NPs, being <100 nm, are one of the novel
promising methods to deal with bacterial infections, including
S. aureus infections (22,23).
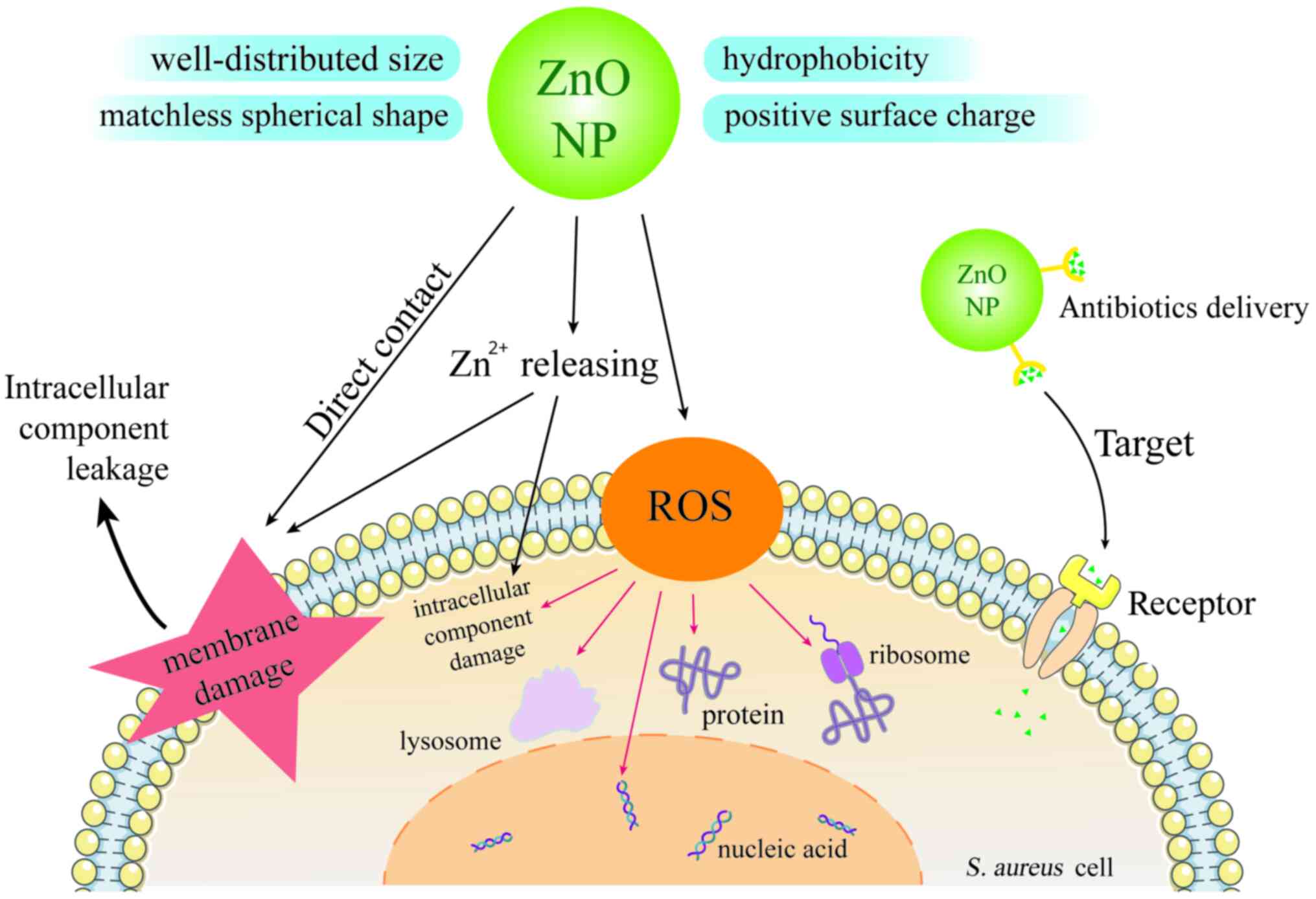
The antibacterial activity of NPs is mostly attributed to their
special characteristics, such as well-distributed size, perfect
spherical shape, positive surface charge and hydrophobicity
(24,25). NPs begin their antibacterial effects
by the direct interplay with cell surface, involving the
destruction of cell wall peptidoglycan and membrane protein and
interference in energy metabolism (ATPase inhibition and electron
transport disruption). Then, NPs can penetrate into cytoplasm and
cause great damage to intracellular components, including nucleic
acids, proteins, lysosomes and ribosomes (26). Additionally, oxidative stress
induced by excess releasing of reactive oxygen species (ROS) also
plays a substantial role in inducing lipid peroxidation on the
bacterial cell membrane (27). As
well as the aforementioned mechanism, metal NPs have specific ways
to resist pathogenic microorganisms by releasing metal ions and
producing different ROS (28).
Several metal (gold, silver, copper and zinc) NPs and their metal
oxide NPs have been reported to have distinctive antimicrobial
properties against S. aureus (29,30)
and they were also shown to be the carriers that can deliver
antibiotics to target sites (22,31).
Fig. 1 shows the properties,
antibacterial mechanism against S. aureus and antibiotics
delivery ability of zinc oxide nanoparticles (ZnO NPs).
There are a number of studies reporting the
antibacterial property of ZnO NPs against S. aureus
(32-34).
ZnO NPs reduce the biofilm of S. aureus by inhibiting
biofilm genes expression, such as ica A, ica D and
fnb A (35). In Kahandal
et al (36), the biofilm
formation of S. aureus was inhibited markedly by 95.39 %
when treated with 125 µg/ml of ZnO NPs for 5 h. Abdelraheem et
al (37) observed that ZnO NPs
presented antibacterial activity against multidrug resistant S.
aureus, such as methicillin, vancomycin and linezolid resistant
S. aureus. Irfan et al (38) confirmed the antibacterial activity
of ZnO NPs against S. aureus and MRSA with the zone of
inhibition (ZOI) of 21±2 and 17±2 mm, respectively. El-Masry et
al (39) also reported that ZnO
NPs (20 nm and concentration of 20 mM) inhibited 105 and
107 CFU/ml S. aureus with ZOI of 26 and 22 mm,
respectively. Currently, there is a lack of a comprehensive review
on ZnO NPs against S. aureus. Therefore, the present study
reviewed the antibacterial activity against S. aureus of ZnO
NPs fabricated by various synthetic ways, especially the green
synthetic ZnO NPs. It also summarized the synergistic antibacterial
effects against S. aureus of ZnO NPs in combination with
antibiotics. Furthermore, it highlighted the enhanced activities
against S. aureus of ZnO nanocomposites, nano-hybrids and
functional ZnO NPs.
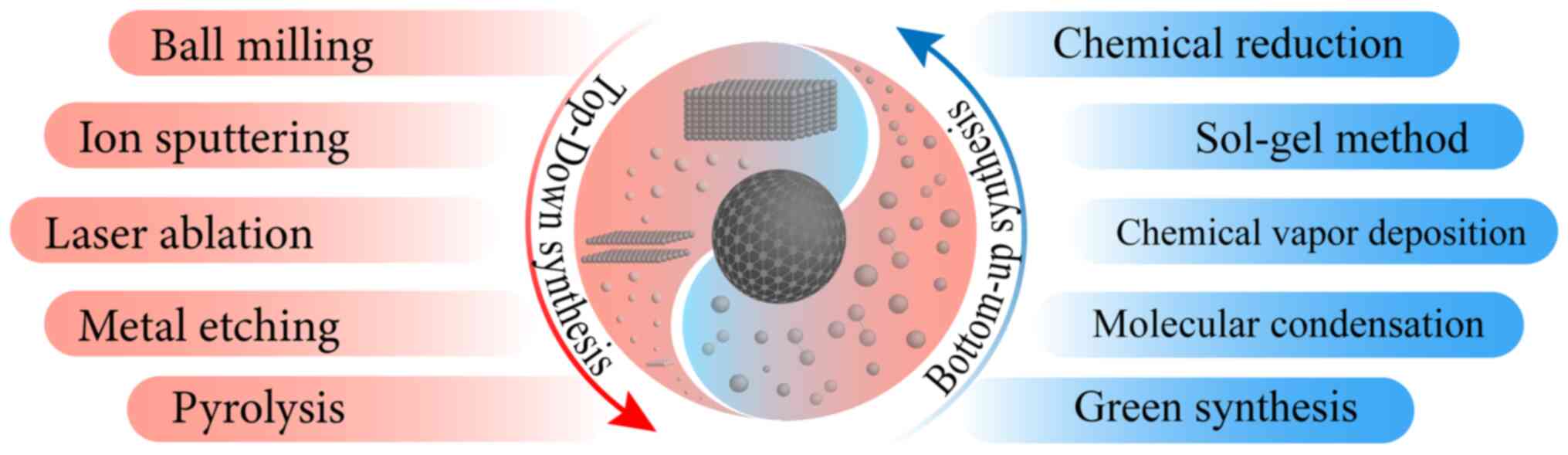
Commonly, ZnO NPs can be synthesized by using
top-down and bottom-up methods that include diverse physical and
chemical ways (40) (Fig. 2). Top-down approaches cut massive
materials into NPs physically, including ball milling, ion
sputtering, laser ablation, metal etching and pyrolysis. According
to Massoudi et al (41)
research, ZnO NPs made by high-speed ball milling inhibit S.
aureus with the largest ZOI of ~13.5±0.5 mm. It was also found
that ZnO NPs synthesized by microwave heating displayed the ZOI of
~16 mm against S. aureus (42). Bottom-up ways fabricated atoms and
molecules into nano-sized particles, which included chemical
reduction, sol-gel method, chemical vapor deposition, molecular
condensation and even green synthesis (43). Different synthesis processes bring
about various physicochemical properties of metal NPs such as size,
shape, dispersity and stabilization diversity, which determine the
antibacterial efficiency (44,45).
Table I shows the characteristics
and anti-S. aureus capacity of ZnO NPs made by several
methods. In Bai et al (46),
small molecule ligand solvothermal synthesized ZnO NPs showed
size-related antibacterial effect and the minimum inhibitory
concentration (MIC) of 4 nm ZnO NPs against S. aureus was
6.25 µg/ml, which is lower than the MIC of 10 nm ZnO NPs at ~25
µg/ml. In an antimicrobial test of solution-polymerization-method
synthesized ZnO NPs, it was discovered that S. aureus was
more susceptible to nanoparticle size than E. coli (47). The co-precipitation method is also
frequently used to synthesize ZnO NPs that show the lowest MIC
against S. aureus compared with other bacteria (48). Moreover, by using an easy chemical
method, diethylene-glycol-mediated ZnO NPs were made and they had
antibacterial activity against S. aureus with the ZOI of 14
mm and showed the excellent S. aureus biofilm control
(49). It was also reported that
S. aureus cell leakage was observed after exposure to
mechano-chemical synthesized ZnO NPs (50). Although a great number of
physicochemical synthetic methods have been found to make ZnO NPs
for S. aureus treatment, some demerits such as high cost,
toxicity and instability still place restrictions on their
large-scale antibacterial applications (43).
