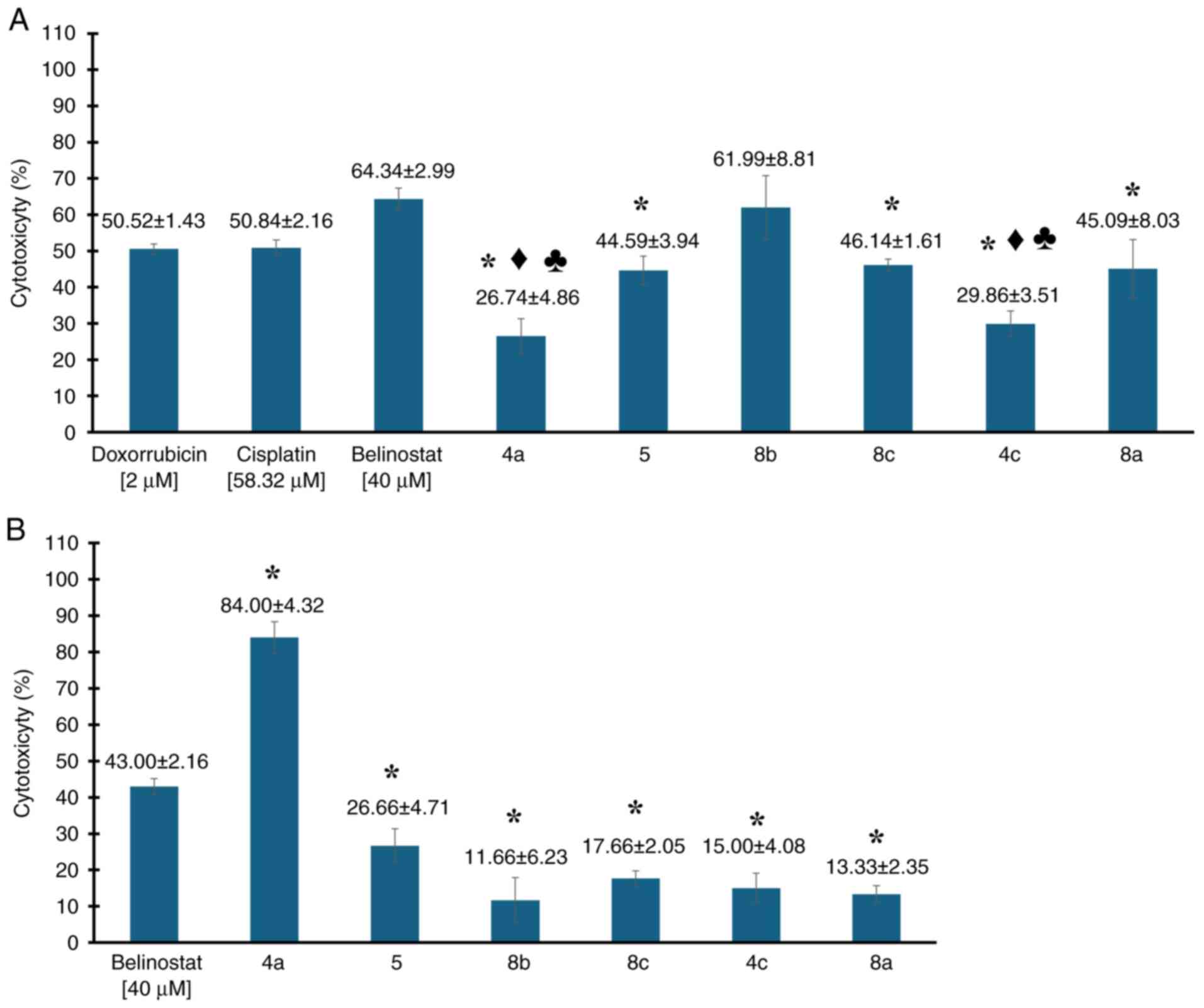
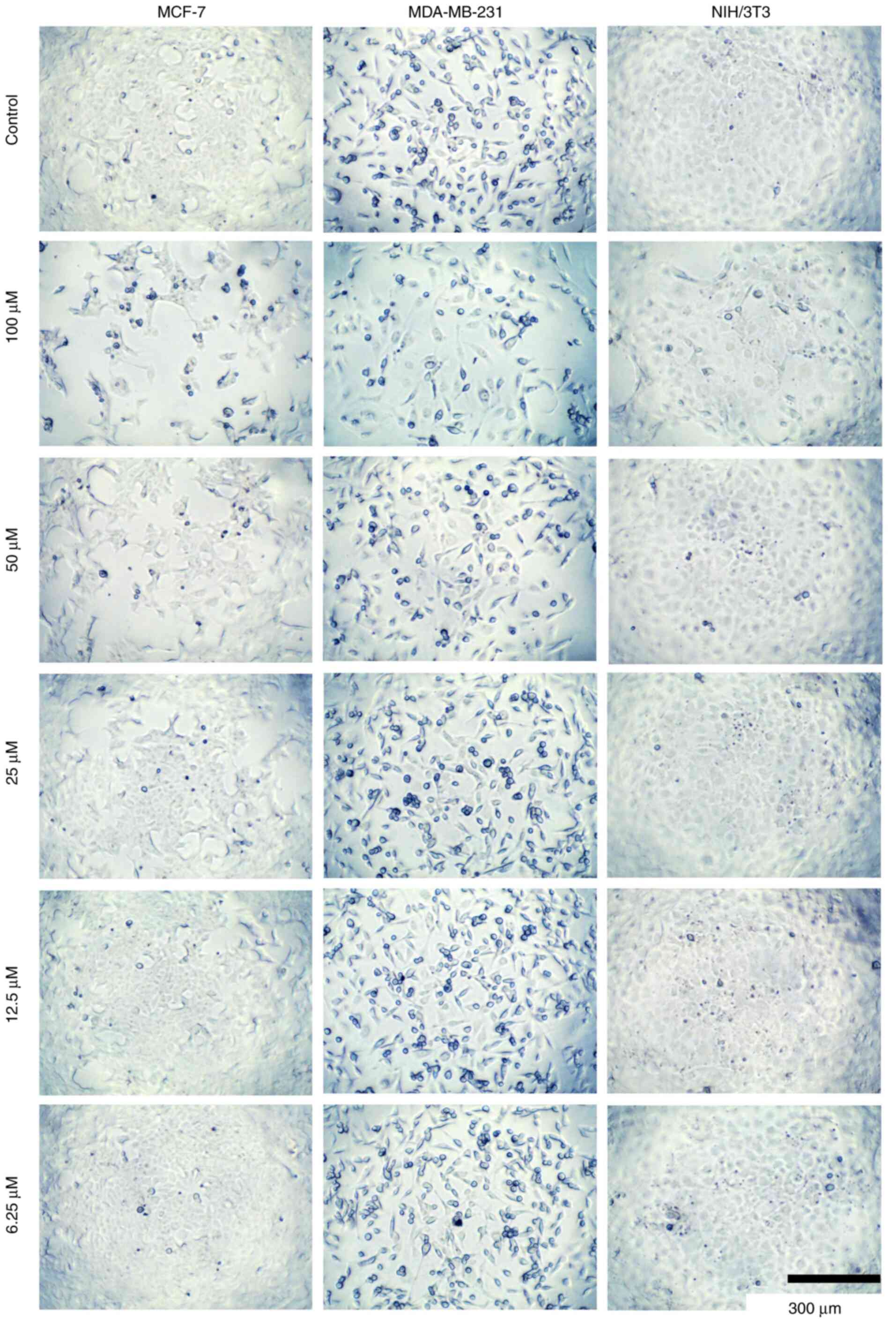
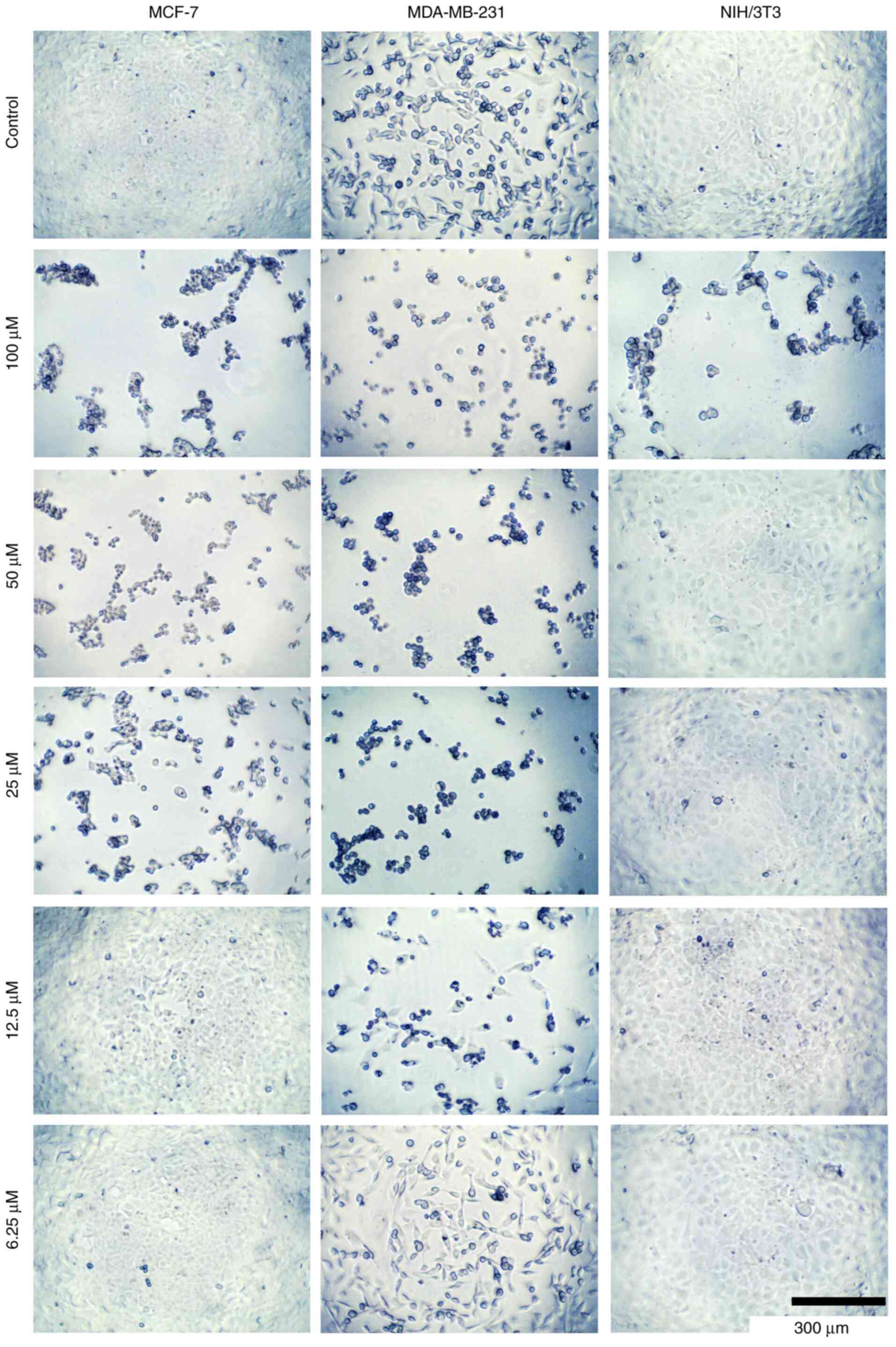
