1. Introduction
Hyperuricemia (HUA) is a metabolic disease
characterized by abnormalities in purine metabolism and elevated
blood uric acid levels, which is often caused by excessive levels
or abnormal secretion of uric acid. Persistent high blood uric acid
levels accelerate deposition of urate crystals in joints, leading
to gout (1). HUA is a metabolic
disorder caused by abnormal purine metabolism. The diagnostic
criteria involve obtaining fasting blood samples on separate days
under usual dietary conditions, and determining blood uric acid
levels using the uricase method. Levels >420 μmol/l for males or
>360 μmol/l for females are considered diagnostic (2). Typical symptoms include inflammation,
oxidative stress and dysbacteriosis. As the economic status and
living standards of individuals have improved, diets high in
protein, purine, fat and sugar are more common. Previous studies
have revealed that HUA is associated with development of chronic
renal disease, diabetes mellitus, hypertension, cardiovascular
events and other disease (3-6).
The incidence of HUA increases annually and it is now recognized as
a metabolic disease. Prevalence of Hua in China has increased from
11.1% in 2009 to 18.7% in 2019. The prevalence of Hua in young men
(31.9%) in 2019 was almost three times that of the same age group
(10.0%) in 2009, and young men were the group with the
fastest-growing prevalence of Hua in the last decade (7). In addition, hyperuricemia is also
becoming more common in young people (8). Formulating an appropriate animal model
is key for further research on HUA.
The final result of the avian purine metabolic
pathway is identical to human uric acid, which may provide an
appropriate animal model for simulating human HUA. To present
review summarizes the literature regarding the establishment of
avian HUA and gout models and model induction methods and detection
indices.
2. Gout in poultry
Avian gout causes
Avian gout is caused by either overproduction or
impaired excretion of avian uric acid in the bloodstream. Uric acid
deposits as urates in the joint capsule, articular cartilage and
periarticular, thoracic and abdominal cavities, as well as on the
surfaces of other mesenchymal tissue and various organs (9). There are numerous factors associated
with avian gout, such as high protein content in feed, excessive
salt, dietary calcium, phosphorus imbalance, insufficient vitamin A
uptake and lack of drinking water. In addition, genetic and disease
factors, viral infection and improper use of drugs and feeding can
lead to avian gout (10). In
nature, spontaneous gout cases have been described in quails,
pigeons, chickens, ducks, geese, peafowls and turkeys (11-17).
Birds and mammals have notable differences in
ammonia excretion; ammonia is toxic to organisms and is produced in
the deamination of protein metabolites and amino acids. Ammonia is
converted into urea via the urea cycle in the liver in most
mammals, or it is excreted by the kidneys via glutamate transport
(18). Certain mammals produce
uricase, which metabolizes uric acid to allantoin; therefore, serum
uric acid levels are not high under normal circumstances (19). However, during evolution, humans and
poultry lost the genes for the uric acid enzyme that promotes
kidney excretion, thus ammonia cannot be transformed into
allantoin. Uric acid is generated by synthesis and decomposition of
purine nucleotides and it reaches the kidney through the blood
circulation. Following glomerular filtering, uric acid reaches the
renal tube. Combined with uric acid, the sodium ions secreted from
the proximal convoluted tubule epithelial cells transform into
urates that are excreted from the kidney, which results in humans
and poultry having higher levels of uric acid than other species.
In addition, lack of urea synthesis arginine in the liver of
poultry means that they cannot remove ammonia via the urea cycle
and as poultry kidneys lack glutamine synthase, they cannot excrete
ammonia through glutamine (20).
Therefore, synthesis and decomposition pathway of purine
nucleotides converts ammonia into uric acid, which is the primary
method of ammonia metabolism in poultry. The decomposition and
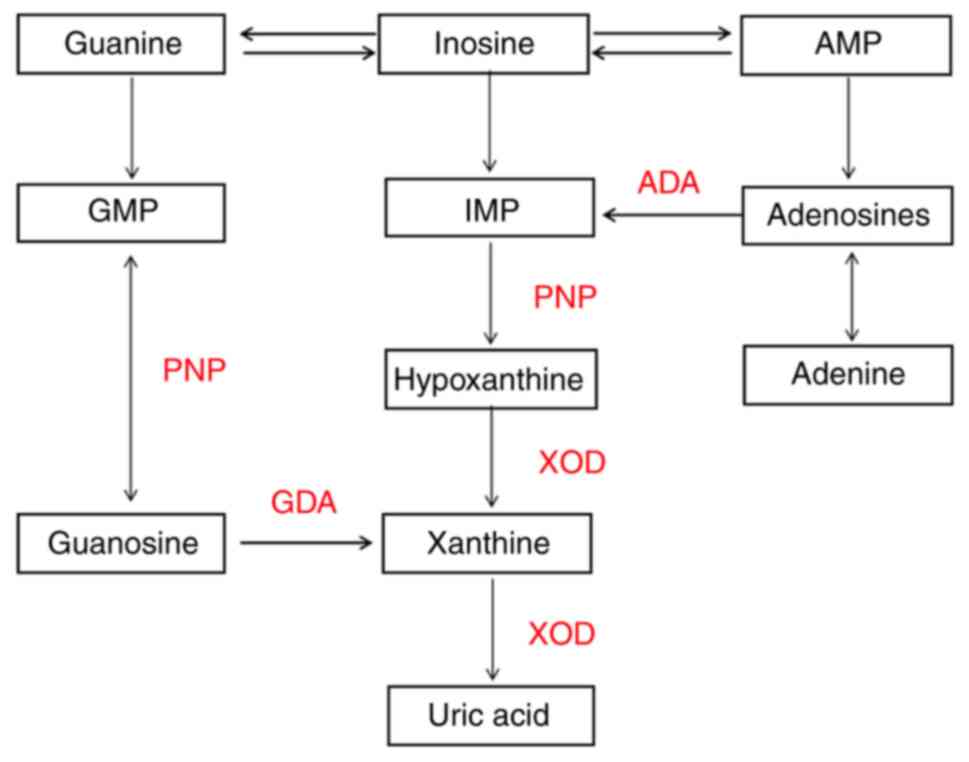
metabolism of poultry purine is as shown in Fig. 1. Under the action of nucleotidase,
purine nucleotides are dephosphorylated to produce the purine
adenosine; adenine produces hypoxanthine under the action of
adenosine deaminase and is then oxidized into xanthine, which is
catalyzed by xanthine oxidase. Guanosine is directly hydrolyzed and
generates guanine and then generates xanthine through deamination.
Ultimately, xanthine produces uric acid catalyzed by xanthine
Oxidase (XOD) (21). Because of the
lack of uricase in humans, uric acid is the final product of purine
nucleotide metabolism in the body, so we think that the metabolism
of uric acid in birds is similar to that in humans, poultry can be
used as a suitable animal model for human uric acid metabolism
(22).
Types of avian gout
The two types of gout in birds are visceral gout and
articular gout. Visceral gout, also called ‘avian urolithiasis’, is
characterized by deposition of urate in the thoracic and abdominal
cavity and on the surface of the viscera such as the kidney, heart,
liver, mesentery and peritoneum, often accompanied by renal
failure, similar to human uremia. Articular gout is characterized
by urate deposition in the joint capsule, articular cartilage and
surrounding tissue (23). Genetic
factors are linked to spontaneous articular gout; notably, gout
develops in New Hampshire chickens as a result of genetic selection
anomalies in the transport of uric acid in renal tubules (24).
Birds with articular gout have a prolonged duration
of disease without internal organ damage. In most cases, clinical
symptoms appear around the joints. In the early stage, the swelling
is soft and painful with no obvious boundaries; the mid-swollen
parts gradually become hard, forming pea- or broad bean-sized
nodules that are either slightly mobile or immobile (19). When swollen joints are cut open,
white, milky urate flows from the joint cavity.
Visceral gout is a common clinical disease in
poultry. Typical clinical symptoms include anorexia, depression,
strong desire to drink, diarrhea and staining of the feathers
around the cloaca with white feces. The disease is often caused by
serious failure of multiple organs, such as the kidney, which can
result in death (20). White urate
crystal deposits, which are detected via microscopic inspection of
needle-like crystals, can be found in the pericardium, liver,
kidneys, intestine, air sacs and the plasma membrane surface of the
ureter (25). The kidney swells and
becomes pale in color. Urate obstruction causes the ureters to
thicken and harden, leading to larger and hardened kidneys that are
pale and have a snowflake pattern on their surface and parenchyma
(26).
3. Techniques for creating gout models and
avian HUA
Increasing synthesis of uric acid.