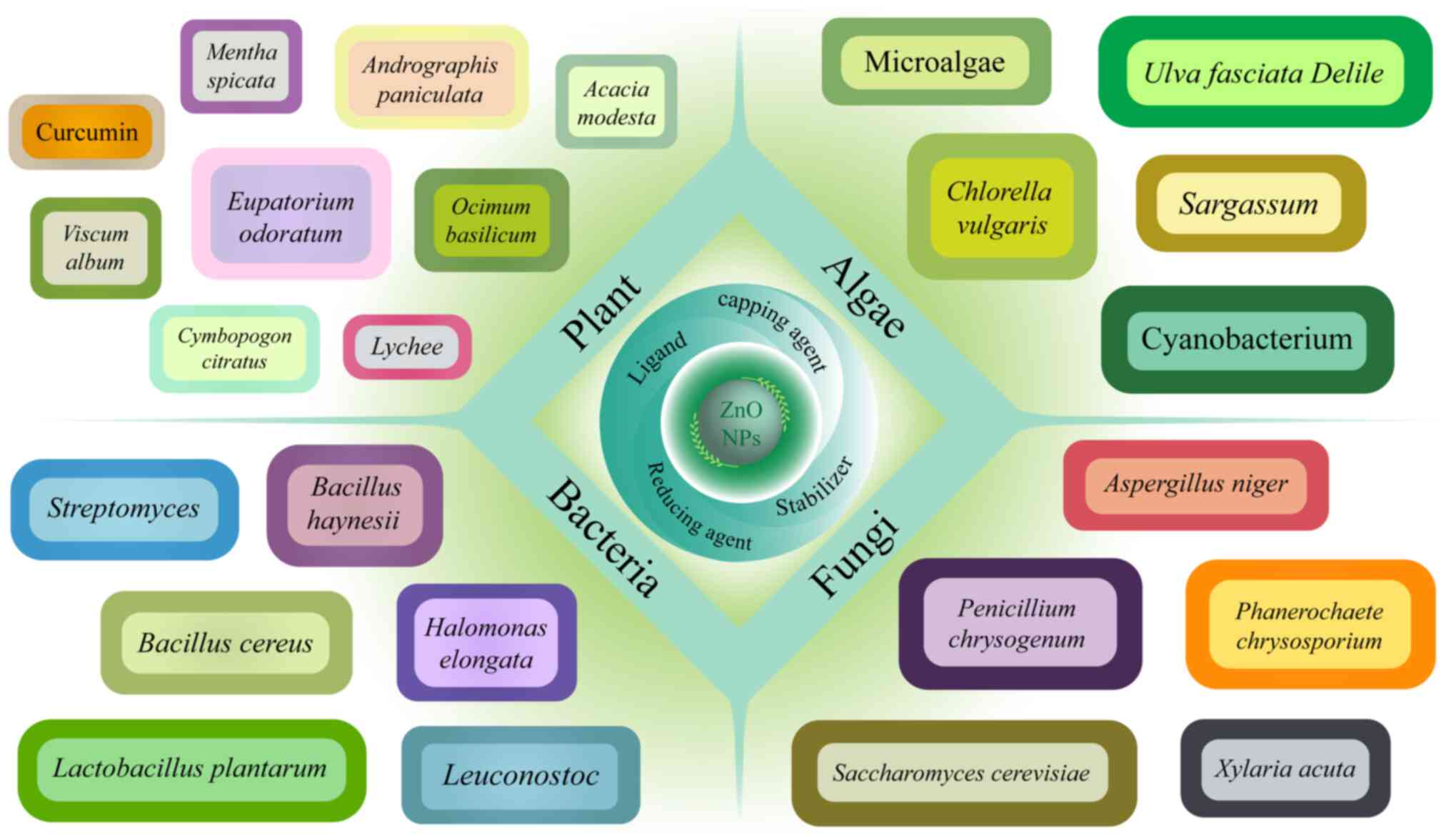
Recently, green biological materials drew much
attention to researchers for their environment-friendly,
cost-effective, low-toxicity and useful properties to make ZnO NPs
(26). There are a number of types
of biological materials such as bacteria, fungi, algae and plant
extracts (51,52) (Fig.
3), which serve as reducing agents, capping agents, stabilizers
and ligands during the synthesis of ZnO NPs (26) and their effects are ion reduction,
size and shape control, NPs surface stabilization, metal
passivation and coating, respectively, which are important to the
antimicrobial properties of ZnO NPs (26,53).
The antibacterial properties of green-synthesized ZnO NPs against
S. aureus are in Table
II.
Due to different synthetic raw materials,
plant-derived ZnO NPs are provided with multifarious
characteristics. Triangle-like M-ZnO-NPs and B-ZnO-NPs were made by
Mentha spicata and Ocimum basilicum acting as
capping, stabilizing and reducing agents with size of 24.5 and 26.7
nm, respectively. These types of ZnO NPs had antibacterial
properties against S. aureus (ATCC 25923) with a 14.73 mm
ZOI with 0.01 g/ml M-ZnO-NPs (54).
In Sachin et al (55), ZnO
NPs synthesized by using lychee peel extract were spherical
and small (<10 nm) and were also proved to combat S.
aureus (ATCC25923) with 15 mm ZOI of 100 µg/ml ZnO NPs. In
Mohammed et al (56), zinc
nitrate hexahydrate and Cymbopogon citratus extracts were
used to synthesize ZnO NPs, which killed S. aureus cells
with a MIC of 88.13±0.35 µg/ml. In Mushtaq et al (57), methanol and water leaf extracts of
Viscum album were applied to fabricate ZnO NPs that were
quasi-spherical with size of 13.5 nm and which showed considerable
inhibitory effects against S. aureus with a ZOI of 39±0.3
and 40±0.3 mm, respectively. Due to having a higher content of DNA
gyrase-B inhibitor, the water extracts of ZnO NPs were proved to be
more effective in limiting bacterial growth. ZnO NPs with
flower-shaped structures were created by a green nanotechnology
facility in Hasan et al (58) and showed 90.9% inhibition against
S. aureus. It is noteworthy that the ZnO NPs showed more
durable antimicrobial activity than Ag NPs in in vivo tests,
which may be attributed to their distinctive morphology and massive
active surface sites. In Irfan et al (59), green-synthesized ZnO NPs by Gum
Acacia modesta expressed antimicrobial ability against MRSA
with a ZOI of 16±2 mm. Alallam et al (60) also observed that ZnO NPs made by
pure curcumin had a great ability to combat MRSA. Notably, these
green-synthesized ZnO NPs showed a minimal cytotoxicity compared
with chemically synthesized ZnO NPs (61). Furthermore, in Ting et al
(53), ZnO NPs biosynthesized by
using the aqueous extract of Andrographis paniculata leaves
demonstrated a high inhibition on S. aureus and then
controlled periimplantitis. ZnO NPs synthesized by using ethanolic
extracts of Eupatorium odoratum are reported to show more
than 97% biofilm inhibition of S. aureus that could be
applied to reduce central venous catheter associated infections
(61).
Algae are known as ‘bio-nano-factories’ due to their
various properties, such as low risk of environmental toxicity,
simple processing methods and the ability to redox metals (62). In addition, algal extracts are full
of bioactive molecules that can be used as reducing and stabilizing
agents. The biosynthesis of ZnO NPs using microalgae was
authenticated to be a cost-effective method and the ZAA2 strain
microalgae-synthesized ZnO NPs showed outstanding antibacterial
activity with the largest ZOI of ~20 mm against S. aureus
(63). In addition, by using
Chlorella vulgaris as green resource, biogenic ZnO NPs were
produced having significant antibacterial activity against MRSA,
attributed to their excellent size distribution and surface energy
(64). Researchers have also
investigated the phyco-synthesis of UFD-ZnO NPs using extract of
Ulva fasciata Delile. The destructive power of UFD-ZnO NPs
against S. aureus (ATCC 25923) was time-dependent, while the
MIC and ZOI were recorded at ~17.5 µg/ml and 24.9±1.5 mm,
respectively (65). In a recent
study, Sargassum extracts have been used to synthesize ZnO
NPs and the ultrasound-assisted green synthesized ZnO NPs showed
the highest inhibition against S. aureus by 99% compared
with ZnO NPs alone (66). As one of
the phototrophic bacteria, cyanobacteria are the source of
bioactive compounds as well as the raw material of ZnO NPs
synthesis. By using cell extract of a new cyanobacterial strain
Desertifilum sp. EAZ03, ZnO NPs have been made that possess
considerable antibiofilm and antimicrobial effects against S.
aureus (ATCC 59223) with an MIC value of 32 µg/ml and the
minimum bactericidal concentration value of 64 µg/ml (67). Similarly, Ebadi et al
(68) synthesized ZnO NPs using the
cell extract of the cyanobacterium Nostoc sp. EA03, which
were also discovered to destroy S. aureus biofilms and had
low cytotoxicity on lung fibroblast cells.
With their lower purification cost and higher
productivity compared with other microorganisms, bacteria are also
considered as the raw materials to create ZnO NPs (69,70).
According to a biosynthesis test of Yusof et al (71), Lactobacillus plantarum TA4, a
microorganism isolated from fermented food, was proved to
synthesize ZnO NPs with concentration- and shape-dependent
antibacterial capacity. In addition, cell-free supernatant (CFS)
and cell-biomass (CB) taken from L. plantarum TA4 were used
as reducing agents to synthesize ZnO NPs, respectively. Although
the MIC value to inhibit S. aureus of ZnO NPs-CB was lower
compared with ZnO NPs-CFS, ZnO NPs were more conveniently purified
by CFS (71). From this, it is
indispensable to weigh up the pros and cons of different synthetic
materials in order to choose the optimal raw material under
different demands and experimental environments. In Rehman et
al (72), Bacillus
haynesii isolated from date palm plant was employed as the
reducing agent to establish an eco-friendly nanobiofactory. ZnO NPs
mediated by Bacillus cereus showed a spherical shape with
median size of 50±5 nm, which damaged S. aureus cell surface
by direct contact (72).
