Introduction
Pancreatic cancer is the fourth leading cause of
cancer-related death in Japan in 2022 and the third leading cause
of cancer-related death in the United States in 2021 (1,2).
Patients with pancreatic cancer have a high mortality rate, with a
5-year relative survival rate of <8% in Japan in 2022(1). Standard chemotherapy regimens for
pancreatic cancer include tegafur, gimeracil and oteracil
potassium, a combination of folinic acid, fluorouracil, irinotecan
and oxaliplatin (3) and gemcitabine
plus nab-paclitaxel (4). However,
the anti-tumor effects of these treatment regimens are weaker than
for other types of solid tumor, such as colon, stomach, lung and
breast tumors. One of the reasons for this disparity is the
inefficient drug delivery to pancreatic tumors, reflecting both the
low blood flow around the pancreatic tumor and the inherent low
vascularity of the pancreas (5,6). In
addition, tumor-associated stroma forms a barrier to drug delivery
from the blood vessels to the tumor (5,6).
Nitric oxide (NO) has broad effects on the
development, progression and metastasis of cancer (7). Over the past 20 years, a number of
studies have reported the cell death-inducing effects of nitro
compounds, showing that these effects are mediated through
intracellular signaling pathways such as Ras, extracellular
signal-regulated kinase and mechanistic target of rapamycin
(8,9). Modification of aspirin, a
non-steroidal anti-inflammatory drug, with a nitro group has been
shown to significantly enhance the induction of apoptosis in human
pancreatic, colon, prostate, lung and tongue cancer cells (9). Similarly, the addition of a nitro
group to doxorubicin weakens the activity of mitochondrial-related
ABC transporters, which leads to decreased drug resistance to
doxorubicin and increased cytotoxicity (10). A previous study by Islam et
al (11) reported the improved
efficacy of anticancer agents when they were combined with
nitroglycerin treatment. Ishima et al (12) also reported that exposure to NO
depletes stroma, which leads to enhanced extravasation and
retention (EPR) effects. These findings showed that nitro compounds
not only enhance the therapeutic effects of other anticancer
agents, but also have therapeutic effects of their own. However,
most NO donors exhibit low blood retention and low tumor
accumulation, which may be the reason for their lack of therapeutic
efficacy (11).
Human serum albumin (HSA) is a type of drug-binding
protein in the blood that controls tissue migration and the blood
retention of bound drugs (13).
Insulin and glucagon-like peptide 1 preparations that bind to HSA
maintain their pharmacological effects by improving their retention
in the blood (14-17).
Therefore, it could be hypothesized that NO donors with a high
affinity to HSA not only improve retention in the blood, but
efficiently reach tumors due to the EPR effect, and the presence of
HSA receptors on the tumor cell surface (SPARC and gp60) promotes
the transfer of HSA-bound NO donors to tumors (18). Ibuprofen (IB), a non-steroidal
anti-inflammatory drug, binds to HSA, and its binding with HSA has
been previously reported (19). In
the present study, two nitrated forms of ibuprofen were synthesized
and their antitumor effects and mechanisms of action were
investigated.
Materials and methods
Cell culture and reagents
The human pancreatic cancer cell line, BxPC3, was
obtained from the American Type Culture Collection. The cells were
cultured in RPMI1640 (FUJIFILM Wako Pure Chemical Corporation),
supplemented with 10% heat-inactivated fetal calf serum (Capricorn
Scientific), penicillin (100 U/ml) and streptomycin (100 µg/ml)
(FUJIFILM Wako Pure Chemical Corporation) and incubated at 37˚C in
95% humidified air with 5% CO2. Ibuprofen (IB) and
warfarin were purchased from FUJIFILM Wako Pure Chemical
Corporation. Dansylsarcosine (DNSS) was purchased from
Sigma-Aldrich (Merck KGaA). All other reagents were of analytical
grade.
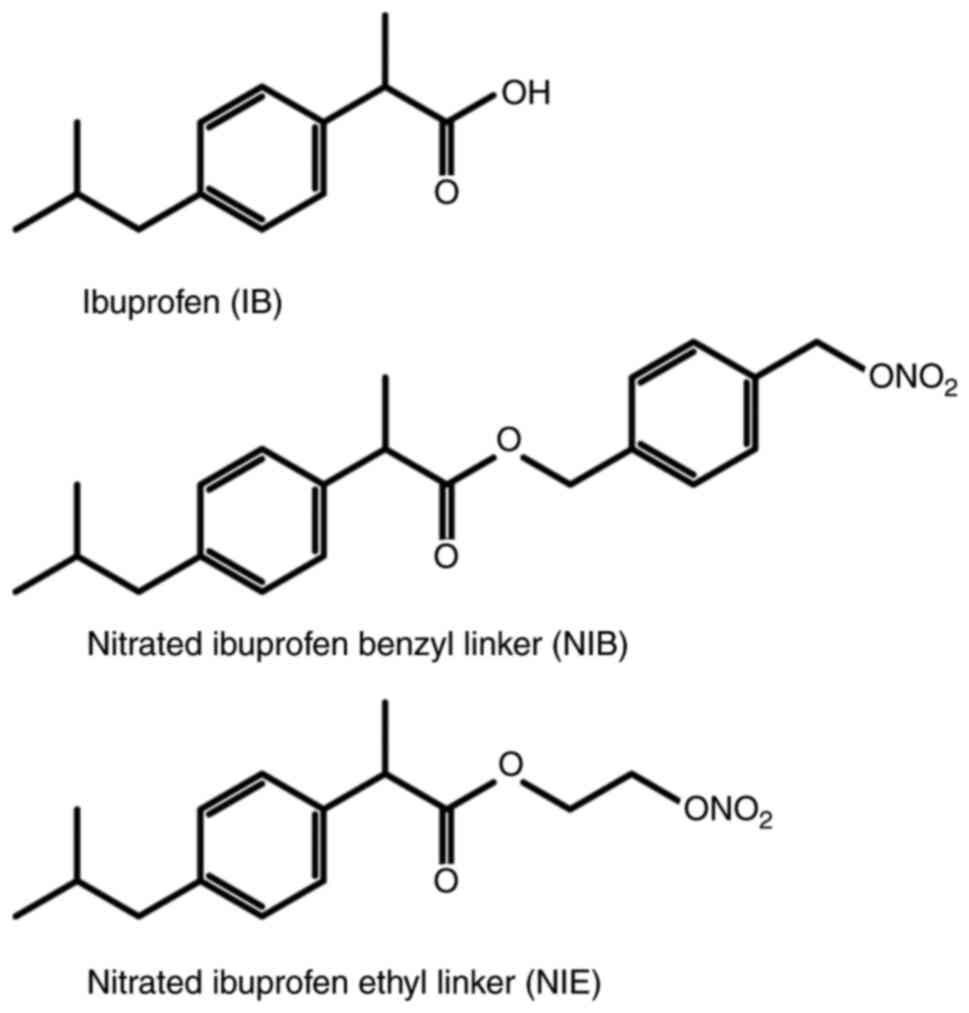
Synthesis of
