Traumatic brain injury (TBI) encompasses a series of
complex pathological processes wherein brain tissue structure and
function are compromised due to mechanical force applied to the
head, stemming from various causes. Of considerable interest is the
potential link between genetic modifications and an augmented
susceptibility to TBI. Specifically, the polymorphisms within the
apolipoprotein E promoter region, the TAU gene (1) and the brain-derived neurotrophic
factor gene have been implicated in elevating the risk of
concussion and contributing to adverse outcomes subsequent to a TBI
episode. Additionally, alterations in the dopamine receptor
D2 gene may potentiate brain injury risk by impacting
cognitive functions and behavioral traits in affected individuals.
Nevertheless, the precise contribution of NEFH gene mutations to
the pathology of concussion remains an area requiring further
elucidation through research (2).
The incidence and mortality rates of TBI are notably
higher in low- and middle-income countries compared with
high-income countries (3),
affecting an estimated 10 million individuals worldwide annually
(4). Falls are the predominant
cause of TBI, especially among individuals >50 years old
(5). This demographic often
presents with comorbidities and may be using anticoagulant
medications prior to injury, factors that amplify the complexity
and mortality risk associated with coagulation abnormalities
post-TBI (6). Coagulopathy in
patients with TBI typically manifests as abnormalities in
conventional coagulation assays. However, the prevalence of early
coagulopathy varies widely due to inconsistent definitions and
differences in the severity of the injury (7).
Most patients with severe TBI demonstrate abnormal
coagulation test results shortly after injury, whereas this is less
common in those with mild injuries (8). The severity of TBI is generally
assessed utilizing the Glasgow Coma Scale, categorizing injuries as
mild (14-15 points), moderate (9-13 points) and severe (3-8 points)
(9). The mortality rate for severe
TBI cases is approximately one-third, and ~60% of survivors suffer
from enduring physical, mental and social deficits (10). Patients with TBI accompanied by
coagulopathy are often closely associated with poor prognosis
(11), thus advancing research and
treatment for coagulopathy following TBI is of paramount
importance.
In the human body, cells are the fundamental units
of structure, function and biological processes. The present review
updated and simplified the series of coagulopathy-related events
occurring after TBI by summarizing the mechanistic changes and
roles of three types of cells [endothelial cells (ECs), neutrophils
and platelets] in the coagulation process post-TBI. Additionally,
by consolidating the pharmacological treatments and potential
therapeutic applications for patients with TBI with coagulation
dysfunction, the present study aimed for this review to provide
more insights into precision medicine by summarizing updated
mechanistic studies and innovative diagnostic and therapeutic
techniques. To facilitate a better understanding of the content for
the readers, definitions for the specialized terminology mentioned
in this article have been provided (Table I).
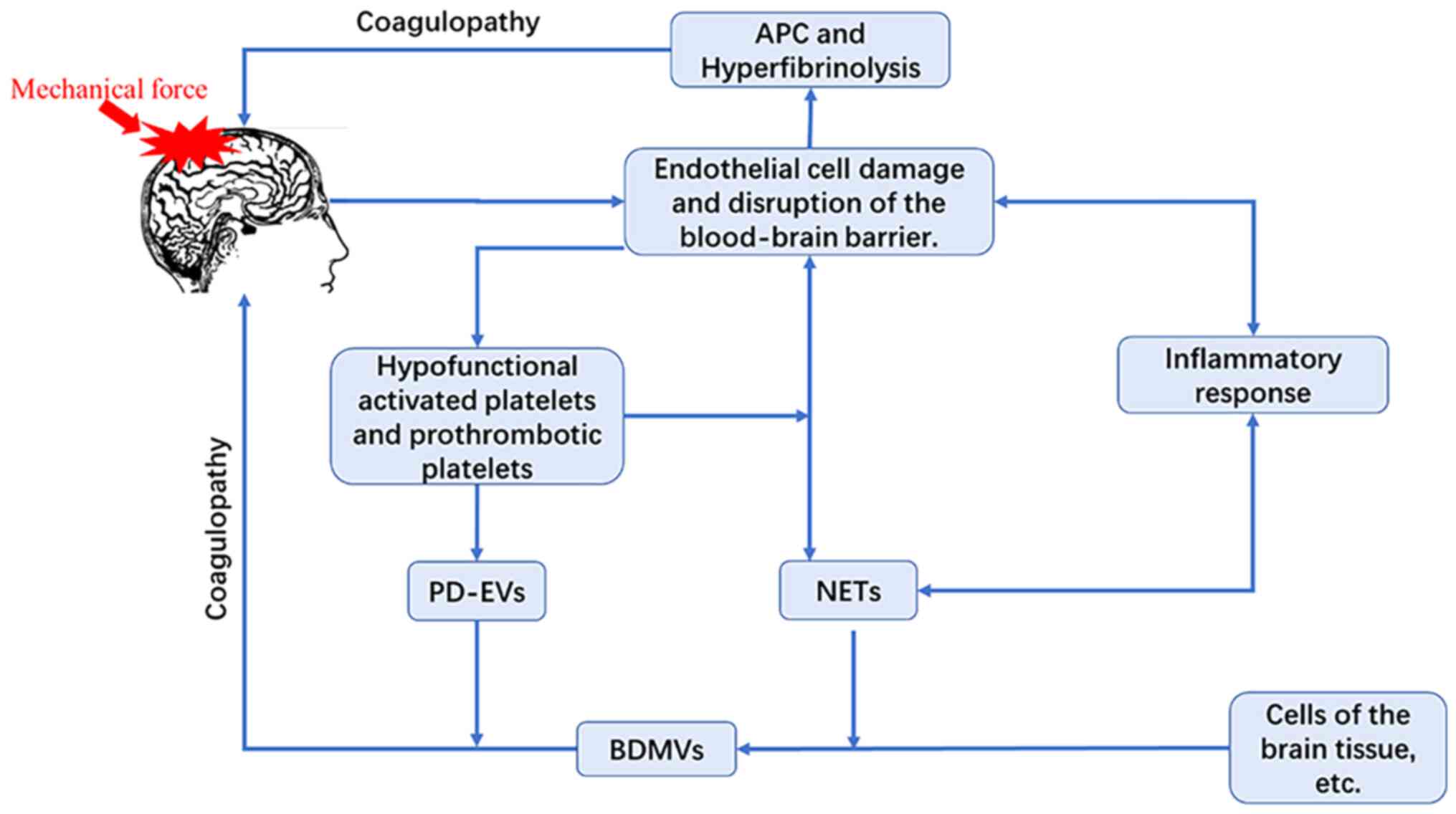
The mechanisms underpinning coagulopathy subsequent
to TBI are multifaceted, encompassing a broad spectrum of cellular
alterations. These primarily include alterations in ECs,
neutrophils and platelets (Fig.
1).
The endothelium and BBB are pivotal in maintaining
hemostatic balance. Under normal conditions, the ECs function as a
barrier to prevent the release of procoagulant factors, such as
cerebral microvesicles, into the circulation (12,13).
However, these cells can sustain rapid damage during the acute
phase of trauma, thereby intensifying coagulation disorders
(14,15). Research from 2003 demonstrated that
systemic coagulopathy can manifest within minutes following TBI
(16), characterized by activation
of protein C and enhanced fibrinolysis (17). Ordinarily, tissue-type plasminogen
activator (tPA) struggles to access fibrin structures shielded by
platelet aggregates, thus impeding the efficiency of subsequent
enzymatic reactions essential for clot resolution (18). Moreover, the mechanical forces
imparted on the brain during TBI mechanically disrupt the BBB in a
pattern akin to a Gaussian distribution (19), precipitating secondary ischemic and
inflammatory injuries. These injuries enhance the permeability of
adjacent BBB segments, exacerbating the condition. Brain-derived
microvesicles (BDMVs) enriched with tissue factor (TF) and
phosphatidylserine (PS) are implicated in the exacerbation of brain
injury, the initiation of early coagulation abnormalities and the
promotion of hyperfibrinolysis (12). The elevated presence of TF and PS
(12) in brain tissue not only
facilitates the expansion of brain injury (20,21)
but also instigates platelet dysfunction and depletion, as well as
disseminated intravascular coagulation (DIC) (22,23).
Lactoferrin, through its interaction with these microvesicles, has
shown promise in mitigating coagulation disturbances and enhancing
prognosis in a mouse model of TBI (24). Nonetheless, the specific mechanisms
through which lactoferrin exerts its effects and the precise
operational definitions of these cellular microvesicles continues
to be elusive.
