Introduction
Diaphragmatic eventration (DE) is a disorder
characterized by diaphragmatic weakness, which is manifested by the
abnormal elevation of the diaphragm due to muscle weakness and/or
phrenic nerve trauma or injury (1).
It most commonly occurs in children, particularly in infants; by
contrast, the onset of DE in adults is relatively rare. Complete DE
in adults is often an acquired condition and the symptoms vary
between patients. Adult DE mostly occurs secondary to thoracic
surgery or mechanical trauma, commonly due to phrenic nerve
dysfunction or injury and muscle atrophy (1,2). The
phrenic nerve provides motor and sensory innervation to the
diaphragm, and any damage to this nerve can result in weakened
diaphragmatic contractions, leading to elevation of the diaphragm
(3). Muscle atrophy refers to the
wasting away of muscle tissue, which can occur due to various
reasons, such as disuse, disease or aging. When the diaphragmatic
muscles undergo atrophy, they lose their strength and ability to
maintain the normal position of the diaphragm, causing it to rise
abnormally (4). In rare cases, DE
has been associated with previous West Nile and dengue viral
infections (5,6).
In the present study, the case of a patient with
congenital localized DE that progressed to complete DE after
infection with severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2) is described, thus supporting the hypothesis that this
virus could exacerbate DE progression. This finding suggested that
SARS-CoV-2 may have a systemic effect that could exacerbate the
symptoms and complications associated with DE. The systemic
inflammatory response triggered by the virus could lead to
additional muscle damage or nerve injury, worsening the condition
of patients with DE. The present case is a significant finding that