Streptomyces purified from waste soil can be used to
biosynthesize ZnO NPs and the antibacterial effects were identified
to combat multiple isolates of S. aureus (73). Taran et al (74) explored the optimum condition to
biosynthesize ZnO NPs by using Halomonas elongata IBRC-M
10214 through the Taguchi method (75). Results showed that these ZnO NPs
were stable, pure and nontoxic, able to fight against multi-drug
resistant bacteria such as S. aureus ATCC 43300. Strain C2
isolated from the genus Leuconostoc of lactic acid bacteria
has been employed to biosynthesize metal NPs, including ZnO NPs and
Au NPs. According to Kang et al (76), the C2-ZnO NPs expressed a lower MIC
value of 512 µg/ml compared with C2-Au NPs (MIC: 1024 µg/ml)
against S. aureus.
A number of studies have reported that fungi can be
used for synthesizing ZnO NPs. Sharma et al (77) used Phanerochaete
chrysosporium to make ZnO NPs with advantages in terms of
stability, simple processing, antimicrobial activity and
non-cytotoxicity. Mohamed et al (78) produced fungal-synthesized ZnO NPs of
9-35 nm by using Penicillium chrysogenum and found that the
ZnO NPs had antibacterial and antibiofilm activities against S.
aureus. ZnO NPs synthesized by a simple, non-toxic method using
fungal filtrate of Xylaria acuta were promising
antimicrobial agents that exhibited an MIC value of 15.6 µg/ml
against S. aureus (79).
Abdelkader et al (80)
synthesized ZnO NPs using Aspergillus niger Endophytic
fungal extract with characteristics of stability and antibiofilm
activity. It was demonstrated that ZnO NPs reduced the number of
biofilm-forming S. aureus from 50-20.83% and the MIC of ZnO
NPs against multiple S. aureus strains ranged from 8-128
µg/ml (80). In Motazedi et
al (81), the extracellular
extract of Saccharomyces cerevisiae was used to create
spherical ZnO NPs with dose-dependent antibacterial ability against
S. aureus.
At present, one of the most serious issues of global
health must be antibiotics resistance. The synergy between
antibiotics and ZnO NPs attracts much attention and would be a
practicable treatment against multi-drug resistant bacteria
(82,83). It has been found that ciprofloxacin
in conjunction with ZnO@Glu-TSC
(thiosemicarbazide-conjugated and glutamic acid-functionalized ZnO
NPs) could significantly inhibit the expression of efflux pump
genes, which is a vital factor towards antibiotics resistance
(84). In addition, ZnO NPs can be
excellent drug carriers to target antibacterial agents to the
action sites and still achieve desired therapeutic effects for a
decreased drug dosage, thus enhancing the antimicrobial efficacy
(22). In Habib et al
(85), using ZnO NPs combined with
ciprofloxacin and imipenem, the ZOI of S. aureus was 17 mm
higher than that of E. coli (12 mm). By using ZnO NPs in
conjunction with antibiotics to defeat S. aureus, the MICs
of six clinical common antibiotics were reduced, which reflected an
effective antibacterial cooperation. Furthermore, the anti-biofilm
efficacy was also investigated and was enhanced from 34-37%
(antibiotics alone) to 65-85% (antibiotics and ZnO NPs combination)
(86).
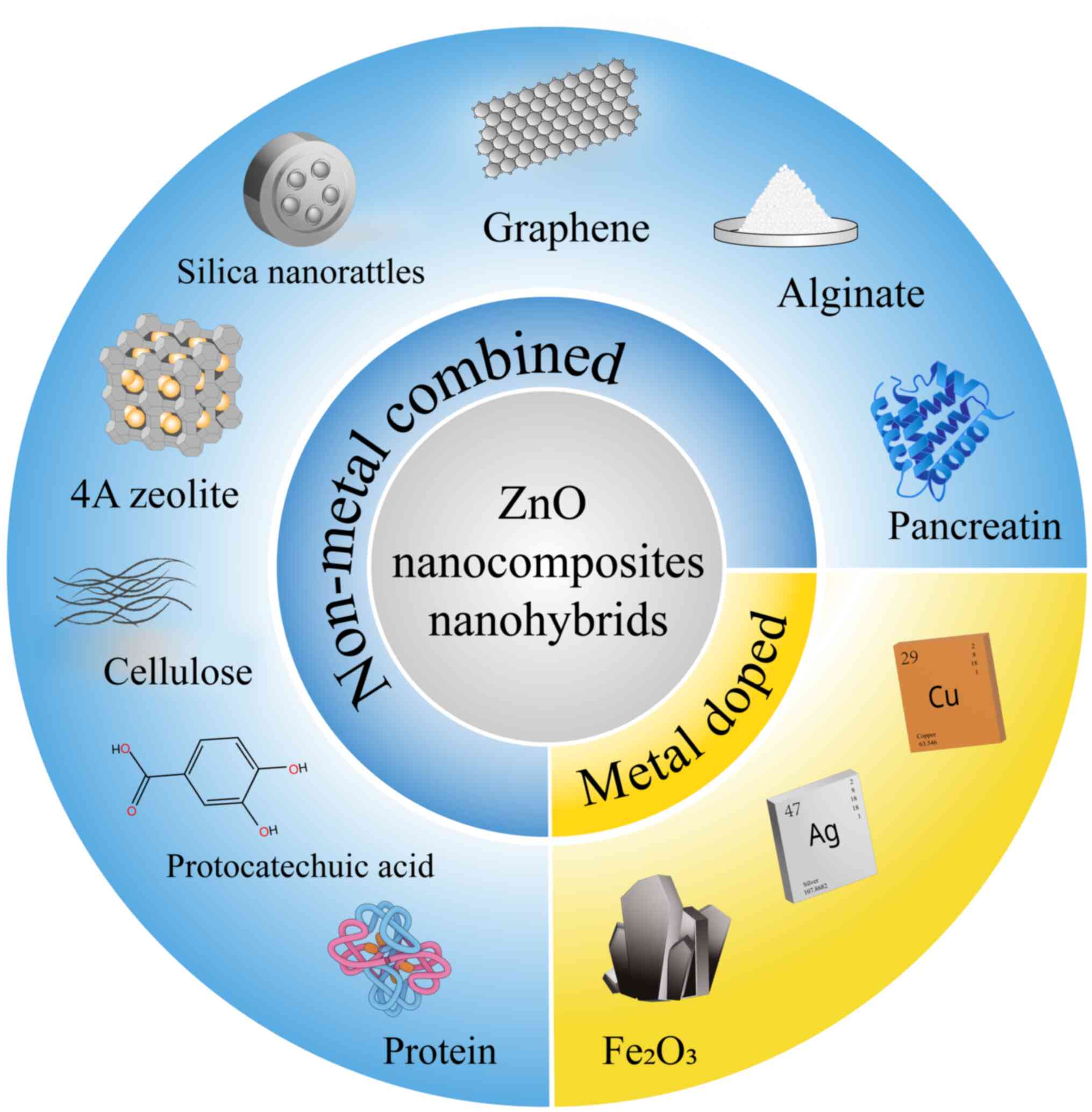
In order to improve the antibacterial activity of
ZnO NPs, various non-metal substances have been used to prepare ZnO
nanocomposites. In Oves et al (91), the combination of graphene, curcumin
and ZnO NPs showed enhanced inhibition against S. aureus
more than five-fold compared with graphene-ZnO NPs and the ZnO
nanocomposites also suppressed MRSA (ATCC 43300) effectively. Zhai
et al (92) designed
ZnO-graphene nanocomposites that could enhance rapid antibiosis due
to the separation of ZnO electron-hole pairs and increased active
sites by transforming the shape of ZnO. Silica nanorattles (SNs)
combined with ZnO NPs were reported to exhibit an improved
antibacterial activity against MRSA with a lower MIC of 6.25 µg/ml
compared with free ZnO NPs in vitro and in vivo.
Since the SNs surface protected and amassed the ZnO NPs, the free
radicals offered by ZnO NPs had an enhanced efficacy in combating
MRSA (93). Vinotha et al
(94) developed the Btp-Ac-ZnO
nanocomposites by using Acorus calamus extract and bacterial
toxic protein (Cry) and they demonstrated the
concentration-dependent biofilm inhibition of the synthesized
nanocomposites against S. aureus (MTCC 9542). ZnO NPs can
also be supported by 4A zeolite, controlling the release of ZnO NPs
and enhancing the antibacterial properties (95). It has been shown that
pancreatin-doped ZnO nanocomposites have improved performances,
such as low-toxicity to human cells and anti-biofilm and
anti-motility abilities against MRSA and increased sensitivity for
vancomycin against MRSA (96).
Canales et al (97)
demonstrated that the electrospun scaffolds based on poly (lactic
acid), bioglass and ZnO NPs showed biocidal properties against
S. aureus with bacteria decreasing by 30%, which may be
useful for tissue engineering. Hydroxypropyl methylcellulose film
combined with ZnO NPs and carboxymethyl starch have been shown to
have excellent antibacterial ability against S. aureus and
no toxicity to human HaCat cells and so can be used for wound
dressing (98). Majeed et al
(99) found that ZnO NPs doped with
selenium showed strong inhibition to MRSA, however, teratogenicity
was also revealed, which means that it is important to use them
cautiously.
Nano-hybrids are also recommended as a good
replacement for conventional antibacterial ZnO NPs and have
enhanced antibacterial efficacy and low-toxicity on normal cells
(100). According to Karthikeyan
et al (101), in order to
develop nanomaterials with high antibacterial ability compared with
antibiotics, the alginate-ZnO hybrid nanomaterials have been
synthesized with good inhibition effects on MRSA and low
cytotoxicity to human cells. Kang et al (102) reported that the dispersibility of
ZnO could be improved by the hybridization of ZnO NPs with
nanocellulose and increasing bacterial inhibition rates were shown
in S. aureus. Furthermore, in the research of AbouAitah
et al (103), a hybrid
nano-formulation was developed from ZnO NPs and protocatechuic acid
and offered a sustained-release antibacterial effect toward S.
aureus (Fig. 4).
The activities of metal ions can be improved by the
amalgamation of metal NPs. For instance, the release of more zinc
and copper ions has been confirmed by ICP-OES analysis in Cu-doped
ZnO nanocomposites, which caused enhanced antibacterial activity
against S. aureus (104).