Low platelet counts and/or functional platelet
defects in platelets markedly enhance the risk of bleeding; a
platelet count below 175x109/l is associated with an
elevated risk of progressive intracranial hemorrhage progression,
and counts below 100x109/l are strongly associated with
increased mortality (38,39). This clinical presentation contrasts
with traumatic coagulopathy (TIC), which is distinct from DIC, the
latter typically characterized by thrombocytopenia (40). Firstly, in patients with TBI a
reduced level of platelet reactivity is positively associated with
improved prognoses (41,42). During TBI, the depletion of von
Willebrand factor hampers platelet aggregation in vitro
(43). This impairment is
exacerbated by diminished platelet responsiveness to agonists such
as adenosine diphosphate (ADP) and/or arachidonic acid (AA),
leading to a specific defect in aggregation defects due to
inhibition of ADP and AA receptors a phenomenon closely linked to
TBI severity (22,44). These defects occur independently of
hemorrhagic shock or the absolute platelet count (22,45).
Additionally, elevated circulating levels of catecholamine platelet
agonists, such as epinephrine and norepinephrine, are associated
with compromised platelet aggregation function in patients with TBI
(46).
This observation elucidates why even patients with
mild injuries may exhibit suppressed platelet function in
vitro (47). Variations in
platelet activity may relate to systemic ischemia-reperfusion and
oxidative stress (48), with
declining platelet counts closely associated with injury severity
and increased mortality risk (42,49).
Overactive platelets may lead to secondary thrombocytopenia,
heightening the risk of bleeding (22). Patients with TBI often exhibit
moderately low platelet counts, with frequent activation of these
cells, which generate microvesicles and display procoagulant
activity (50,51). Secondly, platelet adhesion
dysfunction is recognized as a pivotal factor in trauma response.
Studies have revealed that in the aftermath of severe trauma, a
notable reduction in in platelet adhesion to collagen is observed
(52,53), along with diminished expression of
specific receptors on platelets (54,55).
These alterations may stem from fibrin-induced shedding of
glycoprotein VI (GPVI) receptor (an important receptor on the
surface of platelets that participates in collagen binding)
(56) and interference with its
signaling pathway (57).
Collagen and thrombin, frequently found at sites of
endothelial damage, conjointly stimulate the central mechanism for
the development of procoagulant platelets in vitro,
amplifying thrombin production (63). This process is accompanied by
significant release of extracellular vesicles (EVs) (64). Certain studies have reported
increased levels of platelet-derived EVs in plasma following major
trauma, suggesting that purified platelet-derived EVs could
potentially ameliorate thrombotic events post-trauma (65-68).
Consequently, targeting the emergence of procoagulant platelets
presents a promising therapeutic strategy for TIC.
The early rectification of coagulopathy in patients
with TBI is critically associated with survival rate (69). The predominant strategy for
addressing TBI-induced coagulopathy entails the blood components,
with a growing emphasis on the administration of clotting factors
and related substances. Moreover, hemostatic agents are an
essential element of the therapeutic arsenal employed in these
cases, as their indispensable role in the management of
coagulopathy post-TBI.
Tranexamic acid (TXA), a synthetic derivative of
lysine, has proven effective in reducing active hemorrhage
(70) and mortality (71) in trauma patients when administered
within 3 h of injury, as demonstrated by the CRASH-2 trial
(clinical randomization of an antifibrinolytic in significant
hemorrhage) (71,72). Building on this, the CRASH-3 trial
revealed that early administration of TXA within the same timeframe
significantly reduces mortality risk in patients with
mild-to-moderate TBI (67,73), thus endorsing its immediate use in
such scenarios, whereas its efficacy diminishes in patients with
severe TBI cases. Considering TXA's ability to enhance platelet
function (74), coupled with its
cost-effectiveness and proven efficacy in trauma management, it
continues to be a dependable therapeutic option for patients with
TBI.
Furthermore, desmopressin, as tested in a rat model
of hemorrhagic shock, has been observed to elevate von Willebrand
factor and factor VIII levels, as well as augment platelet
aggregation (75). Given its
short-acting nature and relative safety, desmopressin presents as a
suitable alternative (76).
Additional pharmacological agents such as progesterone, vitamin K2,
Butylphthalide and recombinant interleukin-1 receptor antagonist
(77) have demonstrated potential
in the treatment of TBI. Conversely, administration of high-dose
corticosteroids for 48 h to patients with moderate to severe brain
injury has been associated with an increased mortality rate at two
weeks (78). Amantadine may
accelerate the rate of functional improvement in delirium-rating
scale scores (79,80); however, due to its heterogeneity
when used in patients with TBI (80), the benefits for this patient
population requires further robust investigation. Although
theoretically cytidine diphosphocholine (CDP-choline) might have
positive effects on cell membrane integrity and cellular edema,
studies indicate that its impact on cognitive function improvement
in brain-injured patients appears to be negligible (81,82).
Hemostatic agents can reduce bleeding in trauma patients, but
excessive dosing or prolonged use may lead to acquired thrombosis
(83). Therefore, when planning
treatment, it is important to assess the risk and extent of
thrombosis.
Post-TBI, blood component transfusions typically
refer to the administration of platelets, red blood cells or plasma
based on the individual needs of the patient. In scenarios of
ongoing hemorrhage, platelet transfusions have not been proven
effective in restoring aggregation function (84) nor have they demonstrated
improvements in patient outcomes (85). Notably, the administration of
refrigerated platelets has shown to confer superior hemostatic
benefits compared with those stored at room temperature (86), a protocol now implemented in
numerous trauma centers throughout the United States.
Furthermore, transfusions of packed red blood cells
(pRBCs) have been recognized to enhance cerebral oxygenation;
recent investigations into the combined administration of pRBCs
with plasma in patients with TBI with coagulopathy have associated
this practice with an escalation in adverse reactions and
deteriorated prognoses (87). It is
particularly noteworthy that establishing higher transfusion
thresholds at 10 g/dl has been correlated with an upsurge in
bleeding complications as opposed to a lower threshold of 7 g/dl
(88), indicating that transfusion
decisions should extend beyond mere adherence to rigid hemoglobin
level. Consequently, a restrictive strategy for pRBC transfusion is
advocated, except in instances where patients exhibit intolerance
to anemia (89). The determination
of the optimal timing and volume of transfusions remains a pivotal
focus of ongoing research.
In recent years, advancements in the study of
coagulation factors have significantly progressed the treatment of
trauma patients. Fujiwara et al (90), employing a rat model of controlled
cortical impact, demonstrated that daily intravenous injections of
350 mg/kg of a synthetically derived activated peptide of factor IX
(termed F9-AP) significantly mitigated adjacent neuronal loss
associated with secondary brain injury, markedly reducing both the
volume of brain injury and associated edema. Moreover, recombinant
activated factor VII, which exhibits lesser dependence on platelet
function, appears to offer distinct advantages for patients with
severely compromised platelet function or severe thrombocytopenia,
effectively diminishing the risk of intracranial hemorrhage
(91). Prothrombin complex
concentrate (PCC), an inactivated blend comprising of factors II,
IX, VII and X, has been proven to be highly efficacious in managing
refractory bleeding to conventional treatment methods and in
correcting elevated international normalized ratio (INR) levels
(92). In TBI, where fibrinogen
levels can be depleted, it is imperative to restore these levels to
within normal ranges to alleviate inflammation and reduce
endothelial permeability (93). The
supplementation of factor XIII plays equally a critical role in
inhibiting hyperfibrinolysis, stabilizing clot formations and
minimizing surgical blood loss (94,95).
However, compared with plasma, PCC may reduce hematoma expansion,
yet shows no significant difference in 90-day mortality or Glasgow
Coma Scale scores (96).
With an aging population, the number of patients
with TBI concurrently taking oral anticoagulants is increasing. To
ameliorate adverse outcomes including bleeding in patients
undergoing surgery or those suffering from TBI while on these
therapies, the development of antidotes for the reversal of DOACs
has emerged as a crucial area of research. In 2015, publication of
the first study demonstrating the safety and efficacy of
idarucizumab, a targeted monoclonal antibody fragment for the acute
reversal of dabigatran, a direct thrombin inhibitor, marked a
significant advancement in anticoagulation management (97). This was followed by the introduction
of Andexanet alfa, an antagonist of the facto Xa inhibitors, into
clinical practice (98). Protamine
sulfate is employed for the reversal of both unfractionated heparin
and low molecular weight heparin (LMWH). However, its routine
administration for reversing prophylactic subcutaneous heparin is
not recommended unless there is a significantly prolonged activated
partial thromboplastin time (aPTT) (99).