4-[(nitrooxy)methyl]benzyl 2-(4-isobutylphenyl)propanoate [nitrated
ibuprofen benzyl linker (NIB)] and 2-(nitrooxy)ethyl
2-(4-isobutylphenyl)propanoate [nitrated ibuprofen ethyl linker
(NIE)]
A mixture of 4-(chloromethyl)benzyl alcohol (1.2 g;
7.66 mmol) and AgNO3 (5.2 g; 30.6 mmol) in
CH3CN (12 ml) was stirred at 40˚C for 16 h. After
cooling to room temperature, the white precipitate was removed by
filtration and the filtrate was evaporated. The residue was
dissolved in CH2Cl2, the resulting white
precipitate was removed again by filtration and the filtrate was
evaporated. The resulting residue of 4-(hydroxymethyl)benzyl
nitrate (7.66 mmol) was dissolved in CH2Cl2
(35 ml), and ibuprofen (790 mg; 3.83 mmol),
dicyclohexylcarbodiimide (1.2 g; 5.75 mmol) and
4-dimethylaminopyridne (46 mg; 0.38 mmol) were added to the
aforementioned solution and stirred at room temperature for 17 h.
After dilution with 50 ml of CH2Cl2, the
mixture was washed with saturated aqueous NaHCO3 and
brine (saturated NaCl aqueous solution), the residual water was
removed using MgSO4 and subsequently evaporated. The
residue was purified by flash column chromatography using silica
gel (hexane/ethyl acetate=8/1 v/v) to give NIB (1.08 g; 76%). NIE
was synthesized according to a previous report (20) (Fig.
1). 1H and 13C NMR spectra were recorded
on a JEOL ECA 500 spectrometer operating at room temperature.
Chemical shifts were reported in parts per million (δ) relative to
the residual solvent peak. Multiplicities were described as singlet
(s), doublet (d), doublet of doublets (dd), triplet (t) or
multiplet (m). Coupling constants (J) were reported in hertz
(Hz).
Binding of NIB and NIE to HSA
To obtain the binding parameters of IB to HSA, the
ultrafiltration method was used. Ultrafiltration was performed
using Amicon Ultra-0.5 ml Centrifugal Filters (10 kDa cutoff; 500
µl) from Merck KGaA. After centrifugation at 5,000 x g for 30 min
at 25˚C, the concentration of IB in 50 µl of filtrate, also known
as the concentration of unbound drug to HSA, was measured by HPLC.
The HPLC system used in the present study was a Jasco model
LC-2000Plus HPLC system (JASCO Corporation). YMC-PACK ODS AM-303 (5
µm particle size; 250x5 mm internal diameter; YMC Co. Ltd.) was
used as the stationary phase and was maintained at 40˚C. A total of
two solvents, solvent A (50 mM sodium dihydrogen phosphate) and
solvent B [50 mM sodium dihydrogen phosphate and acetonitrile
(30:70, v/v)] were used as mobile phases at a flow rate of 1
ml/min. Binding parameters were obtained from the Scatchard plot
using the following equation: r/[Df]=-r x Ka
+ n x Ka. Ka, n, [Df] and r were
the binding affinity, the number of binding sites of IB, the
concentration of unbound IB to HSA and the number of moles of IB
bound per mole of HSA, respectively.
Fluorescent probe displacement
Warfarin (WF) and dansylsarcosine (DNSS) were used
as site I and site II fluorescent probes on HSA, respectively
(21). Fluorescence spectra of
probes were measured using a Hitachi F-2500 fluorescence
spectrophotometer at 25˚C (Hitachi, Ltd.). The concentrations of
WF, DNSS and HSA used were 10 µM. The excitation wavelengths used
for WF and DNSS were 320 and 350 nm, respectively.
Evaluation of NO-releasing
properties
NO production in aqueous solution was evaluated by
measuring nitrite (NO2-) and nitrate
(NO3-), the stable end-products of NO
breakdown, using the NO2/NO3 Assay Kit-C II
(Dojindo Laboratories, Inc.). Briefly, NO2-
and NO3- (NOx) were measured according to the
manufacturer's protocol at 1, 2, 3, 24 and 48 h after dissolving
100 µM of NIB or NIE in PBS. For the detection of NO inside cells,
cells were seeded in a 6-well culture plate at 5x105
cells/well and cultured overnight at 37˚C. The medium was replaced
with PBS containing diaminofluorescein-FM diacetate (DAF-FM DA; 10
µM; Goryo Chemical, Inc.) and cultured at 37˚C for 1 h. After
culturing, cells were washed three times with PBS, treated with NIB
or NIE (200 µM) for 5 min at room temperature and imaged using a
fluorescence microscope.