In Rao et al (105),
Na-doped ZnO NPs expressed enhanced inhibition activity against
S. aureus with Na-concentration dependence. By using a
scaled-up green strategy, cellulose-based Ag-ZnO nanocomposites
(AZC) were prepared, which demonstrated good stability. It was also
reported that the AZC films showed greater inhibition against S.
aureus than E. coli (106). Hu et al (107) revealed that ZnO/Ag bimetallic
nanocomposites showed significant inhibition against S.
aureus compared with single metal nanomaterials and the
cytotoxicity to fibroblasts was reduced by a ZnO and Ag complex.
Mohammadi-Aloucheh et al (108) reported that ZnO/CuO nanocomposites
synthesized using fruit extracts could lead to the disruption of
bacterial membranes and enhanced anti-bacterial ability compared
with ZnO NPs alone. Bahari et al (109) synthesized
Fe3O4/ZnO nanocomposite by the sol-gel method
and the molar ratio of 1:10 showed the best antimicrobial
performance against S. aureus with a ZOI of 11.5±0.7 mm.
AlSalhi et al (110) used
the co-precipitation technique to make magnetic
ZnO/ZnFe2O4 nanohybrids that were good
photocatalytic material and it was discovered that the membrane of
S. aureus collapsed after exposure to the nanohybrids. Lee
et al (111) made a
multi-metal oxide nanocomposite including ZrO, ZnO and
TiO2. It was observed that these nanocomposites
demonstrated a killing efficiency of 72.4% against S.
aureus. Poly (vinyl alcohol)-based compositions were developed
with addition of silver, copper and ZnO NPs, which had the feature
of solidifying to be peeled off along with the impaired bacterial
film, thereby decreasing the number of S. aureus (112).
In order to optimize the performance of ZnO NPs to
combat pathogenic microorganisms, researchers have given attention
to functionalized, modified or capped ZnO NPs (34,113).
Choi et al (114) created
novel ZnO NPs functionalized with caffeic acid, which expressed
enhanced antibacterial efficiency against S. aureus and
three MRSA strains. The amino-functionalized hydrophilic ZnO NPs
induced the destruction of respiratory electron transformation,
generation of intracellular ROS and depolarization of cell membrane
in S. aureus (115).
Charoensri et al (116)
prepared polyaniline-functionalized ZnO NPs by a simple
impregnation method; not only did the synthesized ZnO NPs films
show enhanced water hydrophobicity, but also expressed increased
antibacterial ability against S. aureus, which will make it
possible to develop antibacterial biodegradable materials. Lee
et al (117) prepared
gallic acid functionalized ZnO NPs that had high bacterial cell
membrane affinity and showed enhanced bactericidal activity against
S. aureus and higher selective inhibition to MRSA strains
compared with non-functionalized ZnO NPs. According to Chen et
al (118),
photosensitizers-functionalized ZnO NPs demonstrated marked S.
aureus inhibition and showed low-toxicity on endothelial cells
and erythrocyte. In Yuan et al (119), lysozyme-modified ZnO NPs expressed
excellent antibacterial activity against S. aureus and MRSA
due to their small size, membrane permeability and enzyme-mediated
ROS generation and even had lower cytotoxicity than gentamycin at
the same concentration.
In the present study, ZnO NPs synthesized by
different methods and their antibacterial activity against S.
aureus have been summarized. Taken together, the anti-S.
aureus efficacy of ZnO NPs mainly relies on their basic
characteristics, especially size and shape. Spherical shape and
small size are ideal features of ZnO NPs to combat bacteria that
lead to high specific surface areas and more chances to contact
with pathogens. In Babayevska et al (24), ZnO NPs with the highest specific
surface area showed the size <10 nm. It is also reported that
spherical ZnO NPs had the minimal size (31 nm) and higher
anti-S. aureus activity (6-7 log CFU ml−1
reduction) compared with flower-shaped particles (3-4 log CFU
ml−1 reduction for S. aureus) (44). The detail of ZnO NPs synthesized by
various physical and chemical methods has been the subject of
recent research (40). Some studies
also revealed that these traditional synthesized methods had
various shortcomings, such as being environment-contaminating,
expensive and energy-intensive (43,120,121). Green synthesis has been emphasized
due of its environment-friendly, easy-acquired and low-toxicity
features. Some studies also noted that these green materials had
antibacterial abilities already, such as mint (122), aloe (123) and curcumin (124), and they endow ZnO NPs with
enhanced and steady antibacterial activity against S. aureus
(125). Physical or chemical
processes are the indispensable part in NPs synthesis. However, ZnO
NPs made only by physicochemical ways are cannot compare with
biogenic, functional or compound ZnO NPs when they are further
applied to clinical antibacterial situations.
ZnO NPs can be used in a number of pre-clinical and
clinical antimicrobial fields, including surgical operation
(59), post-operative
anti-bacterial therapy (90),
anti-inflammatory (80) and
ophthalmic treatment (126). For
example, suture coated by green synthetic ZnO NPs demonstrated
excellent tensile strength and wound healing ability in an incision
wound rat model (59). An infection
model in mice showed that ZnO NPs originating from fungi could
significantly decrease hepatic inflammatory markers, restrain
congestion and fibrosis in tissues and improve liver function
(80). Sindelo et al
(127) made the phthalocyanines
link to the amino-functionalized ZnO NPs and these nanocomposites
showed considerable activities of photodynamic antimicrobial
chemotherapy and multi-microbial biofilms eradication. Considering
that ZnO NPs had an excellent antibacterial activity against S.
aureus and good biocompatibility, a chitosan-ZnO/selenium
nanoparticles scaffold was developed to be used for infected wound
healing and postoperative treatment of pediatric fractures
(128). Ismail et al
(129) also reported that ZnO NPs
could be used as the hand sanitation in the future, which present
improved anti-MRSA activity compared with the commonly used alcohol
sanitation.
As one of the primary metal oxide NPs, ZnO NPs
express excellent ability against S. aureus, but they still
have drawbacks to be widely used as antibiotics replacements in
clinical contexts. A few trials in vivo suggested that
different metal oxide NPs damage cells to different degrees
(45). Venkatraman et al
(130) noted the toxicity of ZnO
NPs to RAW264 macrophage cells, with half maximal inhibitory
concentration (IC50) of 494 µg/ml. Although electrospun
scaffolds based on ZnO NPs showed increasing antibacterial
activity, it is also reported that cytotoxicity was related to high
ZnO content (97). According to
Pereira et al (131),
erythrocyte changes were also discovered in reptile exposure to ZnO
NPs at the dose of 440 µg/kg. Yang et al (132) reported that ZnO NPs could induce
apoptosis of mouse-derived spermatogonia cell line GC-1 spg cells.
In Al-Zahaby et al (133),
ZnO NPs (0.69 mg/l) mediated ROS that induced cell apoptosis and
caused sensory toxicity effect on zebrafish olfactory organs. ZnO
NPs synthesized by Calotropis procera leaf extract were
reported to exhibit potent antimicrobial ability with
concentration-dependent manner. However, with increasing exposure
to ZnO NPs, deleterious changes (degeneration, swelling and
atrophy) were found in the kidney by histology (85). Nazir et al (134) also discovered liver dysfunction in
mice intraperitoneal injection groups at ZnO NPs doses of 50 and
100 mg/kg. ZnO NPs were also cytotoxic to the human immune system
at doses of 25 and 12 mg/l (135).
Despite ZnO NPs exhibiting outstanding capability in inhibiting
MRSA, the resistance to NPs by microbes remained. If the dosage of
NPs is below the sublethal concentrations, a series of resistance
mechanisms would be initiated stealthily by bacteria, resembling
their antibiotic resistance (136).
ZnO NPs still have potential toxicity when they are
applied to clinical antibacterial treatment, though green-ZnO NPs
have shown lower cytotoxicity than physicochemically synthesized
ZnO NPs (60). Studies mostly pay
close attention to the improving methods for preparation of ZnO NPs
(42,48,54).
They focus on the physical characteristics and antibacterial
abilities, but neglect, to some degree, the cytotoxicity tests
in vivo. Markedly, researchers have developed a predictive
model to evaluate the security of ZnO NPs with different features
and the authors point out that ZnO NPs with larger size, spherical
shape, negative charge and a higher tendency for aggregation are
safer, which is of great value to further toxicity studies
(137). Notably, ZnO NPs <100
µg/ml were biocompatible and the cytotoxicity was parallel with
their antibacterial activity, which meant that the anti-S.
aureus mechanism (direct contact to cells, ROS and
Zn2+ releasing) was also the potential killing process
in normal cells (24). Although a
number of reports explained the antibacterial mechanism against
S. aureus of metal and metal oxide NPs, the studies for ZnO
NPs were still limited compared with other metal NPs such as Ag NPs
(26,138). The majority of ZnO NPs anti-S.
aureus properties lack a comprehensive assessment and were only
analyzed by well or disc diffusion test and bacterial growth curve
and some studies only reported ZOI or MIC or even neither of them
(42,50,126).
A uniform standard of S. aureus strain is also necessary,
which would play a crucial role in comparing antibacterial effects
of ZnO NPs made by different methods.
In order to restrict the immoderate proliferation
and mutation of pathogens, unremitting efforts should be made to
optimize antibacterial strategies. As aforementioned, the synergism
of ZnO NPs and other materials showed complemental effects,
enhanced antibacterial activity and improved properties in clinical
applications. Due to technology developments, there are more
potentials for ZnO NPs preparation and biomedical application. For
instance, apart from the green synthesis aforementioned,
material-saving, safe and even granular NPs can be made by more
novel methods, such as microfluidic (120). Except for using green and easily
obtained raw materials, synthetic methods should be able to
flexibly control the size, shape and dispersity of ZnO NPs, which
means that the key material and procedure of ZnO NPs synthesis must
be identified by modern techniques. Notably, genomic and proteomic
techniques should be devoted to the exploration of the
antibacterial mechanism, synthesis optimization and cytotoxicity of
ZnO NPs. The antibacterial study of effects at the cellular level
in the long term is an essential component to investigate the
dosage and safety of ZnO NPs. It is necessary to focus on ZnO NPs
studies in vivo, especially the biokinetics,
bioavailability, tissue distribution and clearance rate, which are
essential for their antibacterial applications and improved using
as antibiotics replacements. In addition, composite and functional
ZnO NPs could enhance the antibacterial advantages to some degree
and decrease their toxicity and also reduce the excessive exposure
of ZnO NPs that diminish the possibility of antimicrobial
resistance, this is for future researchers.
Not applicable.
Funding: The present study was supported by grants from the
National Natural Science Foundation of China (grant number 82273696
and 81973105). The funders have no role in the preparation of
manuscript and decision to submission.
Not applicable.