underscores the need for heightened awareness and vigilance
regarding the potential impact of viral infections on pre-existing
respiratory conditions.
Case report
A 74-year-old woman was admitted to The Affiliated
People's Hospital of Ningbo University (Ningbo, China) in February
2023 with a persistent cough, chest tightness and abdominal
distension. A total of 2 months prior to hospital admission, the
patient was infected with SARS-CoV-2, accompanied by a persistent
cough, particularly at night, which was sometimes unbearable.
During this period, the patient received dextromethorphan for cough
relief; however, the cough could not be relieved. A total of 1
month later, the patient developed chest tightness and abdominal
distension while laying down or after exertion. These symptoms were
only relieved by standing up. According to the medical history of
the patient, 1 year prior, during a routine physical examination at
a Ningbo Baihe Street Community Health Service Center (Ningbo,
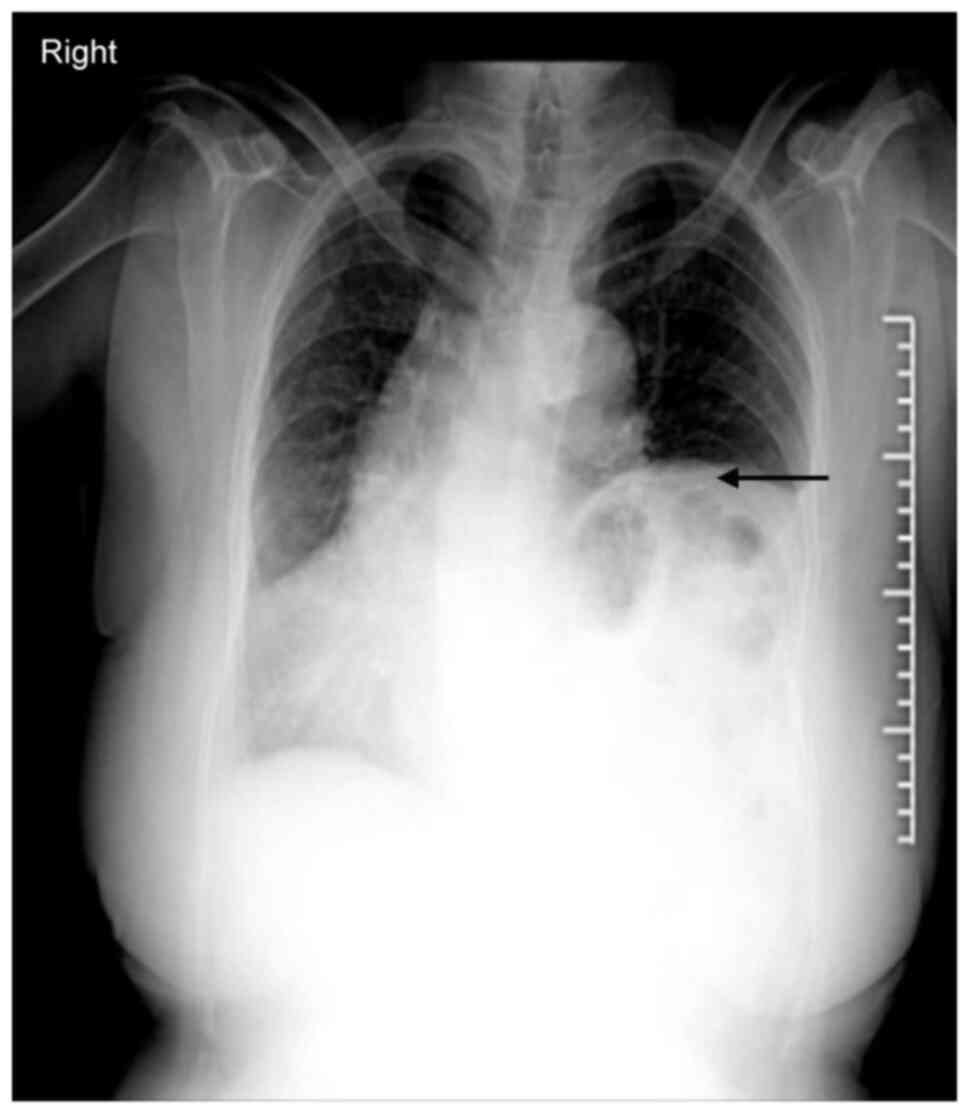
China), the patient underwent a chest radiograph that revealed a
left hemidiaphragmatic eventration extending up to the seventh
posterior rib (Fig. 1). Despite
this finding, the patient did not report any respiratory discomfort
and was ultimately diagnosed with localized DE. Notably, due to
lack of any symptoms or discomfort, the patient was not treated for
DE. The patient also denied any previous history of phrenic nerve
and diaphragm trauma.
Upon admission, the vitals for the patient were as
follows: While at rest, the body temperature was normal, 36.8˚C;
the systolic blood pressure was also normal, 120 mmHg; the heart
rate was 100 beats per minute, which was elevated for an adult; the
respiratory rate was 22 breaths per minute, approaching the upper