Notably, non-specific hemostatic agents such as PCC
and activated prothrombin complex concentrate may also serve to
reverse the effects of DOACs. However, FDA-approved reversal agents
are not applicable to all DOACs or all clinical scenarios where
reversal may be considered. Furthermore, the complexity of clinical
use is compounded by factors such as cost, preparation and the lack
of standardized protocols (97).
Research underscores the complex and heterogeneous
nature of TBI progression, necessitating exploration beyond
conventional treatments toward interventions that may confer
additional benefit to patients. This section delineates several
promising potential therapeutic avenues post-TBI, aiming to foster
groundbreaking advancements in the management during process.
It has been demonstrated that elevated levels of
matrix metalloproteinase-9 (MMP-9) following brain injury
contributes to the dysfunction of the BBB (100). Encouragingly, administration of
MMP inhibitors in rodent models of brain injury has led to improved
outcomes (101), indicating that
MMP inhibition may represent a viable therapeutic strategy for
patients with TBI. Moreover, the integration of medical science and
nanotechnology has facilitated the development of
platelet-mimicking nanovesicles, which incorporate the biological
characteristics of platelet membranes into a lipid-based
nanostructure. Through bioengineering manipulations, these
particles can partially or fully emulate the hemostatic functions
of natural platelets to varying extents (102), thus offering a promising
alternative to platelet transfusion therapy. Additionally, novel
resuscitative agents, known as platelet-derived extracellular
vesicles (103), have surfaced,
exhibiting remarkable hemostatic potency in patients with TBI via
multiple mechanisms. However, current investigational agents remain
in the experimental phase and additional research and data are
required before they can advance to clinical application. As
research advances and technology evolves, it is anticipated that
additional mechanisms will be elucidated and applied to strategies
aimed at ameliorating the outcomes for TBI survivors.
TBI can be accompanied by a wide array of
complications, including but not limited to epilepsy, cerebral
herniation, hydrocephalus and cerebrocardiac syndrome (104-106).
These concomitant complications are intimately associated with a
diminished quality of life and heightened mortality rates.
Specifically, in the period following the initial day post-TBI, the
emergence of multiple organ dysfunction and thrombotic events
become the predominant causes of mortality among critically ill
patients (107). Besides
emphasizing early preventive strategies, it is equally essential to
unravel the mechanisms that underlie the initiation and progression
of these complications is equally indispensable. This underscores
the imperative for a thorough comprehension and the formulation of
precise intervention strategies.
Post-traumatic MODS is widely recognized as a
consequence of deregulated responses to trauma. The primary
pathogenic mechanism likely involves the activation of both
coagulation and inflammatory cascades (108,109). Furthermore, MODS is intimately
associated with extracellular histones, HMGB1 and S100A8/9, among
other factors. Notably, elevated levels of HMGB1 contribute to
organ injury (109). Additionally,
the dynamic interaction between platelets and leukocytes
facilitates leukocyte recruitment to sites of injury, facilitating
tissue repair (110).
Nevertheless, an excessive immune response appears to inflict organ
damage (111). Fortunately,
proactive management of coagulopathy exhibits promising potential
in ameliorating the incidence of organ failure (112).
Thromboembolic events play a significant cause in
increasing morbidity and disability rates among patients,
particularly those who have suffered TBI and are severely injured
or unable to mobilize independently. Considering TBI as an
established independent risk factor for venous thromboembolism
(VTE) (113,114). The present discussion
predominantly focuses on VTE, extending beyond traditional triggers
to explore the pivotal role played by the interaction between
platelets and monocytes in the initiation and propagation of VTE
(115). Moreover, factors
including NETs, platelet-derived microparticles and protein C
depletion (38) are intimately
associated with VTE development. In intensive care units, the
occurrence of VTE among critically ill trauma patients is notably
elevated, with an estimated incidence of up to 35% (116). Even with the implementation of
mechanical prophylaxis measures for deep vein thrombosis (DVT),
residual DVT and pulmonary embolism (PE) rates remain substantial
at 31 and 3% (114), respectively.
A recent large comprehensive observational study emphasized that
de novo pulmonary thrombi are more prevalent than PEs
originating from DVT, often manifesting early in the clinical
course (117). In animal models of
TBI, microthrombi are predominantly observed in the pericontusional
cortex (118), composed of fibrin,
platelets and other components. LMWH has demonstrated superior
efficacy in preventing thromboembolism compared to unfractionated
heparin (119), indicating that,
following a thorough patient evaluation and confirmation of no
contraindications, early initiation of LMWH for thromboprophylaxis
is recommended. Apart from LMWH, factor XI inhibitors, such as
Abelacimab, have shown effectiveness in preventing VTE (120), offering an alternative preventive
strategy in managing thrombotic risks.
Consequently, early detection, prevention and
management of MODS and thrombotic events assume paramount
importance. Identification and assessment of disease severity, as
well as prediction of risks in patients, are facilitated through
monitoring vital signs, imaging changes, laboratory parameters and
scoring systems such as APACHE II, SOFA and qSOFA (121,122). Management of these patients should
be viewed as a dynamic process, necessitating close surveillance
and prompt adjustment of therapeutic strategies as needed. Given
the unique nature of each patient's condition, treatment plans
should be individualized, with the overarching goals of maximizing
organ function recovery, preventing thrombosis and enhancing
quality of life.
Laboratory assessments aimed at detecting
coagulopathy and techniques reflecting brain injury are crucial in
diagnosing and managing coagulation disorders that arise following
TBI (Table II). Conventional
coagulation assays, routinely employed in clinical settings to
evaluate hemostatic function, encompass prothrombin time, aPTT and
INR (123,124), which aid in prognostication
post-cranial injury. Nonetheless, these tests fail to capture the
full complexity of coagulation processes and have limitations in
accurately representing thrombin generation and precision in
hemostatic evaluation (125,126).
More advanced global hemostasis assessments,
including rotational thromboelastometry (ROTEM),
thromboelastography (TEG) and thrombin generation tests, may offer
a superior real-time analysis of hemostatic status. These provide
swift feedback for therapeutic intervention and enable more precise
predictions treatment outcomes (127). These methodologies further
facilitate goal-directed transfusion strategies (128) and guide heparin administration for
thromboembolism prophylaxis (129), with TEG particularly unaffected by
the administration of TXA (130).
A fibrinogen level concentration 2.0 g/l is recognized as a risk
factor for coagulopathy and associated complications post-TBI. Both
ROTEM and TEG have proven effective in swiftly and precisely
measuring fibrinogen levels (127). Moreover, recent research involving
bleeding adult and pediatric patients has demonstrated that
transfusion strategies guided by viscoelastic tests improve
survival rates, decrease blood product usage and reduce the
incidence of renal failure when compared with other methods
(131). Point-of-care platelet
function testing (POC-PFT), exemplified by systems including
VerifyNow, Plateletworks, PFA-100/200, show promise in identifying
platelet dysfunction or guiding antiplatelet therapy (6,20).
However, the absence of a gold standard for POC-PFT and substantial
variation among available analyzers in terms of implementation
technique and platelet agonists utilized hinder widespread
acceptance. Consequently, current European guidelines on massive
hemorrhage and coagulation management in trauma do not advocate for
the routine use of POC-PFT (132).
For patients suffering from moderate to severe brain
injuries, the mere reliance on conventional blood tests is
insufficient, Conventional coagulation tests measure only ~4% of
total thrombin generation and do not assess the overall hemostatic
state. Furthermore, these tests do not reflect the interactions
between multiple coagulation pathway mechanisms (133-135).
Therefore, the incorporation of cutting-edge neurocritical care
monitoring instruments is of utmost importance. In this context,
multimodal monitoring (MMM) encompasses a range of techniques,
including intracranial pressure (ICP)-monitoring, cerebral
microdialysis (CMD), cerebral tissue oxygenation monitoring and
continuous electroencephalography (EEG) (3), amongst others. Through the
amalgamation of these diverse monitoring modalities, MMM provides a
comprehensive, real-time evaluation of the patient's cerebral
pathophysiology. This significantly bolsters the management of
brain disorders, elevates patient outcomes and propels clinical
research forward.
