1. Introduction
Colorectal cancer (CRC) accounts for ~10% of all new
cases of cancer detected worldwide (1). CRC treatment is commonly based on a
combination of chemotherapy, radiotherapy and, more recently,
immunotherapy (2). However, the
need for new drugs remains constant given the primary
chemoresistance associated with CRC, which results in poor overall
survival and prognosis, even in patients treated with combined
therapy (3).
The aetiology of CRC is closely associated with
chronic inflammation of the colonic epithelium, which can originate
from genetic mutations or extrinsic factors, leading to the
constant activation of different signalling pathways associated
with the immune response. During the inflammatory process, the
production of pro-inflammatory cytokines, such as IL-1β, IL-6,
IL-18 and tumour necrosis factor-α (TNF-α), induces the activation
of signalling pathways associated with proliferation,
vascularisation and inhibition of apoptosis through signal
transducer and activator of transcription 3 (STAT3), which is a
master regulator of pro-inflammatory pathways (4).
STAT3 has been reported to be upregulated in a high
proportion of tumour types (5);
thus, STAT3 is one of the most studied signal intermediaries in
tumour development. STAT3 regulates the transcription of various
genes implicated in the inhibition of apoptosis; among them,
BIRC5, a member of the inhibitor of apoptosis protein (IAP)
family that encodes the protein survivin, is of particular interest
because it has a dual role as a regulator of apoptosis and cell
mitosis (6). Survivin is a subunit
of the chromosomal passenger complex (CPC), along with the mitotic
kinase Aurora-B, Borealin and inner centromere protein. The CPC
serves an essential role for adequate segregation of chromosomes
and cytokinesis during cell mitosis (6). In addition, survivin controls
apoptosis mediated by caspase-3 and -7(7). Although survivin expression is
restricted to foetal tissues in healthy individuals, it has been
found to be upregulated in various types of cancer, including CRC
(6).
Because of the relevant participation of STAT3 and
survivin in the control of molecular events promoting and
maintaining the oncogenic phenotype in CRC, they have emerged as
promising potential targets, given their selective expression in
cancerous tissues. Additionally, since survivin is
transcriptionally regulated by STAT3, and studies have demonstrated
the relevance of their interaction for the acquisition of
chemoresistance and the evasion of apoptosis, they may be
considered targets for future therapeutic approaches (6,8).
In the present review, the function of the
STAT3-survivin axis in CRC is discussed, with particular emphasis
on the relationship between both molecules and CRC progression.
This review also summarizes recent proposals for the clinical
applications of STAT3 and survivin inhibitors.
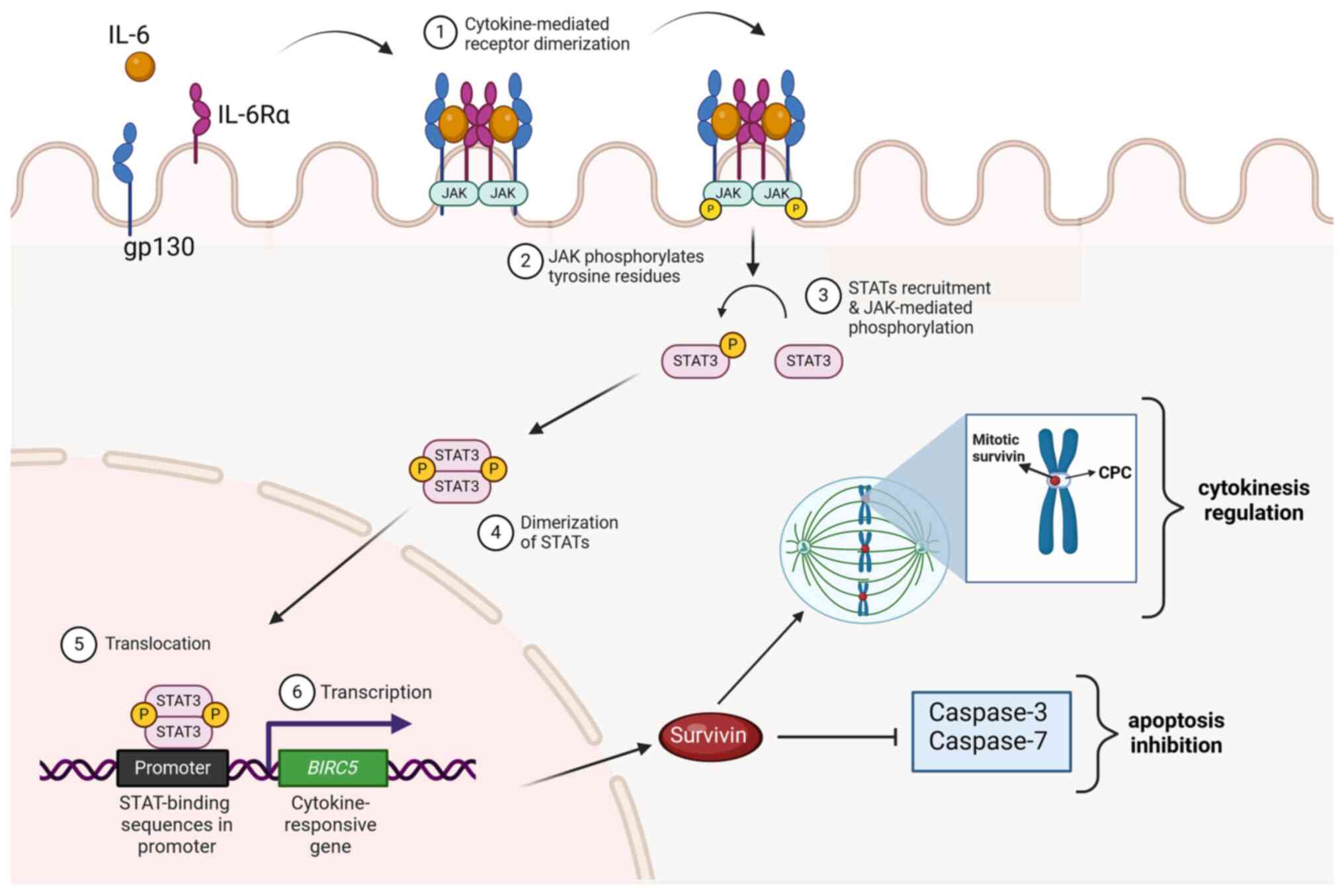
2. STAT3-survivin axis
STAT3 is a member of the STAT family of
transcription factors, which includes seven proteins: STAT1, STAT2,
STAT3, STAT4, STAT5A, STAT5B and STAT6. These proteins transduce
signals downstream of >50 ILs, hormones and growth factors
(8,9). After being phosphorylated by Janus
kinases, activated STAT3 [phosphorylated-STAT3 (pSTAT3)] molecules
translocate into the cell nucleus to regulate the transcription of
genes involved in the cell cycle, differentiation, proliferation,
inflammation, immune response and apoptosis (10,11).