limit of the normal range; and thee oxygen saturation when
breathing ambient air was 92%, which was slightly low. The
respiratory dynamics of the left lung were weak, with absent
respiratory sounds of the left lower lung and low respiratory
sounds in the left upper lung. Bowel sounds were auscultated in the
left hemithorax. There was no obvious abnormality in the right
lung. The abdomen was flat and soft, with no palpable abdominal
masses. The blood gas analysis results were as follows: pH, 7.42
(normal range: 7.350-7.450); PaCO2, 43 mmHg (normal
range: 35.0-45.0 mmHg); PaO2, 65 mmHg (normal range:
80-100 mmHg); HCO3-, 22.2 mmol/l (normal
range: 21-27 mmol/l); and base excess, -3 mmol/l (normal range:
-3-3 mmol/l). Blood testing procedures revealed no abnormalities in
the complete blood count, and levels of inflammatory proteins
(C-reactive protein) and tumor markers [cancer antigen (CA)72-4,
neuron-specific enolase, α-fetoprotein, carcinoembryonic antigen,
CA19-9, CA125 and squamous cell carcinoma antigen]. The pulmonary
function test revealed moderate restrictive ventilation
dysfunction; however, the bronchodilator reversibility test was
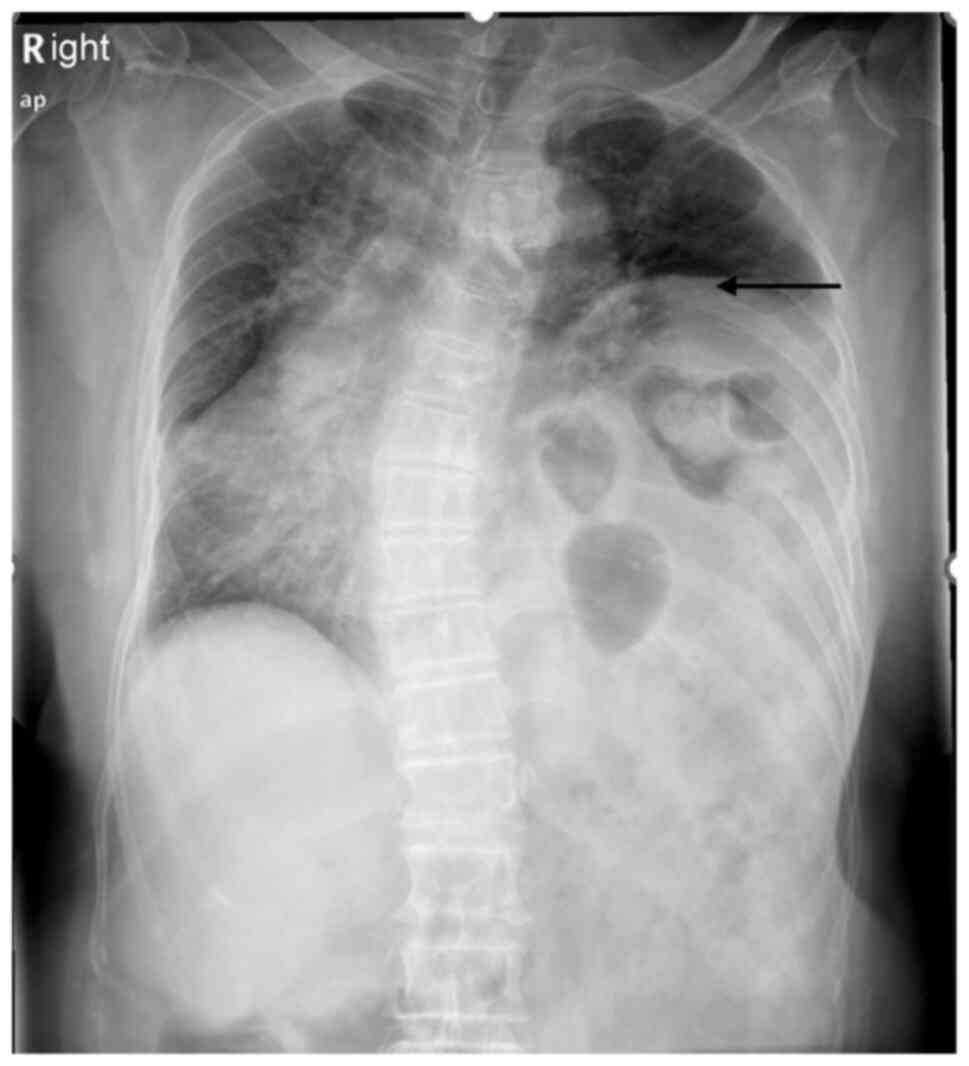
negative. A chest X-ray showed notable elevation of the left
diaphragm, extending up to the fourth posterior rib, compared with
the previous examinations (Fig. 2).
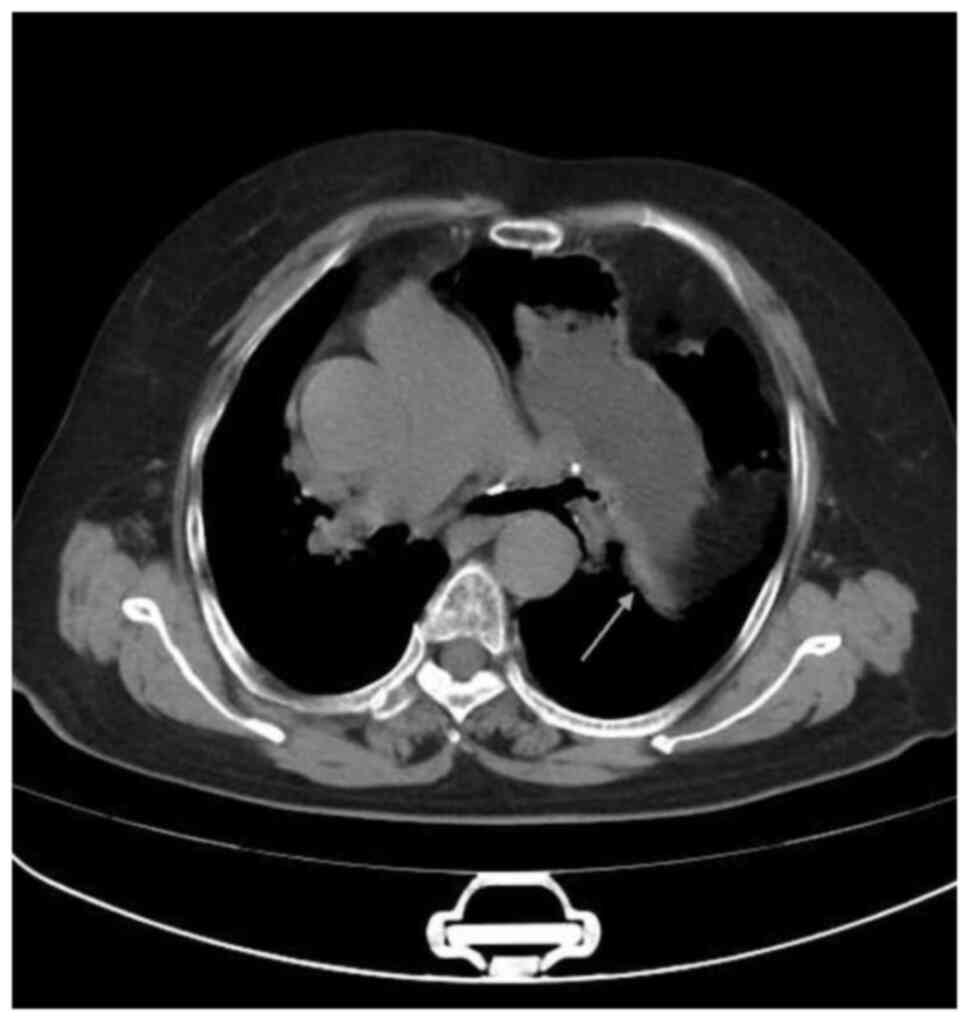
Furthermore, the chest computed tomography (CT) results revealed
deviation of the trachea to the right, significant swelling of the
left diaphragm, displacement of the abdominal contents upwards,
compression of the left lung tissue and displacement of the
mediastinum to the right; however, disruption of diaphragmatic
continuity was not detected (Fig.
3). The patient was diagnosed with left-sided complete DE and