HY and GD conceived the present study. YH, YW, LZ,
FL, YJ, JL and SC performed the literature search, drafted the
manuscript and drew the figures. YH wrote the manuscript. All
authors read and approved the final manuscript. Data authentication
is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Tong SY, Davis JS, Eichenberger E, Holland
TL and Fowler VJ: Staphylococcus aureus infections:
Epidemiology, pathophysiology, clinical manifestations, and
management. Clin Microbiol Rev. 28:603–661. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang Y, Zhang P, Wu J, Chen S, Jin Y, Long
J, Duan G and Yang H: Transmission of livestock-associated
methicillin-resistant Staphylococcus aureus between animals,
environment, and humans in the farm. Environ Sci Pollut Res Int.
30:86521–86539. 2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Willyard C: Drug-resistant bacteria
ranked. Nature (London). 543(15)2017.
|
|
4
|
Hou H, Li Y, Jin Y, Chen S, Long J, Duan G
and Yang H: The crafty opponent: The defense systems of
Staphylococcus aureus and response measures. Folia Microbiol
(Praha). 67:233–243. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lakhundi S and Zhang K:
Methicillin-zesistant Staphylococcus aureus: Molecular
characterization, evolution, and epidemiology. Clin Microbiol Rev.
31:e00020–18. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gong C, Guan W, Liu X, Zheng Y, Li Z,
Zhang Y, Zhu S, Jiang H, Cui Z and Wu S: Biomimetic
bacteriophage-like particles formed from probiotic extracts and NO
donors for eradicating multidrug-resistant Staphylococcus
aureus. Adv Mater. 34(e2206134)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Plumet L, Ahmad-Mansour N, Dunyach-Remy C,
Kissa K, Sotto A, Lavigne JP, Costechareyre D and Molle V:
Bacteriophage therapy for Staphylococcus aureus infections:
A Review of animal models, treatments, and clinical trials. Front
Cell Infect Microbiol. 12(907314)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chand U, Priyambada P and Kushawaha PK:
Staphylococcus aureus vaccine strategy: Promise and
challenges. Microbiol Res. 271(127362)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Miller LS, Fowler VG, Shukla SK, Rose WE
and Proctor RA: Development of a vaccine against Staphylococcus
aureus invasive infections: Evidence based on human immunity,
genetics and bacterial evasion mechanisms. FEMS Microbiol Rev.
44:123–153. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Super M, Doherty EJ, Cartwright MJ, Seiler
BT, Langellotto F, Dimitrakakis N, White DA, Stafford AG, Karkada
M, Graveline AR, et al: Biomaterial vaccines capturing
pathogen-associated molecular patterns protect against bacterial
infections and septic shock. Nat Biomed Eng. 6:8–18.
2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
de Vor L, van Dijk B, van Kessel K,
Kavanaugh JS, de Haas C, Aerts PC, Viveen MC, Boel EC, Fluit AC,
Kwiecinski JM, et al: Human monoclonal antibodies against
Staphylococcus aureus surface antigens recognize in vitro
and in vivo biofilm. Elife. 11(e67301)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Raafat D, Otto M, Reppschlager K, Iqbal J
and Holtfreter S: Fighting Staphylococcus aureus biofilms
with monoclonal antibodies. Trends Microbiol. 27:303–322.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Haddad Kashani H, Schmelcher M,
Sabzalipoor H, Seyed Hosseini E and Moniri R: Recombinant
endolysins as potential therapeutics against antibiotic-resistant
Staphylococcus aureus: Current status of research and novel
delivery strategies. Clin Microbiol Rev. 31:e00071–17.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Defraine V, Fauvart M and Michiels J:
Fighting bacterial persistence: Current and emerging anti-persister
strategies and therapeutics. Drug Resist Updat. 38:12–26.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ganesan N, Mishra B, Felix L and Mylonakis
E: Antimicrobial peptides and small molecules targeting the cell
membrane of Staphylococcus aureus. Microbiol Mol Biol Rev.
87(e0003722)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hou X, Li J, Tang H, Li Q, Shen G, Li S,
Chen A, Peng Z, Zhang Y, Li C and Zhang Z: Antibacterial peptide
NP-6 affects Staphylococcus aureus by multiple modes of
action. Int J Mol Sci. 23(7812)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li J, Liu D, Tian X, Koseki S, Chen S, Ye
X and Ding T: Novel antibacterial modalities against methicillin
resistant Staphylococcus aureus derived from plants. Crit
Rev Food Sci Nutr. 59 (Suppl 1):S153–S161. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pirzadeh M, Caporaso N, Rauf A, Shariati
MA, Yessimbekov Z, Khan MU, Imran M and Mubarak MS: Pomegranate as
a source of bioactive constituents: A review on their
characterization, properties and applications. Crit Rev Food Sci
Nutr. 61:982–999. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Vestergaard M and Ingmer H: Antibacterial
and antifungal properties of resveratrol. Int J Antimicrob Agents.
53:716–723. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rai M, Ingle AP, Pandit R, Paralikar P,
Gupta I, Chaud MV and Dos Santos CA: Broadening the spectrum of
small-molecule antibacterials by metallic nanoparticles to overcome
microbial resistance. Int J Pharm. 532:139–148. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zahran M and Marei AH: Innovative natural
polymer metal nanocomposites and their antimicrobial activity. Int
J Biol Macromol. 136:586–596. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Makabenta JMV, Nabawy A, Li CH,
Schmidt-Malan S, Patel R and Rotello VM: Nanomaterial-based
therapeutics for antibiotic-resistant bacterial infections. Nat Rev
Microbiol. 19:23–36. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yuan Z, Lin C, Dai L, He Y, Hu J, Xu K,
Tao B, Liu P and Cai K: Near-infrared light-activatable dual-action
nanoparticle combats the established biofilms of
methicillin-resistant Staphylococcus aureus and its
accompanying inflammation. Small. 17(e2007522)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Babayevska N, Przysiecka Ł, Iatsunskyi I,
Nowaczyk G, Jarek M, Janiszewska E and Jurga S: ZnO size and shape
effect on antibacterial activity and cytotoxicity profile. Sci Rep.
12(8148)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang Y, Yang Y, Shi Y, Song H and Yu C:
Antibiotic-free antibacterial strategies enabled by nanomaterials:
Progress and perspectives. Adv Mater. 32(e1904106)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Maťátková O, Michailidu J, Miškovská A,
Kolouchová I, Masák J and Čejková A: Antimicrobial properties and
applications of metal nanoparticles biosynthesized by green
methods. Biotechnol Adv. 58(107905)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang L, Hu C and Shao L: The antimicrobial
activity of nanoparticles: Present situation and prospects for the
future. Int J Nanomedicine. 12:1227–1249. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Alzahrani KE, Niazy AA, Alswieleh AM,
Wahab R, El-Toni AM and Alghamdi HS: Antibacterial activity of
trimetal (CuZnFe) oxide nanoparticles. Int J Nanomedicine.
13:77–87. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Roszczenko P, Szewczyk OK, Czarnomysy R,
Bielawski K and Bielawska A: Biosynthesized gold, silver,
palladium, platinum, copper, and other transition metal
nanoparticles. Pharmaceutics. 14(2286)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Slavin YN, Asnis J, Häfeli UO and Bach H:
Metal nanoparticles: Understanding the mechanisms behind
antibacterial activity. J Nanobiotechnology. 15(65)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Huang X, Zheng X, Xu Z and Yi C: ZnO-based
nanocarriers for drug delivery application: From passive to smart
strategies. Int J Pharm. 534:190–194. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Dadi R, Azouani R, Traore M, Mielcarek C
and Kanaev A: Antibacterial activity of ZnO and CuO nanoparticles
against gram positive and gram negative strains. Mater Sci Eng C
Mater Biol Appl. 104(109968)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kalpana VN and Devi Rajeswari V: A Review
on green synthesis, biomedical applications, and toxicity studies
of ZnO NPs. Bioinorg Chem Appl. 2018(3569758)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lallo da Silva B, Caetano BL,
Chiari-Andréo BG, Pietro RCLR and Chiavacci LA: Increased
antibacterial activity of ZnO nanoparticles: Influence of size and
surface modification. Colloids Surf B Biointerfaces. 177:440–447.
2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Bianchini Fulindi R, Domingues Rodrigues
J, Lemos Barbosa TW, Goncalves Garcia AD, de Almeida La Porta F,
Pratavieira S, Chiavacci LA, Pessoa Araújo Junior J, da Costa PI
and Martinez LR: Zinc-based nanoparticles reduce bacterial biofilm
formation. Microbiol Spectr. 11(e0483122)2023.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
36
|
Kahandal A, Chaudhary S, Methe S, Nagwade
P, Sivaram A and Tagad CK: Galactomannan polysaccharide as a
biotemplate for the synthesis of zinc oxide nanoparticles with
photocatalytic, antimicrobial and anticancer applications. Int J
Biol Macromol. 253(126787)2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Abdelraheem WM, Khairy RMM, Zaki AI and
Zaki SH: Effect of ZnO nanoparticles on methicillin, vancomycin,
linezolid resistance and biofilm formation in Staphylococcus
aureus isolates. Ann Clin Microbiol Antimicrob.
20(54)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Irfan M, Munir H and Ismail H: Moringa
oleifera gum based silver and zinc oxide nanoparticles: Green
synthesis, characterization and their antibacterial potential
against MRSA. Biomater Res. 25(17)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
El-Masry RM, Talat D, Hassoubah SA,
Zabermawi NM, Eleiwa NZ, Sherif RM, Abourehab MSA, Abdel-Sattar RM,
Gamal M, Ibrahim MS and Elbestawy A: Evaluation of the
antimicrobial activity of ZnO nanoparticles against enterotoxigenic
Staphylococcus aureus. Life (Basel). 12(1662)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bommakanti V, Banerjee M, Shah D, Manisha
K, Sri K and Banerjee S: An overview of synthesis,
characterization, applications and associated adverse effects of
bioactive nanoparticles. Environ Res. 214(113919)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Massoudi I, Hamdi R, Ababutain I,
Alhussain E and Kharma A: HSBM-produced zinc oxide nanoparticles:
Physical properties and evaluation of their antimicrobial activity
against human pathogens. Scientifica (Cairo).
2022(9989282)2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yusof NAA, Zain NM and Pauzi N: Synthesis
of ZnO nanoparticles with chitosan as stabilizing agent and their
antibacterial properties against Gram-positive and Gram-negative
bacteria. Int J Biol Macromol. 124:1132–1136. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Asif N, Amir M and Fatma T: Recent
advances in the synthesis, characterization and biomedical
applications of zinc oxide nanoparticles. Bioprocess Biosyst Eng.