Purine-rich diet
Changes in food, including the addition of uric acid
precursors or supplements, can elevate blood uric acid levels in
chickens. Increased consumption of adenine-rich meals can
facilitate synthesis of uric acid. Purine- and protein-rich yeast
can hydrolyze in vivo to form purine and pyrimidine bases,
which interfere with purine metabolism, increase XOD activity and
thus increase uric acid production. Lin et al (27) induced a quail model of HUA via a
high-purine diet ordinary feed mixed with yeast dried powder (15
g/kg/d). On days 14 and 21, levels of serum uric acid in the model
significantly increased and the oxidation levels also increased.
This strategy was also employed by Wang et al (28) to generate a quail HUA model. After
14 days, serum uric acid levels in the model group were
substantially greater than that in the normal group. Wu et
al (29) created a gout model
in quail via high-purine diet. By day 30, the quail exhibited gout
symptoms: XOD activity and uric acid levels were elevated,
oxidative stress balance was disrupted, NLRP3 inflammatory vesicles
were activated and an inflammatory response was produced. Li et
al (30) created a mixed diet
using yeast powder to formulate a quail model of HUA. From day 4,
blood uric acid levels gradually increased in the quail; the levels
peaked on days 7 or 10 and stayed high for the next 28 days. Kuang
et al (31) fed chickens
high-purine diet (formulated through a ratio of 90% basal feed, 10%
yeast leachate supplemented with 0.4% adenine) with a daily water
limit of 50 ml/chicken for 3 weeks, resulting in high uric acid
levels (617.54±16.36 µmol/l).
Protein-rich diet. High-protein feed
materials mainly include animal offal, meat and bone meal, fish
meal, etc. After the body decomposition can produce a large number
of purine nucleotides and converted into excess uric acid (32). Li et al (33) discovered that feeding chicks 24%
crude protein content could cause gout symptoms. Furthermore, by
feeding 1-day-old geese high-protein diet (23% crude protein
content), Wang et al (34)
established a HUA model that resulted in elevated blood protein
content and uric acid production. Hong et al (35) raised 24-week-old male Henleigh
chickens on a high-protein diet (34.88% crude protein content) and
observed ankle joints were curved and deformed. The synovial fluid,
tissue fluid and liver produced sodium urate crystals and their
kidneys displayed different degrees of damage. In addition, serum
uric acid levels increased within 2 weeks and remained high
throughout. Liu (36) fed
50-day-old male Roman chickens high-protein diet (50% soybean meal
and 50% basal diet) and limited water to 100 cc/chicken/day. On day
28, blood uric acid increased (>417 µmol/l), HUA model was
established successfully. Wang et al (37) fed male Huainan sparrow chickens
high-protein diet (50% soybean meal and 50% basal diet) and limited
water to 100 ml. High levels of uric acid (>476.57 µmol/l) were
observed. In addition, it has been reported that the average
abundance of enterococci and anaplastic bacilli is positively
correlated with serum uric acid concentration and that there is a
dose-dependent association between the incidence of gout in
goslings and levels of dietary protein, as determined by assessing
degree of damage to the kidney and cecum microbiota (38).
As discovered by Lumeij et al (39) that eating meat can increase the
levels of uric acid in the blood of birds, spontaneous HUA emerges
in birds of prey after a meal. In another study, after feeding
quail meat to peregrine falcons, plasma uric acid concentrations
peaked at 1,881 µmol/l after 3-8 h and plasma uric acid and urea
concentrations were considerably increased up to 15 h (40). As reported by Bollman and
Schlotthauer (41) turkeys fed
diets supplemented with horse meat also exhibit elevated blood uric
acid levels, indicating that blood uric acid levels are influenced
by the protein or urea of the diet.
Induction by uric acid and sodium urate
administration. Since avian species lack uricase, high uric
acid levels can be induced by directly administering uric acid
monomers in vivo. According to Li et al (42), broilers were gavaged with saline or
ethambutol hydrochloride (200 mg/kg) and adenine (AD, 250 mg/kg),
or yeast powder (YEP, 10 g/kg) and AD (100 mg/kg) to map a stable
model of broiler HUA. The model exhibited a rise in blood uric acid
levels in 49 days. HUA model animals did not lose weight or suffer
renal impairment, making them safer and more appropriate for
short-term HUA observation. Gout was caused by a buildup of urate
in the joints, which resulted in inflammation and impaired joint
function. A model of gout was created by injecting sodium urate
(0.2 ml; 40 mg/ml) into the left ankle joint of chickens; hens in
this model had edematous and stiff ankle joints; pain, indicated by
crouching, standing on one foot and lameness, appeared 1-3 h after
injection and disappeared after 4 h (43). Uric acid-induced models of HUA and
gout in poultry are summarized in Table
I.
 | Table ISummary of uric acid-induced poultry
hyperuricemia and gout models. |
Table I
Summary of uric acid-induced poultry
hyperuricemia and gout models.
| Model animal | Modeling
method | Time of peak uric
acid levels, days | Experiment
duration, days | Performance | (Refs.) |
|---|
| Korea Coturnix-2
male quail | High-purine feed
(15 g/kg per quail per day) | 14 | 21 | Increased serum UA
levels at 14 and 21 days; increased XOD, ADA, G6PD, G6P and NADPH
content; decreased SOD activity, increased activity of GSH-PX,
GSH-GR, and content of MDA, GSH | (27) |
| 4-week-old male
quail | High-purine diet
(yeast and bone extraction powder and 10% fruit sugar; drinking
water 15 ml per chicken | 30 | 40 | Increased XOD and
UA; disrupted oxidation stimulus; NLRP3 inflammation activation;
inflammatory response | (29) |
| Yellow feather
female quail | Yeast powder hybrid
feed (yeast powder: feed, 1:4) | 7 | 28 | Increased UA
levels | (30) |
| 1-day-old
geese | High-protein die
(protein mass, 24%) | 14 | 14 | UA increased; liver
and kidney damage; urate deposition | (33) |
| 1-day-old
geese | High-protein diet
(protein mass, 23%) | 7 | 14 | Increased levels of
AlT, AST transport, LDH, TP, UA and GLB | (34) |
| 24-week-old male
white Henglai chicken | High-protein feed
(34.88% crude protein content) | 14 | 73 | Bent and deformed
foot join; synovial fluid, joint tissue fluid and liver produce
sodium uric acid crystals; kidney damage | (35) |
| 50-day-old male
Roman egg chicken | High-protein feed
(50% basic diet, 50% soybean meal), 100 ml water/chicken | 28 | 42 | Increased blood UA
levels (>417 µmol/l) and XOD, ADA and GD activity | (36) |
| 50-day-old male
Huainan hemp chicken | High-protein diet
(50% basic diet, 50% soybean meal), 100 ml water | 28 | 42 | High UA levels
(>476.57 µmol/l); increased calcium content in serum | (37) |
| Male and female
20-week-old Silky chicken | Injection of sodium
uric acid (0.2 ml, 40 mg/ml) in the ankle joint | - | 7 | Pain 1-3 h
post-injection, indicated by sitting and lying, standing on one
foot, and recovery after 4 h; ankle edema and rigidity | (43) |
Uric acid excretion disorder.
Calcium-rich diet
The primary byproduct of avian protein metabolism is
uric acid, which increases serum levels when uric acid excretion is
inhibited. High-calcium diets have also been proven to impair renal
function. Increased calcium intake is linked to renal impairment
and development of gout among birds (44). According to previous studies,
high-protein diets develop the model fastest, the replication model
of high calcium and high protein diet had the highest success rate
(36,45). Guo et al (46) fed chickens meals with up to 3.78%
calcium, which caused kidney damage and markedly increased serum
uric acid levels on day 17 of the trial. In addition, a
high-protein diet (consisting of 36.59% crude protein) increased
the serum uric acid level of 1-day-old male Sanhua geese, according
to Song et al (47). The
level of serum uric acid in all groups was higher than that in
healthy individuals at 15 days of age, according to Xi et al
(48); in chicks fed high-protein
(22% protein and 1% calcium) and -calcium diets (16% protein and 3%
calcium), kidney injury caused by a high-calcium diet was more
serious than that caused by a high-protein diet. Zheng et al
(49) fed 40-day-old quails with
yeast (15 g/kg), and the levels of UA, XOD, CR and BUN increased on
the 14th day; quails in the HUA model group exhibited reduced
activity, dull coat color, reduced appetite, and depression.
Induction via adenine. Adenine administration
can disrupt regular purine metabolism, leading to increased uric
acid production. It also changes adenine to dihydroxyadenine in the
body by the action of xanthine oxidase, deposition of which in the
renal tubules causes kidney damage and decreases uric acid
excretion (50). By injecting
adenine into basic feed, Li et al (51) produced a model of HUA in quail. In a
study by Ding et al (52),
intraperitoneal UA injection was used to increase the level of SUA.