Primarily, ICP monitoring stands as a pivotal
component, as elevated ICP can diminish cerebral perfusion
[cerebral perfusion pressure=mean arterial pressure (MAP)-ICP],
subsequently augmenting the peril of ischemia and herniation
(136,137). In accordance with the established
guidelines, maintaining ICP at 20-25 mmHg (138), with MAP kept at 60-70 mmHg in
severe patients with TBI, is paramount in effectively mitigating
adverse outcomes. Furthermore, CMD emerges as an invasive yet
invaluable technique. It involves hourly sampling and analysis of
cerebral extracellular fluid metabolites, offering an unprecedented
glimpse into the biochemical shifts within the brain (139). Complementing this, cerebral tissue
oxygenation monitoring employs near-infrared spectroscopy for a
non-invasive, continuous assessment of regional concentrations of
oxygenated and deoxygenated hemoglobin in the brain (140). This, in turn, aids in evaluating
the risk of cerebral hypoxia and shaping individualized therapeutic
strategies. However, it's worth noting that its application remains
primarily confined to superficial areas such as the frontal cortex,
owing to technological limitations.
In the realm of severe TBI, post-traumatic seizures
affect roughly one in ten patients, with asymptomatic seizure
activity prevalence potentially escalating to 20-25% (141). Continuous EEG enables the early
detection of these cerebral pathologies and guides antiepileptic
therapy. As previously reported, the successful management of a
25-year-old comatose patient following a car accident was achieved
through the utilization of the MMM monitoring and management
system. This case underscores the practical utility of MMM. The
system is capable of providing real-time updates on physiological
changes and offering early indications of potential issues before
they escalate, thereby guiding preemptive interventions (142). In conclusion, with the progression
of technology and the accumulation of clinical experience, the
application of MMM is likely to evolve into a more refined and
widespread practice. IF so, it would be an indispensable component
of modern neurointensive care.
TBI triggers swift and substantial modifications in
cellular behaviors, thereby amplifying the intricacy of
coagulopathy mechanisms and complicating treatment options by
altering both intra- and intercellular connections. The diverse
changes induced by TBI are still inadequately targeted by current
therapeutic approaches. As a result, there is an urgent necessity
for deeper investigation to clarify the fundamental mechanisms
underlying coagulopathy of post-TBI and to refine precision
therapies accordingly. This involves establishing diagnostic
thresholds and developing targeted therapeutic interventions aimed
at effectively managing coagulation disorders. Current research
lacks prospective diagnostic modalities that can accurately
determine the timepoint of hemostatic failure, making it
challenging to define the optimal timing for initiating
anticoagulation therapy in patients with coagulopathy. The aging
population of patients with TBI, coupled with the widespread use of
antiplatelet medications and anticoagulants, poses a considerable
challenge in comprehending the distinct impacts of various
antiplatelet medications and anticoagulants on post-TBI
coagulopathy. Tackling these challenges is paramount for improving
clinical outcomes and minimizing the morbidity and mortality
associated with this condition. Hence, there is a critical need for
more expeditious and efficacious techniques to restore coagulation,
thereby limiting the progression of brain injury in patients on
anticoagulant and antiplatelet medications, with the ultimate aim
of improving outcomes. Moreover, future research focusing on the
management of coagulopathies in both critically and non-critically
ill patients post-TBI should prioritize not just survival rates,
but also enhancements in quality of life for patients.
The authors are grateful to Ms. Du Pengchao (Binzhou
Medical College, Yantai, China) for her insightful suggestions and
constructive feedback on the manuscript.
Funding: No funding was received.
Not applicable.
HH and ZQ performed the literature search and
co-wrote the manuscript. RL, BJ, LW and AL participated in the
literature search and reviewed the literature and the manuscript.
HH, ZQ, RL, BJ, LW and AL made substantial contributions to the
conception and revision of the manuscript. Data authentication is
not applicable. All authors read and approved the final version of
the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Tierney RT, Mansell JL, Higgins M,
McDevitt JK, Toone N, Gaughan JP, Mishra A and Krynetskiy E:
Apolipoprotein E genotype and concussion in college athletes. Clin
J Sport Med. 20:464–468. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Panenka WJ, Gardner AJ, Dretsch MN, Crynen
GC, Crawford FC and Iverson GL: Systematic review of genetic risk
factors for sustaining a mild traumatic brain injury. J
Neurotrauma. 34:2093–2099. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Khellaf A, Khan DZ and Helmy A: Recent
advances in traumatic brain injury. J Neurol. 266:2878–2889.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Maegele M, Aversa J, Marsee MK, McCauley
R, Chitta SH, Vyakaranam S and Walsh M: Changes in coagulation
following brain injury. Semin Thromb Hemost. 46:155–166.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Roozenbeek B, Maas AI and Menon DK:
Changing patterns in the epidemiology of traumatic brain injury.
Nat Rev Neurol. 9:231–236. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Prinz V, Finger T, Bayerl S, Rosenthal C,
Wolf S, Liman T and Vajkoczy P: High prevalence of
pharmacologically induced platelet dysfunction in the acute setting
of brain injury. Acta Neurochir (Wien). 158:117–123.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shehata M, Afify M, El-Shafie M and Khaled
M: Prevalence and clinical implications of coagulopathy in patients
with isolated head trauma. Med J Cairo Univ. 79:131–137. 2011.
|
|
8
|
Harhangi BS, Kompanje EJ, Leebeek FW and
Maas AI: Coagulation disorders after traumatic brain injury. Acta
Neurochir (Wien). 150:165–175; discussion 175. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hartings JA, Bullock MR, Okonkwo DO,
Murray LS, Murray GD, Fabricius M, Maas AI, Woitzik J, Sakowitz O,
Mathern B, et al: Spreading depolarisations and outcome after
traumatic brain injury: A prospective observational study. Lancet
Neurol. 10:1058–1064. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mena JH, Sanchez AI, Rubiano AM, Peitzman
AB, Sperry JL, Gutierrez MI and Puyana JC: Effect of the modified
Glasgow Coma Scale score criteria for mild traumatic brain injury
on mortality prediction: Comparing classic and modified Glasgow
Coma Scale score model scores of 13. J Trauma. 71:1185–1192;
discussion 1193. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Maegele M, Schöchl H, Menovsky T, Maréchal
H, Marklund N, Buki A and Stanworth S: Coagulopathy and
haemorrhagic progression in traumatic brain injury: Advances in
mechanisms, diagnosis, and management. Lancet Neurol. 16:630–647.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tian Y, Salsbery B, Wang M, Yuan H, Yang
J, Zhao Z, Wu X, Zhang Y, Konkle BA, Thiagarajan P, et al:
Brain-derived microparticles induce systemic coagulation in a
murine model of traumatic brain injury. Blood. 125:2151–2159.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhao Z, Wang M, Tian Y, Hilton T, Salsbery
B, Zhou EZ, Wu X, Thiagarajan P, Boilard E, Li M, et al:
Cardiolipin-mediated procoagulant activity of mitochondria
contributes to traumatic brain injury–associated coagulopathy in
mice. Blood. 127:2763–2772. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Albert V, Subramanian A, Agrawal D, Pati
HP, Gupta SD and Mukhopadhyay AK: Acute Traumatic endotheliopathy
in isolated severe brain injury and its impact on clinical outcome.
Med Sci (Basel). 6(5)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sillesen M, Rasmussen LS, Jin G, Jepsen
CH, Imam A, Hwabejire JO, Halaweish I, DeMoya M, Velmahos G,
Johansson PI and Alam HB: Assessment of coagulopathy, endothelial
injury, and inflammation after traumatic brain injury and
hemorrhage in a porcine model. J Trauma Acute Care Surg. 76:12–20.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Maegele M: Coagulopathy after traumatic
brain injury: Incidence, pathogenesis, and treatment options.
Transfusion. 53 (Suppl 1):28S–37S. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hulka F, Mullins RJ and Frank EH: Blunt
brain injury activates the coagulation process. Arch Surg.
131:923–928. 1996.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Brommer EJ: The level of extrinsic
plasminogen activator (t-PA) during clotting as a determinant of
the rate of fibrinolysis; Inefficiency of activators added
afterwards. Thromb Res. 34:109–115. 1984.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Haltmeier T, Benjamin E, Gruen JP, Shulman
IA, Lam L, Inaba K and Demetriades D: Decreased mortality in
patients with isolated severe blunt traumatic brain injury
receiving higher plasma to packed red blood cells transfusion
ratios. Injury. 49:62–66. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fleck RA, Rao LV, Rapaport SI and Varki N:
Localization of human tissue factor antigen by immunostaining with
monospecific, polyclonal anti-human tissue factor antibody. Thromb
Res. 59:421–437. 1990.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Eddleston M, de la Torre JC, Oldstone MB,
Loskutoff DJ, Edgington TS and Mackman N: Astrocytes are the
primary source of tissue factor in the murine central nervous
system. A role for astrocytes in cerebral hemostasis. J Clin
Invest. 92:349–358. 1993.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Castellino FJ, Chapman MP, Donahue DL,
Thomas S, Moore EE, Wohlauer MV, Fritz B, Yount R, Ploplis V, Davis
P, et al: Traumatic brain injury causes platelet adenosine
diphosphate and arachidonic acid receptor inhibition independent of
hemorrhagic shock in humans and rats. J Trauma Acute Care Surg.