46:1377–1398. 2023.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Mendes AR, Granadeiro CM, Leite A, Pereira
E, Teixeira P and Poças F: optimizing antimicrobial efficacy:
Investigating the impact of zinc oxide nanoparticle shape and size.
Nanomaterials (Basel). 14(638)2024.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Stankic S, Suman S, Haque F and Vidic J:
Pure and multi metal oxide nanoparticles: Synthesis, antibacterial
and cytotoxic properties. J Nanobiotechnology.
14(73)2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Bai X, Li L, Liu H, Tan L, Liu T and Meng
X: Solvothermal synthesis of ZnO nanoparticles and anti-infection
application in vivo. ACS Appl Mater Interfaces. 7:1308–1317.
2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Navarro-López DE, Garcia-Varela R,
Ceballos-Sanchez O, Sanchez-Martinez A, Sanchez-Ante G,
Corona-Romero K, Buentello-Montoya DA, Elías-Zuñiga A and
López-Mena ER: Effective antimicrobial activity of ZnO and Yb-doped
ZnO nanoparticles against Staphylococcus aureus and
Escherichia coli. Mater Sci Eng C Mater Biol Appl.
123(112004)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Manyasree D, Kiranmayi P and Kolli VR:
Characterization and antibacterial activity of zno nanoparticles
synthesized by co precipitation method. Int J Appl Pharm.
10(224)2018.
|
|
49
|
Mahamuni PP, Patil PM, Dhanavade MJ,
Badiger MV, Shadija PG, Lokhande AC and Bohara RA: Synthesis and
characterization of zinc oxide nanoparticles by using polyol
chemistry for their antimicrobial and antibiofilm activity. Biochem
Biophys Rep. 17:71–80. 2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Manzoor U, Siddique S, Ahmed R, Noreen Z,
Bokhari H and Ahmad I: Antibacterial, structural and optical
characterization of mechano-chemically prepared ZnO nanoparticles.
PLoS One. 11(e0154704)2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Paiva-Santos AC, Herdade AM, Guerra C,
Peixoto D, Pereira-Silva M, Zeinali M, Mascarenhas-Melo F, Paranhos
A and Veiga F: Plant-mediated green synthesis of metal-based
nanoparticles for dermopharmaceutical and cosmetic applications.
Int J Pharm. 597(120311)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Salem SS and Fouda A: Green synthesis of
metallic nanoparticles and their prospective biotechnological
applications: An overview. Biol Trace Elem Res. 199:344–370.
2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ting BYS, Fuloria NK, Subrimanyan V, Bajaj
S, Chinni SV, Reddy LV, Sathasivam KV, Karupiah S, Malviya R,
Meenakshi DU, et al: Biosynthesis and response of zinc oxide
nanoparticles against periimplantitis triggering pathogens.
Materials (Basel). 15(3170)2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Doğaroğlu ZG, Uysal Y, çaylalı Z and
Karakulak DS: Green nanotechnology advances: Green manufacturing of
zinc nanoparticles, characterization, and foliar application on
wheat and antibacterial characteristics using Mentha spicata
(mint) and Ocimum basilicum (basil) leaf extracts. Environ
Sci Pollut Res Int. 30:60820–60837. 2023.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Sachin Jaishree, Singh N, Singh R, Shah K
and Pramanik BK: Green synthesis of zinc oxide nanoparticles using
lychee peel and its application in anti-bacterial properties and CR
dye removal from wastewater. Chemosphere.
327(138497)2023.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Mohammed YHI, Alghamdi S, Jabbar B,
Marghani D, Beigh S, Abouzied AS, Khalifa NE, Khojali WMA, Huwaimel
B, Alkhalifah DHM and Hozzein WN: Green synthesis of zinc oxide
nanoparticles using Cymbopogon citratus extract and its
antibacterial activity. ACS Omega. 8:32027–32042. 2023.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Mushtaq W, Ishtiaq M, Maqbool M, Mazhar
MW, Casini R, Abd-Elgawad AM and Elansary HO: Green synthesis of
zinc oxide nanoparticles using Viscum album extracts:
Unveiling bioactive compounds, antibacterial potential, and
antioxidant activities. Plants (Basel). 12(2130)2023.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Hasan M, Zafar A, Imran M, Iqbal KJ, Tariq
T, Iqbal J, Shaheen A, Hussain R, Anjum SI and Shu X: Crest to
trough cellular drifting of green-synthesized zinc oxide and silver
nanoparticles. ACS Omega. 7:34770–34778. 2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Irfan M, Munir H and Ismail H:
Characterization and fabrication of zinc oxide nanoparticles by gum
Acacia modesta through green chemistry and impregnation on
surgical sutures to boost up the wound healing process. Int J Biol
Macromol. 204:466–475. 2022.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Alallam B, Doolaanea AA, Alfatama M and
Lim V: Phytofabrication and characterisation of zinc oxide
nanoparticles using pure curcumin. Pharmaceuticals (Basel).
16(269)2023.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Malhotra A, Chauhan SR, Rahaman M,
Tripathi R, Khanuja M and Chauhan A: Phyto-assisted synthesis of
zinc oxide nanoparticles for developing antibiofilm surface
coatings on central venous catheters. Front Chem.
11(1138333)2023.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Khanna P, Kaur A and Goyal D: Algae-based
metallic nanoparticles: Synthesis, characterization and
applications. J Microbiol Methods. 163(105656)2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Jamil Z, Naqvi STQ, Rasul S, Hussain SB,
Fatima N, Qadir MI and Muhammad SA: Antibacterial activity and
characterization of zinc oxide nanoparticles synthesized by
microalgae. Pak J Pharm Sci. 33:2497–2504. 2020.PubMed/NCBI
|
|
64
|
Morowvat MH, Kazemi K, Jaberi MA, Amini A
and Gholami A: Biosynthesis and antimicrobial evaluation of zinc
oxide nanoparticles using Chlorella vulgaris biomass against
multidrug-resistant pathogens. Materials (Basel).
16(842)2023.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Alsaggaf MS, Diab AM, Elsaied BEF, Tayel
AA and Moussa SH: Application of ZnO nanoparticles phycosynthesized
with Ulva fasciata extract for preserving peeled shrimp
quality. Nanomaterials (Basel). 11(385)2021.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Lopez-Miranda JL, Molina GA,
González-Reyna MA, España-Sánchez BL, Esparza R, Silva R and
Estévez M: Antibacterial and anti-inflammatory properties of ZnO
nanoparticles synthesized by a green method using Sargassum
extracts. Int J Mol Sci. 24(1474)2023.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Ebadi M, Zolfaghari MR, Aghaei SS, Zargar
M and Noghabi KA: Desertifilum sp. EAZ03 cell extract as a
novel natural source for the biosynthesis of zinc oxide
nanoparticles and antibacterial, anticancer and antibiofilm
characteristics of synthesized zinc oxide nanoparticles. J Appl
Microbiol. 132:221–236. 2022.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Ebadi M, Zolfaghari MR, Aghaei SS, Zargar
M, Shafiei M, Zahiri HS and Noghabi KA: A bio-inspired strategy for
the synthesis of zinc oxide nanoparticles (ZnO NPs) using the cell
extract of cyanobacterium Nostoc sp. EA03: From biological
function to toxicity evaluation. RSC Adv. 9:23508–23525.
2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Barani M, Masoudi M, Mashreghi M,
Makhdoumi A and Eshghi H: Cell-free extract assisted synthesis of
ZnO nanoparticles using aquatic bacterial strains: Biological
activities and toxicological evaluation. Int J Pharm.
606(120878)2021.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Dikshit P, Kumar J, Das AK, Sadhu S,
Sharma S, Singh S, Gupta PK and Kim BS: Green synthesis of metallic
nanoparticles: Applications and limitations. Catalysts.
11(902)2021.
|
|
71
|
Mohd Yusof H, Abdul Rahman NA, Mohamad R,
Zaidan UH and Samsudin AA: Biosynthesis of zinc oxide nanoparticles
by cell-biomass and supernatant of Lactobacillus plantarum
TA4 and its antibacterial and biocompatibility properties. Sci Rep.
10(19996)2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Rehman S, Jermy BR, Akhtar S, Borgio JF,
Abdul AS, Ravinayagam V, Al Jindan R, Alsalem ZH, Buhameid A and
Gani A: Isolation and characterization of a novel thermophile;
Bacillus haynesii, applied for the green synthesis of ZnO
nanoparticles. Artif Cells Nanomed Biotechnol. 47:2072–2082.
2019.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Shaaban M and El-Mahdy AM: Biosynthesis of
Ag, Se, and ZnO nanoparticles with antimicrobial activities against
resistant pathogens using waste isolate Streptomyces
enissocaesilis. IET Nanobiotechnol. 12:741–747. 2018.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Taran M, Rad M and Alavi M: Biosynthesis
of TiO2 and ZnO nanoparticles by Halomonas
elongata IBRC-M 10214 in different conditions of medium.
Bioimpacts. 8:81–89. 2018.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Taran M and Amirkhani H: Strategies of
poly(3-hydroxybutyrate) synthesis by Haloarcula sp. IRU1 utilizing
glucose as carbon source: Optimization of culture conditions by
Taguchi methodology. Int J Biol Macromol. 47:632–634.
2010.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Kang MG, Khan F, Jo DM, Oh D, Tabassum N
and Kim YM: Antibiofilm and antivirulence activities of gold and
zinc oxide nanoparticles synthesized from kimchi-isolated
Leuconostoc sp. Strain C2. Antibiotics (Basel).
11(1524)2022.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Sharma JL, Dhayal V and Sharma RK:
White-rot fungus mediated green synthesis of zinc oxide
nanoparticles and their impregnation on cellulose to develop
environmental friendly antimicrobial fibers. 3 Biotech.
11(269)2021.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Mohamed AA, Abu-Elghait M, Ahmed NE and
Salem SS: Eco-friendly mycogenic synthesis of ZnO and CuO
nanoparticles for in vitro antibacterial, antibiofilm, and
antifungal applications. Biol Trace Elem Res. 199:2788–2799.
2021.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Sumanth B, Lakshmeesha TR, Ansari MA,
Alzohairy MA, Udayashankar AC, Shobha B, Niranjana SR, Srinivas C
and Almatroudi A: Mycogenic synthesis of extracellular zinc oxide
nanoparticles from Xylaria acuta and its nanoantibiotic
potential. Int J Nanomedicine. 15:8519–8536. 2020.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Abdelkader DH, Negm WA, Elekhnawy E, Eliwa
D, Aldosari BN and Almurshedi AS: Zinc oxide nanoparticles as
potential delivery carrier: Green synthesis by Aspergillus niger
Endophytic fungus, characterization, and in vitro/in vivo
antibacterial activity. Pharmaceuticals (Basel).