In this study, the diet of chickens was supplemented with fishmeal
to increase the dietary protein level, but it did not increase the
the level of SUA in chickens. HUA and gout models induced by
inhibition of uric acid secretion are summarized in Table II.
 | Table IISummary of poultry hyperuricemia and
gout models induced by inhibiting uric acid excretion. |
Table II
Summary of poultry hyperuricemia and
gout models induced by inhibiting uric acid excretion.
| Model animal | Modeling
method | Time to peak uric
acid, days | Experiment
duration, days | Performance | (Refs.) |
|---|
| 35-day-old Isa egg
hens | High calcium diet
(calcium content, 3.78%) | 17 | 17 | Weight loss,
water-like feces, dehydration and death. Decreased serum inorganic
phosphorus levels; increased serum calcium, UA, creatinine and urea
nitrogen, levels; visible kidney damage | (46) |
| 1-day-old Yangzhou
goose goslings (male:female, 1:1) | High-protein diet
(22% protein, 1% calcium), high-calciumdiet (16% protein, 3%
calcium) | 15 | 21 | Kidney damage;
increased serum UA and levels of harmful flora such as
Enterococcus and Proteus | (48) |
| 4-week-old French
male quails | 4 g/kg/day adenine
in basic feed | 28 | 28 | Increased UA kidney
damage | (51) |
Multi-pathway induction
Protein- and calcium-rich diet. Composite
induction instead of single modeling techniques results in faster
development of HUA and rapid increase in blood uric acid levels
(50). By incorporating yeast and
fish meal into regular diets, Zhao et al (53) created a model of chicken HUA;
high-protein (30.0% protein and 1.0% calcium) and high-protein and
-calcium (30 protein and 3% calcium) groups displayed anorexia,
weight loss, water-like feces and standing instability and autopsy
revealed urate deposition on the surface of heart, liver, spleen
and mesentery organ surfaces, thickening of the ureter, enlargement
of the kidney and hallmark piebald nephropathy. It was confirmed
that a diet high in protein and calcium results in an increase in
blood uric acid levels. Huanga (54) discovered that diets high in calcium
and protein cause typical visceral gout in chickens; diets high in
calcium and high/normal in protein cause severe renal damage and
diets high in protein and normal for calcium do not raise plasma
uric acid and inorganic phosphorus concentrations but cause renal
damage. After feeding 1-day-old Yangzhou white geese (male/female
ratio, 1:1) high-protein and -calcium food (22% protein and 3%
calcium), Xi et al (48)
observed that, in 15-day-old goslings, serum uric acid, creatinine,
urea nitrogen content, XOD activity and Enterococcus spp.
abundance were increased, renal damage appeared and bacterial
species were malformed, such as Enterococcus spp. and
Bacillus. In addition, other dangerous bacterial flora
disrupt the microecological equilibrium and harm the kidneys. Fu
et al (55) demonstrated
that a high-calcium and -protein diet (24.03% protein and 3.04%
calcium) interferes with chick intestinal flora via the
intestine/liver/kidney axis, inducing HUA. The high-calcium and
-protein diet decreased the relative abundance of
Lachnospiraceae, Butyricicoccus pullicaecorum, Ruminococcus
torques, Ruminococcus gnavus, and Dorea and increased the
relative abundance of Collinsella and
Desulfovibrionales.
Protein- and calcium-rich diet and water
restriction. Severe dehydration or inadequate water intake is a
key cause of visceral urate deposition in poultry (56). The solubility of urate increases
with the amount of water taken. Water is key for physiological and
biochemical processes in the animal body. Notably, the body
temperature of poultry is 2-3 higher than that of human. Urate is
soluble in poultry and can be excreted easily (31).
Chu et al (57) revealed that a diet high in purines
and calcium (basal feed:yeast extract powder:cow bone meal, 5:2:3)
integrated with a 150 ml/day water restriction increases blood uric
acid levels and causes urate deposition in the kidney, which
results in notable pathological alterations. The etiology and
pathology of this model resemble those of gout. Kuang et al
(31) discovered that a chicken
model of HUA could be established through high-calcium and protein
diet with water restriction; after 3 weeks of persistent HUA, the
circumference of the ankle and tarsus increased significantly;
additionally, persistent HUA caused gout, although this was not
consistently associated with uric acid levels. Wang et al
(37) used high-protein and
-calcium diet integrated with water restriction to replicate HUA in
chickens; high-protein diet increased uric acid production and
could also cause renal damage, accelerating the buildup of uric
acid and resulting in the typical characteristics of gout. Yan
et al (58) reported gout
symptoms in experimental chickens on day 14. Notably, elevated
blood uric acid levels, loss of vitality, feather shedding, a
preference for lying down, loss of appetite, kidney damage occurred
in response to high-protein and -calcium diets. Wu et al
(59) discovered that in the HUA
group, in which quail were fed with a commercial formulation with
added 20% yeast extract powder (high-purine diet), UA was increased
and the quails exhibited kidney damage. Qi et al (60) developed a model of gout in chickens
by mimicking the progress of HUA in humans; high-protein diet and
controlling the intake of 100ml water per day, the excretion of
uric acid was reduced while the intake of exogenous purine was
increased, resulting in an increase in serum uric acid level,
eventually leading to gout. Liu (36) fed 50-day-old male Roman chicken
high-protein and -calcium diet (35% basal diet, 15% stone meal and
50% soybean meal) alongside 100 ml water limit and observed that
the blood uric acid levels increased on day 42, the levels of blood
uric acid reached 681.68±508.76 µmol/l, which was significantly
higher than that in normal group. Multi-pathway induction models of
poultry HUA and gout are summarized in Table III.
 | Table IIISummary of hyperuricemia and gout
models multi-pathway induction. |
Table III
Summary of hyperuricemia and gout
models multi-pathway induction.
| Model animal | Modeling
method | Time to increased
uric acid, days | Experiment
duration, days | Performance | (Refs.) |
|---|
| 1-day-old male
Hl-line brown chicken | High-protein and
-calcium feed (protein, 30%; calcium, 3.0%) | 8 | 40 | Increased levels of
UA and activity of XOD; notable symptoms of gout, such as urate
deposition on the surface of viscera, enlargement of the ureter and
kidney; kidney damage | (53) |
| 35-day-old
chicken | High-calcium and
-protein diet (245 g/kg protein; 36.8 g/kg calcium) | 30 | 30 | Increased levels of
plasma uric acid, calcium and sodium; kidney injury-induced
visceral gout; increased urine volume and 24 h urinary acid,
calcium, magnesium, inorganic phosphorus and potassium excretion;
decreased 24 h urinary sodium excretion | (54) |
| 1-day-old Yangzhou
White Goose (male:female, 1:1) | High-protein and
-calcium diet (22% protein, 3% calcium) | 15 | 21 | Increased levels of
UA, CR and Bun and activity of XOD; kidney damage; increased
abundance of harmful flora such as Enterococcus and
Proteus | (48) |
| 1-day-old male
Magang geese | High-calcium and -
protein diet (24.03% protein, 3.04% calcium) | 28 | 28 | HCP diet interfered
with intestinal flora via intestinal/hepatic/renal axis, which
induced systemic inflammation | (55) |
| 40-day-old male
Dafak quails | High-purine and
-calcium diet (basic feed:yeast powder: bovine bone powder, 5:2:3)
and water limit 150 ml/day | 10 | 40 | Visceral gout in
quails was induced by increase of UA, CR and BUN levels, ADA
activity and renal urate deposition in renal tubules | (57) |
| 21-day-old male
Lingnan chickens | High-protein and
-calcium feed (50% corn powder, 30% soybean meal, 6% imported fish
powder, 7% calcium powder, 7% calcium phosphate powder), water
limit 50 ml/day | 7 | 105 | Gout arthritis
diagnosed after 3 weeks of poor general condition, elevated serum
uric acid, rapid weight gain and increased tarsus ankle
circumference in chickens with uric acid >476.57 µmol/l | (31) |
| 50-day-old male in
Huainan hemp chickens | High-protein and
-calcium diet (35% basic diet, 15% calcium powder, 50% soybean
meal) + water limit 100 ml/day | 17 | 42 | Poor general
condition; increased blood uric acid levels; loose feathers,
decreased eating, water-like stool, joint swelling, pain | (37) |
| 30-day-old male
Xiang Yellow chickens | High-protein diet
(50% protein, 9.17% calcium), limited drinking water (100
ml/day) | 7 | 21 | Poor general
condition, ankle swelling, elevated serum uric acid levels,
synovial inflammation of the ankle joint | (60) |
| 50-day-old, male
Roman egg chickens | High-calcium and
-protein feed (35% basal diet, 15% calcium powder, 50% soybean
meal) and limited water (100 ml) | 28 | 42 | UA increased
slightly on the 42nd day, the level of serum uric acid reached
681.68±508.76 µmol/l, which was much higher than normal group;
whole blood viscosity, hematocrit, ESR and erythrocyte aggregation
increased, erythrocyte deformability decreased | (36) |
Other induction techniques. Fat-rich
diet
Epidemiological and clinical studies have shown a
pathological link between abdominal obesity, hyperlipidemia and HUA
(61,62). Purine-rich foods may contribute to
development of HUA, hypertriglyceridemia and abdominal obesity
(63). Lin et al (64) fed Difak quails high-fat diet (85%
regular diet, 14% cooked lard and 1% cholesterol) and suggested
that a high-fat diet may lead to increased adenosine deaminase and
xanthine oxidase activity, activate purine metabolism and
significantly increase serum uric acid levels. High-fat diet may
induce changes in adenosine deaminase and xanthine oxidase activity
and increase uric acid production. Simultaneously, lipoprotein
lipase, hepatic lipase and total esterase activity increased and
lipid metabolism was abnormal.
