao
Y, Li Y, Wu M, Chen Y, Wang W, et al: CRP gene polymorphism
contributes genetic susceptibility to dyslipidemia in Han Chinese
population. Mol Biol Rep. 41:2335–2343. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Todendi PF, Klinger EI, Ferreira MB,
Reuter CP, Burgos MS, Possuelo LG and Valim AR: Association of IL-6
and CRP gene polymorphisms with obesity and metabolic disorders in
children and adolescents. An Acad Bras Cienc. 87:915–924.
2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Solé X, Guinó E, Valls J, Iniesta R and
Moreno V: SNPStats: A web tool for the analysis of association
studies. Bioinformatics. 22:1928–1929. 2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Libby P: Mechanisms of acute coronary
syndromes and their implications for therapy. N Engl J Med.
368:2004–2013. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hackam DG and Anand SS: Emerging risk
factors for atherosclerotic vascular disease: A critical review of
the evidence. JAMA. 290:932–940. 2003.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Miller DT, Zee RY, Suk Danik J, Kozlowski
P, Chasman DI, Lazarus R, Cook NR, Ridker PM and Kwiatkowski DJ:
Association of common CRP gene variants with CRP levels and
cardiovascular events. Ann Hum Genet. 69:623–638. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Danesh J, Wheeler JG, Hirschfield GM, Eda
S, Eiriksdottir G, Rumley A, Lowe GDO, Pepys MB and Gudnason V:
C-reactive protein and other circulating markers of inflammation in
the prediction of coronary heart disease. N Engl J Med.
350:1387–1397. 2004.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Greenland P, Knoll MD, Stamler J, Neaton
JD, Dyer AR, Garside DB and Wilson PW: Major risk factors as
antecedents of fatal and nonfatal coronary heart disease events.
JAMA. 290:891–897. 2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Schulz S, Rehm S, Schlitt A, Lierath M,
Lüdike H, Hofmann B, Bitter K and Reichert S: C-reactive protein
level and the genetic variant rs1130864 in the CRP gene as
prognostic factors for 10-year cardiovascular outcome. Cells.
12(1775)2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Task Force on diabetes, pre-diabetes and
cardiovascular diseases of the European Society of Cardiology
(ESC); European Association for the Study of Diabetes (EASD). Rydén
L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C,
Escaned J, et al: ESC guidelines on diabetes, pre-diabetes, and
cardiovascular diseases developed in collaboration with the
EASD-summary. Diabetes Vasc Dis Res. 11:133–173. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Babes EE, Bustea C, Behl T, Abdel-Daim MM,
Nechifor AC, Stoicescu M, Brisc CM, Moisi M, Gitea D, Iovanovici
DC, et al: Acute coronary syndromes in diabetic patients, outcome,
revascularization, and antithrombotic therapy. Biomed Pharmacother.
148(112772)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Reynoso-Villalpando GL, Padilla-Gutiérrez
JR, Valdez-Haro A, Casillas-Muñoz F, Muñoz-Valle JF,
Castellanos-Nuñez E, Chávez-Herrera JC and Valle Y: Relationship
between C-reactive protein serum concentration and the 1846 C>T
(rs1205) polymorphism in patients with acute coronary syndrome from
Western Mexico. Genet Test Mol Biomarkers. 21:334–340.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Suk Danik J, Chasman DI, Cannon CP, Miller
DT, Zee RY, Kozlowski P, Kwiatkowski DJ and Ridker PM: Influence of
genetic variation in the C-reactive protein gene on the
inflammatory response during and after acute coronary ischemia. Ann
Hum Genet. 70:705–716. 2006.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kardys I, de Maat MP, Uitterlinden AG,
Hofman A and Witteman JC: C-reactive protein gene haplotypes and
risk of coronary heart disease: The rotterdam study. Eur Heart J.
27:1331–1337. 2006.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ridker PM, Cannon CP, Morrow D, Rifai N,
Rose LM, McCabe CH, Pfeffer MA and Braunwald E: Pravastatin or
Atorvastatin Evaluation and Infection Therapy-Thrombolysis in
Myocardial Infarction 22 (PROVE IT-TIMI 22) Investigators.
C-reactive protein levels and outcomes after statin therapy. N Engl
J Med. 352:20–28. 2005.
|
|
43
|
Russell AI, Cunninghame Graham DS,
Shepherd C, Roberton CA, Whittaker J, Meeks J, Powell RJ, Isenberg
DA, Walport MJ and Vyse TJ: Polymorphism at the C-reactive protein
locus influences gene expression and predisposes to systemic lupus
erythematosus. Hum Mol Genet. 13:137–147. 2004.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hernández-Díaz Y, Tovilla-Zárate CA,
Juárez-Rojop I, Baños-González MA, Torres-Hernández ME,
López-Narváez ML, Yañez-Rivera TG and González-Castro TB: The role
of gene variants of the inflammatory markers CRP and TNF-α in
cardiovascular heart disease: Systematic review and meta-analysis.
Int J Clin Exp Med. 15:11958–11984. 2015.PubMed/NCBI
|
|
45
|
Grammer TB, März W, Renner W, Böhm BO and
Hoffmann MM: C-reactive protein genotypes associated with
circulating C-reactive protein but not with angiographic coronary
artery disease: the LURIC study. Eur Heart J. 30:170–182.