The structure of STAT3 includes a C-terminal
transactivation domain, a coiled-coil domain, a DNA-binding domain,
a linker, a Src homology 2 (SH2) domain and an N-terminal domain
which mediates binding to gene promoters and enhancers (12,13).
STAT3 induces the transcription of genes whose products are
regulators of cell survival, such as the anti-apoptotic protein
survivin. Survivin is a member of the IAP family; it contains a
baculoviral IAP repeat domain, which mediates protein-protein
interactions (PPIs) (14-16).
Survivin is mainly expressed in foetal tissue, but it has also been
found to be upregulated in multiple tumours (17).
Survivin is a small protein (142 amino acids; 16.5
kDa) encoded by the BIRC5 gene. STAT3 is the main
transcription factor activating BIRC5 expression; however,
hypoxia-inducible factor 1 and nuclear factor kB are also able to
induce BIRC5 transcription (18). Survivin is a bifunctional protein
that participates in both the inhibition of apoptosis and the
regulation of cell division, serving an important role during
chromosome segregation and cytokinesis (19,20).
Survivin prevents cells from apoptotic death by decreasing the
activity of effector caspases (21); by interacting with other apoptosis
regulators, such as XIAP, hepatitis B virus X-interacting protein
(22), cIAP1 and cIAP2(23); and by interfering with inhibitors of
IAP activity (24). Notably,
survivin has also been reported to participate in angiogenesis
(25), autophagy (26) and stem cell maintenance (27).
3. STAT3-survivin axis in CRC
Given that STAT3 regulates the expression of an
extensive number of genes associated with carcinogenic processes,
the activity of this transcription factor has been studied in
several tumour types. Immunohistochemical evaluation of tumour
samples and the analysis of transcriptomic data from publicly
available datasets demonstrated that STAT3 was upregulated in ~70%
of cancer types, including CRC (28). The study of pSTAT3 expression in CRC
and colorectal adenoma tissue samples by immunohistochemistry
demonstrated that the level of pSTAT3 in CRC was significantly
higher than that in adenoma, and it was associated with tumour
progression (29). Furthermore,
evaluation of pSTAT3 levels by immunohistochemistry in a
prospective cohort of 724 patients with CRC demonstrated that
pSTAT3 upregulation was negatively associated with a good prognosis
in patients with CRC (30).
Using systems biology approaches, Erdogan et
al (28) analysed the global
STAT interactome. The authors collected data using the Integrated
Interactions Database, which gathers PPI data from various
databases, and allows segregation of these interactions into
disease and tissue contexts. Specific STAT interactions in the
context of CRC were confirmed when particular PPIs were reported in
two or more independent publications, databases or experimental
assays. The results suggested that STAT3 may be one of the most
relevant molecules during CRC progression (28). Notably, STAT3 has been found to
contribute to CRC malignancy by establishing cascades of
interactions with other molecules. Atypical activation of STAT3 may
be related to excessive concentrations of cytokines in the
surrounding tumour microenvironment. Notably, CRC has been strongly
associated with chronic inflammation, and IL-6 is produced by gut
immune cells as a response to local inflammation, further inducing
the activation of STAT3, creating a key circuit to stimulate
colonic malignant transformation. Thus, among upstream STAT3
inductor factors, IL-6 has been widely recognised as a major
activator in CRC, creating a pathway that stimulates proliferation,
invasion and metastasis (31).
Moreover, the detection of high levels of serum IL-6, measured
using electrochemiluminescence assays, along with a positive
immunohistochemical staining of IL-6 in tumour and stromal cells,
have been shown to be correlated with poor prognosis and a local
immunosuppressive state in patients with CRC (32). Although it is known that the number
of cancer-associated STAT3 target genes is large, those encoding
for inhibitors of apoptosis are of particular importance. Thus,
survivin has long been considered a key participant in the
STAT3-associated carcinogenic process (Fig. 1).
Immunohistochemical analyses of large numbers of CRC
tumours demonstrated that expression of active pSTAT3 in the
cytoplasmic and nuclear compartments was associated with higher
mortality, increased reactive lymph nodes (30), clinical stage, tumour invasion depth
and reduced overall survival (29).
Simultaneously, studies of relative survivin mRNA expression
levels, as determined by semi-quantitative PCR (33) and quantitative PCR (34,35),
and evaluation of survivin expression by western blotting and
immunostaining (33), have been
performed in CRC cohorts showing a higher expression in tumour
tissue as compared with that in paired adjacent tissue samples. As
expected, survivin expression was revealed to be associated with
lower apoptotic index in tumours (33). Survivin expression has also been
associated with CRC progression. Kawasaki et al (36) evaluated survivin in colon
hyperplastic polyp, low dysplastic adenoma, adenoma with high
dysplasia and carcinoma samples by immunohistochemistry, finding
that immunoreactivity of survivin was significantly augmented in
the transition from adenoma with low dysplasia to high dysplasia
and carcinoma. Further studies demonstrated that survivin encoding
gene expression was elevated in more advanced CRC samples (37), and that positive cytoplasmic
survivin immunostaining was strongly associated with lymph node
metastasis (38). Notably, when CRC
cases were stratified by age, survivin mRNA expression measured by
quantitative PCR was revealed to be higher in elderly patients
(>70 years) than that observed in younger patients (<70
years), suggesting that survivin might also be associated with
age-related characteristics of colon cancer (39).