Induction via injectable drugs and high-fat
diet. A quail model of HUA was created by Ma et al
(65) via high-fat diet combined
with adenine (50 mg) gavage for 7 days. Following modeling, serum
uric acid, creatinine and urea nitrogen levels increased, joints
were enlarged, and kidneys were damaged. It is also associated with
dyslipidemia, with an increase in triglyceride, low-density
lipoprotein and xanthine oxidase, and a decrease in serum total
cholesterol, lipoprotein esterase, adenosine deaminase and
high-density lipoprotein. Zhang et al (66) reported that blood uric acid levels
quickly increase and remain high for 4 weeks when the quails are
fed high-fat and -hypoxanthine food (8% soybean meal, 50% corn
meal, 16% flour, 10% cooked lard, 1% cholesterol, 5% whole egg
powder, 9% sugar and 1% hypoxanthine; 419 kcal/100 g) and
subcutaneously administered potassium oxazepate (200 mg/kg). After
8 weeks, the high-fat diet only could not sustain elevated uric
acid levels.
High-calcium and low-phosphorus diet.
According to Chen and Fan (67),
gout in chickens can be caused by high-calcium and low-phosphorus
feeds, as well as high-calcium and normal-phosphorus feeds.
Visceral urate deposition and urolithiasis are observed, with high
calcium serving a key role and low phosphorus promoting occurrence
of gout. The kidneys were pale in color and patterned, with varying
degrees of enlargement or atrophy; the ureter was thickened and
contained white urate crystals. After 50 days, 16 chickens died of
gout (10 in the high-calcium and low-phosphorus group; six in the
high-calcium and normal-phosphorus group).
Induced by toxins. Viruses and toxins cause
of avian gout (68). Renal
excretory dysfunction is associated with pathophysiology of gout
produced by ovine toxin poisoning, according to Pegram and Wyatt
(69). The virus causing chicken
infectious bronchitis can multiply in renal epithelial cells,
impairing renal function and triggering visceral gout (70). The goose-derived astrovirus (GoAstV)
has been suggested to be the cause of renal disease and visceral
gout (71,72), as discovered by Yin et al
(73) through oral and subcutaneous
injection of GoAstV and observation of dynamic distribution of the
virus in chicks. The virus could replicate in tissues and cause
pathological damage, particularly in the kidney, liver, heart and
spleen, and typical visceral gout (74). Ali et al (75) added 2.5 and 5.0% sodium bicarbonate
to drinking water, causing gout in 72-day-old broiler chickens.
Biochemical analyses in week 3 revealed significant elevation of
blood (hemoglobin, stacked cell volume, total erythrocyte and
leukocyte count and heterophilic granulocytes) and biochemical
parameters (aspartate and alanine transferase, uric acid, urea
nitrogen, creatinine, total protein and albumin levels) in the
sodium bicarbonate group, along with renal damage. In addition to
hepatotoxicity and nephrotoxicity, acetyl chloride phenolic acids
induce visceral gout in laying hens (76). A previous study also revealed that
diclofenac sodium results in uric acid crystal deposition, necrosis
of hepatocytes and myocytes, leukocyte infiltration of the liver
and heart parenchyma and visceral gout in male broiler chickens
(77). Using diclofenac sodium,
Jiao (78) developed a model of
chicken gout with visceral-type gout lesions. Methods of inducing
avian HUA and gout are summarized in Table IV.
 | Table IVSummary of other methods to induce
avian hyperuricemia and gout models. |
Table IV
Summary of other methods to induce
avian hyperuricemia and gout models.
| Model animal | Modeling
method | Time to increased
uric acid, days | Experiment
duration, days | Performance | (Refs.) |
|---|
| Male Difak
quails | High-fat diet (85%
common diet, 14% cooked lard, 1% cholesterol) | 7 | 14 | Increased ADA and
XOD activity, purine metabolism and UA levels and activities of
LPL, HL; abnormal lipid metabolism | (64) |
| 30-day-old male
Diphac quails | High-fat diet (54%
corn, 2% bran, 7% fish meal, 30% soybean meal) and adenine
suspension (50 mg/kg) by gavage | 11 | 22 | Increased UA, CRE,
BUN, TG and LDL levels and XOD activity; decreased CHO, LPL, ADA
and HDL levels; joint swelling; decreased toe bone density;
synovial hyperplasia; uric acid deposition in kidney; lipid
metabolism disorder | (65) |
| 5-week-old Chinese
white-feathered quail (male:female, 1:1) | High-fat and
-hypoxanthine diet (8% soybean meal, 50% corn meal, 16% flour, 10%
cooked lard, 1% cholesterol, 5% whole egg meal, 9% sugar, 1%
hypoxanthine) combined with subcutaneous injection of potassium
oxazate (200 mg/kg) | 7 | 63 | Increased levels of
UA, TG, insulin, AlT, AST, BUN and CRE and peak blood glucose;
liver, kidney and aorta damaged | (66) |
| 21-day-old Hessex
and Roth hybrid chicken | High-calcium and
low-phosphorus diet (3.1% calcium and 0.19% absorbable phosphorus).
High calcium and phosphorus (containing 3.1% calcium and 0.39%
absorbable phosphorus) | 21 | 91 | Uric acid salt with
visceral deposition and urolithiasis; kidney pale and patterned;
thick ureter; white uric acid salt crystals; death due to gout | (67) |
| 72-day-old broiler
chickens | 2.5 and 5.0% sodium
bicarbonate in drinking water | 7 | 21 | Significantly
increased hemoglobin, AST, ALT, UA, BUN, CRE, TP and ALB levels,
packed cell volume, total red and white blood cells and
heterophils; renal damage | (75) |
| 357-day-old White
Leghorn chickens (The sex of the animal is not stated) | Sodium diclofenac
(300, 500 and 700 mg/kg) in basal feed | 1 | 7 | Poor general
condition; visceral uric acid deposition, including the kidney,
liver, heart, pericardium, mesentery | (78) |
4. Metrics from post-model induction
assays
To assess whether animal models of HUA have been
established, it is important to detect related indices. Animal
models of HUA are generated by various methods but there is no
unified method of evaluation. Of 281 HUA models, 98.22% examined
biochemical indicators, 48.75% assessed pathological indicators and
18.15% investigated epigenetic indicators (79).
General observation indicators
Body mass, joint swelling, mental state, changes in
activity, food and water intake, diarrhea, pain and other bodily
changes have been observed in animal models since they are the
common indicators of HUA and gout (29,54,60,80).
However, because researchers may introduce subjective elements
(such as human error in observing and recording indicators), these
alone cannot be used to evaluate models but must be used in
conjunction with other methods of evaluation (79).
Biochemical indices
Biochemical markers serve as key indicators for
tracking alterations in tissue and organ function since they
demonstrate the degree of the pathological change at the molecular
level. The most common method to assess the success of modeling is
dynamic measurement of blood uric acid levels (31,57,59,81).
Development of gout due to high blood uric acid levels is
associated with inflammation and oxidative stress damage (82). The excretion of uric acid could be
indicated by the amount of uric acid in the feces and urine of
birds, owing to their unique physiology, including the ureter,
reproductive ducts and digestive tract converging at the end of the
cloaca (59). The primary clinical
biochemical markers of HUA include alterations in the activity of
XOD, adenosine deaminase and guanosine deaminase, which impact uric
acid metabolism. Aspartate and alanine aminotransferase are markers
of liver function and urea nitrogen and creatinine levels indicate
renal impairment associated with HUA (82). Both the degree of modeling and
therapeutic and adverse effects of medication can be assessed
through kidney and liver function (83). Blood glucose, blood lipids,
triglycerides, total cholesterol, high-density lipoprotein
cholesterol, and low-density lipoprotein cholesterol are indicators
that can be used to observe HUA combined with metabolic syndrome.
They are also commonly tested clinical indicators in patients with
gout (82). Oxidative
stress-induced variations in the capacity of the body to scavenge
free radicals and repair damage can be inferred from changes in the
activity of glutathione peroxidase and reductase, reduced
glutathione, malondialdehyde and peroxide dismutase (84). The increase in lactic acid affect
the excretion of uric acid (85).