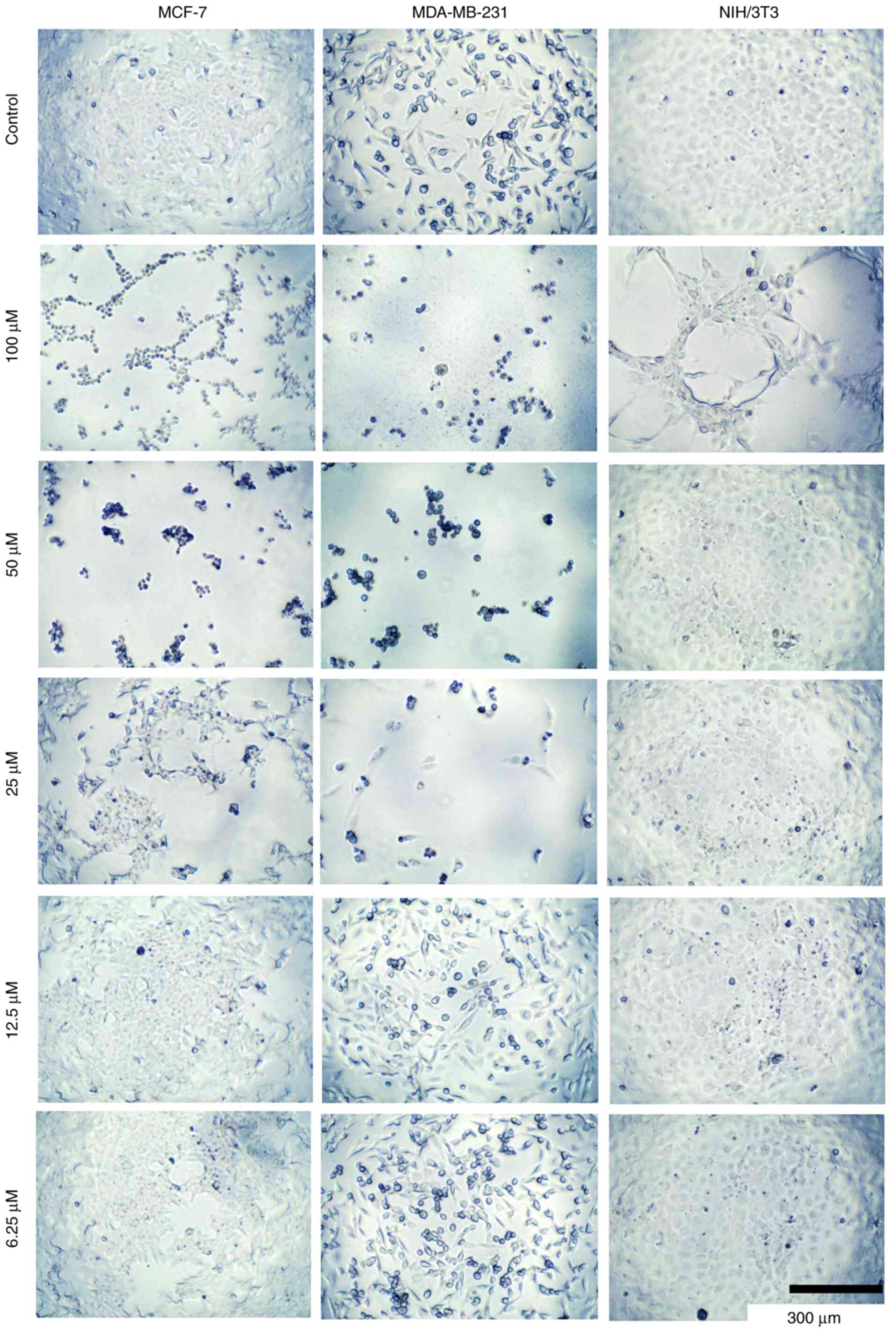
|
1
|
Lüönd F, Tiede S and Christofori G: Breast
cancer as an example of tumour heterogeneity and tumour cell
plasticity during malignant progression. Br J Cancer. 125:164–175.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Akram M, Iqbal M, Daniyal M and Khan AU:
Awareness and current knowledge of breast cancer. Biol Res.