Annexin V and dead cell assay
Live and apoptotic cell numbers were determined
using the Muse® Annexin V and Dead Cell kit (Cytek
Biosciences) according to the manufacturer's instructions. Briefly,
cells were seeded at a density of 2x105 cells/well in
6-well plates. After 12 h, 50, 100 or 200 µM of NIE or NIB was
added to each well and the plates were incubated for 6, 12 and 24 h
at 37˚C. The cells were then washed twice with PBS, trypsinized
(FUJIFILM Wako Pure Chemical Corporation) at 37˚C until cells were
detached and mixed well with the Muse® Annexin V and
Dead Cell Assay kit reagents. Samples were measured using a
Muse® Cell Analyzer (MuseSoft; Version 1.5.0.0; Cytek
Biosciences).
Lactose dehydrogenase (LDH) assay
LDH is a cytoplasmic enzyme that it is released into
the cell culture medium when the cell membrane is damaged. As the
released LDH is stable in cell culture medium, it can be used as an
indicator of the number of dead cells or cells with damaged cell
membranes (8). Cell toxicity was
evaluated using the Cytotoxicity LDH Assay Kit-WST (Dojindo
Laboratories, Inc.) according to the manufacturer's protocol.
Briefly, cells were seeded at a density of 3x103
cells/well in 96-well white, flat-bottom plates. After overnight
incubation at 37˚C, the cells were incubated with NIB (200 µM) or
NIE (200 µM) at 37˚C for 72 h. Subsequently, the cells were
incubated for 1 h at room temperature with the LDH reagent and
luminescence was measured. Cytotoxicity (%) was estimated using the
ratio between the concentration of LDH in the culture medium and
the whole cell.
Caspase 3/7 activity assay
Cellular caspase 3/7 activity was measured using the
Caspase-Glo 3/7 Reagent (Promega Corporation). Briefly, cells were
seeded at a density of 1x104 cells/well in 96-well
plates. After incubation with NIB (200 µM), NIE (200 µM) or
staurosporine (1 µM) for 6 h at 37˚C, Caspase-Glo 3/7 Reagent was
added to each well. After incubation at room temperature for 1 h,
the luminescence of each sample was measured using a plate-reading
luminometer.
Statistical analysis
The mean values of each group were compared using a
one-way ANOVA followed by Tukey's multiple comparison test.
P<0.05 was considered to indicate a statistically significant
difference. Assays were conducted in triplicate.
Results
Synthesis of NIB and NIE
NIB and NIE were successfully synthesized. The
chemical structure of NIB was confirmed by NMR (Fig. S1). 1H-NMR results were