2009.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Schulz S, Lüdike H, Lierath M, Schlitt A,
Werdan K, Hofmann B, Gläser C, Schaller HG and Reichert S:
C-reactive protein levels and genetic variants of CRP as prognostic
markers for combined cardiovascular endpoint (cardiovascular death,
death from stroke, myocardial infarction, and stroke/TIA).
Cytokine. 88:71–76. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chen Z, Yu D, Xu ZW, Li SS, Li XF, Li J
and Yang X: C-reactive protein gene polymorphisms and
gene-environment interactions in ischaemic stroke. Neurol Res.
37:979–984. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lee CC, You NC, Song Y, Hsu YH, Manson J,
Nathan L, Tinker L and Liu S: Relation of genetic variation in the
gene coding for C-reactive protein with its plasma protein
concentrations: Findings from the women's health initiative
observational cohort. Clin Chem. 55:351–360. 2009.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Markt SC, Rider JR, Penney KL, Schumacher
FR, Epstein MM, Fall K, Sesso HD, Stampfer MJ and Mucci LA: Genetic
variation across C-reactive protein and risk of prostate cancer.
Prostate. 74:1034–1042. 2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Chen XL, Liao YQ and Liu JR: Genotype CC
of rs1800947 in the C-reactive protein gene may increase
susceptibility to colorectal cancer: a meta-analysis. Asian Pac J
Cancer Prev. 15:2663–2667. 2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kuhlenbaeumer G, Huge A, Berger K, Kessler
C, Voelzke H, Funke H, Stoegbauer F, Stoll M and Ringelstein EB:
Genetic variants in the C-reactive protein gene are associated with
microangiopathic ischemic stroke. Cerebrovasc Dis. 30:476–482.
2010.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Dai DF, Chiang FT, Lin JL, Huang LY, Chen
CL, Chang CJ, Lai LP, Hsu KL, Tseng CD, Tseng YZ and Hwang JJ:
Human C-reactive protein (CRP) gene 1059G>C polymorphism is
associated with plasma CRP concentration in patients receiving
coronary angiography. J Formos Med Assoc. 106:347–354.
2007.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kolz M, Koenig W, Müller M, Andreani M,
Greven S, Illig T, Khuseyinova N, Panagiotakos D, Pershagen G,
Salomaa V, et al: DNA variants, plasma levels and variability of
C-reactive protein in myocardial infarction survivors: Results from
the AIRGENE study. Eur Heart J. 29:1250–1258. 2008.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Rizzello V, Liuzzo G, Giannuario GD,
Trabetti E, Brugaletta S, Santamaria M, Piro M, Pignatti PF, Maseri
A, Biasucci LM and Crea F: 1059G/C polymorphism within the exon 2
of the C-reactive protein gene: Relationship to C-reactive protein
levels and prognosis in unstable angina. Coron Artery Dis.
18:533–538. 2007.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Slatkin M: Linkage
disequilibrium-understanding the evolutionary past and mapping the
medical future. Nat Rev Genet. 9:477–485. 2008.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Martínez-Cortés G, Salazar-Flores J,
Fernández-Rodríguez LG, Rubi-Castellanos R, Rodríguez-Loya C,
Velarde-Félix JS, Muñoz-Valle JF, Parra-Rojas I and
Rangel-Villalobos H: Admixture and population structure in
Mexican-Mestizos based on paternal lineages. J Hum Genet.
57:568–574. 2012.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Obisesan TO, Leeuwenburgh C, Phillips T,
Ferrell RE, Phares DA, Prior SJ and Hagberg JM: C-reactive protein
genotypes affect baseline, but not exercise training-induced
changes, in C-reactive protein levels. Arterioscler Thromb Vasc
Biol. 24:1874–1879. 2004.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Wang Q, Hunt SC, Xu Q, Chen YE, Province
MA, Eckfeldt JH, Pankow JS and Song Q: Association study of CRP
gene polymorphisms with serum CRP level and cardiovascular risk in
the NHLBI family heart study. Am J Physiol Heart Circ Physiol.
291:H2752–H2757. 2006.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Szalai AJ, Wu J, Lange EM, McCrory MA,
Langefeld CD, Williams A, Zakharkin SO, George V, Allison DB,
Cooper GS, et al: Single-nucleotide polymorphisms in the C-reactive
protein (CRP) gene promoter that affect transcription factor
binding, alter transcriptional activity, and associate with
differences in baseline serum CRP level. J Mol Med (Berl).
83:440–447. 2005.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Shipe ME, Deppen SA, Farjah F and Grogan
EL: Developing prediction models for clinical use using logistic
regression: An overview. J Thorac Dis. 11 (Suppl 4):S574–S584.
2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Van Smeden M, de Groot JAH, Moons KG,
Collins GS, Altman DG, Eijkemans MJ and Reitsma JB: No rationale
for 1 variable per 10 events criterion for binary logistic
regression analysis. BMC Med Res Methodol. 16(163)2016.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Van Smeden M, Moons KG, de Groot JA,
Collins GS, Altman DG, Eijkemans MJ and Reitsma JB: Sample size for
binary logistic prediction models: Beyond events per variable
criteria. Stat Methods Med Res. 28:2455–2474. 2019.PubMed/NCBI View Article : Google Scholar
|