Additionally, the pathophysiology of HUA is connected to
inflammatory factors, uric acid transporter protein levels,
pathways and changes in gene expression. Monitoring these
indicators may contribute to research on the mechanism of
decreasing uric acid and serve as targets for research on drug
interventions.
Signs of pathology
X-ray examination is a common examination of gout in
humans. It can show changes in the bones and joints of gout and the
presence or absence of urate deposits. Ma et al (65) examined the swollen toes of HUA
quails by X-ray and histomorphology. X-ray results show decreased
toe bone density, osteolytic articular surface destruction and soft
tissue changes of the toe, which can be combined with biochemical
indexes as a method to establish the detection model. Hong et
al (35) used X-rays to examine
joints and phalanges of chickens with high-protein diet-induced HUA
and discovered that the structure of joint sections was unclear,
similar to images of joints of patients with mild gout, while urate
crystals in synovial fluid of chicken joints were detected by
optical microscopy, which is also used as a test for human gout.
Furthermore, the damage induced by modeling was primarily assessed
by examining morphological changes, inflammatory cell infiltration,
urate deposition and organelle changes in the heart, liver, kidney
and joints (85). Furthermore,
pathological section directly reflects the extent of target organ
damage. In experimental investigations, the modeling is mostly
assessed through biochemical and pathological indicators (29,35,54).
Chu et al (57) fed the
quails with a high purine diet and induced quail HUA. Biochemical
tests showed that the levels of creatinine, urea nitrogen and
adenosine deaminase activity were increased. The renal
histopathology was observed, it was found that black urate crystals
were deposited in the renal tubules in the model group, and both
proximal and distal tubules were distributed. Renal tubular
obstruction can aggravate uric acid excretion disorders, and
eventually induce the occurrence of gout. In order to evaluate the
renal injury of this model more comprehensively. Meng et al
(86) combined conventional renal
function indexes: increased levels of creatinine and urea nitrogen,
and early markers of kidney injury: elevated levels of kidney
injury molecule 1(KIM-1) and neutrophil gelatinase-associated
lipocalin (NGAL); pathological indices: congestion of the kidney,
dilation of the renal tubules, epithelial cell shedding; The
accumulation of urate in the kidney was observed by inflammatory
cell infiltration and fibrosis, and by renal examination with
silver staining.
Intestinal flora indicators
Numerous studies have shown that development of HUA
and gout is directly associated with alteration of the gut
microbiota and decreased gut barrier function (87,88).
The human body obtains a notable amounts of uric acid from the
intestinal microflora. The intestine excretes approximately
one-third of uric acid and bacteria such as Lactobacillus,
Bacteroides and Bifidobacterium are key to this
process. Changes in the relevant intestinal microbiological indices
may provide new targets and approaches for the treatment of HUA
(89,90). Guo et al (91) reported a decrease in
Lactobacillus and Pseudomonas among the intestinal
flora of patients with gout and diagnosis via 17 gout-associated
bacteria reached 88.9% accuracy. Compared with serum uric acid as a
reference index, this method is more sensitive in the diagnosis of
gout As well as structural alterations in intestinal flora, Huang
et al (92) found that a
quail model of high-purine diet-induced HUA was associated with ~3
times the level of lipopolysaccharide (LPS) in peripheral blood
compared with that in quail fed normal diet. LPS is considered to
be responsible for metabolic disorders and elevated XOD activity,
which may increase uric acid levels (93,94).
According to Xi et al (95),
geese with gout have considerably higher serum levels of LPS and
increased loss of intestinal barrier as a result of gut dysbiosis
causes the translocation of gut-derived LPS, may put chicks at risk
for developing gout.
Traditional Chinese Medicine (TCM)
indicators
TCM emphasizes the relationship between ‘disease’
and ‘evidence’. From this perspective, gout belongs to the
categories of ‘paralysis’ ‘dampness, heat and steam embedded in the
meridians’ and ‘paralyzing disease’ (96,97).
Lin et al (98) used a
high-purine diet to induce HUA in quail and measured the indices of
questioning, smelling and looking, observed changes in the shape of
urine and feces, along with changes in the tongue. Certain
pathological changes such as stool is loose and greasy were similar
to the symptoms of ‘spleen deficiency, phlegm and dampness’
triggered by poor diet.
5. Issues in the modeling of gout and HUA in
poultry
Methodology for model selection
The ideal HUA and gout model should show stability,
repeatability, applicability and affordability. The model should
mimic the progressive pathological process of gout and should be
able to be replicated in a short time. Moreover, the state of HUA
should be maintained, whereas tissue damage unrelated to HUA and
gout should be decreased (99).
Current models of HUA and gout focus on rodents,
which have urate oxidase that break down uric acid into more
water-soluble allantoin (90,100-102).
Mice are unlikely to undergo the persistent, progressive
pathological changes that lead to gout when high uric acid levels
arise in the body. Mouse models of HUA are primarily divided into
environment-(drug- or diet-induced) and gene-induced (103). The use of urate Oxidase (UOX)
inhibitors, such as potassium oxazate, is a common method of
modeling since they block the further metabolism of uric acid
(104). However, in rodents,
uricase exists, which breaks down uric acid into allantoin and
excretes it. Uric acid rarely accumulates in the body; therefore it
is difficult to replicate a sustained model of HUA in experiments
(105,106).
Concentration of uric acid is much lower in mice
than that in human. In addition, drugs can cause renal functional
damage unrelated to gout (30,107,108). Uricase-deficient mice developed by
gene knockout, abrogated a major difference in the inability of
uric acid to accumulate in vivo due to the presence of
uricase in mice, more in line with the human uric acid metabolic
pathway; however, complex techniques and high animal mortality
suggest that the model is less economically viable for basic
research (100,109).
The pathway of purine nucleotide metabolism in birds
is similar to that in humans. Purine nucleotides are metabolized,
and uric acid is excreted as an end product. When purine content in
the diet is too high, it can lead to an increase in uric acid
production in the body, which can accurately reflect the metabolic
levels of purine nucleotides (66).
Notably, similar to in humans, birds lack uricase and uric acid is
the end product of their purine metabolism. When birds are selected
to generate a model of HUA, there is no need to use an inhibitor of
uricase, which can decrease the visceral damage caused by inducers
(such as a high purine diet). Studies have shown that stable yet
persistent serum uric acid levels and mild renal lesions in avian
models of HUA are required to observe the effects of drug therapy
for a long time (42,85). Compared with mouse models, avian
models of gout have a longer survival time and are considered more
suitable for long-term observation of drug effects (30,52).
Furthermore, gout in poultry is affected by genetic factors,
dietary changes and environmental factors, similar to the etiology
of gout in humans, and the joint symptoms of gout in poultry are
akin to those in humans (18).
Therefore, due to physiological and pathological characteristics,
birds may be a suitable model for exploring human HUA and gout
since they simulate the whole pathological process from HUA to gout
onset in the human body (9,57,110).
Animal selection for poultry
models
In terms of HUA models, factors such as incidence of
gout in poultry, feeding management and experimental feasibility
should be considered. Medium and small-sized poultry, such as
chickens, pigeons and quails, are easy be raise and manage in large
quantities. The large size of chickens, ducks and geese is
convenient for experimental observation and observation of
experimental indexes. Poultry have a short growth cycle, hatching
can be controlled and they are low cost. Common species of
experimental poultry include chickens, ducks, quails, pigeons and
geese; however, only specific pathogen-free (SPF) chickens and
ducks have been developed. Other species of birds have not yet been
standardized, making it difficult to use them in laboratory
studies. Notably, the avian model of HUA requires further
exploration.
To the best of our knowledge, there is no uniform
conclusion regarding the effect of sex on modeling. The sex of most
animal models of HUA has been male, with fewer studies using female
HUA models (79,110,111).
6. Conclusion
In conclusion, the most common approach to induce
avian HUA and gout is diet. The majority of researchers induce HUA
by increasing uric acid production and inhibiting uric acid
excretion, because preparing the model is easy, affordable and it
produces a stable model that is convenient to operate and allows
for the dynamic observation of blood biochemical indices. The
progression of human gout and its etiology is similar to that in
avian models. Nevertheless, the applicability of these models is
restricted by the strains, species and feeding circumstances of
experimental animals. Evaluation indexes include apparent index,
biochemical index, pathological index and intestinal microflora
index; however, there is no consistent standard for creation and
assessment of animal models of HUA in gout research.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Key Projects of
Natural Science Foundation of Heilongjiang (grant no. ZD2022H006),
Basic Scientific Research Expenses Scientific Research Projects of
Universities in Heilongjiang Province(2022-KYYWF-0651), key
Laboratory of Gout Research of Heilongjiang Province
(TFYJ202301).