underwent a thoracic surgery that lasted ~90 min under general
anesthesia. Anesthetics administered included intravenous propofol
(induction dose, 70 mg), intravenous cisatracurium besylate
(induction dose, 10 mg) and intravenous citric acid sufentanil
(induction dose, 20 µg). For intraoperative maintenance, the
patient was administered propofol injected at a rate of 6 mg/min,
remifentanil hydrochloride injected at a rate of 5 µg/min, and
rocuronium bromide injected at a rate of 0.24 mg/min, all delivered
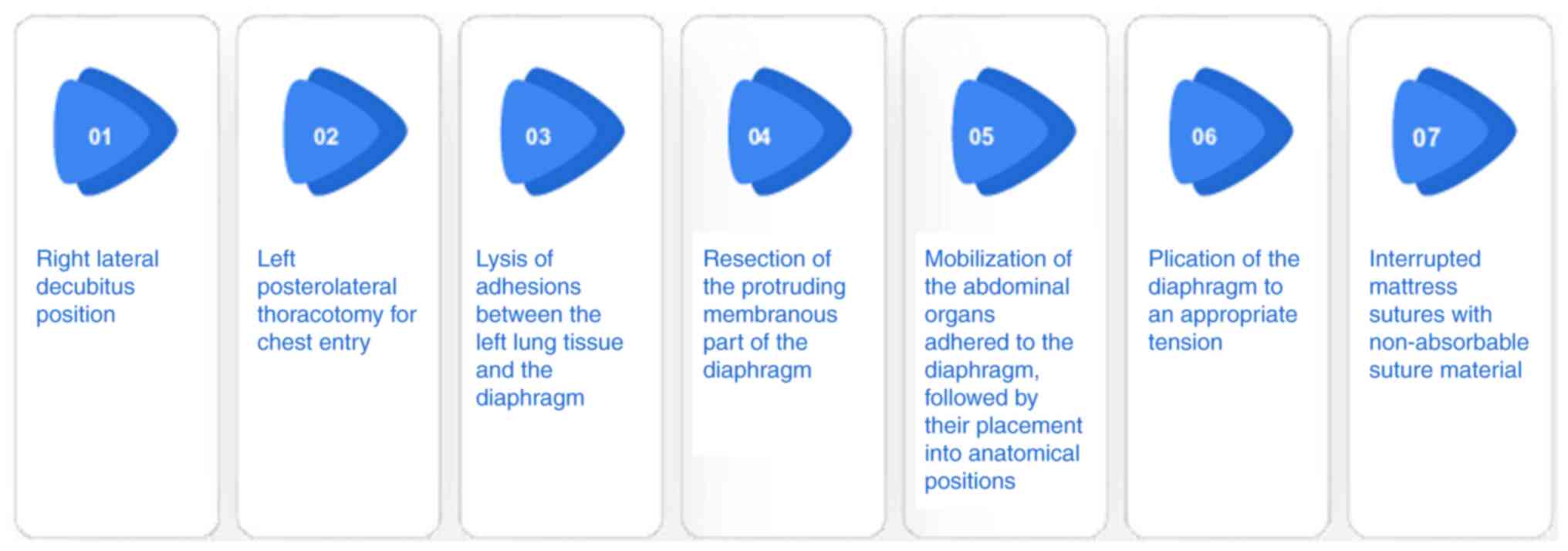
via micro-pumps. During the surgery, the left diaphragm was noted
to be significantly elevated into the left hemithorax. The
adhesions between the left lung tissue and the diaphragm were
lysed, and the abdominal organs protruding into the thoracic cavity
were placed to their anatomical positions. Subsequently, plication
of the protruding diaphragmatic tissue was performed to an
appropriate tension and secured with interrupted mattress sutures.
The schematic diagram of the surgical procedures is shown in
Fig. 4. The condition of the
patient remained stable after surgery and they were discharged
after 7 days. At the 6-month follow-up visit, the patient did not
report any significant discomfort.
Discussion
DE refers to the elevation or upward protrusion of
one or both sides of the diaphragm due to positive intra-abdominal
pressure and negative intra-thoracic pressure. This could be caused
by an underdeveloped diaphragm, phrenic nerve trauma, dysfunction,
or paralysis and muscle fiber atrophy, and is accompanied by the
protrusion of the abdominal organs into the thoracic cavity
(7,8). DE is categorized as congenital or
acquired based on the etiology, and complete or localized based on
its anatomical features. Congenital DE is caused by weakness of the