The concurrent expression of STAT3 and survivin in
CRC has been detected in matching sections of normal colonic
epithelium and invasive tumours by quantitative PCR and
immunohistochemistry. Both pSTAT3 and survivin expression was shown
to be significantly increased in CRC, and the presence of pSTAT3 at
the nuclear compartment was directly associated with the expression
of survivin (40). In vitro
studies demonstrated the presence of an active STAT3-survivin axis
in CRC-derived cells. Silencing the expression of STAT3 produced a
reduction in cell proliferation, a significantly higher rate of
apoptosis (41), and reduced colony
formation and cell migration (42),
which were associated with a significant suppression of survivin
expression. Notably, inhibition of Smad7, a positive regulator of
STAT3, significantly reduced the phosphorylation of STAT3 in CRC
cells stimulated with IL-6, along with a decreased level of
survivin (43), suggesting that an
active STAT3-survivin axis, induced by CRC-associated cytokines,
such as IL-6, serves a role in maintaining the malignant phenotype
of CRC cells, and might also be important during CRC
progression.
4. STAT3 and survivin as therapeutic
targets
The relevance of the STAT3-survivin axis in CRC
progression has prompted the development of drugs targeting
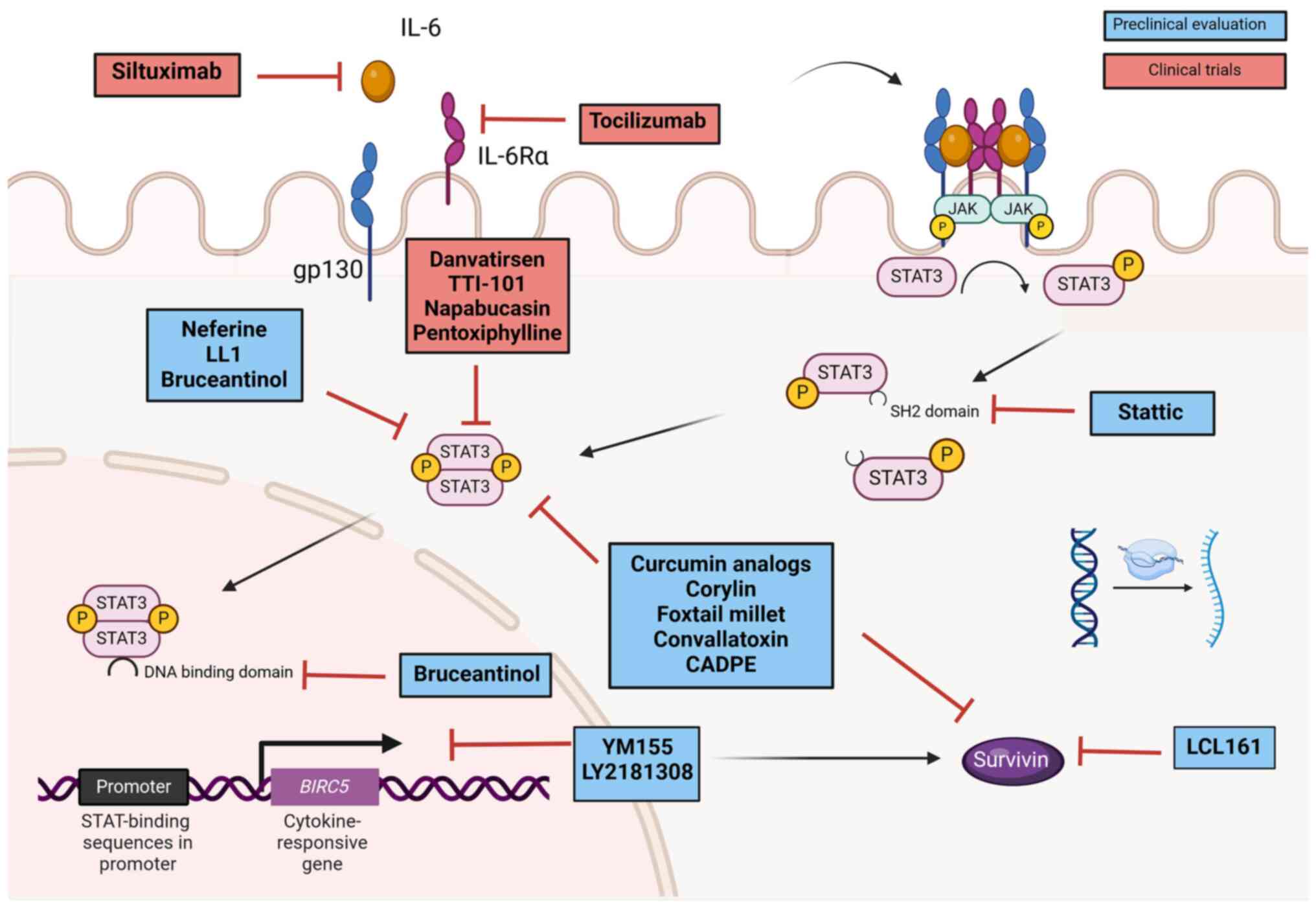
molecular elements activating this pathway (Fig. 2). The pro-inflammatory cytokine IL-6
is considered a major regulator of the STAT3-survivin axis in CRC
(31,32); therefore, efforts to block this
upstream element have been performed. Clinical trials using
siltuximab (Sylvant®; Janssen Biotech), a chimeric
monoclonal antibody that targets and neutralises IL-6, have shown
that although siltuximab monotherapy was well tolerated in patients
with CRC, no objective response occurred in a phase II study
(44). In addition, human trials
using tocilizumab, an antagonist monoclonal antibody to IL-6
receptor (IL-6R), have shown that clinical efficacy is still
uncertain (45). Altogether, these
observations suggest that targeting upstream elements may not be
enough to control the STAT3-survivin axis in CRC.
STAT3 and survivin have been considered therapeutic
targets for the development of diverse approaches to control and
treat CRC, from plant derivatives to molecular therapies (Fig. 2). Phytochemicals have been widely
explored as a potential source of STAT3-survivin axis regulators.