76:1169–1176. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hoffman M and Monroe DM: Tissue factor in
brain is not saturated with factor VIIa: Implications for factor
VIIa dosing in intracerebral hemorrhage. Stroke. 40:2882–2884.
2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhou Y, Cai W, Zhao Z, Hilton T, Wang M,
Yeon J, Liu W, Zhang F, Shi FD, Wu X, et al: Lactadherin promotes
microvesicle clearance to prevent coagulopathy and improves
survival of severe TBI mice. Blood. 131:563–572. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kolaczkowska E and Kubes P: Neutrophil
recruitment and function in health and inflammation. Nat Rev
Immunol. 13:159–175. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sollberger G, Tilley DO and Zychlinsky A:
Neutrophil extracellular traps: The biology of chromatin
externalization. Dev Cell. 44:542–553. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Allen C, Thornton P, Denes A, McColl BW,
Pierozynski A, Monestier M, Pinteaux E, Rothwell NJ and Allan SM:
Neutrophil cerebrovascular transmigration triggers rapid
neurotoxicity through release of proteases associated with
decondensed DNA. J Immunol. 189:381–392. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jin J, Wang F, Tian J, Zhao X, Dong J,
Wang N, Liu Z, Zhao H, Li W, Mang G and Hu S: Neutrophil
extracellular traps contribute to coagulopathy after traumatic
brain injury. JCI Insight. 8(e141110)2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Maugeri N, Capobianco A, Rovere-Querini P,
Ramirez GA, Tombetti E, Valle PD, Monno A, D'Alberti V, Gasparri
AM, Franchini S, et al: Platelet microparticles sustain
autophagy-associated activation of neutrophils in systemic
sclerosis. Sci Transl Med. 10(eaao3089)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jiao Y, Li W, Wang W, Tong X, Xia R, Fan
J, Du J, Zhang C and Shi X: Platelet-derived exosomes promote
neutrophil extracellular trap formation during septic shock. Crit
Care. 24(380)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Frangou E, Chrysanthopoulou A, Mitsios A,
Kambas K, Arelaki S, Angelidou I, Arampatzioglou A, Gakiopoulou H,
Bertsias GK, Verginis P, et al: REDD1/autophagy pathway promotes
thromboinflammation and fibrosis in human systemic lupus
erythematosus (SLE) through NETs decorated with tissue factor (TF)
and interleukin-17A (IL-17A). Ann Rheum Dis. 78:238–248.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Stakos DA, Kambas K, Konstantinidis T,
Mitroulis I, Apostolidou E, Arelaki S, Tsironidou V, Giatromanolaki
A, Skendros P, Konstantinides S and Ritis K: Expression of
functional tissue factor by neutrophil extracellular traps in
culprit artery of acute myocardial infarction. Eur Heart J.
36:1405–1414. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Folco EJ, Mawson TL, Vromman A,
Bernardes-Souza B, Franck G, Persson O, Nakamura M, Newton G,
Luscinskas FW and Libby P: Neutrophil extracellular traps induce
endothelial cell activation and tissue factor production through
Interleukin-1α and Cathepsin G. Arterioscler Thromb Vasc Biol.
38:1901–1912. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhu K, Zhu Y, Hou X, Chen W, Qu X, Zhang
Y, Li Z, Wang C, Chen J, Lv L, et al: NETs Lead to sympathetic
hyperactivity after traumatic brain injury through the
LL37-Hippo/MST1 Pathway. Front Neurosci. 15(621477)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vaibhav K, Braun M, Alverson K, Khodadadi
H, Kutiyanawalla A, Ward A, Banerjee C, Sparks T, Malik A, Rashid
MH, et al: Neutrophil extracellular traps exacerbate neurological
deficits after traumatic brain injury. Sci Adv.
6(eaax8847)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jassam YN, Izzy S, Whalen M, McGavern DB
and El Khoury J: Neuroimmunology of Traumatic Brain Injury: Time
for a Paradigm Shift. Neuron. 95:1246–1265. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Noubouossie DF, Reeves BN, Strahl BD and
Key NS: Neutrophils: Back in the thrombosis spotlight. Blood.
133:2186–2197. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Laroche M, Kutcher ME, Huang MC, Cohen MJ
and Manley GT: Coagulopathy after traumatic brain injury.
Neurosurgery. 70:1334–1345. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Joseph B, Aziz H, Zangbar B, Kulvatunyou
N, Pandit V, O'Keeffe T, Tang A, Wynne J, Friese RS and Rhee P:
Acquired coagulopathy of traumatic brain injury defined by routine
laboratory tests: Which laboratory values matter? J Trauma Acute
Care Surg. 76:121–125. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wada H, Thachil J, Di Nisio M, Mathew P,
Kurosawa S, Gando S, Kim HK, Nielsen JD, Dempfle CE, Levi M, et al:
Guidance for diagnosis and treatment of DIC from harmonization of
the recommendations from three guidelines. J Thromb Haemost: Feb 4,
2013 (Epub ahead of print).
|
|
41
|
McCully BH, Connelly CR, Fair KA, Holcomb
JB, Fox EE, Wade CE, Bulger EM and Schreiber MA: PROPPR Study
Group. Onset of coagulation function recovery is delayed in
severely injured trauma patients with venous thromboembolism. J Am
Coll Surg. 225:42–51. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Solomon C, Traintinger S, Ziegler B, Hanke
A, Rahe-Meyer N, Voelckel W and Schöchl H: Platelet function
following trauma. A multiple electrode aggregometry study. Thromb
Haemost. 106:322–330. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kornblith LZ, Robles AJ, Conroy AS,
Hendrickson CM, Calfee CS, Fields AT, Callcut RA and Cohen MJ:
Perhaps it's not the platelet: Ristocetin uncovers the potential
role of von Willebrand factor in impaired platelet aggregation
following traumatic brain injury. J Trauma Acute Care Surg.
85:873–880. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Davis PK, Musunuru H, Walsh M, Cassady R,
Yount R, Losiniecki A, Moore EE, Wohlauer MV, Howard J, Ploplis VA,
et al: Platelet dysfunction is an early marker for traumatic brain
injury-induced coagulopathy. Neurocrit Care. 18:201–208.
2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Briggs A, Gates JD, Kaufman RM, Calahan C,
Gormley WB and Havens JM: Platelet dysfunction and platelet
transfusion in traumatic brain injury. J Surg Res. 193:802–806.
2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Matthay ZA, Fields AT, Nunez-Garcia B,
Park JJ, Jones C, Leligdowicz A, Hendrickson CM, Callcut RA,
Matthay MA and Kornblith LZ: Importance of catecholamine signaling
in the development of platelet exhaustion after traumatic injury. J
Thromb Haemost. 20:2109–2118. 2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Sirajuddin S, Valdez C, DePalma L, Maluso
P, Singhal R, Schroeder M and Sarani B: Inhibition of platelet
function is common following even minor injury. J Trauma Acute Care
Surg. 81:328–332. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kutcher ME, Redick BJ, McCreery RC, Crane
IM, Greenberg MD, Cachola LM, Nelson MF and Cohen MJ:
Characterization of platelet dysfunction after trauma. J Trauma
Acute Care Surg. 73:13–19. 2012.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Brown LM, Call MS, Margaret Knudson M and
Cohen MJ: Trauma Outcomes Group. Holcomb JB, Wade CE, Brasel KJ,
Vercruysse G, MacLeod J, et al: A normal platelet count may not be
enough: The impact of admission platelet count on mortality and
transfusion in severely injured trauma patients. J Trauma. 71 (2
Suppl 3):S337–S342. 2011.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ploplis VA, Donahue DL, Sandoval-Cooper
MJ, MorenoCaffaro M, Sheets P, Thomas SG, Walsh M and Castellino
FJ: Systemic platelet dysfunction is the result of local
dysregulated coagulation and platelet activation in the brain in a
rat model of isolated traumatic brain injury. J Neurotrauma.