diaphragm because of incomplete muscularization or
non-muscularization of the thoracic and abdominal membranes during
the embryonic period, and is more common on the left side. Acquired
DE is caused by phrenic nerve paralysis because of surgery- or
trauma-induced phrenic nerve damage (9). Complete DE is characterized by
symptoms of the respiratory, digestive and circulatory systems
caused by compression of the mediastinum by protruding abdominal
organs, whereas localized DE can be asymptomatic (10). The diagnostic tests conducted in the
present case included a chest X-ray and a chest CT scan, both of
which are standard procedures for diagnosing DE (11). In a typical respiratory cycle, upon
inspiration, the dome of the diaphragm is typically situated at the
level of the tenth posterior rib or the sixth anterior rib. The
right hemidiaphragm is typically elevated 1-2 cm above the left
hemidiaphragm, which can be attributed to the underlying liver
anatomy on the right side. When the affected side of the diaphragm
is noticeably higher than normal in the chest X-ray, the patient
can be diagnosed with DE. The incidence of DE is ~1:10,000 in
adults in Europe (11), and the
ratio of left/right DE is 8-9:1 in Europe based on chest X-ray
examination (11).
There is a well-documented association between
congenital DE and some viruses. Becroft (12) described a case of DE caused by
prenatal cytomegalovirus infection. In addition, Mitsiakos et
al (13) reported on a case of
congenital DE in a preterm female neonate that was associated with
parvovirus B19. Furthermore, congenital DE has been associated with
fetal rubella (14). Idiopathic
phrenic nerve paralysis and DE in adults can be caused by
subclinical viral infection. Previous studies have demonstrated
that SARS-CoV-2 infection may lead to phrenic paralysis (15-17).