One of the most studied natural dietary phytochemicals is curcumin,
a polyphenol extracted from Curcuma longa (turmeric)
rhizomes. Curcumin analogues have been demonstrated to inhibit
STAT3 activation and survivin expression in CRC cells (46), and to increase caspase-3-mediated
apoptosis in pre-clinical models (47). Moreover, different reports have
indicated that the STAT3-survivin axis can be suppressed by other
natural derivatives, such as corylin, foxtail millet (Setaria
italica) and neferine. Corylin, an isoflavone isolated from
Cullen corylifolium (L.) Medik, was found to inhibit STAT3
phosphorylation and survivin production, and significantly reduced
tumour size in an animal model of CRC (48). Similarly, a previous evaluation of
S. italica, a coarse cereal, in a murine model of
azoxymethane/dextran sodium sulphate-induced CRC showed that S.
italica diet supplementation reduced tumour burden by
decreasing IL-6, pSTAT3 and survivin levels, alongside regulation
of mice microbiota (49). Using the
same murine model of colonic carcinogenesis, Zhou et al
(50) showed that neferine, a
natural bisbenzylisoquinoline alkaloid extracted from Nelumbinis
plumula, was able to reduce the development of colorectal
tumours by decreasing pSTAT3, among other molecules, and was shown
to interact with STAT3 by molecular docking analysis (50). In addition, two more plant-derived
molecules have been shown to be efficient inhibitors of the
STAT3-survivin axis in CRC animal models. Convallatoxin, a cardiac
glycoside derived from Adonis amurensis Regel & Radde
(51), and caffeic acid
3,4-dihydroxyphenethylester extracted from Sarcandra glabra
(Thunb) (52), have been reported
to decrease CRC viability based on inhibition of the STAT3-survivin
axis.
Alternatively, classical chemistry and innovative
cellular approaches are being explored to develop new treatments
for CRC, targeting the STAT3-survivin axis. In this regard, Xu
et al (53) reported the
design and synthesis of 27 cyanopyridines. Their anticancer
activities were screened using CRC-derived cell lines, and the
results indicated that at least one compound resulted in a
significant reduction of CRC cell migration and colony formation.
Notably, cyanopyridine-induced inhibition of cell migration and
colony formation was mediated by a significant reduction of STAT3
activation and survivin expression (53). Finally, an innovative approach using
macrophage-produced extracellular vesicles as nanocarriers of
oxaliplatin, retinoic acid and Libidibia ferrea-derived
polyphenols to modulate CRC progression was recently published
(54). This previous study reported
that extracellular vesicles loaded with antitumour reagents induced
a significant reduction in tumour size and metastasis formation in
an allographic mouse model, and that the cytotoxic effect was
mediated by downregulation of STAT3 and survivin, among other
cancer related factors (54). These
observations reinforce the importance of the STAT3-survivin axis as
a potential target for the development of new treatment strategies
for patients with CRC.
5. Clinical trials targeting the
STAT3-survivin axis
Being at the centre of the oncogenic STAT3-survivin
axis, the transcription factor is a major target for the
development of new drugs for the treatment of CRC (Fig. 2). Several STAT3 inhibitor compounds
have been developed. However, at least three highly selective
inhibitors have shown enough evidence in pre-clinical studies to be
considered promising drugs to be included in further clinical
trials: Stattic, which binds to the SH2 domain thus resulting in
the retention of STAT3 in the cytoplasmic compartment (55); LL1, which blocks STAT3 dimerization
(56); and bruceantinol, which
hampers STAT3 binding to target DNA (57).
A number of STAT3 inhibitors are currently being
tested in human clinical trials. In the particular case of CRC
there are four specific inhibitors: TTI-101, danvatirsen,
pentoxiphylline and napabucasin, which have entered clinical trials
(Fig. 2).
TTI-101 is a small molecule that competitively
inhibits STAT3 by targeting the phospho-Y-peptide binding site
within the SH2 domain, thus blocking STAT3 recruitment to activated
receptors and further homodimerization (58). TTI-101 is being tested as an oral
treatment for patients with histologically confirmed diagnosis of
locally-advanced, inoperable, metastatic and/or treatment
refractory CRC, among other types of tumours, in an active phase I
trial (NCT03195699) to establish safety, tolerability,
pharmacodynamic effects and efficacy. The study started in 2017; at
present, the trial remains active and is no longer recruiting
participants. The estimated completion date is December 2024.
Danvatirsen is a 16-nucleotide antisense
oligonucleotide that specifically targets STAT3 RNA, downregulating
the production of the protein (59). Danvatirsen is being evaluated as a
co-treatment with durvalumab (IMFINZI™; AstraZeneca), a fully human
antibody that blocks programmed cell death ligand 1. Data from the
phase I trial have already been published (60). The authors assessed the safety,
tolerability, pharmacokinetics, pharmacodynamics and preliminary
antitumour activity of danvatirsen and durvalumab in two cohorts of
patients with advanced CRC, among other types of solid tumours.
Patients in cohort one received danvatirsen as monotherapy, while
patients in cohort two were treated with a combination of
danvatirsen and durvalumab. The results demonstrated that
danvatirsen was well tolerated as a monotherapy and when combined
with durvalumab. It was also reported that STAT3 expression was
decreased in patients of both cohorts (60). A phase II trial (NCT02983578)
including patients with advanced and refractory pancreatic,
non-small cell lung cancer and mismatch repair-deficient CRC was
subsequently started. Researchers estimate that the study will be
completed by April 2025.
In 2023, a clinical trial using pentoxiphylline
(NCT06115174) was registered. Pentoxiphylline is a non-specific
phosphodiesterase inhibitor that was initially approved by the FDA
for the treatment of peripheral vascular disease (61). Subsequently, pentoxiphylline was
demonstrated to inhibit melanoma growth by targeting STAT3(62). Notably, pentoxiphylline was first
evaluated as a protective agent against chemotherapy-induced
toxicities in patients with CRC. Administration of pentoxiphylline
induced significantly improved survival rates, weight gain and
reduced the occurrence of stomatitis (63). The beneficial effects detected were
further demonstrated to correlate with a significant reduction of
systemic inflammatory cytokines, including IL-1β, IL-6, IL-8 and
TNF-α (64). Given that STAT3
regulates the expression of most inflammatory cytokines, it may be
hypothesized that the aforementioned effects were mediated by the
capacity of pentoxiphylline to inhibit STAT3. However, the authors
did not evaluate the level of pSTAT3 expression. A further clinical
trial (NCT06115174) to evaluate the anticancer effect of
pentoxiphylline in patients with metastatic colorectal cancer was
recently registered. The authors estimate that the study will be
completed by November 2024.