15(1057)2022.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Motazedi R, Rahaiee S and Zare M:
Efficient biogenesis of ZnO nanoparticles using extracellular
extract of Saccharomyces cerevisiae: Evaluation of
photocatalytic, cytotoxic and other biological activities. Bioorg
Chem. 101(103998)2020.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Akbar N, Aslam Z, Siddiqui R, Shah MR and
Khan NA: Zinc oxide nanoparticles conjugated with
clinically-approved medicines as potential antibacterial molecules.
AMB Express. 11(104)2021.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Ghaffar N, Javad S, Farrukh MA, Shah AA,
Gatasheh MK, Al-Munqedhi BMA and Chaudhry O: Metal nanoparticles
assisted revival of Streptomycin against MDRS Staphylococcus
aureus. PLoS One. 17(e0264588)2022.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Nejabatdoust A, Zamani H and Salehzadeh A:
Functionalization of ZnO nanoparticles by glutamic acid and
conjugation with thiosemicarbazide alters expression of efflux pump
genes in multiple drug-resistant Staphylococcus aureus
strains. Microb Drug Resist. 25:966–974. 2019.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Habib S, Rashid F, Tahir H, Liaqat I,
Latif AA, Naseem S, Khalid A, Haider N, Hani U, Dawoud RA, et al:
Antibacterial and cytotoxic effects of biosynthesized zinc oxide
and titanium dioxide nanoparticles. Microorganisms.
11(1363)2023.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Abdelghafar A, Yousef N and Askoura M:
Zinc oxide nanoparticles reduce biofilm formation, synergize
antibiotics action and attenuate Staphylococcus aureus
virulence in host; an important message to clinicians. BMC
Microbiol. 22(244)2022.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Hemmati F, Salehi R, Ghotaslou R, Kafil
HS, Hasani A, Gholizadeh P and Rezaee MA: The assessment of
antibiofilm activity of chitosan-zinc oxide-gentamicin
nanocomposite on Pseudomonas aeruginosa and
Staphylococcus aureus. Int J Biol Macromol. 163:2248–2258.
2020.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Saddik MS, Elsayed MMA, El-Mokhtar MA,
Sedky H, Abdel-Aleem JA, Abu-Dief AM, Al-Hakkani MF, Hussein HL,
Al-Shelkamy SA, Meligy FY, et al: Tailoring of novel
azithromycin-loaded zinc oxide nanoparticles for wound healing.
Pharmaceutics. 14(111)2022.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Khan SA, Shahid S, Mahmood T and Lee CS:
Contact lenses coated with hybrid multifunctional ternary
nanocoatings (phytomolecule-coated ZnO nanoparticles:Gallic
acid:Tobramycin) for the treatment of bacterial and fungal
keratitis. Acta Biomater. 128:262–276. 2021.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Rath G, Hussain T, Chauhan G, Garg T and
Goyal AK: Development and characterization of cefazolin loaded zinc
oxide nanoparticles composite gelatin nanofiber mats for
postoperative surgical wounds. Mater Sci Eng C Mater Biol Appl.
58:242–253. 2016.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Oves M, Rauf MA, Ansari MO, Aslam Parwaz
Khan A, A Qari H, Alajmi MF, Sau S and Iyer AK: Graphene decorated
zinc oxide and curcumin to disinfect the methicillin-resistant
Staphylococcus aureus. Nanomaterials (Basel).
10(1004)2020.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Zhai F, Luo Y, Zhang Y, Liao S, Cheng J,
Meng X, Zeng Y, Wang X, Yang J, Yin J and Li L: Viscosity
simulation of glass microfiber and an unusual air filter with
high-efficiency antibacterial functionality enabled by
ZnO/graphene-modified glass microfiber. ACS Omega. 7:14211–14221.
2022.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Chai Q, Wu Q, Liu T, Tan L, Fu C, Ren X,
Yang Y and Meng X: Enhanced antibacterial activity of silica
nanorattles with ZnO combination nanoparticles against
methicillin-resistant Staphylococcus aureus. Sci Bull
(Beijing). 62:1207–1215. 2017.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Vinotha V, Yazhiniprabha M, Jeyavani J and
Vaseeharan B: Synthesis and characterization of cry protein coated
zinc oxide nanocomposites and its assessment against bacterial
biofilm and mosquito vectors. Int J Biol Macromol. 208:935–947.
2022.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Azizi-Lalabadi M, Ehsani A, Divband B and
Alizadeh-Sani M: Antimicrobial activity of titanium dioxide and
zinc oxide nanoparticles supported in 4A zeolite and evaluation the
morphological characteristic. Sci Rep. 9(17439)2019.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Banerjee S, Vishakha K, Das S, Dutta M,
Mukherjee D, Mondal J, Mondal S and Ganguli A: Antibacterial,
anti-biofilm activity and mechanism of action of pancreatin doped
zinc oxide nanoparticles against methicillin resistant
Staphylococcus aureus. Colloids Surf B Biointerfaces.
190(110921)2020.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Canales DA, Piñones N, Saavedra M, Loyo C,
Palza H, Peponi L, Leonés A, Baier RV, Boccaccini AR, Grünelwald A
and Zapata PA: Fabrication and assessment of bifunctional
electrospun poly(l-lactic acid) scaffolds with bioglass and zinc
oxide nanoparticles for bone tissue engineering. Int J Biol
Macromol. 228:78–88. 2023.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Pitpisutkul V and Prachayawarakorn J:
Hydroxypropyl methylcellulose/carboxymethyl starch/zinc oxide
porous nanocomposite films for wound dressing application.
Carbohydr Polym. 298(120082)2022.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Majeed A, Javed F, Akhtar S, Saleem U,
Anwar F, Ahmad B, Nadhman A, Shahnaz G, Hussain I, Hussain SZ and
Sohail MF: Green synthesized selenium doped zinc oxide
nano-antibiotic: Synthesis, characterization and evaluation of
antimicrobial, nanotoxicity and teratogenicity potential. J Mater
Chem B. 8:8444–8458. 2020.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Zabihi E, Arab-Bafrani Z, Hoseini SM,
Mousavi E, Babaei A, Khalili M, Hashemi MM and Javid N: Fabrication
of nano-decorated ZnO-fibrillar chitosan exhibiting a superior
performance as a promising replacement for conventional ZnO.
Carbohydr Polym. 274(118639)2021.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Karthikeyan C, Tharmalingam N, Varaprasad
K, Mylonakis E and Yallapu MM: Biocidal and biocompatible hybrid
nanomaterials from biomolecule chitosan, alginate and ZnO.
Carbohydr Polym. 274(118646)2021.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Kang J, Hu C, Dichiara A, Guan L, Yun H
and Gu J: Facile preparation of cellulose nanocrystals/ZnO hybrids
using acidified ZnCl2 as cellulose hydrolytic media and
ZnO precursor. Int J Biol Macromol. 227:863–871. 2023.PubMed/NCBI View Article : Google Scholar
|
|
103
|
AbouAitah K, Piotrowska U, Wojnarowicz J,
Swiderska-Sroda A, El-Desoky AHH and Lojkowski W: Enhanced activity
and sustained release of protocatechuic acid, a natural
antibacterial agent, from hybrid nanoformulations with zinc oxide
nanoparticles. Int J Mol Sci. 22(5287)2021.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Naserian F and Mesgar AS: Development of
antibacterial and superabsorbent wound composite sponges containing
carboxymethyl cellulose/gelatin/Cu-doped ZnO nanoparticles.
Colloids Surf B Biointerfaces. 218(112729)2022.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Nageswara Rao B, Tirupathi Rao P, Vasudha
K, Esub Basha S, Prasanna DSL, Bhushana Rao T, Samatha K and
Ramachandra RK: Physiochemical characterization of sodium doped
zinc oxide nano powder for antimicrobial applications. Spectrochim
Acta A Mol Biomol Spectrosc. 291(122297)2023.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Fu F, Gu J, Zhang R, Xu X, Yu X, Liu L,
Liu X, Zhou J and Yao J: Three-dimensional cellulose based
silver-functionalized ZnO nanocomposite with controlled geometry:
Synthesis, characterization and properties. J Colloid Interface
Sci. 530:433–443. 2018.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Hu M, Li C, Li X, Zhou M, Sun J, Sheng F,
Shi S and Lu L: Zinc oxide/silver bimetallic nanoencapsulated in
PVP/PCL nanofibres for improved antibacterial activity. Artif Cells
Nanomed Biotechnol. 46:1248–1257. 2018.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Mohammadi-Aloucheh R, Habibi-Yangjeh A,
Bayrami A, Latifi-Navid S and Asadi A: Enhanced anti-bacterial
activities of ZnO nanoparticles and ZnO/CuO nanocomposites
synthesized using Vaccinium arctostaphylos L. fruit extract. Artif
Cells Nanomed Biotechnol. 46 (Suppl 1):S1200–S1209. 2018.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Bahari A, Roeinfard M, Ramzannezhad A,
Khodabakhshi M and Mohseni M: Nanostructured Features and
Antimicrobial Properties of Fe3O4/ZnO Nanocomposites. Natl Acad Sci
Lett. 42:9–12. 2019.
|
|
110
|
AlSalhi MS, Devanesan S, Asemi N and
Ahamed A: Concurrent fabrication of
ZnO-ZnFe2O4 hybrid nanocomposite for
enhancing photocatalytic degradation of organic pollutants and its
bacterial inactivation. Chemosphere. 318(137928)2023.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Lee M, Han SI, Kim C, Velumani S, Han A,
Kassiba AH and Castaneda H: ZrO2/ZnO/TiO2
nanocomposite coatings on stainless steel for improved corrosion
resistance, biocompatibility, and antimicrobial activity. ACS Appl
Mater Interfaces. 14:13801–13811. 2022.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Pulit-Prociak J, Staroń A, Staroń P,
Chmielowiec-Korzeniowska A, Drabik A, Tymczyna L and Banach M:
Preparation and of PVA-based compositions with embedded silver,
copper and zinc oxide nanoparticles and assessment of their
antibacterial properties. J Nanobiotechnology.
18(148)2020.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Akhil K, Jayakumar J, Gayathri G and Khan
SS: Effect of various capping agents on photocatalytic,
antibacterial and antibiofilm activities of ZnO nanoparticles. J
Photochem Photobiol B. 160:32–42. 2016.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Choi KH, Nam KC, Lee SY, Cho G, Jung JS,
Kim HJ and Park BJ: Antioxidant potential and antibacterial
efficiency of caffeic acid-functionalized ZnO nanoparticles.