Additionally, four reports have described the association between
DE and SARS-CoV-2 infection (15,16,18,19),
as follows: All individuals were male, with three cases of
right-sided DE and one case of left-sided DE. All the four patients
had a history of hypertension, three had a history of diabetes
mellitus, two had a history of obesity and one patient had
undergone pelvic surgery. Following SARS-CoV-2 infection, all
patients exhibited cough and dyspnea and were diagnosed with DE
through chest X-ray and CT. Two patients underwent endotracheal
intubation and mechanical ventilation and three underwent surgical
treatment of the diaphragm; finally, one patient died, and three
recovered well (15,16,18,19).
The aforementioned studies suggested that SARS-CoV-2 could possibly
exacerbate DE. The patient in the present study was found to have a
protrusion of the left diaphragm to the seventh posterior rib
during a chest X-ray examination 1 year prior to hospital
admission. At that time, the patient had no respiratory discomfort,
no history of diaphragmatic trauma, and no previous history of
trauma or surgery, suggesting a congenital condition. The diagnosis
was left-sided congenital localized DE. After contracting
SARS-CoV-2, the patient suffered from a persistent and severe
cough, chest tightness and abdominal distension, which was
especially noticeable after lying down or engaging in activity. A
subsequent chest X-ray revealed that the left diaphragm had further
extended up to the fourth posterior rib. Chest CT scan results
excluded diaphragmatic hernia, leading to the diagnosis of left
complete DE. Compared with previous similar cases, several
distinctive features are noted in the present study: i) The patient
was an elderly woman; ii) the patient had no prior history of
diabetes, and had not experienced prolonged intubation or
mechanical ventilation, which are factors that could potentially
impair diaphragmatic function; and iii) a unique progression from
localized to complete DE was observed following SARS-CoV-2
infection, which has not been previously reported, to the best of
our knowledge.
It has been reported that SARS-CoV-2 infection
exerts multifaceted effects on the neuromuscular and
musculoskeletal systems (20), and
it can also trigger inflammatory myopathies, including
rhabdomyolysis, a serious condition where muscle tissue breaks down
(21). In a previous retrospective
study, chest X-ray examination showed diaphragmatic swelling in
several patients after SARS-CoV-2 infection (22). In children, SARS-CoV-2 infection can
also lead to the development of Grisel's Syndrome (23), thus indicating that coronavirus
disease 2019 (COVID-19) can have significant and varied impacts on
the musculoskeletal system in children.
Cough is one of the most common symptoms of
SARS-CoV-2 infection, and can last for weeks or months after the
infection (24). The persistent
cough in long COVID-19 is a multifaceted symptom arising from
respiratory tract injury, immune system overactivity, neurological
effects and post-viral immune responses. It is further complicated
by several other factors, such as mucus disruption,
angiotensin-converting enzyme 2 receptor binding, airway
hyperresponsiveness, viral persistence, autoimmune reactions, and
in severe cases, pulmonary fibrosis (25-27).
Persistent severe cough can lead to diaphragm damage, rupture or
diaphragmatic hernia (24,28,29).
The forceful and repetitive contractions of the diaphragm during
coughing could cause strain on the muscle fibers, which over time,
could result in microscopic tears or more significant injuries
(29). It was hypothesized that the
SARS-CoV-2 infection in the present case may have directly damaged
the phrenic nerve and the diaphragm. Subsequently, persistent and
severe cough after SARS-CoV-2 infection could cause further damage
to the weakened diaphragm on the left side due to spasming. At the
same time, the pressure difference between the chest and the
abdominal cavity of the patient may have changed due to the
continuous cough and further aggravated the swelling in the left
diaphragm. The abdominal organs thus moved further upward and
compressed the left lung tissue and the mediastinum, thereby
resulting in chest tightness and other symptoms.
To the best of our knowledge, the progression from
localized to complete DE following SARS-CoV-2 infection has not
been previously reported, highlighting a potentially new and
significant impact of COVID-19 on the diaphragm. This case suggests
that the SARS-CoV-2 virus may have a direct or indirect effect on
the diaphragmatic muscle or its innervation, exacerbating the
condition. This observation warrants further investigation to
understand the mechanisms underlying this phenomenon and to assess
the prevalence of such complications in patients with pre-existing
diaphragmatic conditions who contract COVID-19. When comparing this
case with similar previous cases, it is essential to consider the
unique progression to complete DE following COVID-19 infection.