31:1672–1675. 2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Prodan CI, Vincent AS and Dale GL:
Coated-Platelet levels increase with number of injuries in patients
with mild traumatic brain injury. J Neurotrauma. 33:818–824.
2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Vulliamy P, Montague SJ, Gillespie S, Chan
MV, Coupland LA, Andrews RK, Warner TD, Gardiner EE, Brohi K and
Armstrong PC: Loss of GPVI and GPIbα contributes to trauma-induced
platelet dysfunction in severely injured patients. Blood Adv.
4:2623–2630. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Li R, Elmongy H, Sims C and Diamond SL: Ex
vivo recapitulation of trauma-induced coagulopathy and preliminary
assessment of trauma patient platelet function under flow using
microfluidic technology. J Trauma Acute Care Surg. 80:440–449.
2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Fields AT, Matthay ZA, Nunez-Garcia B,
Matthay EC, Bainton RJ, Callcut RA and Kornblith LZ: Good platelets
gone bad: The effects of trauma patient plasma on healthy platelet
aggregation. Shock. 55:189–197. 2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Verni CC, Davila A Jr, Balian S, Sims CA
and Diamond SL: Platelet dysfunction during trauma involves diverse
signaling pathways and an inhibitory activity in patient-derived
plasma. J Trauma Acute Care Surg. 86:250–259. 2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Montague SJ, Delierneux C, Lecut C, Layios
N, Dinsdale RJ, Lee CS, Poulter NS, Andrews RK, Hampson P, Wearn
CM, et al: Soluble GPVI is elevated in injured patients: shedding
is mediated by fibrin activation of GPVI. Blood Adv. 2:240–251.
2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Lee MY, Verni CC, Herbig BA and Diamond
SL: Soluble fibrin causes an acquired platelet glycoprotein VI
signaling defect: Implications for coagulopathy. J Thromb Haemost.
15:2396–2407. 2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Montague SJ, Hicks SM, Lee CS, Coupland
LA, Parish CR, Lee WM, Andrews RK and Gardiner EE: Fibrin exposure
triggers αIIbβ3-independent platelet aggregate formation, ADAM10
activity and glycoprotein VI shedding in a charge-dependent manner.
J Thromb Haemost. 18:1447–1458. 2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Gardiner EE, Karunakaran D, Shen Y, Arthur
JF, Andrews RK and Berndt MC: Controlled shedding of platelet
glycoprotein (GP)VI and GPIb-IX-V by ADAM family
metalloproteinases. J Thromb Haemost. 5:1530–1537. 2007.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Agbani EO, van den Bosch MT, Brown E,
Williams CM, Mattheij NJ, Cosemans JM, Collins PW, Heemskerk JW,
Hers I and Poole AW: Coordinated membrane ballooning and
procoagulant spreading in human platelets. Circulation.
132:1414–1424. 2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Kulkarni S, Woollard KJ, Thomas S, Oxley D
and Jackson SP: Conversion of platelets from a proaggregatory to a
proinflammatory adhesive phenotype: Role of PAF in spatially
regulating neutrophil adhesion and spreading. Blood. 110:1879–1886.
2007.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Vulliamy P, Gillespie S, Armstrong PC,
Allan HE, Warner TD and Brohi K: Histone H4 induces platelet
ballooning and microparticle release during trauma hemorrhage. Proc
Natl Acad Sci USA. 116:17444–17449. 2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Agbani EO and Poole AW: Procoagulant
platelets: Generation, function, and therapeutic targeting in
thrombosis. Blood. 130:2171–2179. 2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Boilard E, Duchez AC and Brisson A: The
diversity of platelet microparticles. Curr Opin Hematol.
22:437–444. 2015.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Curry N, Raja A, Beavis J, Stanworth S and
Harrison P: Levels of procoagulant microvesicles are elevated after
traumatic injury and platelet microvesicles are negatively
correlated with mortality. J Extracell Vesicles.
3(25625)2014.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Matijevic N, Wang YW, Holcomb JB, Kozar R,
Cardenas JC and Wade CE: Microvesicle phenotypes are associated
with transfusion requirements and mortality in subjects with severe
injuries. J Extracell Vesicles. 4(29338)2015.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Lopez E, Srivastava AK, Pati S, Holcomb JB
and Wade CE: Platelet-Derived microvesicles: A potential therapy
for trauma-induced coagulopathy. Shock. 49:243–248. 2018.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Price J, Gardiner C and Harrison P:
Platelet-enhanced plasma: Characterization of a novel candidate
resuscitation fluid's extracellular vesicle content, clotting
parameters, and thrombin generation capacity. Transfusion.
61:2179–2194. 2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Epstein DS, Mitra B, Cameron PA,
Fitzgerald M and Rosenfeld JV: Normalization of coagulopathy is
associated with improved outcome after isolated traumatic brain
injury. J Clin Neurosci. 29:64–69. 2016.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Dewan Y, Komolafe EO, Mejía-Mantilla JH,
Perel P, Roberts I and Shakur H: CRASH-3 Collaborators.
CRASH-3-tranexamic acid for the treatment of significant traumatic
brain injury: study protocol for an international randomized,
double-blind, placebo-controlled trial. Trials.
13(87)2012.PubMed/NCBI View Article : Google Scholar
|
|
71
|
CRASH-2 trial collaborators. Shakur H,
Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, El-Sayed H,
Gogichaishvili T, Gupta S, et al: Effects of tranexamic acid on
death, vascular occlusive events, and blood transfusion in trauma
patients with significant haemorrhage (CRASH-2): A randomised,
placebo-controlled trial. Lancet. 376:23–32. 2010.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Roberts I, Shakur H, Coats T, Hunt B,
Balogun E, Barnetson L, Cook L, Kawahara T, Perel P, Prieto-Merino
D, et al: The CRASH-2 trial: A randomised controlled trial and
economic evaluation of the effects of tranexamic acid on death,
vascular occlusive events and transfusion requirement in bleeding
trauma patients. Health Technol Assess. 17:1–79. 2013.PubMed/NCBI View Article : Google Scholar
|
|
73
|
CRASH-3 trial collaborators. Effects of
tranexamic acid on death, disability, vascular occlusive events and
other morbidities in patients with acute traumatic brain injury
(CRASH-3): A randomised, placebo-controlled trial. Lancet.
394:1713–1723. 2019.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Weber CF, Görlinger K, Byhahn C, Moritz A,
Hanke AA, Zacharowski K and Meininger D: Tranexamic acid partially
improves platelet function in patients treated with dual
antiplatelet therapy. Eur J Anaesthesiol. 28:57–62. 2011.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Naidech AM, Maas MB, Levasseur-Franklin
KE, Liotta EM, Guth JC, Berman M, Rosenow JM, Lindholm PF, Bendok
BR, Prabhakaran S, et al: Desmopressin improves platelet activity
in acute intracerebral hemorrhage. Stroke. 45:2451–2453.
2014.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Kim DY, O'Leary M, Nguyen A, Kaji A,
Bricker S, Neville A, Bongard F, Putnam B and Plurad D: The effect
of platelet and desmopressin administration on early radiographic
progression of traumatic intracranial hemorrhage. J Neurotrauma.
32:1815–1821. 2015.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Helmy A, Guilfoyle MR, Carpenter KL,
Pickard JD, Menon DK and Hutchinson PJ: Recombinant human
interleukin-1 receptor antagonist in severe traumatic brain injury:
A phase II randomized control trial. J Cereb Blood Flow Metab.
34:845–851. 2014.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Roberts I, Yates D, Sandercock P, Farrell
B, Wasserberg J, Lomas G, Cottingham R, Svoboda P, Brayley N,
Mazairac G, et al: Effect of intravenous corticosteroids on death
within 14 days in 10008 adults with clinically significant head
injury (MRC CRASH trial): Randomised placebo-controlled trial.
Lancet. 364:1321–1328. 2004.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Mizoguchi K, Yokoo H, Yoshida M, Tanaka T
and Tanaka M: Amantadine increases the extracellular dopamine
levels in the striatum by re-uptake inhibition and by
N-methyl-D-aspartate antagonism. Brain Res. 662:255–258.
1994.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Giacino JT, Whyte J, Bagiella E, Kalmar K,
Childs N, Khademi A, Eifert B, Long D, Katz DI, Cho S, et al:
Placebo-controlled trial of amantadine for severe traumatic brain
injury. N Engl J Med. 366:819–826. 2012.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Secades JJ: Role of citicoline in the
management of traumatic brain injury. Pharmaceuticals (Basel).