Napabucasin is a STAT3 inhibitor that has been used
in numerous clinical trials. Napabucasin is a natural
naphthoquinone produced by various plants, including Newbouldia
laevis, Ekmanianthe longiflora and Handroanthus
impetiginosus. It is a small molecule targeting STAT3 that has
been shown to possess an extensive range of inhibitory activities
in diverse types of cancer (65).
Napabucasin has been incorporated in various clinical trials,
including those on patients with CRC; a phase III trial
demonstrated that in pSTAT3-positive patients with CRC the overall
survival was longer in the napabucasin group than that in the
placebo group (66). In addition,
two phase I trials testing napabucasin as a monotherapy (67), or in combination with fluorouracil,
l-leucovorin, irinotecan and bevacizumab (68), in patients with advanced CRC
demonstrated that the selected doses were well tolerated, with a
manageable safety profile. Similarly, a multicentre phase I/II
trial to assess the safety and efficacy of napabucasin in
combination with pembrolizumab, an anti-PD1 blocking antibody
(Keytruda®; Merck Sharp & Dohme) was performed in 55
patients with metastatic CRC. The combined therapy showed
antitumour activity with tolerable toxicity levels; however, the
therapy did not meet the primary endpoint of the study
(immune-related objective response rate) (69). Finally, data from a multi-centre,
open-label, phase III clinical trial (CanStem303C) has recently
been published (70). The study
included 1,253 patients with histologically confirmed metastatic
CRC, who were randomized to napabucasin plus fluoropyrimidine,
oxaliplatin and bevacizumab (FOLFIRI) or FOLFIRI alone groups. The
primary endpoint was overall survival. Notably, the addition of
napabucasin to FOLFIRI did not improve overall survival in the
evaluated population (70).
Strategies for the development of survivin
inhibitors have provided molecules to block either survivin
production or activity. YM155 (sepantronium bromide) was the first
developed small molecule that specifically targets the survivin
gene promoter, thus inhibiting its transcription. In vitro
studies have demonstrated that YM155 is a promising treatment for
CRC, since YM155 alone or in combination with 5-fluorouracil (5-FU)
was more effective than 5-FU alone to treat CRC cells (71). Although other molecules, for example
the antisense oligonucleotide LY2181308(72), and the SMAC/Diablo mimetic
LCL161(73), which inhibit survivin
transcription and activity, respectively, have shown promising
results in pre-clinical assays, clinical trials have not reached
objective responses.
6. Conclusions
The increasing numbers of cases and deaths due to
CRC worldwide contributes to the urgent need for developing new,
more efficient drugs. The STAT3-survivin axis is a regulator of CRC
development, progression and response to treatment. Thus, molecular
elements participating in this pathway have been considered
potential therapeutic targets. Although pre-clinical studies have
provided a number of potential drugs, only a limited number of them
have reached clinical trials. In particular, blockade of upstream
activators IL-6 and IL-6R using monoclonal antibodies has shown
uncertain clinical efficacy. In line with the former observations,
targeting survivin by means of small molecules and oligonucleotides
to specifically hamper survivin expression has resulted in poor
results in clinical trials. A rational explanation might be that
the STAT3-survivin axis can be activated by a wide number of
cytokines and growth factors present in the tumour
microenvironment, as a consequence, inhibition of IL-6/IL-6R may
not be enough to stop upregulation of the axis. Furthermore, STAT3
has several transcriptional targets that have important functions
to maintain the tumour phenotype. Regarding apoptosis, STAT3
induces transcription of survivin, but also other proteins, such as
Bcl-2 and Bcl-xL, which control apoptosis independently of survivin
activity, suggesting that targeting survivin alone may have little
impact in STAT3-associated inhibition of cell apoptosis. In
general, pre-clinical and clinical trials targeting STAT3 have
produced more promising results, particularly when the new drugs
are used in combination with already tested treatments. However, to
the best of our knowledge, no protocol has yet studied the joint
effect of molecules targeting STAT3 and survivin; therefore, the
potential clinical usefulness of inhibiting the complete
STAT3-survivin axis has not been explored. Thus, we consider that
combined therapeutic strategies that translate into a stronger
inhibition of the STAT3-survivin axis deserve further investigation
to potentially provide benefit to patients with CRC in the near
future.
Acknowledgements
Liliana Cortés Ballinas is a student of Programa de
Doctorado en Ciencias Bioquímicas, Universidad Nacional Autónoma de
México (UNAM).
Funding
Funding: The present study was financed by a grant from PAPIIT,
UNAM (grant no. IN206022). LCB received a fellowship from Consejo
Nacional de Ciencia y Tecnología (CVU: 626746).
Availability of data and materials
Not applicable.
Authors' contributions
LCB, TVLP and LRZ participated in the design,
conception, investigation, writing and editing of the manuscript.
Data authentication is not applicable. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Marin JJG, Macias RIR, Monte MJ, Herraez
E, Peleteiro-Vigil A, Blas BS, Sanchon-Sanchez P, Temprano AG,
Espinosa-Escudero RA, Lozano E, et al: Cellular mechanisms
accounting for the refractoriness of colorectal carcinoma to
pharmacological treatment. Cancers (Basel). 12(2605)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Markham MJ, Wachter K, Agarwal N,
Bertagnolli MM, Chang SM, Dale W, Diefenbach CSM, Rodriguez-Galindo
C, George DJ, Gilligan TD, et al: Clinical cancer advances 2020:
Annual report on progress against cancer from the American Society
of Clinical Oncology. J Clin Oncol. 38(1081)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yu H, Pardoll D and Jove R: STATs in
cancer inflammation and immunity: A leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting theIL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin
Oncol. 15:234–248. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ge QX, Li YY, Nie YQ, Zuo WG and Du YL:
Expression of survivin and its four splice variants in colorectal
cancer and its clinical significances. Med Oncol.