Availability of data and materials
Not applicable.
Authors' contributions
QH and WJ conceived the study and revised the
manuscript. WL and WB wrote manuscript. LJ and YX reviewed the
manuscript. Data authentication is not applicable. All authors read
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huang J, Yang T, Wang Y and Zhang B:
Progress and thinking on the treatment of gout disease at home and
abroad. World Chin Med. 16:1–7. 2021.(In Chinese).
|
|
2
|
No authors listed. Dietary guide for
hyperuricemia and gout patients (WS/T 560-2017). Biomed Environ
Sci. 36:897–898. 2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sun LM, Xia XL and Ma HP: Research
progress on the relationship between hyperuricemia and hypertension
and cardiovascular diseases. Shanxi Med J. 53:286–289. 2024.
|
|
4
|
Jiang J, Zhang T, Liu Y, Chang Q, Zhao Y,
Guo C and Xia Y: Prevalence of diabetes in patients with
hyperuricemia and gout:A systematic review and meta-analysis. Curr
Diab Rep. 23:103–117. 2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhou H and Liang W: Chronic kidney disease
and hyperuricemia. J Clin Internal Med. 40:374–378. 2023.(In
Chinese).
|
|
6
|
Wang X, Hou Y, Wang X, Li Z, Wang X, Li H,
Shang L, Zhou J and Zhang Y, Ren M and Zhang Y: Relationship
between serum uric acid levels and different types of atrial
fibrillation:An updated meta-analysis. Nutr Metab Cardiovasc Dis.
31:2756–2765. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
She D, Wang Y, Liu J, Luo N, Feng S, Li Y,
Xu J, Xie S, Zhu Y, Xue Y and Zhang Z: Changes in the prevalence of
hyperuricemia in clients of health examination in Eastern China,
2009 to 2019. BMC Endocr Disord. 22(202)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tang YS, Liu H and Liu BC: Reflections on
the basis of changes: the epidemiological data of hyperuricemia.
Drugs and Clinic. 12:8–13. 2015.(In Chinese).
|
|
9
|
Liu C, Sun M and Liao M: A review of
emerging goose astrovirus causing gout. Biomed Res Int.
2022(1635373)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bulbule NR, Kapgate SS and Chawak MM:
Infectious causes of gout in chickens. Adv Anim Vet Sci. 2:255–260.
2014.
|
|
11
|
Zhou YZ and Wu SK: Comprehensive diagnosis
and treatment of a case of gout in quails. Anim Husbandry Poult
Industry. 6:89–90. 2009.(In Chinese).
|
|
12
|
Herbert JD, Coulson JO and Coulson TD:
Quantification of tissue uric acid levels in a Harris's hawk with
visceral gout. Avian Dis. 55:513–515. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang ZG: Diagnosis and treatment of avian
gout. Jilin Anim Husbandry Vet Med. 44:89–90. 2023.(In
Chinese).
|
|
14
|
Xing LJ: Diagnosis and treatment of duck
gout. Poult Farming Poult Dis Control. 12:43–44. 2010.(In
Chinese).
|
|
15
|
Guo HW: Etiological analysis and diagnosis
and treatment of goose gout. Anim Sci Technology Information.
1:174–176. 2023.(In Chinese).
|
|
16
|
Liu B: The diagnosis and treatment of
peacock gout. Anim Husbandry Vet Sci Tech Inf. 8:207–209. 2023.(In
Chinese).
|
|
17
|
Chu B: Diagnosis and treatment of gout in
Turkey. Chin J Poult Ind. 8(29)2003.(In Chinese).
|
|
18
|
McFarland DC and Coon CN: Purine
metabolism studies in the high and low uric acid containing lines
of chickens: De novo uric acid synthesis and xanthine
dehydrogenase activities. Poult Sci. 59:2250–2255. 1980.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Roman YM: The role of uric acid in human
health: Insights from the uricase gene. J Pers Med.
13(1409)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
McFarland DC and Coon CN: Purine
metabolism in high- and low-uric acid lines of chickens:
hypoxanthine/guanine phosphoribosyltransferase activities. Proc Soc
Exp Biol Med. 173:41–47. 1983.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Guo X, Huang K and Tang J:
Clinicopathology of gout in growing layers induced by high calcium
and high protein diets. Br Poult Sci. 46:641–646. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wu H, Wang Y, Ren Z, Li Y, Huang J, Lin Z
and Zhang B: Overnutrition-induced gout: An immune response to
NLRP3 inflammasome dysregulation by XOD activity increased in
quail. Front Immunol. 13(1074867)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Austic RE and Cole RK: Impaired renal
clearance of uric acid in chickens having hyperuricemia and
articular gout. Am J Physiol. 223:525–530. 1972.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cole RK and Austic RE: Hereditary uricemia
and articular gout in chickens. Poult Sci. 59:951–960.
1980.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lakshmi Namratha M, Kumar YR and Lakshman
M: Pathology of visceral gout in layer chicken. Int J Recent Sci
Res. 10:35546–35548. 2019.https://www.researchgate.net/publication/338898873.
|
|
26
|
Rahimi M, Minoosh Z and Haghighi S:
Visceral urate deposition in a little bittern (Ixobrychus
minutus). Vet Res Forum. 6:177–180. 2015.PubMed/NCBI
|
|
27
|
Lin ZJ, Zhang B, Li LY and Zhu CS: Study
on oxidative stress condition of hyperuricemia quail model. J Clin
Exp Med. 14:1665–1668. 2015.(In Chinese).
|
|
28
|
Wang Y, Lin ZJ, Huang J, Chu MZ, Ding XL,
Li WJ, Mao QY and Zhang B: An integrated study of Shenling Baizhu
San against hyperuricemia: Efficacy evaluation, core target
identification and active component discovery. J Ethnopharmacol.
295(115450)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wu H, Wang Y, Huang J, Li Y, Lin Z and
Zhang B: Rutin ameliorates gout via reducing XOD
activity,inhibiting ROS production and NLRP3 inflammasome
activation in quail. Biomed Pharmacother.
158(114175)2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li F, Zhang GW, Li XH, et al: Comparison
of the uric acid-lowering effect of
3,4-dihydroxy-5-nitrobenzaldehyde on mouse and quail hyperuricemia
models. J Xi'an Jiaotong Univ (Med Sci). 43:827–832. 2022.(In
Chinese).
|
|
31
|
Kuang HY, Cheng TP, Hu JB, Cao Z, Kou W
and Rong J: Constructing method of chicken model with persistent
hyperuricemia model. Sichuan J Zool. 4:554–558. 2008.(In
Chinese).
|
|
32
|
Hevia P and Clifford AJ: Protein intake,
uric acid metabolism and protein efficiency ratio in growing
chicks. J Nutr. 107:959–964. 1977.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li MM, Ding XD, Rong XL, Fang TY, Qian SF,
He MC, Li Y, Li JC and Wu JJ: Clinical pathological study of
gosling gout induced by high protein diet. J South China Agr Univ.
40:46–52. 2019.(In Chinese).
|
|
34
|
Wang Z, Hu ZH, Li ST, Gui X, Feng S, Li Y,
Wang X, Li J and Wu J: Study on serum metabonomics of goslings with
hyperuricemia induced by high protein diet. J Nanjing Agr Univ.
44:1169–1176. 2021.(In Chinese).
|
|
35
|
Hong F, Zheng A, Xu P, Wang J, Xue T, Dai
S, Pan S, Guo Y, Xie X, Li L, et al: High-protein diet induces
hyperuricemia in a new animal model for studying human gout. Int J
Mol Sci. 21(2147)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liu W: The expression of VIP in immune
organs and the investigation of hemorheology of hyperuricemia model
chickens. Anhui Agr Univ, 2010 (In Chinese).
|
|
37
|
Wang SZ, Li FB, Wu CJ, Liu W, Yang J, Luo
YX, Liu XB and Wu JJ: Serology test on experimental hyperuricemia
chicken. Anim Husbandry Feed Sci. 31:10–12. 2010.(In Chinese).
|
|
38
|
Xi Y, Huang Y, Li Y, Yan J and Shi Z:
Fermented feed supplement relieves caecal microbiota dysbiosis and
kidney injury caused by high-protein diet in the development of
gosling gout. Animals (Basel). 10(2139)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Lumeij JT, Sprang EP and Redig PT: Further
studies on allopurinol-induced hyperuricaemia and visceral gout in
red-tailed hawks (Buteo jamaicensis). Avian Pathol.
27:390–393. 1998.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lumeij JT and Remple JD: Plasma
urea,creatinine and uric acid concentrations in relation to feeding
in peregrine falcons (Falco peregrinus). Avian Pathol.