14(410)2021.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Zafonte RD, Bagiella E, Ansel BM, Novack
TA, Friedewald WT, Hesdorffer DC, Timmons SD, Jallo J, Eisenberg H,
Hart T, et al: Effect of citicoline on functional and cognitive
status among patients with traumatic brain injury: Citicoline Brain
Injury Treatment Trial (COBRIT). JAMA. 308:1993–2000.
2012.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Narayan RK, Maas AI, Marshall LF, Servadei
F, Skolnick BE and Tillinger MN: rFVIIa Traumatic ICH Study Group.
Recombinant factor VIIA in traumatic intracerebral hemorrhage:
Results of a dose-escalation clinical trial. Neurosurgery.
62:776–786; discussion 786-778. 2008.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Vulliamy P, Gillespie S, Gall LS, Green L,
Brohi K and Davenport RA: Platelet transfusions reduce fibrinolysis
but do not restore platelet function during trauma hemorrhage. J
Trauma Acute Care Surg. 83:388–397. 2017.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Anglin CO, Spence JS, Warner MA, Paliotta
C, Harper C, Moore C, Sarode R, Madden C and Diaz-Arrastia R:
Effects of platelet and plasma transfusion on outcome in traumatic
brain injury patients with moderate bleeding diatheses. J
Neurosurg. 118:676–686. 2013.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Nair PM, Pidcoke HF, Cap AP and
Ramasubramanian AK: Effect of cold storage on shear-induced
platelet aggregation and clot strength. J Trauma Acute Care Surg.
77 (3 Suppl 2):S88–S93. 2014.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Etemadrezaie H, Baharvahdat H, Shariati Z,
Lari SM, Shakeri MT and Ganjeifar B: The effect of fresh frozen
plasma in severe closed head injury. Clin Neurol Neurosurg.
109:166–171. 2007.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Robertson CS, Hannay HJ, Yamal JM,
Gopinath S, Goodman JC and Tilley BC: Epo Severe TBI Trial
Investigators. Baldwin A, Rivera Lara L, Saucedo-Crespo H, et al:
Effect of erythropoietin and transfusion threshold on neurological
recovery after traumatic brain injury: A randomized clinical trial.
JAMA. 312:36–47. 2014.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Lelubre C, Bouzat P, Crippa IA and Taccone
FS: Anemia management after acute brain injury. Crit Care.
20(152)2016.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Fujiwara Y, Kitano H, Yamamoto T, Kokubun
S and Hidai C: Activation peptide of coagulation factor IX improves
the prognosis after traumatic brain injury. Biochem Biophys Res
Commun. 569:35–40. 2021.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Kramer AH, Deis N, Ruddell S, Couillard P,
Zygun DA, Doig CJ and Gallagher C: Decompressive craniectomy in
patients with traumatic brain injury: Are the usual indications
congruent with those evaluated in clinical trials? Neurocrit Care.
25:10–19. 2016.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Spahn DR, Bouillon B, Cerny V, Duranteau
J, Filipescu D, Hunt BJ, Komadina R, Maegele M, Nardi G, Riddez L,
et al: The European guideline on management of major bleeding and
coagulopathy following trauma: Fifth edition. Crit Care.
23(98)2019.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Muradashvili N and Lominadze D: Role of
fibrinogen in cerebrovascular dysfunction after traumatic brain
injury. Brain Inj. 27:1508–1515. 2013.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Theusinger OM, Baulig W, Asmis LM, Seifert
B and Spahn DR: In vitro factor XIII supplementation increases clot
firmness in Rotation Thromboelastometry (ROTEM). Thromb Haemost.
104:385–391. 2010.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Gerlach R, Raabe A, Zimmermann M,
Siegemund A and Seifert V: Factor XIII deficiency and postoperative
hemorrhage after neurosurgical procedures. Surg Neurol. 54:260–264;
discussion 264-265. 2000.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Steiner T, Poli S, Griebe M, Hüsing J,
Hajda J, Freiberger A, Bendszus M, Bösel J, Christensen H, Dohmen
C, et al: Fresh frozen plasma versus prothrombin complex
concentrate in patients with intracranial haemorrhage related to
vitamin K antagonists (INCH): A randomised trial. Lancet Neurol.
15:566–573. 2016.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Pollack CV Jr, Reilly PA, Eikelboom J,
Glund S, Verhamme P, Bernstein RA, Dubiel R, Huisman MV, Hylek EM,
Kamphuisen PW, et al: Idarucizumab for dabigatran reversal. N Engl
J Med. 373:511–520. 2015.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Connolly SJ, Crowther M, Eikelboom JW,
Gibson CM, Curnutte JT, Lawrence JH, Yue P, Bronson MD, Lu G,
Conley PB, et al: Full study report of andexanet alfa for bleeding
associated with factor Xa Inhibitors. N Engl J Med. 380:1326–1335.
2019.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Frontera JA, Lewin JJ III, Rabinstein AA,
Aisiku IP, Alexandrov AW, Cook AM, Del Zoppo GJ, Kumar M, Peerschke
EI, Stiefel MF, et al: Guideline for reversal of antithrombotics in
intracranial hemorrhage: Executive summary. A statement for
healthcare professionals from the neurocritical care society and
the society of critical care medicine. Crit Care Med. 44:2251–2257.
2016.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Asahi M, Wang X, Mori T, Sumii T, Jung JC,
Moskowitz MA, Fini ME and Lo EH: Effects of matrix
metalloproteinase-9 gene knock-out on the proteolysis of
blood-brain barrier and white matter components after cerebral
ischemia. J Neurosci. 21:7724–7732. 2001.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Zhao BQ, Wang S, Kim HY, Storrie H, Rosen
BR, Mooney DJ, Wang X and Lo EH: Role of matrix metalloproteinases
in delayed cortical responses after stroke. Nat Med. 12:441–445.
2006.PubMed/NCBI View
Article : Google Scholar
|
|
102
|
Shukla M, Sekhon UD, Betapudi V, Li W,
Hickman DA, Pawlowski CL, Dyer MR, Neal MD, McCrae KR and Sen Gupta
A: In vitro characterization of SynthoPlate™ (synthetic platelet)
technology and its in vivo evaluation in severely thrombocytopenic
mice. J Thromb Haemost. 15:375–387. 2017.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Fitzpatrick GM, Cliff R and Tandon N:
Thrombosomes: A platelet-derived hemostatic agent for control of
noncompressible hemorrhage. Transfusion. 53 (Suppl 1):100S–106S.
2013.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Ahmed S, Venigalla H, Mekala HM, Dar S,
Hassan M and Ayub S: Traumatic brain injury and neuropsychiatric
complications. Indian J Psychol Med. 39:114–121. 2017.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Chen H, Yuan F, Chen SW, Guo Y, Wang G,
Deng ZF and Tian HL: Predicting posttraumatic hydrocephalus:
Derivation and validation of a risk scoring system based on
clinical characteristics. Metab Brain Dis. 32:1427–1435.
2017.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Wang XC, Gao SJ, Zhuo SL, Weng CL, Feng
HW, Lin J, Lin XS and Huang L: Predictive factors for
cerebrocardiac syndrome in patients with severe traumatic brain
injury: A retrospective cohort study. Front Neurol.
4(1192756)2023.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Campwala I, Guyette FX, Brown JB, Yazer
MH, Daley BJ, Miller RS, Harbrecht BG, Claridge JA, Phelan HA,
Eastridge B, et al: Evaluation of critical care burden following
traumatic injury from two randomized controlled trials. Sci Rep.
13(1106)2023.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Nicolai L and Massberg S: Platelets as key
players in inflammation and infection. Curr Opin Hematol. 27:34–40.
2020.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Nicolai L, Leunig A, Brambs S, Kaiser R,
Weinberger T, Weigand M, Muenchhoff M, Hellmuth JC, Ledderose S,
Schulz H, et al: Immunothrombotic Dysregulation in COVID-19
pneumonia is associated with respiratory failure and coagulopathy.
Circulation. 142:1176–1189. 2020.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Passacquale G, Vamadevan P, Pereira L,
Hamid C, Corrigall V and Ferro A: Monocyte-platelet interaction
induces a pro-inflammatory phenotype in circulating monocytes. PLoS
One. 6(e25595)2011.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Bozza FA, Shah AM, Weyrich AS and
Zimmerman GA: Amicus or adversary: Platelets in lung biology, acute
injury, and inflammation. Am J Respir Cell Mol Biol. 40:123–134.
2009.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Cole E, Davenport R, Willett K and Brohi
K: Tranexamic acid use in severely injured civilian patients and
the effects on outcomes: A prospective cohort study. Ann Surg.