30(535)2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC,
Hwang JI, Chung CW, Jung YK and Oh BH: An anti-apoptotic protein
human survivin is a direct inhibitor of caspase-3 and -7.
Biochemistry. 40:1117–1123. 2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Darnell JE Jr: STATs and gene regulation.
Science. 277:1630–1635. 1997.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ehret GB, Reichenbach P, Schindler U,
Horvath CM, Fritz S, Nabholz M and Bucher P: DNA binding
specificity of different STAT proteins. Comparison of in vitro
specificity with natural target sites. J Biol Chem. 276:6675–6688.
2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Furtek SL, Backos DS, Matheson CJ and
Reigan P: Strategies and approaches of targeting STAT3 for cancer
treatment. ACS Chem Biol. 11:308–318. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu LJ, Leung KH, Chan DSH, Wang YT, Ma DL
and Leung CH: Identification of a natural product-like STAT3
dimerization inhibitor by structure-based virtual screening. Cell
Death Dis. 5:e1293. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sgrignani J, Garofalo M, Matkovic M,
Merulla J, Catapano CV and Cavalli A: Structural Biology of STAT3
and its implications for anticancer therapies development. Int J
Mol Sci. 19(1591)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hillmer EJ, Zhang H, Li HS and Watowich
SS: STAT3 signaling in immunity. Cytokine Growth Factor Rev.
31:1–15. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Donepudi M and Grütter MG: Structure and
zymogen activation of caspases. Biophys Chem. 101-102:145–153.
2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Thornberry NA and Lazebnik Y: Caspases:
Enemies within. Science. 281:1312–1316. 1998.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fukuda S and Pelus LM: Survivin, a cancer
target with an emerging role in normal adult tissues. Mol Cancer
Ther. 5:1087–1098. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Boidot R, Vegran F and Lizard-Nacol S:
Transcriptional regulation of the survivin gene. Mol Biol Rep.
41:233–240. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lens SM, Wolthuis RM, Klompmaker R, Kauw
J, Agami R, Brummelkamp T, Kops G and Medema RH: Survivin is
required for a sustained spindle checkpoint arrest in response to
lack of tension. EMBO J. 22:2934–2947. 2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rosa J, Canovas P, Islam A, Altieri DC and
Doxsey SJ: Survivin modulates microtubule dynamics and nucleation
throughout the cell cycle. Mol Biol Cell. 17:1483–1493.
2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pavlyukov MS, Antipova NV, Balashova MV,
Vinogradova TV, Kopantzev EP and Shakhparonov MI: Survivin monomer
plays an essential role in apoptosis regulation. J Biol Chem.
286:23296–23307. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Marusawa H, Matsuzawa S, Welsh K, Zou H,
Armstrong R, Tamm I and Reed JC: HBXIP functions as a cofactor of
survivin in apoptosis suppression. EMBO J. 22:2729–2740.
2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Verhagen AM, Coulson EJ and Vaux DL:
Inhibitor of apoptosis proteins and their relatives: IAPs and other
BIRPs. Genome Biol. 2(REVIEWS3009)2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Song Z, Liu S, He H, Hoti N, Wang Y, Feng
S and Wu M: A single amino acid change (Asp53-Ala53) converts
survivin from anti-apoptotic to pro-apoptotic. Mol Biol Cell.
15:1287–1296. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sanhueza C, Wehinger S, Castillo Bennett
J, Valenzuela M, Owen GI and Quest AF: The twisted survivin
connection to angiogenesis. Mol Cancer. 14(198)2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cheung CHA, Chang YC, Lin TY, Cheng SM and
Leung E: Anti-apoptotic proteins in the autophagic world: An update
on fucntions of XIAP, Survivin, and BRUCE. J Biomed Sci.
27(31)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mull AN, Klar A and Navara CS:
Differential localization and high expression of SURVIVIN splice
variants in human embryonic stem cells but not in differentiated
cells implicate a role for SURVIVIN in pluripotency. Stem Cell Res.
12:539–549. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Erdogan F, Radu TB, Orlova A, Qadree AK,
de Araujo ED, Israelian J, Valent P, Mustjoki SM, Herling M,
Moriggl R and Gunning PT: JAK-STAT core cancer pathway: An
integrative cancer interactome analysis. J Cell Mol Med.
26:2049–2062. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kusaba T, Nakayama T, Yamazumi K, Yakata
Y, Yoshizaki A, Inoue K, Nagayasu T and Sekine I: Activation of
STAT3 is a marker of poor prognosis in human colorectal cancer.
Oncol Rep. 15:1445–1451. 2006.PubMed/NCBI
|
|
30
|
Morikawa T, Baba Y, Yamauchi M, Kuchiba A,
Nosho K, Shima K, Tanaka N, Huttenhower C, Frank DA, Fuchs CS and
Ogino S: STAT3 expression, molecular features, inflammation
patterns, and prognosis in a database of 724 colorectal cancers.
Clin Cancer Res. 17:1452–1462. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rokavec M, Öner MG, Li H, Jackstadt R,
Jiang L, Lodygin D, Kaller M, Horst D, Ziegler PK, Schwitalla S, et
al: IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated
colorectal cancer invasion and metastasis. Clin Invest.
124:1853–1867. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Yamamoto T, Tsunedomi R, Nakajima M,
Suzuki N, Yoshida S, Tomochika S, Xu M, Nakagami Y, Matsui H,
Tokumitsu Y, et al: IL-6 levels correlate with prognosis and
immunosuppressive stromal cells in patients with colorectal cancer.
Ann Surg Oncol. 30:5267–5277. 2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen WC, Liu Q, Fu JX and Kang SY:
Expression of survivin and its significance in colorectal cancer.