20:79–83. 1991.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bollman JL and Schlotthauer CF:
Experimental gout in turkeys. Am J Digest Dis. 3:483–488. 1936.
|
|
42
|
Li D, Zhang M, Teng Zhu La A, Lyu Z, Li X,
Feng Y, Liu D, Guo Y and Hu Y: Quercetin-enriched Lactobacillus
aviarius alleviates hyperuricemia by hydrolase-mediated
degradation of purine nucleosides. Pharmacol Res.
196(106928)2023.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu RH, Shi W, Zhang YX, Zhuo M and Li XH:
Selective inhibition of adenylyl cyclase subtype 1 reduces
inflammatory pain in chicken of gouty arthritis. Mol Pain.
17(17448069211047863)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Li XM, Deng MX, Li QY, Jia RY and Wang J:
Experimental study on clinical pathology deposition of urate in
chickens. Chin J Vet Sci. 18:50–52. 1998.
|
|
45
|
Ren YY: Establishment of an experimental
chicken hyperuricemia model. J Huaibei Professional Tech Coll.
12:133–135. 2013.(In Chinese).
|
|
46
|
Guo XQ, Huang KH, Chen F and Luo JB: Renal
injury and apoptosis in growers with gout induced by high dietary
calcium. Chin J Vet Sci. 28:1461–1463, 1479. 2008.(In Chinese).
|
|
47
|
Song N, Wang M, Zhong G, Zhu K, Chen P,
Zhang N, Liu X and Zhang W: Bacteroides xylanisolvens
possesses a potent anti-hyperuricemia effect in goslings fed on a
high-protein diet. Front Microbiol. 14(1173856)2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Xi YM, Yan JS, Ying SJ, et al: Effects of
high protein and high calcium diet on visceral gout, renal function
and intestinal microflora in goslings. Chin J of Anim Nutr.
31:612–621. 2019.(In Chinese).
|
|
49
|
Zheng L, Bai Y, Wan Y, Liu F, Xie Y, He J
and Guo P: Ameliorative action of "daitongxiao" against
hyperuricemia includes the "uric acid transporter group". Front
Pharmacol. 15(1300131)2024.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lin ZJ, Li F and Zhang B: Advances in
research on hyperuricemia in avians. Acta Lab Anim Sci Sin.
25:572–576. 2017.
|
|
51
|
Li CD, Gao L, You GS, et al: Effect of
ultrafine powder of Mazhu powder on quail hyperuricemia. Hebei Med
J. 31:490–491. 2009.(In Chinese). DOI:
10.3969/j.issn.1002-7386.2009.04.054.
|
|
52
|
Ding X, Peng C, Li S, Li M, Li X, Wang Z,
Li Y, Wang X, Li J and Wu J: Chicken serum uric acid level is
regulated by glucose transporter 9. Anim Biosci. 34:670–679.
2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zhao ZS, Kong T and Zhou BH: Establishment
of chicken hyperuircemia model and its inhibition by allopurine.
Chin J Vet Sci. 34:1345–1348. 2014.(In Chinese).
|
|
54
|
Huanga KH: Clinicopathology of Gout in
growing layers induced by high calcium and high protein diets.
Proceedings of the International Symposium on Metabolic Diseases in
Poultry Nutrition, 2006.
|
|
55
|
Fu Y, Chen YS, Xia DY, Luo XD, Luo HT, Pan
J, Ma WQ, Li JZ, Mo QY, Tu Q, et al: Lactobacillus rhamnosus GG
ameliorates hyperuricemia in a novel model. NPJ Biofilms
Microbiomes. 10(25)2024.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Rexhepi A, Brown C, Sherifi K and Behluli
B: Visceral gout (Uricosis) and urolithiasis caused by dehydration
in laying hen farm, necropsy and histopathology findings. Kafkas
Üniversitesi Veteriner Fakültesi Dergisi. 21:291–294. 2015.
|
|
57
|
Chu MZ, Lin ZJ, Zhang B, et al: Induction
of visceral gout model in quail. Chin Pharmacol Bull. 36:879–883.
2020.(In Chinese). DOI: 10.3969/j.issn.1001-1978.2020.06.026.
|
|
58
|
Yan M, Zheng X, Lin Y, Zheng X, Xi K, Gao
Y, Wang H, Li Y and Liu C: Effects of Smilax China L. extracts on
Hyperuricemia chicken model via inhibiting xanthine oxidase
activity. Poult Sci. 103(103887)2024.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Wu J, Aga L, Tang L, Li H, Wang N, Yang L,
Zhang N and Wang X and Wang X: Lacticaseibacillus paracasei
JS-3 isolated from "Jiangshui" ameliorates hyperuricemia by
regulating gut microbiota and iTS metabolism. Foods.
13(1371)2024.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Qi XY, Xiang LL, Xiong H, Li TL, Zhou B,
Guo YX and Lu XL: A dose-effect relationship study of gouty
arthritis chicken model built by high-protein diet. J Trad Chin
Orthop Trauma. 27:1–6. 2015.(In Chinese).
|
|
61
|
Wang CY and He JH: Relationship between
Hyperuricemia and hypertension, obesity, hyperlipidemia and
diabetes. J Applied Med. 5:819–821. 2010.(In Chinese).
|
|
62
|
Lin ZJ, Zhang B, Huang SN and Li LY:
Advances in research of animal models of hyperuricemia combined
with abdominal obesity. Acta Lab Anim Sci Sin. 4:81–85. 2014.(In
Chinese).
|
|
63
|
Lin Z, Zhang B, Liu X, Jin R and Zhu W:
Effects of chicory inulin on serum metabolites of uric acid,
lipids, glucose, and abdominal fat deposition in quails induced by
purine-rich diets. J Med Food. 17:1214–1221. 2014.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Lin ZJ, Zhang B, Liu XQ and Yang HL:
Abdominal fat accumulation with hyperuricemia and
hypercholesterolemia quail model induced by high fat diet. Chin Med
Sci J. 24:191–194. 2009.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Ma L, Zeng GR, Zhang MH, Deng Q, Zhang D,
Pan SQ, Jiang DJ and Wang XQ: Hunan Center for Drug Safety
Evaluation, Institute of Medicinal Plant Beijing Union Medical
College. Lipid metabolism study quail high uric acid hematic
disease animal model. Chin J Comp Med. 11:17–20+5. 2015.
|
|
66
|
Zhang Y, Ma LM and Wu GZ: High fat high
purine diet combined oteracil potassium induced quail glucose and
lipid metabolic disorders and complications. Chin J Comp Med.
1:58–64. 2016.(In Chinese).
|
|
67
|
Chen JK and Fan GX: Pathological study on
gout induced by high calcium and low phosphorus diet in chickens.
Acta Veterinaria et Zootechnica Sinica. 1:80–86. 1992.(In
Chinese).
|
|
68
|
Jian GL, Qing YF and Zhang QB: Research
status and progress of animal models of gout. Medical Review.
27:2397–2401. 2021.
|
|
69
|
Pegram RA and Wyatt RD: Avian gout caused
by oosporein,a mycotoxin produced by Chaetomium trilaterale. Poult
Sci. 60:2429–2440. 1981.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
70
|
Timurkaan N, Öngör H, Kalender H,
Karabulut B, Çöven F, Çevik A, Eröksüz H and Çetinkaya B:
Pathological and molecular findings of visceral gout caused by
Israel variant 2 (IS/1494/06) genotype of infectious bronchitis
virus in chickens. Ankara Univ Vet Fak Derg. 70:149–156. 2023.
|
|
71
|
Li L, Sun M, Zhang Y and Liao M: A review
of the emerging poultry visceral gout disease linked to avian
Astrovirus infection. Int J Mol Sci. 23(10429)2022.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Xiang Y, Tang Z, Li Ll, et al: Progress in
the study of a new type of goose astrovirus causing goslings gout.
Guangdong Anim Husbandry Vet Sci Tech. 49:1–7. 2024.(In
Chinese).
|
|
73
|
Yin D, Tian J, Yang J, Tang Y and Diao Y:
Pathogenicity of novel goose-origin astrovirus causing gout in
goslings. BMC Vet Res. 17(40)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Ejaz S, Kim BS and Lim CW: Gout induced by
intoxication of sodium bicarbonate in Korean native broilers. Drug
Chem Toxicol. 28:245–261. 2005.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Ali R, Kamil SA, Mir MS, et al: Disease
modifying activity of methanolic extract of Colchicum luteum
against experimental gout in broiler chicken. Indian Journal of
Natural Products and Resources (IJNPR)[Formerly Natural Product
Radiance (NPR)]. 13:223–229. 2022.DOI:
10.56042/ijnpr.v13i2.41191.
|
|
76
|
Patel NJ, Joshi BP, Prajapati KS and Patil
VM: Pathomorphological changes of aceclofenac toxicity in layer
chicks. Veterinary World. 7:90–94. 2014.
|
|
77
|
Sandhyarani K, Madhuri D, Jeevanalatha M
and Dhanalakshmi K: Histopathological changes in liver and heart of
diclofenac induced visceral gout in broilers and its amelioration
with ayurvet product. Pharma Innovat. 8:250–255. 2019.
|
|
78
|
Jiao HJ: Establishment and application of
a rapid diagnostic method for chicken gout strip[D]. Hebei Normal
Univ Sci Tech, 2020 (In Chinese).
|
|
79
|
Qiu GN, Li ZD and Miao MS: Analysis of
application characteristics of hyperuricemia animal model based on
data mining. Trad Chin Drug Res & Clin Pharmacol. 34:222–227.