50(33)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
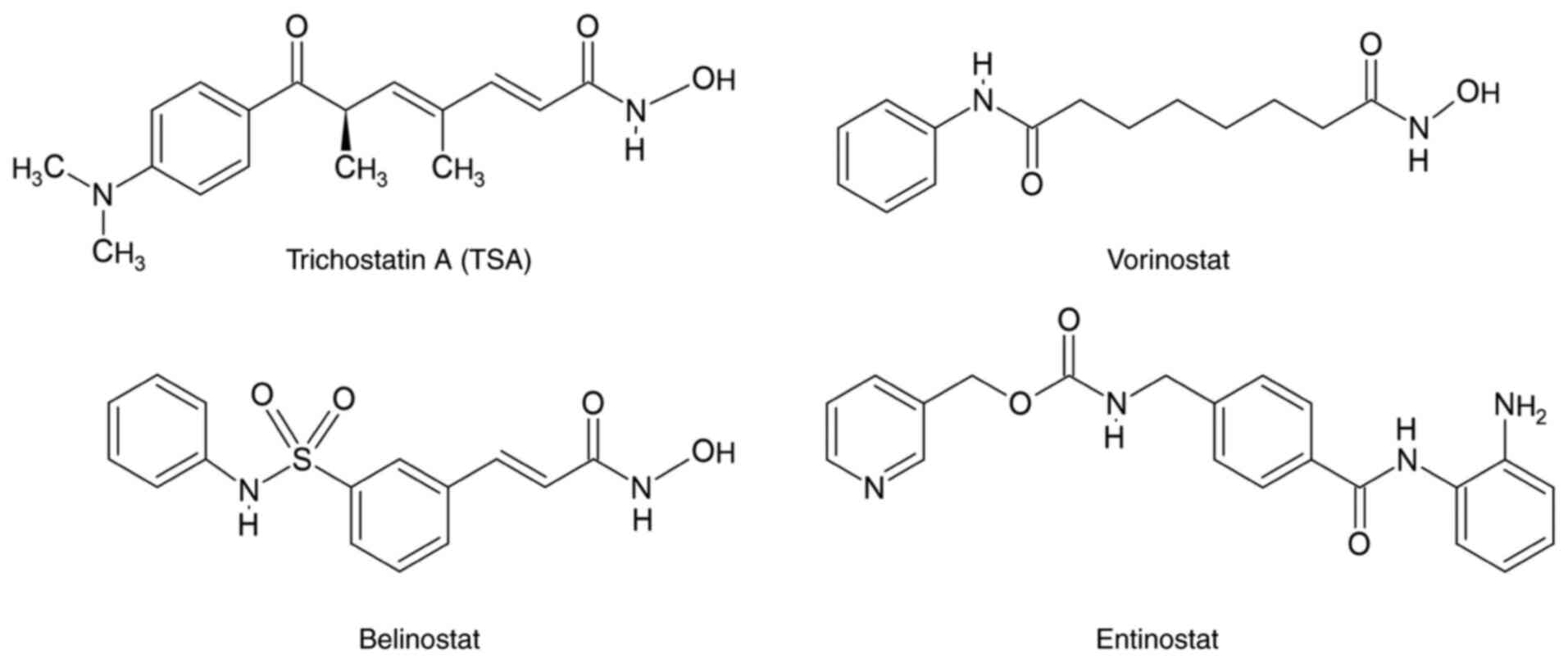
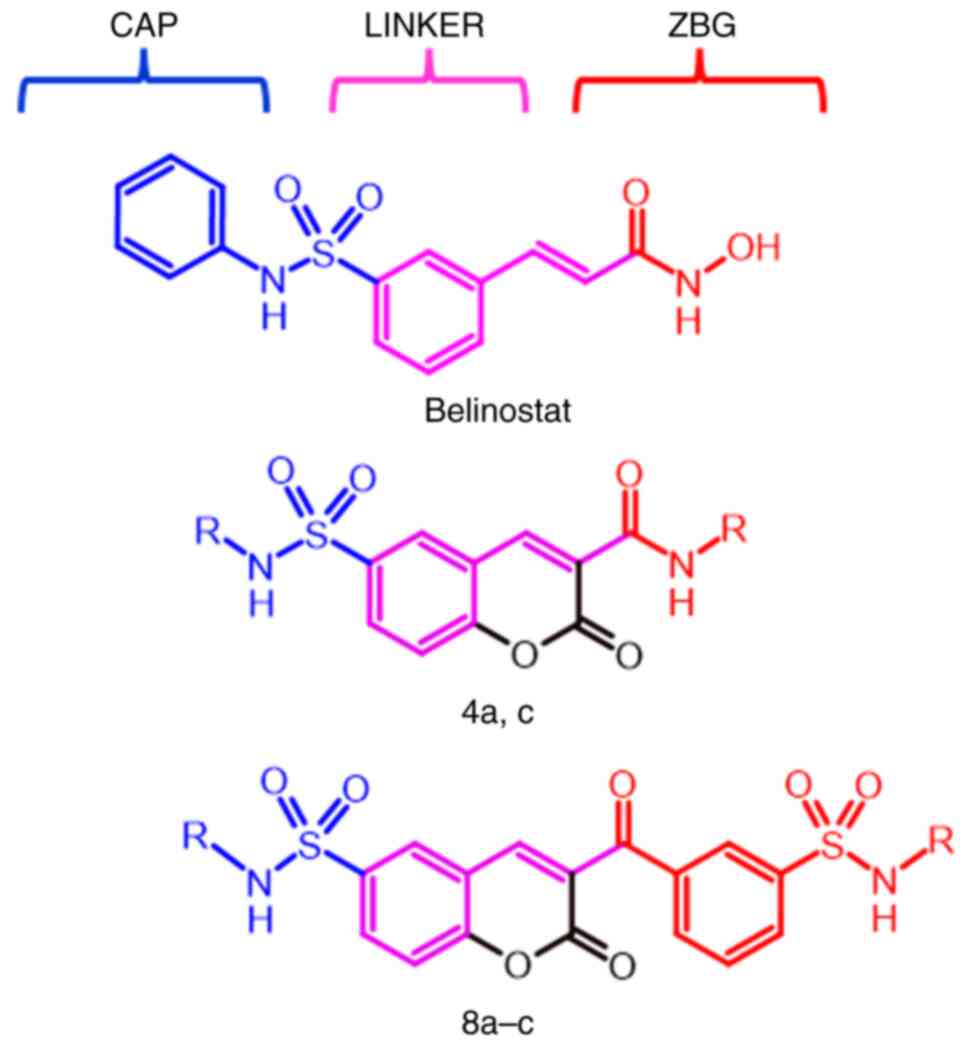
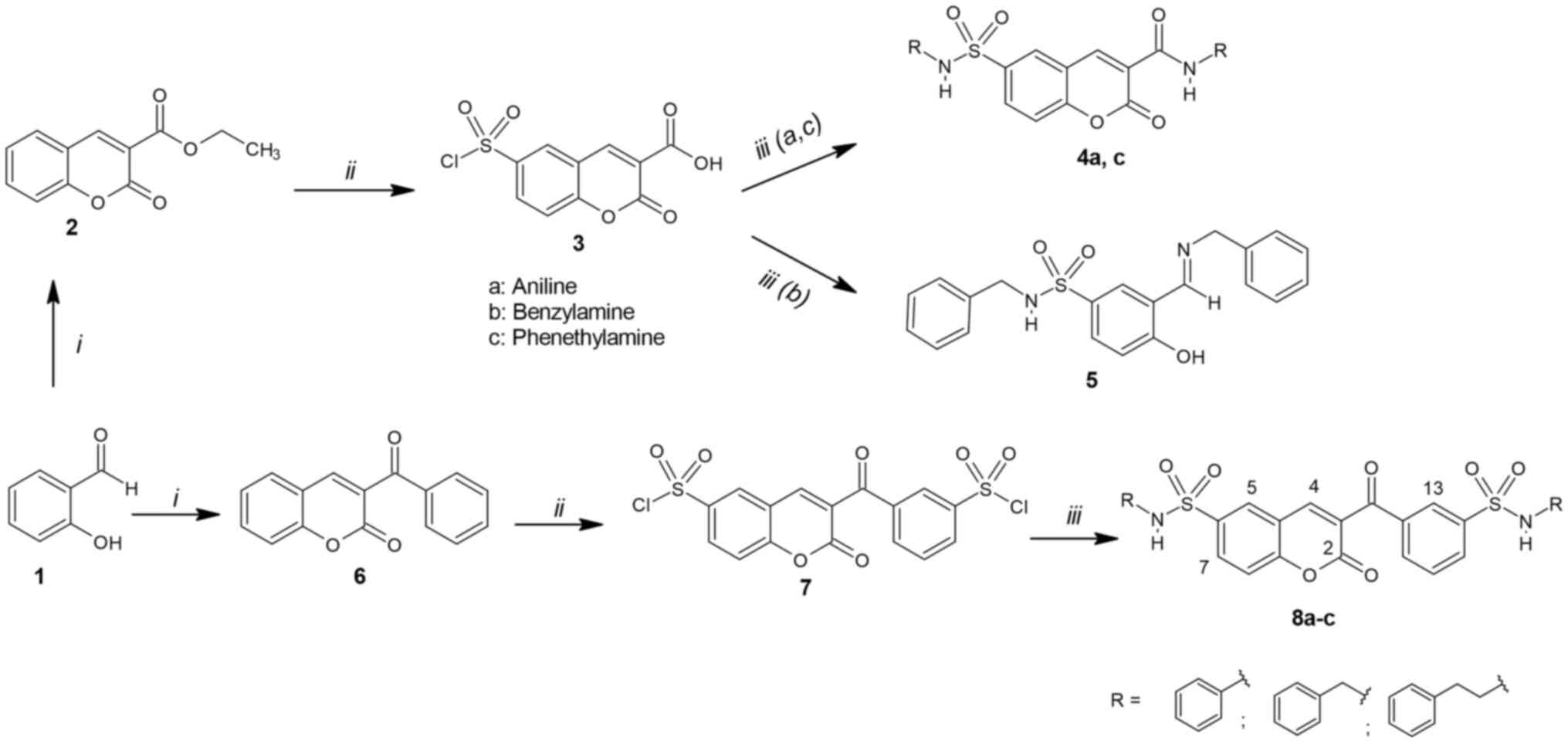
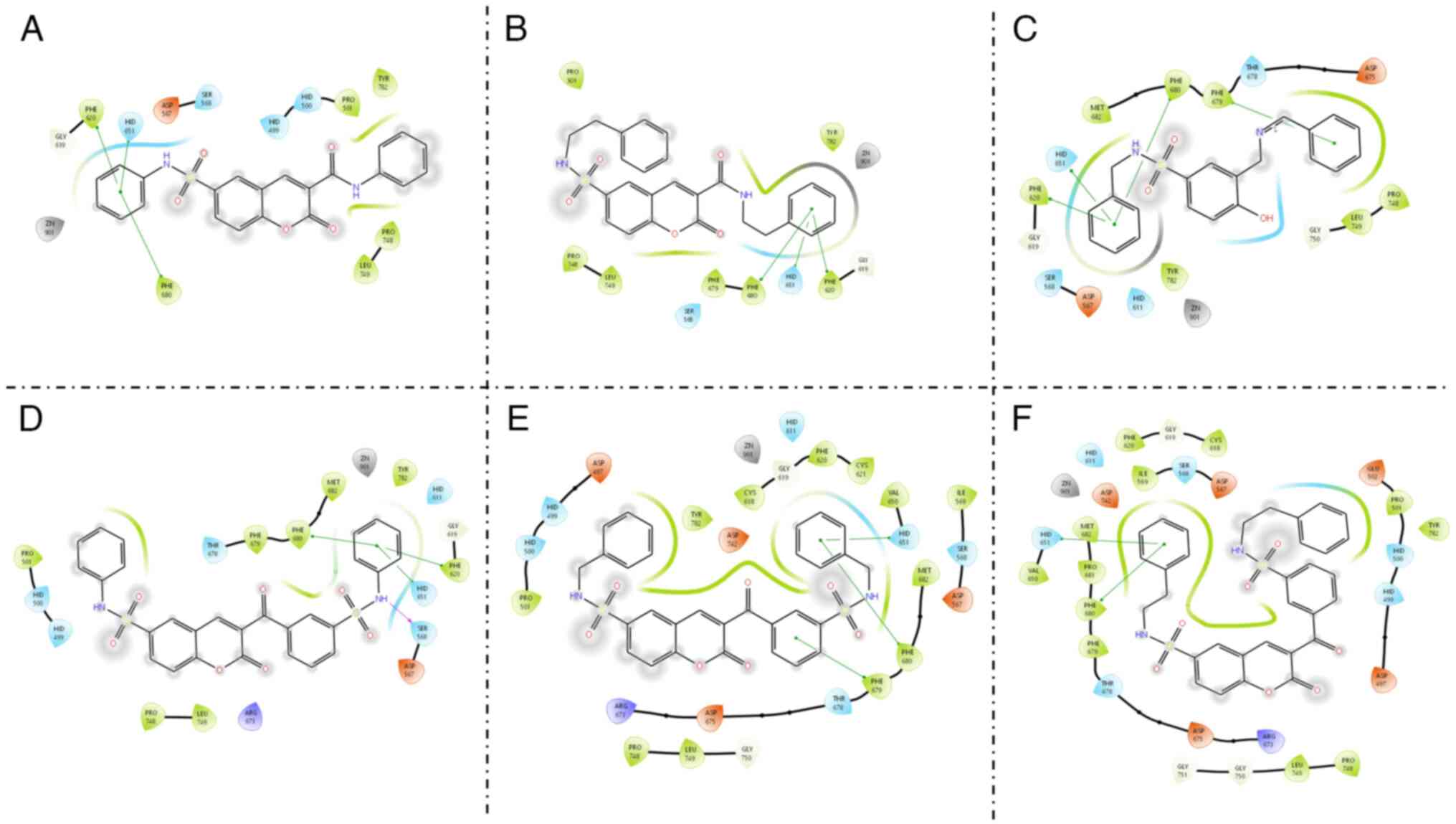
van den Boogaard WMC, Komninos DSJ and
Vermeij WP: Chemotherapy side-effects: Not All DNA damage is equal.
Cancers (Basel). 14(627)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Eckschlager T, Plch J, Stiborova M and
Hrabeta J: Histone deacetylase inhibitors as anticancer drugs. Int