World J Gastroenterol. 10:2886–2889. 2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Suga K, Yamamoto T, Yamada Y, Miyatake S,
Nakagawa T and Tanigawa N: Correlation between transcriptional
expression of survivin isoforms and clinicopathological findings in
human colorectal carcinomas. Oncol Rep. 13:891–897. 2005.PubMed/NCBI
|
|
35
|
Wang H, Li S, Luo X, Song Z, Long X and
Zhu X: Knockdown of PARP6 or survivin promotes cell apoptosis and
inhibits cell invasion of colorectal adenocarcinoma cells. Oncol
Rep. 37:2245–2251. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kawasaki H, Toyoda M, Shinohara H, Okuda
J, Watanabe I, Yamamoto T, Tanaka K, Tenjo T and Tanigawa N:
Expression of survivin correlates with apoptosis, proliferation,
and angiogenesis during human colorectal tumorigenesis. Cancer.
91:2026–2032. 2001.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Waniczek D, Nowak M, Lorenc-Góra J,
Muc-Wierzgoń M, Mazurek U, Bichalska-Lach M and Lorenc Z: The
transcriptional activity profile of inhibitor apoptosis protein
encoding genes in colon cancer patients: A STROBE-compliant study.
Medicine (Baltimore). 100(e27882)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tuncel H, Shimamoto F, Kaneko Guangying Qi
H, Aoki E, Jikihara H, Nakai S, Takata T and Tatsuka M: Nuclear
aurora B and cytoplasmic survivin expression is involved in lymph
node metastasis of colorectal cancer. Oncol Lett. 3:1109–1114.
2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Endo T, Abe S, Seidlar HB, Nagaoka S,
Takemura T, Utsuyama M, Kitagawa M and Hirokawa K: Expression of
IAP family proteins in colon cancers from patients with different
age groups. Cancer Immunol Immunother. 53:770–776. 2004.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lassmann S, Schuster I, Walch A, Göbel H,
Jütting U, Makowiec F, Hopt U and Werner M: STAT3 mRNA and protein
expression in colorectal cancer: Effects on STAT3-inducible targets
linked to cell survival and proliferation. J Clin Pathol.
60:173–179. 2007.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Li J, Liu YY, Yang KF, Shen DF, Sun HZ,
Huang KQ and Zheng HC: Effects and mechanism of STAT3 silencing on
the growth and apoptosis of colorectal cancer cells. Oncol Lett.
16:5575–5582. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Li W, Lee MR, Kim T, Kim YW and Cho MY:
Activated STAT3 may participate in tumor progression through
increasing CD133/survivin expression in early stage of colon
cancer. Biochem Biophys Res Commun. 497:354–361. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Maresca C, Di Maggio G, Stolfi C, Laudisi
F, Colella M, Pacifico T, Di Grazia A, Di Fusco D, Congiu D, Guida
AM, et al: Smad7 sustains STAT3 expression and signaling in colon
cancer cells. Cancers (Basel). 14(4993)2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Angevin E, Tabernero J, Elez E, Cohen SJ,
Bahleda R, van Laethem JL, Ottensmeier C, Lopez-Martin JA, Clive S,
Joly F, et al: A phase I/II, multiple-dose, dose-escalation study
of siltuximab, an anti-interleukin-6 monoclonal antiboy, in
patients with advanced solid tumors. Cancer Res. 20:2192–2204.
2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kampan NC, Xiang SD, McNally OM, Stephens
AN, Quinn MA and Plebanski M: Immunotherapeutic interleukin-6 or
interleukin-6 receptor blockade in cancer: Challenges and
opportunities. Curr Med Chem. 25:4785–4806. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lin L, Liu Y, Li H, Li PK, Fuchs J,
Shibata H, Iwabuchi Y and Lin J: Targeting colon cancer stem cells
using a new curcumin analogue, GO-Y030. Br J Cancer. 105:212–220.
2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Chung SS, Dutta P, Chard N, Wu Y, Chen QH,
Chen G and Vadgama J: A novel curcumin analog inhibits canonical
and non-canonical functions of telomerase through STAT3 and NK-kB
inactivation in colorectal cancer cells. Oncotarget. 10:4516–4531.
2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Yang L, Yao Y, Bai Y, Zheng D, Zhou F,
Chen L, Hu W, Xiang Y, Zhao H, Liu Z, et al: Effect of the
isoflavone corylin from Cullen corylifolium on colorectal cancer
growth, by targeting the STAT3 signaling pathway. Phytomedicine.
80(153366)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhang B, Xu Y, Liu S, Lv H, Hu Y, Wang Y,
Li Z, Wang J, Ji X, Ma H, et al: Dietary supplementation of foxtail
millet ameliorates colitis-associated colorectal cancer in mice via
activation of gut receptors and suppression of the STAT3 pathway.
Nutrients. 12(2367)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhou Y, Xiang S, Zheng H, Hou Y, Wang Y,
Li CC, Wu Q, Shi J and Chen X: Neferine suppresses experimental
colitis-associated colorectal cancer by inhibition of NF-[Formula:
see text] B p65 and STAT3. Am J Chin Med. 50:1387–1400.
2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zhang ZH, Li MY, Wang Z, Zuo HX, Wang JY,
Xing Y, Jin C, Xu G, Piao L, Piao H, et al: Convallatoxin promotes
apoptosis and inhibits proliferation and angiogenesis through
crosstalk between JAK2/STAT3 (T705) and mTOR/STAT3 (S727) signaling
pathways in colorectal cancer. Phytomedicine.
68(153172)2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Zheng GW, Tang MM, Shu CY, Xin WX, Zhang
YH, Chi BB, Shi MR, Guo X, Zhang ZZ and Lian XY: A small neural
molecule CADPE kills residual colorectal cancer cells by inhibiting
key transcription factors and translation initiation factors. Cell
Death Dis. 11(982)2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Xu L, Shi L, Qiu S, Chen S, Lin M, Xiang
Y, Zhao C, Zhu J, Shen L and Zuo Z: Design, synthesis, and
evaluation of cyanopyridines as anti-colorectal cancer agents via
inhibiting STAT3 pathway. Drug Des Devel Ther. 13:3369–3381.