as follows: (500 MHz, CHLOROFORM-D) δ 7.32 (d, J=8.0 Hz,
2H), 7.24 (d, J=8.0 Hz, 2H), 7.19 (d, J=8.0 Hz, 2H),
7.09 (d, J=8.0 Hz, 2H), 5.40 (s, 2H), 5.11 (s, 2H), 3.76 (q,
J=7.3 Hz, 1H), 2.46 (d, J=7.4 Hz, 2H), 1.88-1.83 (m,
1H), 1.51 (d, J=7.4 Hz, 3H) and 0.91 (d, J=6.9 Hz,
6H). 13C-NMR results were as follows: (126 MHz,
CHLOROFORM-D) δ 174.6, 140.8, 137.7, 137.6, 132.1, 129.5, 129.3,
128.2, 127.3, 74.5, 65.8, 45.3, 45.1, 30.3, 22.5 and 18.5.
Binding of NIB and NIE to HSA
To evaluate the binding properties of NIB and NIE to
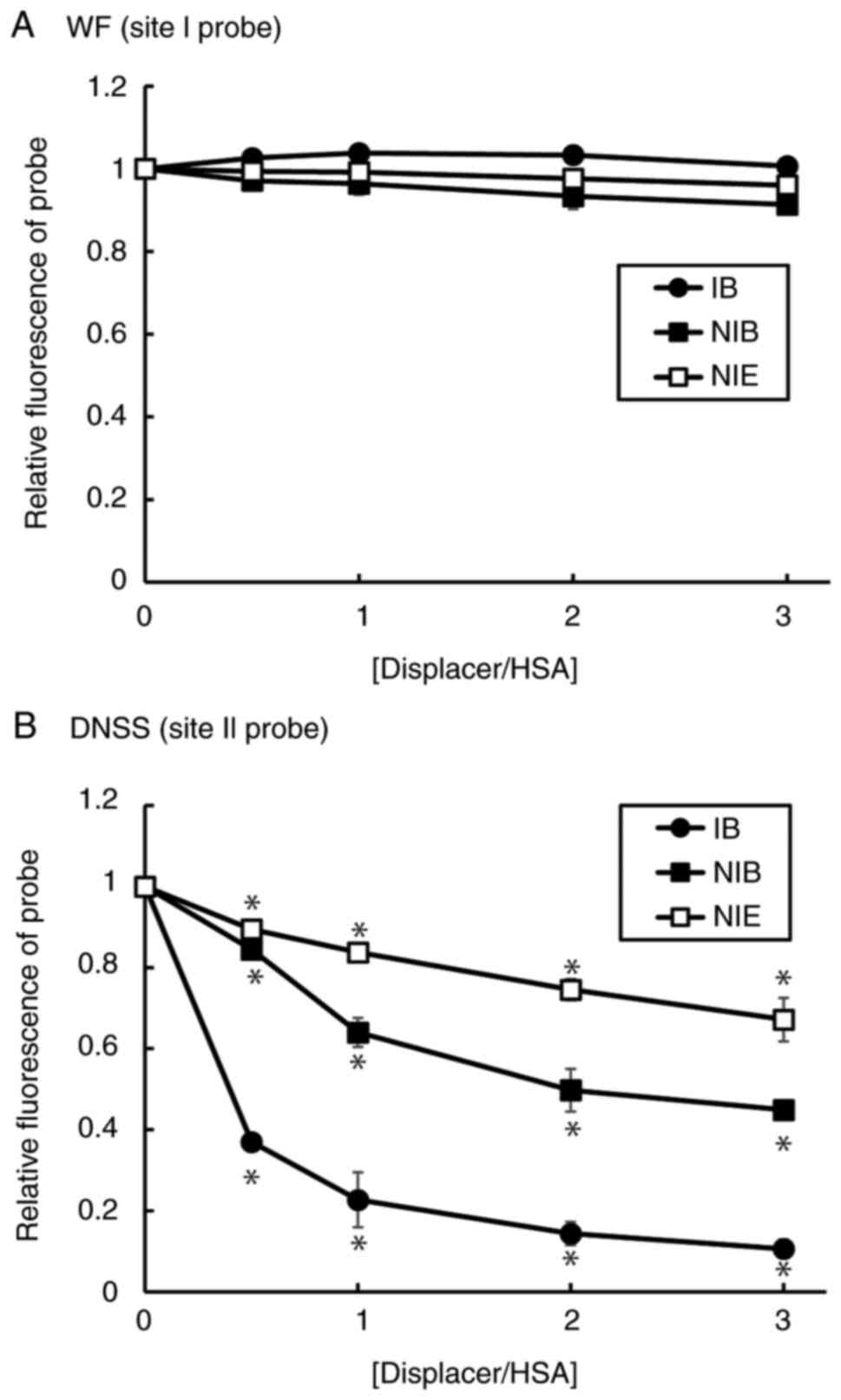
HSA, a quantitative displacement experiment was performed (Table I). Both NIB and NIE significantly
decreased the binding constant of IB to HSA, but they had no
significant effect on the number of binding sites. These results
demonstrated that both NIB and NIE competitively inhibited the
binding of IB to HSA. Fluorescence displacement experiments were
performed using WF and DNSS, which are fluorescent probes for the
drug binding sites I and II, respectively (Fig. 2). Although the degree of
substitution was different, only DNSS was significantly substituted
with both NIB and NIE. These results suggested that both NIB and
NIE bind specifically to site II of HSA, as does IB.
 | Table IBinding constants and the number of
binding sites of IB, IB + NIB and IB + NIE. |
Table I
Binding constants and the number of
binding sites of IB, IB + NIB and IB + NIE.
| | IB | IB + NIB | IB + NIE |
|---|
| Ka (x106
M-1) | 2.11±0.65 | 0.49±0.12 | 1.32±0.17 |
| n | 0.98±0.07 | 1.30±0.15 | 1.00±0.03 |
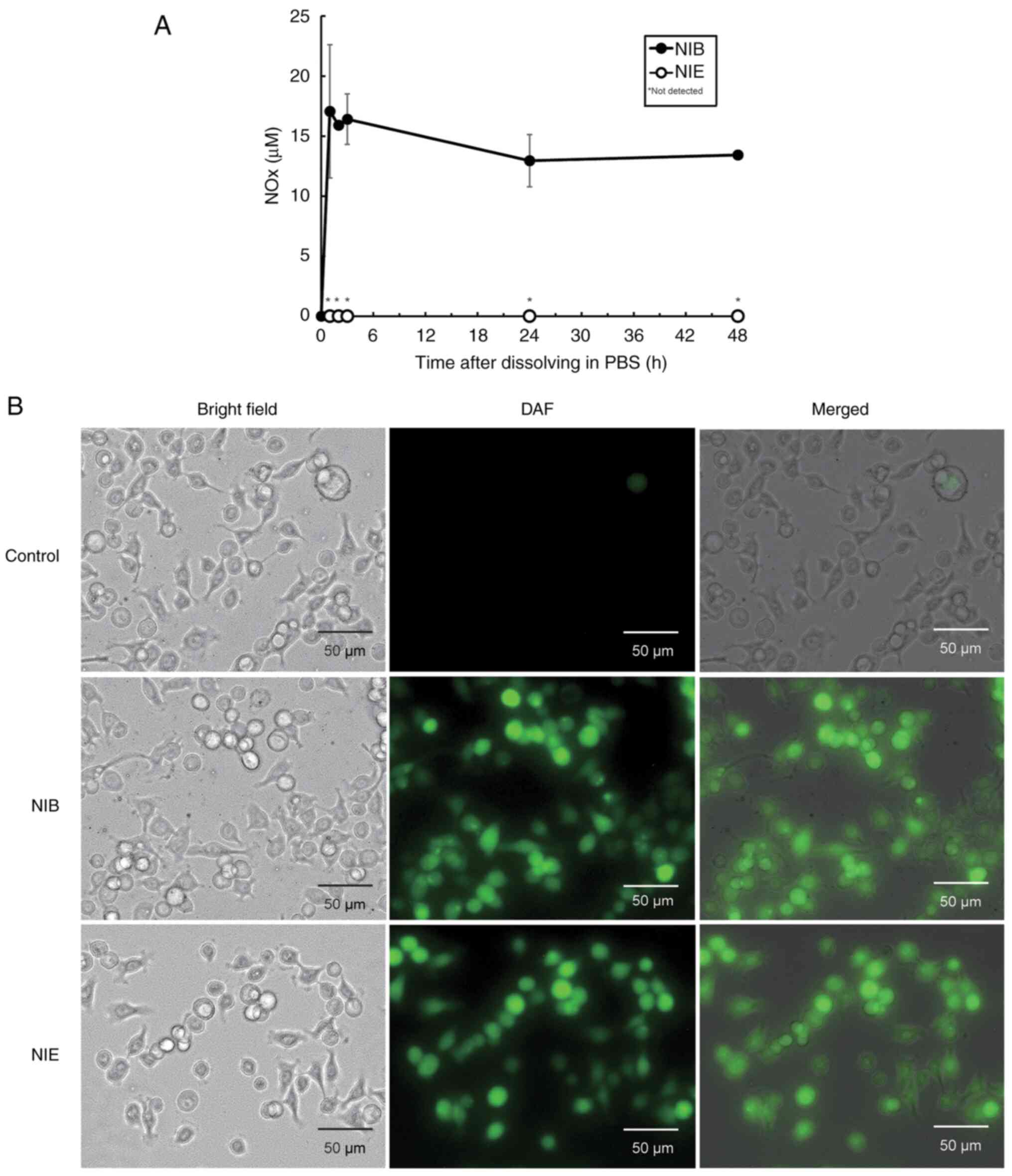
NO release from NIB and NIE
Generally, NO donors liberate NOx by hydrolysis in
aqueous solution (22). Therefore,
the release of NOx from NIB and NIE in PBS was measured (Fig. 3A). Although the release of NOx from
NIB was observed immediately after dissolution, no release of NOx
from NIE was observed over the 48 h time period measured. Since the
RPMI1640 medium used for culturing BxPC cells contains a large
amount of nitrate ions, it is difficult to measure intracellular
NO. Therefore, whether NIB and NIE released NO within cells was
investigated. NO from NIB or NIE in BxPC3 cells was confirmed using
DAF-FM DA, a cell membrane-permeable, NO-specific fluorescence
probe. It was demonstrated that both NIB and NIE released NO within
the cells (Fig. 3B). This result
potentially suggests that a factor, such as an intracellular
hydrolase, may be involved in the release of NO from NIE.