261:390–394. 2015.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Einersen PM, Moore EE, Chapman MP, Moore
HB, Gonzalez E, Silliman CC, Banerjee A and Sauaia A: Rapid
thrombelastography thresholds for goal-directed resuscitation of
patients at risk for massive transfusion. J Trauma Acute Care Surg.
82:114–119. 2017.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Ekeh AP, Dominguez KM, Markert RJ and
McCarthy MC: Incidence and risk factors for deep venous thrombosis
after moderate and severe brain injury. J Trauma. 68:912–915.
2010.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Ivanov II, Apta BHR, Bonna AM and Harper
MT: Platelet P-selectin triggers rapid surface exposure of tissue
factor in monocytes. Sci Rep. 9(13397)2019.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Teichman AL, Cotton BA, Byrne J, Dhillon
NK, Berndtson AE, Price MA, Johns TJ, Ley EJ, Costantini T and Haut
ER: Approaches for optimizing venous thromboembolism prevention in
injured patients: Findings from the consensus conference to
implement optimal venous thromboembolism prophylaxis in trauma. J
Trauma Acute Care Surg. 94:469–478. 2023.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Knudson MM, Moore EE, Kornblith LZ, Shui
AM, Brakenridge S, Bruns BR, Cipolle MD, Costantini TW, Crookes BA,
Haut ER, et al: Challenging traditional paradigms in posttraumatic
pulmonary thromboembolism. JAMA Surg. 157(e216356)2022.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Stein SC, Chen XH, Sinson GP and Smith DH:
Intravascular coagulation: A major secondary insult in nonfatal
traumatic brain injury. J Neurosurg. 97:1373–1377. 2002.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Chelladurai Y, Stevens KA, Haut ER,
Brotman DJ, Sharma R, Shermock KM, Kebede S, Singh S and Segal JB:
Venous thromboembolism prophylaxis in patients with traumatic brain
injury: A systematic review. F1000Res. 2(132)2013.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Verhamme P, Yi BA, Segers A, Salter J,
Bloomfield D, Büller HR, Raskob GE and Weitz JI: ANT-005 TKA
Investigators. Abelacimab for prevention of venous thromboembolism.
N Engl J Med. 385:609–617. 2021.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Dübendorfer C, Billeter AT, Seifert B,
Keel M and Turina M: Serial lactate and admission SOFA scores in
trauma: An analysis of predictive value in 724 patients with and
without traumatic brain injury. Eur J Trauma Emerg Surg. 39:25–34.
2013.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Raj R, Siironen J, Kivisaari R,
Hernesniemi J and Skrifvars MB: Predicting outcome after traumatic
brain injury: Development of prognostic scores based on the IMPACT
and the APACHE II. J Neurotrauma. 31:1721–1732. 2014.PubMed/NCBI View Article : Google Scholar
|
|
123
|
MacLeod JB, Lynn M, McKenney MG, Cohn SM
and Murtha M: Early coagulopathy predicts mortality in trauma. J
Trauma. 55:39–44. 2003.PubMed/NCBI View Article : Google Scholar
|
|
124
|
McCully SP, Fabricant LJ, Kunio NR, Groat
TL, Watson KM, Differding JA, Deloughery TG and Schreiber MA: The
International Normalized Ratio overestimates coagulopathy in stable
trauma and surgical patients. J Trauma Acute Care Surg. 75:947–953.
2013.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Mann KG, Butenas S and Brummel K: The
dynamics of thrombin formation. Arterioscler Thromb Vasc Biol.
23:17–25. 2003.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Haas T, Fries D, Tanaka KA, Asmis L, Curry
NS and Schöchl H: Usefulness of standard plasma coagulation tests
in the management of perioperative coagulopathic bleeding: Is there
any evidence? Br J Anaesth. 114:217–224. 2015.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Schöchl H, Solomon C, Traintinger S,
Nienaber U, Tacacs-Tolnai A, Windhofer C, Bahrami S and Voelckel W:
Thromboelastometric (ROTEM) findings in patients suffering from
isolated severe traumatic brain injury. J Neurotrauma.
28:2033–2041. 2011.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Inaba K, Rizoli S, Veigas PV, Callum J,
Davenport R, Hess J and Maegele M: Viscoelastic Testing in Trauma
Consensus Panel. 2014 Consensus conference on viscoelastic
test-based transfusion guidelines for early trauma resuscitation:
Report of the panel. J Trauma Acute Care Surg. 78:1220–1229.
2015.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Connelly CR, Van PY, Hart KD, Louis SG,
Fair KA, Erickson AS, Rick EA, Simeon EC, Bulger EM, Arbabi S, et
al: Thrombelastography-Based dosing of enoxaparin for
thromboprophylaxis in trauma and surgical patients: A Randomized
clinical trial. JAMA Surg. 151(e162069)2016.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Chang R, Cardenas JC, Wade CE and Holcomb
JB: Advances in the understanding of trauma-induced coagulopathy.
Blood. 128:1043–1049. 2016.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Wikkelsø A, Wetterslev J, Møller AM and
Afshari A: Thromboelastography (TEG) or thromboelastometry (ROTEM)
to monitor haemostatic treatment versus usual care in adults or
children with bleeding. Cochrane Database Syst Rev.
2016(Cd007871)2016.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Rossaint R, Afshari A, Bouillon B, Cerny
V, Cimpoesu D, Curry N, Duranteau J, Filipescu D, Grottke O,
Grønlykke L, et al: The European guideline on management of major
bleeding and coagulopathy following trauma: Sixth edition. Crit
Care. 27(80)2023.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Woolley T, Gwyther R, Parmar K, Kirkman E,
Watts S, Midwinter M, Lucca JD and Hunt BJ: A prospective
observational study of acute traumatic coagulopathy in traumatic
bleeding from the battlefield. Transfusion. 60 (Suppl 3):S52–S61.
2020.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Dunbar NM and Chandler WL: Thrombin
generation in trauma patients. Transfusion. 49:2652–2660.
2009.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Davenport R, Manson J, De'Ath H, Platton
S, Coates A, Allard S, Hart D, Pearse R, Pasi KJ, MacCallum P, et
al: Functional definition and characterization of acute traumatic
coagulopathy. Crit Care Med. 39:2652–2658. 2011.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Steiner LA, Czosnyka M, Piechnik SK,
Smielewski P, Chatfield D, Menon DK and Pickard JD: Continuous
monitoring of cerebrovascular pressure reactivity allows
determination of optimal cerebral perfusion pressure in patients
with traumatic brain injury. Crit Care Med. 30:733–738.
2002.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Depreitere B, Güiza F, Van den Berghe G,
Schuhmann MU, Maier G, Piper I and Meyfroidt G: Pressure
autoregulation monitoring and cerebral perfusion pressure target
recommendation in patients with severe traumatic brain injury based
on minute-by-minute monitoring data. J Neurosurg. 120:1451–1457.
2014.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Chesnut RM, Marshall LF, Klauber MR, Blunt
BA, Baldwin N, Eisenberg HM, Jane JA, Marmarou A and Foulkes MA:
The role of secondary brain injury in determining outcome from
severe head injury. J Trauma. 34:216–222. 1993.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Bernard F, Barsan W, Diaz-Arrastia R,
Merck LH, Yeatts S and Shutter LA: Brain Oxygen Optimization in
Severe Traumatic Brain Injury (BOOST-3): A multicentre, randomised,
blinded-endpoint, comparative effectiveness study of brain tissue
oxygen and intracranial pressure monitoring versus intracranial
pressure alone. BMJ Open. 12(e060188)2022.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Okonkwo DO, Shutter LA, Moore C, Temkin
NR, Puccio AM, Madden CJ, Andaluz N, Chesnut RM, Bullock MR, Grant
GA, et al: Brain oxygen optimization in severe traumatic brain
injury phase-II: A phase II Randomized trial. Crit Care Med.
45:1907–1914. 2017.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Zeiler FA, Thelin EP, Helmy A, Czosnyka M,
Hutchinson PJA and Menon DK: A systematic review of cerebral
microdialysis and outcomes in TBI: Relationships to patient
functional outcome, neurophysiologic measures, and tissue outcome.
Acta Neurochir (Wien). 159:2245–2273. 2017.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Robinson MB, Shin P, Alunday R, Cole C,
Torbey MT and Carlson AP: Decision-making for decompressive
craniectomy in traumatic brain injury aided by multimodality
monitoring: Illustrative case. J Neurosurg Case Lessons.
1(CASE2197)2021.PubMed/NCBI View Article : Google Scholar
|