This progression provides insights into the potential mechanisms of
the effects of SARS-CoV-2 on the diaphragm, and may aid in
identifying risk factors and developing strategies for monitoring
and managing such patients. It is also crucial to examine the
clinical presentation, diagnostic approaches and treatment
strategies in these cases to better understand the full spectrum of
COVID-19-related musculoskeletal complications. It is important for
clinicians to be aware of these potential complications when
treating patients with persistent cough, especially in the context
of post-COVID-19 recovery. Appropriate imaging examinations, such
as chest X-rays or CT scans, can identify diaphragmatic injuries.
Treatment could involve conservative management with rest, pain
control and physical therapy to strengthen the diaphragm, or in
more severe cases, surgical intervention could be necessary to
repair the diaphragm. In the present case report, the patient was
diagnosed with complete DE on the left side. Therefore, treatment
with left-sided diaphragmatic plication significantly improved the
postoperative symptoms of the patient.
In conclusion, the current case report illuminated
how SARS-CoV-2 infection could intensify some chronic and
congenital conditions, such as DE, thus leading to exacerbated
symptoms. While DE can be aggravated by several factors, such as
thoracic surgery or mechanical trauma, its association with
COVID-19 is a recent and uncommon observation. This association
underscores the necessity for timely medical diagnosis and
treatment to address the intensified symptoms. The present case
report also highlighted the significance of clinical vigilance in
monitoring patients with underlying conditions for potential
COVID-19-related complications, even those not typically associated
with the disease.
Acknowledgements
Not applicable.
Funding
Funding: This work was funded by the Zhejiang Provincial
Education Scientific Research Project (grant no. Y202044078). The
funding body had no role in the study design, collection, analysis,
interpretation of data, and the preparation of the manuscript.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TX wrote the original draft and WJY reviewed and
edited the manuscript. Both authors contributed to the study
conception and design. WJY and TX confirm the authenticity of all
the raw data. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
individual, for the publication of any potentially identifiable
images or data included in this article.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pradhan P, Karmacharya RM, Vaidya S, Singh
AK, Thapa P, Dhakal P, Dahal S, Bade S and Bhandari N: Case report
of eventration of diaphragm due to an unknown febrile illness
causing phrenic nerve palsy and other multiple nerve palsies. Ann
Med Surg (Lond). 54:74–78. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhao S, Pan Z, Li Y, An Y, Zhao L, Jin X,
Fu J and Wu C: Surgical treatment of 125 cases of congenital
diaphragmatic eventration in a single institution. BMC Surg.
20(270)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vivier E, Roussey A, Doroszewski F,
Rosselli S, Pommier C, Carteaux G and Mekontso Dessap A: Atrophy of
diaphragm and pectoral muscles in critically Ill patients.
Anesthesiology. 131:569–579. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Soták M, Roubík K, Henlín T and Tyll T:
Phrenic nerve stimulation prevents diaphragm atrophy in patients
with respiratory failure on mechanical ventilation. BMC Pulm Med.
21(314)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kokatnur L and Rudrappa M: Diaphragmatic
palsy. Diseases. 6(16)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kapoor K, Jain S, Jajoo M and Talukdar B:
A rare neurological complication of typhoid fever: Guillain-Barre'
syndrome. J Pediatr Neurosci. 9:148–149. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cai Y, Wu Y, Wu Z, Liu X and Pan W:
Comparative study of thoracoscopic and modified small incision
repair for congenital diaphragmatic eventration in children. J
Laparoendosc Adv Surg Tech A. 31:1079–1083. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Groth SS and Andrade RS: Diaphragm
plication for eventration or paralysis: A review of the literature.
Ann Thorac Surg. 89:S2146–S2150. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
McLean TR: Phrenic nerve injury. Chest.