Effects of NIB and NIE on induction of
cell death
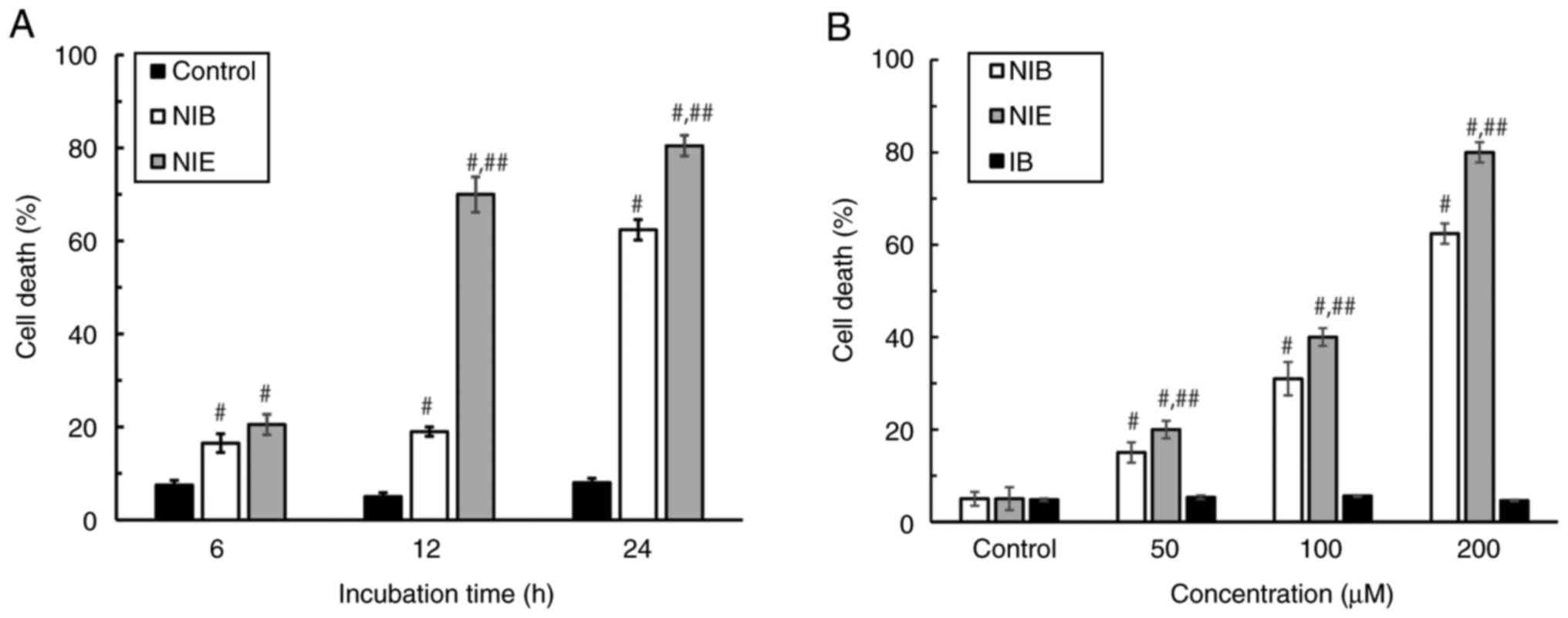
To evaluate the cytotoxicity of NIB and NIE against
the BxPC3 pancreatic cancer cell line, annexin-positive cells were
detected after the addition of NIB or NIE. It was demonstrated that
both NIB and NIE significantly induced cell death in both a
time-(Figs. 4A, S2) and concentration-dependent manner
(Figs. 4B, S2), whereas IB had no significant effect
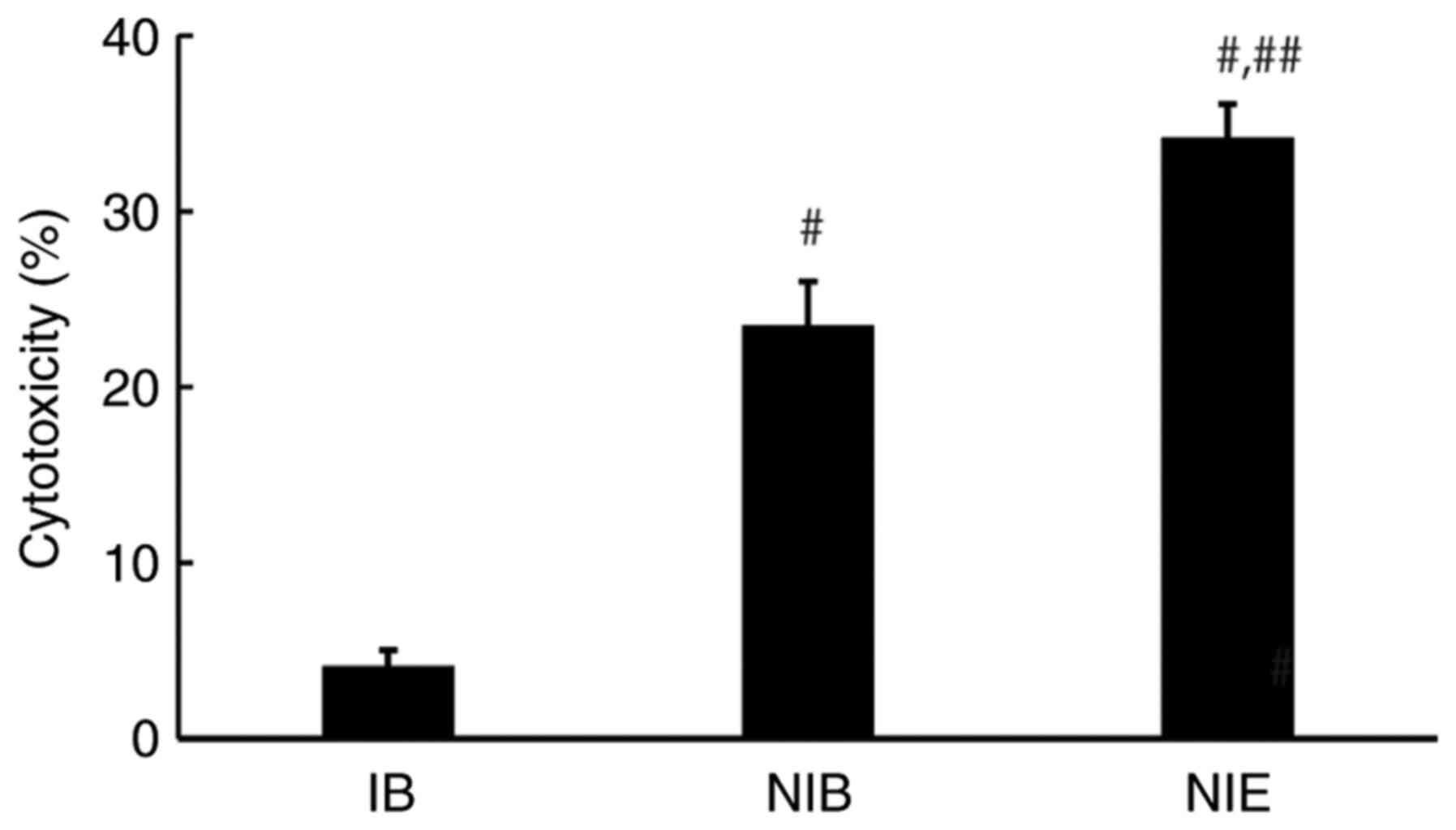
on cell death. Next, an LDH assay was performed to evaluate cell
membrane damage caused by NIB or NIE (Fig. 5).
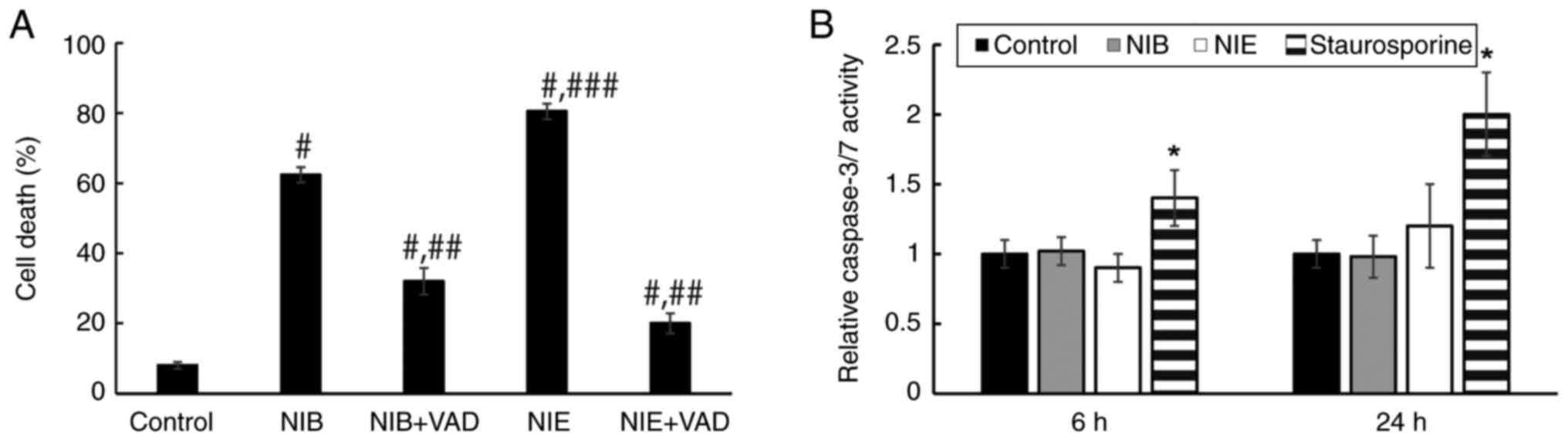
To investigate the mechanism of cell death induction
by NIB and NIE, the effect of the nonspecific caspase inhibitor
z-VAD FMK on cell death was investigated (Fig. 6A). Cell death induced by both NIB
and NIE was significantly suppressed in the presence of z-VAD FMK
compared with cells not treated with z-VAD FMK. However, no
significant activation of caspase 3 and 7, which serve important
roles in apoptosis, was observed in cells treated with NIB and NIE
at 6 and 24 h, despite treatment with staurosporine, a
representative activator of caspase 3/7 (Figs. 6B and S1). These results suggested that NIB and
NIE induced cell death through a non-caspase 3/7 pathway.