2023.(In Chinese).
|
|
80
|
Cao J, Bu Y, Hao H, Liu Q, Wang T, Liu Y
and Yi H: Effect and potential mechanism of Lactobacillus
plantarum Q7 on hyperuricemia in vitro and in
vivo. Front Nutr. 9(954545)2022.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Cao J, Liu Q, Hao H, Bu Y, Tian X, Wang T
and Yi H: Lactobacillus paracasei X11 ameliorates
hyperuricemia and modulates gut microbiota in mice. Front Immunol.
13(940228)2022.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Kang L, Miao MS, Liu HJ and Li N: Analysis
of animal model based on clinical symptoms of gout. Zhongguo Zhong
Yao Za Zhi. 43:4547–4552. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
83
|
Yue YS, Zhang W, Xie YF, Qin XM and Du GH:
Research progress on experimental animal models of hyperuricemia.
Chin Pharmacol Bull. 39:201–206. 2023.(In Chinese).
|
|
84
|
Liang H, Deng P, Ma YF, Wu Y, Ma ZH, Zhang
W, Wu JD, Qi YZ, Pan XY and Huang FS: , et al: Advances in
experimental and clinical research of the gouty arthritis treatment
with Traditional Chinese Medicine. Evid Based Complement Alternat
Med. 2021(8698232)2021.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Tan WB and Li J: Evaluation of different
modeling methods to establish hyperuricemia animal models. Acad J
Chin PLA Med Sch. 45:12–17. 2024.
|
|
86
|
Meng J, Tian JZ, Wang LM, Zhao Y, Li CY,
Yi Y, Zhang YS, Han JY, Pan C, Liu SY, et al: Adequate Animal
Models of Hyperuricemia for Traditional Chinese Medicine Screening.
Chin J Exp Trad Med Formulae. 27:46–56. 2021.(In Chinese).
|
|
87
|
Bian M, Wang J, Wang Y, Nie A, Zhu C, Sun
Z, Zhou Z and Zhang B: Chicory ameliorates hyperuricemia via
modulating gut microbiota and alleviating LPS/TLR4 axis in quail.
Biomed Pharmacother. 131(110719)2020.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Wang Z, Li Y, Liao W, Huang J, Liu Y, Li Z
and Tang J: Gut microbiota remodeling: A promising therapeutic
strategy to confront hyperuricemia and gout. Front Cell Infect
Microbiol. 12(935723)2022.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Lin S, Zhang T, Zhu L, Pang K, Lu S, Liao
X, Ying S, Zhu L, Xu X, Wu J and Wang X: Characteristic dysbiosis
in gout and the impact of a uric acid-lowering treatment,
febuxostat on the gut microbiota. J Genet Genomics. 48:781–791.
2021.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Zhang QZ, Zhang JR, Li X, Yin JL, Jin LM,
Xun ZR, Xue H, Yang WQ, Zhang H, Qu J, et al: Fangyukangsuan
granules ameliorate hyperuricemia and modulate gut microbiota in
rats. Front Immunol. 15(1362642)2024.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Guo Z, Zhang J, Wang Z, Ang KY, Huang S,
Hou Q, Su X, Qiao J, Zheng Y, Wang L, et al: Intestinal microbiota
distinguish gout patients from healthy humans. Sci Rep.
6(20602)2016.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Huang SN, Lin ZJ, Zhang B, Geng D, Niu H,
Zhu C, Wang X and Sun B: Effects of chicory on gut microflora of
hyperuricemia quail. Trad Chin Drug Res Clin Pharm. 26:447–451.
2015.(In Chinese).
|
|
93
|
Qin DE, Liang W, Yu Y, Whelan EC, Yuan X,
Wang ZL, Wu XW, Cao ZR, Hua SY, Yin L, et al: Modified Simiaowan
prevents and treats gouty arthritis via the Nrf2/NLRP3 inflammasome
signaling pathway. J Ethnopharmacol. 318(Pt
A)(116906)2024.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Xu MX, Wang M and Yang WW: Gold-quercetin
nanoparticles prevent metabolic endotoxemia-induced kidney injury
by regulating TLR4/NF-κB signaling and Nrf2 pathway in high fat
diet fed mice. Int J Nanomedicine. 12:327–345. 2017.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Xi Y, Yan J, Li M, Ying S and Shi Z: Gut
microbiota dysbiosis increases the risk of visceral gout in
goslings through translocation of gut-derived lipopolysaccharide.
Poult Sci. 98:5361–5373. 2019.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Si K and Wang YG: Current situation and
prospect of diagnosis and treatment of gout and hyperuricemia with
integrated traditional Chinese and western medicine. Chin J Clin
Health. 26:606–609. 2023.(In Chinese).
|
|
97
|
Zhou Z and Yu D: Research progress on
mechanism of gouty arthritis treated by traditional Chinese
medicine. Chinese medicine information. 41:71–79. 2024.(In
Chinese).
|
|
98
|
Lin ZJ, Liu XQ, Zhang B, Xue CM, Liu XL,
Wu LL and Zhu WJ: Study on syndrome categorization of hyperuricemia
quail model base on quantitative analysis. Chi J Tradit Chin Med
Pharm. 26:1072–1076. 2011.(In Chinese).
|
|
99
|
Zhou ZZ, Xu L and Gao JD: Research
progress of Hyperuricemia animal model. Med Rev. 26:1462–1466.
2020.(In Chinese).
|
|
100
|
Gao Y, Yu Y, Qin W, Fan N, Qi Y, Chen H
and Duan W: Uricase-deficient rats with similarly stable serum uric
acid to human's are sensitive model animals for studying
hyperuricemia. PLoS One. 17(e0264696)2022.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Wu X, Wakamiya M, Vaishnav S, Geske R,
Montgomery C Jr, Jones P, Bradley A and Caskey CT: Hyperuricemia
and urate nephropathy in urate oxidase-deficient mice. Proc Natl
Acad Sci U S A. 91:742–746. 1994.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Zhu Y, Peng X and Ling G: An update on the
animal models in hyperuricaemia research. Clin Exp Rheumatol.
35:860–864. 2017.PubMed/NCBI
|
|
103
|
Lu J, Dalbeth N, Yin H, Li C, Merriman TR
and Wei WH: Mouse models for human hyperuricaemia: A critical
review. Nat Rev Rheumatol. 15:413–426. 2019.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Xu Y, Chen P, Sun L, Yan Zou, Lixing
Zhang, Wanghai Tang, Tingji Zhang, Huo J and Zhou J: Effect and
mechanism of Yiqing decoction on hyperuricemia rats. Cell Mol Biol
(Noisy-le-grand). 70:217–224. 2024.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Zhou H, Yang J, Yuan X, Song X, Zhang X,
Cao T and Zhang J: Hyperuricemia research progress in model
construction and traditional Chinese medicine interventions. Front
Pharmacol. 15(1294755)2024.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Yu SY and Miao MS: Studies on the
screening methods and mechanisms of anti-gout drugs. Chin J Trad
Chin Med. 28:1875–1878. 2013.(In Chinese).
|
|
107
|
Wang L, Tao Y, Wang X, Gan Y, Zeng Y, Li S
and Zhu Q: Aqueous extract of Phellinus igniarius ameliorates
hyperuricemia and renal injury in adenine/potassium oxonate-treated
mice. Biomed Pharmacother. 177(116859)2024.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Wang M, Zhao J, Zhang N and Chen J:
Astilbin improves potassium oxonate-induced hyperuricemia and
kidney injury through regulating oxidative stress and inflammation
response in mice. Biomed Pharmacother. 83:975–988. 2016.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Lu J, Hou X, Yuan X, Cui L, Liu Z, Li X,
Ma L, Cheng X, Xin Y, Wang C, et al: Knockout of the urate oxidase
gene provides a stable mouse model of hyperuricemia associated with
metabolic disorders. Kidney Int. 93:69–80. 2018.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Aynur A, Buviayxem N, Adila A, et al:
Dynamic comparison of hyperuricemia animal model in male and female
rats. Advances in cardiovascular disease. 44:277–282. 2023.
|
|
111
|
Zhang N, Hu XY, Dong XX, et al: Research
progress on animal models of hyperuricemia. Acta Kunming Med Univ.
40:129–134. 2019.(In Chinese).
|