105(1618)1994.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dres M, Goligher EC, Dubé BP, Morawiec E,
Dangers L, Reuter D, Mayaux J, Similowski T and Demoule A:
Diaphragm function and weaning from mechanical ventilation: An
ultrasound and phrenic nerve stimulation clinical study. Ann
Intensive Care. 8(53)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rodgers BM and Hawks P: Bilateral
congenital eventration of the diaphragms: Successful surgical
management. J Pediatr Surg. 21:858–864. 1986.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Becroft DM: Prenatal cytomegalovirus
infection and muscular deficiency (eventration) of the diaphragm. J
Pediatr. 94:74–75. 1979.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mitsiakos G, Gavras C, Katsaras GN,
Chatziioannidis I, Mouravas V, Mitsiakou C, Lampropoulos V and
Nikolaidis N: Parvovirus B19 intrauterine infection and eventration
of the diaphragm. Prague Med Rep. 123:48–55. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Barakat NA, Maaty SH and Al-Koly A:
Outcome of congenital diaphragmatic defects: 3 Years experience.
Int J Acad Res. 2:183–187. 2010.
|
|
15
|
Lowenkamp MN, Vercauteren M, Levesque RL
and Dhupar R: Unilateral diaphragm paralysis following COVID-19
infection: A case report. Ann Intern Med Clin Cases.
2(e221180)2023.
|
|
16
|
FitzMaurice TS, McCann C, Walshaw M and
Greenwood J: Unilateral diaphragm paralysis with COVID-19
infection. BMJ Case Rep. 14(e243115)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Maurier F, Godbert B and Perrin J:
Respiratory distress in SARS-CoV-2 without lung damage: Phrenic
paralysis should be considered in COVID-19 infection. Eur J Case
Rep Intern Med. 7(001728)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Baby BP and Mittal N: Right hemi
diaphragmatic eventration-A rare post SARS-COV-2 infection
complication. Lung India. 40:462–464. 2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Adekanmi AJ, Baiyewu LA, Osobu BE and
Atalabi OM: Where COVID-19 testing is challenging: A case series
highlighting the role of thoracic imaging in resolving management
dilemma posed by unusual presentation. Pan Afr Med J.
37(284)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hasan LK, Deadwiler B, Haratian A, Bolia
IK, Weber AE and Petrigliano FA: Effects of COVID-19 on the
musculoskeletal system: Clinician's guide. Orthop Res Rev.
13:141–150. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wu MJ and Sun YT: The impact of SARS-CoV-2
on neuromuscular disorders. Acta Neurol Taiwan. 32:88–99.
2023.PubMed/NCBI
|
|
22
|
Law SM, Scott K, Alkarn A, Mahjoub A,
Mallik AK, Roditi G and Choo-Kang B: COVID-19 associated phrenic
nerve mononeuritis: A case series. Thorax. 77:834–838.
2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hashimoto K, Nishimura S, Shinyashiki Y
and Goto K: Grisel's syndrome after COVID-19 in a pediatric
patient: A case report. Cureus. 16(e62028)2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Song WJ, Hui CKM, Hull JH, Birring SS,
McGarvey L, Mazzone SB and Chung KF: Confronting
COVID-19-associated cough and the post-COVID syndrome: role of
viral neurotropism, neuroinflammation, and neuroimmune responses.
Lancet Respir Med. 9:533–544. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wan D, Du T, Hong W, Chen L, Que H, Lu S
and Peng X: Neurological complications and infection mechanism of
SARS-COV-2. Signal Transduct Target Ther. 6(406)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Payus AO, Jeffree MS, Ohn MH, Tan HJ,
Ibrahim A, Chia YK and Raymond AA: Immune-mediated neurological
syndrome in SARS-CoV-2 infection: A review of literature on
autoimmune encephalitis in COVID-19. Neurol Sci. 43:1533–1547.
2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Davis HE, McCorkell L, Vogel JM and Topol
EJ: Long COVID: Major findings, mechanisms and recommendations. Nat
Rev Microbiol. 21:133–146. 2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cloche E, Dessertenne G, Callahan JC,
Pinquie F and Barbieux J: Diaphragmatic rupture and right
ipsilateral intercostal hernia in chronic cough. Rev Mal Respir.
39:561–565. 2022.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
29
|
Farinacci-Vilaró M, Gerena-Montano L,
Nieves-Figueroa H, Garcia-Puebla J, Fernández R, Hernández R,
Fernández R, González M and Quintana C: Chronic cough causing
unexpected diaphragmatic hernia and chest wall rupture. Radiol Case
Rep. 15:15–18. 2019.PubMed/NCBI View Article : Google Scholar
|