Discussion
Pancreatic cancer tumors have low blood flow and
abundant interstitial tissue, which makes drug delivery difficult
to these tumors, resulting in a low response rate to chemotherapy
(5,6). The aim of the present study was to
address these problems by focusing on NO donors that had
vasodilatory and cytotoxic effects on tumors and the surrounding
stromal tissues. To efficiently reach the tumor environment,
nitrated forms of IB, an HSA-binding drug, were synthesized to take
advantage of the ability of albumin to remain in blood for a long
time and accumulate in pancreatic tumors (18).
To confirm the albumin-binding properties of NIB and
NIE, quantitative and qualitative substitution experiments for IB
were performed. Both NIB and NIE significantly and competitively
inhibited the binding of IB to HSA, reducing its binding constant,
without affecting the number of binding sites. In fluorescence
displacement experiments, IB, NIB and NIE significantly displaced
the site II fluorescent probe DNSS. These results suggested that
IB, NIB and NIE bound to site II. However, the degree of
substitution with NIE was smaller compared to that with NIB. This
result showed the same trend as the quantitative substitution
experimental data. As the binding of HSA to IB may to involve an
interaction between the carboxyl group of IB and HSA, some steric
hindrance may have occurred in the case of NIE.
Release of NO from NO donors generally occurs in
aqueous solutions (22). Released
NO is immediately oxidized under aerobic conditions to
NO2- and NO3-. In a
weakly acidic and anaerobic environment, such as a tumor, released
NO is not oxidized and exerts its cytotoxic effect (11). Although NIE did not release NO in
aqueous solution in the present study, NO was demonstrated to be
released in cells. This suggested that NO release from NIE may
occur only in the intracellular environment, where the presence of
hydrolytic enzymes and other factors may be involved. Similarly,
NONOate prodrug is stable in solution, but releases NO following
esterase activity within cells, causing apoptosis of human leukemia
cell lines (23). In addition, NIE
could be considered to have superior characteristics to NIB because
NIE does not release NO in aqueous solutions, such as the blood. If
NO is released in the blood, it will quickly be removed from the
blood. However, since NIE may release NO only inside cells, NIE may
be able to reach tumors without releasing NO in the blood, liberate
NO after being absorbed into the cells and exert its antitumor
activity.
In the present study, the cell death-inducing
effects of NIB and NIE were both significantly inhibited by the
caspase non-specific inhibitor, z-VAD FMK, but no activation of
caspase 3/7 was observed. Necroptosis and pyroptosis are two
regulated cell death pathways that exhibit morphological
characteristics of necrosis, such as cell membrane pore formation,
and membrane collapse. Therefore, several caspases, such as caspase
1, 4, 5, 8 and 11, which are involved in cell death events, may be
involved in the induction of cell death in NIB and NIE (24). Further investigation is needed to
clarify the involvement of these caspases and the mechanisms of
cell death. Furthermore, the details of the mechanism of cell death
induction by other NO donors have also not been clarified. It has
been reported that nitrated aspirin increases oxidative stress by
nitrosylating the cysteine residues of reduced glutathione in cells
(25). It has also been reported
that NO donors induce cell death by nitrosylating the tyrosine
residues of proteins that are important for cell survival, such as
p50, NF-kB, cytochrome C and ribonucleotide reductase (26,27).
In conclusion, newly synthesized NIB and NIE
exhibited HSA-binding properties equivalent to IB. Although both
NIB and NIE had NO-release properties, their release mechanisms
were significantly different and NIE was stable in aqueous
solution. Furthermore, it was shown that both NIB and NIE
significantly induced the cell death of human pancreatic cancer
cells in a caspase 3/7-independent manner. The present study
suggested that NO could be effective in cancer therapy and both NIB
and NIE may potentially be future candidate compounds for
pancreatic cancer therapeutics.
Supplementary Material
1H-NMR and
13C-NMR spectra of NIB. 1H and 13C
NMR spectra were recorded on a JEOL ECA 500 spectrometer operating
at room temperature. NMR, nuclear magnetic resonance.
Representative flow cytometry dot
plots of BxPC cells treated with NIB or NIE. BxPC cells were
cultured with 200 μM of NIB or NIE for 6, 12 and 24 h and
with 0, 50, 100 or 200 μM of IB, NIB or NIE for 24 h. NIB
and NIE group plots that were presented in Figure 6 were the same as those for the 24
h groups presented in Fig. 4. NIB,
nitrated ibuprofen benzyl linker; NIE, nitrated ibuprofen ethyl
linker; IB, ibuprofen. VAD, z-VAD FMK.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by JSPS KAKENHI (grant no.