2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
De Carvalho TG, Lara P, Jorquera-Cordero
C, Aragao CFS, de Santana Oliveira A, Garcia VB, de Paiva Souza SV,
Schomann T, Soares LAL, da Matta Guedes PM and de Araújo Júnior RF:
Inhibition of murine colorectal cancer metastasis by targeting
M2-TAM through STAT3/NF-kB/AKT signaling using macrophage 1-derived
extracellular vesicles loaded with oxaliplatin, retinoic acid, and
Libidibia ferrea. Biomed Pharmacother. 168(115663)2023.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Spitzner M, Roesler B, Bielfeld C, Emons
G, Gaedcke J, Wolff HA, Rave-Fränk M, Kramer F, Beissbarth T, Kitz
J, et al: STAT3 inhibitor sensitizes colorectal cancer to
chemoradiotherapy in vitro and in vivo. Int J Cancer. 134:997–1007.
2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Liu Z, Wang H, Guan L, Lai C, Yu W and Lai
M: LL1, a novel highly selective STAT3 inhibitor, displays
anti-colorectal cancer activities in vitro and in vivo. Br J
Pharmacol. 177:298–313. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Wei N, Li J, Fang C, Chang J, Xirou V,
Syrigos NK, Marks BJ, Chu E and Schmitz JC: Targeting colon cancer
with the novel STAT3 inhibitor bruceantinol. Oncogene.
38:1676–1687. 2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Bharadwaj U, Eckols TK, Xu X, Kasembeli
MM, Chen Y, Adachi M, Song Y, Mo Q, Lai SY and Tweardy DJ:
Small-molecule inhibition of STAT3 in radioresistant head and neck
squamous cell carcinoma. Oncotarget. 7:26307–26330. 2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Seth PP, Vasquez G, Allerson CA, Berdeja
A, Gaus H, Kinberger GA, Prakash TP, Migawa MT, Bhat B and Swayze
EE: Synthesis and biophysical evaluation of 2',4'-constrained
2'O-methoxyethyl and 2',4'-constrained 2'O-ethyl nucleic acid
analogues. J Org Chem. 75:1569–1581. 2010.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Nishina T, Fujita T, Yoshizuka N,
Sugibayashi K, Murayama K and Kuboki Y: Safety, tolerability,
pharmacokinetics and preliminary antitumor activity of an antisense
oligonucleotide targeting STAT3 (danvatirsen) as monotherapy and in
combination with durvalumab in Japanese patients with advanced
solid malignancies: A phase 1 study. BMJ Open.
12(e055718)2022.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Frampton JE and Brogden RN: Pentoxifylline
(oxpentifylline). A review of its therapeutic efficacy in the
management of peripheral vascular and cerebrovascular disorders.
Drugs Aging. 7:480–503. 1995.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Kamran MZ and Gude RP: Pentoxifylline
inhibits melanoma tumor growth and angiogenesis by targeting STAT3
signaling pathway. Biomed Pharmacother. 67:399–405. 2013.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Meirovitz A, Baider L, Peretz T, Stephanos
S and Barak V: Effect of pentoxifylline on colon cancer patients
treated with chemotherapy (Part I). Tumour Biol. 43:341–349.
2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Meirovitz A, Baider L, Peretz T, Stephanos
S and Barak V: PTX treatment of colon cancer: Mode of action based
on tumor marker and cytokine kinetics. Anticancer Res.
42:5487–5496. 2022.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Shao Z, Wang H, Ren H, Sun Y and Chen X:
The anticancer effect of Napabucasin (BBI608), a natural
naphthoquinone. Molecules. 28(5678)2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Jonker DJ, Nott L, Yoshino T, Gill S,
Shapiro J, Ohtsu A, Zalcberg J, Vickers MM, Wei AC, Gao Y, et al:
Napabucasin versus placebo in refractory advanced colorectal
cancer: A randomised phase 3 trial. Lancet Gastroenterol Hepatol.
3:263–270. 2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Kawazoe A, Kuboki Y, Bando H, Fukuoka S,
Kojima T, Naito Y, Iino S, Yodo Y, Doi T, Shitara K and Yoshino T:
Phase 1 study of napabucasin, a cancer stemness inhibitor, in
patients with advanced solid tumors. Cancer Chemother Pharmacol.
85:855–862. 2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Taniguchi H, Masuishi T, Kawazoe A, Muro
K, Kadowaki S, Bando H, Iino S, Kageyama R and Yoshino T: Phase I
study of napabucasin in combination with FOLFIRI + bevacizumab in
Japanese patients with metastatic colorectal cancer. Int J Clin
Oncol. 26:2017–2024. 2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Kawazoe A, Kuboki Y, Shinozaki E, Hara H,
Nishina T, Komatsu Y, Yuki S, Wakabayashi M, Nomura S, Sato A, et
al: Multicenter phase I/II trial of napabucasin and pembrolizumab
in patients with metastasic colorectal cancer (EPOC1503/SCOOP
trial). Clin Cancer Res. 26:5887–5894. 2020.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Shah MA, Yoshino T, Tebbutt NC, Grothey A,
Tabernero J, Xu RH, Cervantes A, Oh SC, Yamaguchi K, Fakih M, et
al: Napabucasin plus FOLFIRI in patients with previously treated
metastasic colorectal cancer: Results from the open-label,
randomized, phase III CanStem303C study. Clin Colorectal Cancer.
22:100–110. 2023.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Li WL, Lee MR and Cho MY: The small
molecule survivin inhibitor YM155 may be an effective treatment
modality for colon cancer through increasing apoptosis. Biochem
Biophys Res Commun. 471:309–314. 2016.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Rödel F, Frey B, Leitmann W, Capalbo G,
Weiss C and Rödel C: Survivin antisense oligonucleotides
effectively radiosensitize colorectal cancer cells in both tissue
culture and murine xenograft models. Int J Radiat Oncol Biol Phys.
71:247–255. 2008.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Chang YC, Kondapuram SK, Yang TH, Syed SB,
Cheng SM, Lin TY, Lin YC, Coumar MS, Chang JY, Leung E and Cheung
CHA: The SMAC mimetic LCL161 is a direct ABCB1/MDR1-ATPase activity
modulator and BIRC5/Survivin expression down-regulator in cancer
cells. Toxicol Appl Pharmacol. 401(115080)2020.PubMed/NCBI View Article : Google Scholar
|