Nanomaterials (Basel). 7(148)2017.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Du M, Zhao W, Ma R, Xu H, Zhu Y, Shan C,
Liu K, Zhuang J and Jiao Z: Visible-light-driven photocatalytic
inactivation of S. aureus in aqueous environment by
hydrophilic zinc oxide (ZnO) nanoparticles based on the interfacial
electron transfer in S. aureus/ZnO composites. J Hazard
Mater. 418(126013)2021.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Charoensri K, Rodwihok C, Wongratanaphisan
D, Ko JA, Chung JS and Park HJ: Investigation of functionalized
surface charges of thermoplastic starch/zinc oxide nanocomposite
films using polyaniline: The potential of improved antibacterial
properties. Polymers (Basel). 13(425)2021.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Lee J, Choi KH, Min J, Kim HJ, Jee JP and
Park BJ: Functionalized ZnO nanoparticles with gallic acid for
antioxidant and antibacterial activity against
methicillin-resistant S. aureus. Nanomaterials (Basel).
7(365)2017.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Chen J, Jing Q, Xu Y, Lin Y, Mai Y, Chen
L, Wang G, Chen Z, Deng L, Chen J, et al: Functionalized zinc oxide
microparticles for improving the antimicrobial effects of skin-care
products and wound-care medicines. Biomater Adv.
135(212728)2022.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Yuan K, Liu X, Shi J, Liu W, Liu K, Lu H,
Wu D, Chen Z and Lu C: Antibacterial properties and mechanism of
lysozyme-modified ZnO nanoparticles. Front Chem.
9(762255)2021.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Popa ML, Preda MD, Neacșu IA, Grumezescu
AM and Ginghină O: Traditional vs microfluidic synthesis of ZnO
nanoparticles. Int J Mol Sci. 24(1875)2023.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Ahmed S, Annu Chaudhry SA and Ikram S: A
review on biogenic synthesis of ZnO nanoparticles using plant
extracts and microbes: A prospect towards green chemistry. J
Photochem Photobiol B. 166:272–284. 2017.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Kapp K, Orav A, Roasto M, Raal A, Püssa T,
Vuorela H, Tammela P and Vuorela P: Composition and antibacterial
effect of mint flavorings in candies and food supplements. Planta
Med. 86:1089–1096. 2020.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Deng Z, Bheemanaboina RRY, Luo Y and Zhou
CH: Aloe emodin-conjugated sulfonyl hydrazones as novel type of
antibacterial modulators against S. aureus 25923 through
multifaceted synergistic effects. Bioorg Chem.
127(106035)2022.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Hussain Y, Alam W, Ullah H, Dacrema M,
Daglia M, Khan H and Arciola CR: Antimicrobial potential of
curcumin: Therapeutic potential and challenges to clinical
applications. Antibiotics (Basel). 11(322)2022.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Singh P, Garg A, Pandit S, Mokkapati VRSS
and Mijakovic I: Antimicrobial effects of biogenic nanoparticles.
Nanomaterials (Basel). 8(1009)2018.PubMed/NCBI View Article : Google Scholar
|
|
126
|
El-Gendy AO, Nawaf KT, Ahmed E, Samir A,
Hamblin MR, Hassan M and Mohamed T: Preparation of zinc oxide
nanoparticles using laser-ablation technique: Retinal epithelial
cell (ARPE-19) biocompatibility and antimicrobial activity when
activated with femtosecond laser. J Photochem Photobiol B.
234(112540)2022.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Sindelo A, Nene L and Nyokong T:
Photodynamic antimicrobial chemotherapy with asymmetrical cationic
or neutral metallophthalocyanines conjugated to
amino-functionalized zinc oxide nanoparticles (spherical or
pyramidal) against planktonic and biofilm microbial cultures.
Photodiagnosis Photodyn Ther. 40(103160)2022.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Ruan Q, Yuan L, Gao S, Ji X, Shao W, Ma J
and Jiang D: Development of ZnO/selenium nanoparticles embedded
chitosan-based anti-bacterial wound dressing for potential healing
ability and nursing care after paediatric fracture surgery. Int
Wound J. 20:1819–1831. 2023.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Ismail A, Raya NR, Orabi A, Ali AM and
Abo-Zeid Y: Investigating the antibacterial activity and safety of
zinc oxide nanoparticles versus a commercial alcohol-based
hand-sanitizer: Can zinc oxide nanoparticles be useful for hand
sanitation? Antibiotics (Basel). 11(1606)2022.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Venkatraman G, Mohan PS, Abdul-Rahman PS,
Sonsudin F, Muttiah B, Hirad AH, Alarfaj AA and Wang S: Morinda
citrifolia leaf assisted synthesis of ZnO decorated Ag
bio-nanocomposites for in-vitro cytotoxicity, antimicrobial and
anticancer applications. Bioprocess Biosyst Eng. 47:1213–1226.
2024.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Pereira da Costa Araújo A, Lima VS,
Emmanuela de Andrade Vieira J, Mesak C and Malafaia G: First report
on the mutagenicity and cytotoxicity of Zno nanoparticles in
reptiles. Chemosphere. 235:556–564. 2019.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Yang D, Zhang M, Gan Y, Yang S, Wang J, Yu
M, Wei J and Chen J: Involvement of oxidative stress in ZnO
NPs-induced apoptosis and autophagy of mouse GC-1 spg cells.
Ecotoxicol Environ Saf. 202(110960)2020.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Al-Zahaby SA, Farag MR, Alagawany M, Taha
HSA, Varoni MV, Crescenzo G and Mawed SA: Zinc oxide nanoparticles
(ZnO-NPs) induce cytotoxicity in the zebrafish olfactory organs via
activating oxidative stress and apoptosis at the ultrastructure and
genetic levels. Animals (Basel). 13(2867)2023.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Nazir S, Rabbani A, Mehmood K, Maqbool F,
Shah GM, Khan MF and Sajid M: Antileishmanial activity and
cytotoxicity of ZnO-based nano-formulations. Int J Nanomedicine.
14:7809–7822. 2019.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Czyzowska A and Barbasz A: Cytotoxicity of
zinc oxide nanoparticles to innate and adaptive human immune cells.
J Appl Toxicol. 41:1425–1437. 2021.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Niño-Martínez N, Salas Orozco MF,
Martínez-Castañón GA, Torres Méndez F and Ruiz F: Molecular
mechanisms of bacterial resistance to metal and metal oxide
nanoparticles. Int J Mol Sci. 20(2808)2019.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Alsmadi MM, Al-Nemrawi NK, Obaidat R, Abu
Alkahsi AE, Korshed KM and Lahlouh IK: Insights into the mapping of
green synthesis conditions for ZnO nanoparticles and their
toxicokinetics. Nanomedicine (Lond). 17:1281–1303. 2022.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Marinescu L, Ficai D, Oprea O, Marin A,
Ficai A, Andronescu E and Holban AM: Optimized Synthesis approaches
of metal nanoparticles with antimicrobial applications. J
Nanomater. 2020:1–14. 2020.
|
|
139
|
Sajjad A, Bhatti SH, Ali Z, Jaffari GH,
Khan NA, Rizvi ZF and Zia M: Photoinduced fabrication of zinc oxide
nanoparticles: Transformation of morphological and biological
response on light irradiance. ACS Omega. 6:11783–11793.
2021.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Al-Mosawi RM, Jasim HA and Haddad A: Study
of the antibacterial effects of the starch-based zinc oxide
nanoparticles on methicillin resistance Staphylococcus
aureus isolates from different clinical specimens of patients
from Basrah, Iraq. AIMS Microbiol. 9:90–107. 2023.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Kim I, Viswanathan K, Kasi G, Sadeghi K,
Thanakkasaranee S and Seo J: Preparation and characterization of
positively surface charged zinc oxide nanoparticles against
bacterial pathogens. Microb Pathog. 149(104290)2020.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Hozyen HF, Ibrahim ES, Khairy EA and
El-Dek SI: Enhanced antibacterial activity of capped zinc oxide
nanoparticles: A step towards the control of clinical bovine
mastitis. Vet World. 12:1225–1232. 2019.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Khan MF, Hameedullah M, Ansari AH, Ahmad
E, Lohani MB, Khan RH, Alam MM, Khan W, Husain FM and Ahmad I:
Flower-shaped ZnO nanoparticles synthesized by a novel approach at
near-room temperatures with antibacterial and antifungal
properties. Int J Nanomedicine. 9:853–864. 2014.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Gharpure S, Jadhav T, Ghotekar C, Jagtap
A, Vare Y and Ankamwar B: Non-antibacterial and antibacterial ZnO
nanoparticles composed of different surfactants. J Nanosci
Nanotechnol. 21:5945–5959. 2021.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Wahab R, Mishra A, Yun SI, Kim YS and Shin
HS: Antibacterial activity of ZnO nanoparticles prepared via
non-hydrolytic solution route. Appl Microbiol Biotechnol.
87:1917–1925. 2010.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Al-Askar AA, Hashem AH, Elhussieny NI and
Saied E: Green biosynthesis of zinc oxide nanoparticles using
Pluchea indica leaf extract: Antimicrobial and
photocatalytic activities. Molecules. 28(4679)2023.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Manojkumar U, Kaliannan D, Srinivasan V,
Balasubramanian B, Kamyab H, Mussa ZH, Palaniyappan J, Mesbah M,
Chelliapan S and Palaninaicker S: Green synthesis of zinc oxide
nanoparticles using Brassica oleracea var. botrytis leaf extract:
Photocatalytic, antimicrobial and larvicidal activity. Chemosphere.
323(138263)2023.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Irfan M, Naz MY, Saleem M, Tanawush M,
Głowacz A, Glowacz W, Rahman S, Mahnashi MH, Alqahtani YS, Alyami
BA, et al: Statistical study of nonthermal plasma-assisted ZnO
coating of cotton fabric through ultrasonic-assisted green
synthesis for improved self-cleaning and antimicrobial properties.
Materials (Basel). 14(6998)2021.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Abdo AM, Fouda A, Eid AM, Fahmy NM,
Elsayed AM, Khalil AMA, Alzahrani OM, Ahmed AF and Soliman AM:
Green synthesis of zinc oxide nanoparticles (ZnO-NPs) by
Pseudomonas aeruginosa and their activity against pathogenic
microbes and common house mosquito, culex pipiens. Materials
(Basel). 14(6983)2021.PubMed/NCBI View Article : Google Scholar
|