20K07193). The funder had no role in study design, in the
collection, analysis and interpretation of data, writing of the
report and in the decision to submit the article for
publication.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KN contributed to the design of this study, data
collection and interpretation and wrote the initial draft of the
manuscript. YA, NS, TB, KT, AT, RK and KT contributed to data
collection. SI and HM contributed to the synthesis and the
structural validation of NIB and NIE. MO and KY contributed to the
design of this study, interpretation and critically reviewed the
manuscript. All authors approved the final version of the
manuscript. KN and KY confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cancer Registry and Statistics: Cancer
Information Service. National Cancer Center, 2020 (Vital Statistics
of Japan). https://ganjoho.jp/en/professional/statistics/table_download.html.
|
|
2
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: Folfirinox versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Michl P and Gress TM: Improving drug
delivery to pancreatic cancer: Breaching the stromal fortress by
targeting hyaluronic acid. Gut. 61:1377–1379. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Provenzano PP, Cuevas C, Chang AE, Goel
VK, Von Hoff DD and Hingorani SR: Enzymatic targeting of the stroma
ablates physical barriers to treatment of pancreatic ductal
adenocarcinoma. Cancer Cell. 21:418–429. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ishima Y, Kragh-Hansen U, Maruyama T and
Otagiri M: Albumin as a nitric oxide-traffic protein:
Characterization, biochemistry and possible future therapeutic
applications. Drug Metab Pharmacokinet. 24:308–317. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Estrada C, Gómez C, Martín C, Moncada S
and González C: Nitric oxide mediates tumor necrosis factor-alpha
cytotoxicity in endothelial cells. Biochem Biophys Res Commun.
186:475–482. 1992.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kashfi K, Rayyan Y, Qiao LL, Williams JL,
Chen J, Del Soldato P, Traganos F, Rigas B and Ryann Y: Nitric
oxide-donating nonsteroidal anti-inflammatory drugs inhibit the
growth of various cultured human cancer cells: Evidence of a tissue
type-independent effect. J Pharmacol Exp Ther. 303:1273–1282.
2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Riganti C, Rolando B, Kopecka J, Campia I,
Chegaev K, Lazzarato L, Federico A, Fruttero R and Ghigo D:
Mitochondrial-targeting nitrooxy-doxorubicin: A new approach to
overcome drug resistance. Mol Pharm. 10:161–174. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Islam W, Fang J, Imamura T, Etrych T, Subr
V, Ulbrich K and Maeda H: Augmentation of the enhanced permeability
and retention effect with nitric oxide-generating agents improves
the therapeutic effects of nanomedicines. Mol Cancer Ther.
17:2643–2653. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ishima Y, Chen D, Fang J, Maeda H, Minomo
A, Kragh-Hansen U, Kai T, Maruyama T and Otagiri M: S-Nitrosated
human serum albumin dimer is not only a novel anti-tumor drug but
also a potentiator for anti-tumor drugs with augmented EPR effects.
Bioconjug Chem. 23:264–271. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Peters T: All about albumin: Biochemistry,
genetics, and medical application, acad. Press, Orlando, FL, pp42,
1966.
|
|
14
|
Whittingham JL, Havelund S and Jonassen I:
Crystal structure of a prolonged-acting insulin with
albumin-binding properties. Biochemistry. 36:2826–2831.
1997.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kurtzhals P, Havelund S, Jonassen I and
Markussen J: Effect of fatty acids and selected drugs on the
albumin binding of a long-acting, acylated insulin analogue. J
Pharm Sci. 86:1365–1368. 1997.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sisson EM: Liraglutide: Clinical
pharmacology and considerations for therapy. Pharmacotherapy.
31:896–911. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tiessen RG, Castaigne JP, Dreyfus JF,
Nemansky M, Kruizinga HH and van Vliet AA: Pharmacokinetics and
tolerability of a novel long-acting glucagon-like peptide-1 analog,
CJC-1131, in healthy and diabetic subjects. Int J Clin Pharmacol
Ther. 46:443–452. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Ishima Y, Maruyama T, Otagiri M, Chuang
VTG and Ishida T: The new delivery strategy of albumin carrier
utilizing the interaction with albumin receptors. Chem Pharm Bull
(Tokyo). 70:330–333. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rahman MH, Yamasaki K, Shin YH, Lin CC and
Otagiri M: Characterization of high affinity binding sites of
non-steroidal anti-inflammatory drugs with respect to site-specific
probes on human serum albumin. Biol Pharm Bull. 16:1169–1174.
1993.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Theodosis-Nobelos P, Papagiouvanis G,
Pantelidou M, Kourounakis PN, Athanasekou C and Rekka EA: Design,
synthesis and study of nitrogen monoxide donors as potent
hypolipidaemic and anti-inflammatory agents. Molecules.
25(19)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sakai T, Yamasaki K, Sako T, Kragh-Hansen
U, Suenaga A and Otagiri M: Interaction mechanism between indoxyl
sulfate, a typical uremic toxin bound to site II, and ligands bound
to site I of human serum albumin. Pharm Res. 18:520–524.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Huang Z, Fu J and Zhang Y: Nitric oxide
donor-based cancer therapy: Advances and prospects. J Med Chem.
60:7617–7635. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Saavedra JE, Shami PJ, Wang LY, Davies KM,
Booth MN, Citro ML and Keefer LK: Esterase-sensitive nitric oxide
donors of the diazeniumdiolate family: In vitro antileukemic
activity. J Med Chem. 43:261–269. 2000.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shi J, Gao W and Shao F: Pyroptosis:
Gasdermin-mediated programmed necrotic cell death. Trends Biochem
Sci. 42:245–254. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gao J, Liu X and Rigas B: Nitric
oxide-donating aspirin induces apoptosis in human colon cancer
cells through induction of oxidative stress. Proc Natl Acad Sci
USA. 102:17207–17212. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bonavida B, Khineche S, Huerta-Yepez S and
Garbán H: Therapeutic potential of nitric oxide in cancer. Drug
Resist Updat. 9:157–173. 2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bonavida B, Baritaki S, Huerta-Yepez S,
Vega MI, Jazirehi AR and Berenson J: Nitric oxide donors are a new
class of anti-cancer therapeutics for the reversal of resistance
and inhibition of metastasis. In: Nitric oxide (NO) and cancer:
Prognosis, prevention, and therapy. Bonavida B (ed). Springer, New
York, NY, pp459-477, 2010.
|