J Mol Sci. 18(1414)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ružić D, Đoković N, Nikolić K and Vujić Z:
Medicinal chemistry of histone deacetylase inhibitors. Arh Farm.
71:73–100. 2021.
|
|
6
|
Yang H, Salz T, Zajac-Kaye M, Liao D,
Huang S and Qiu Y: Overexpression of histone deacetylases in cancer
cells is controlled by interplay of transcription factors and
epigenetic modulators. FASEB J. 28:4265–4279. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sakamoto KM and Aldana-Masangkay GI: The
role of HDAC6 in cancer. J Biomed Biotechnol.
2011(875824)2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Matthews GM, Newbold A and Johnstone RW:
Intrinsic and extrinsic apoptotic pathway signaling as determinants
of histone deacetylase inhibitor antitumor activity. Adv Cancer
Res. 116:165–197. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bondarev AD, Attwood MM, Jonsson J,
Chubarev VN, Tarasov VV and Schiöth HB: Recent developments of HDAC
inhibitors: Emerging indications and novel molecules. Br J Clin
Pharmacol. 87:4577–4597. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Campbell P and Thomas CM: Belinostat for
the treatment of relapsed or refractory peripheral T-cell lymphoma.
J Oncol Pharm Pract. 23:143–147. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
El Omari N, Bakrim S, Khalid A, Albratty
M, Abdalla AN, Lee LH, Goh KW, Ming LC and Bouyahya A: Anticancer
clinical efficiency and stochastic mechanisms of belinostat. Biomed
Pharmacother. 165(115212)2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Han XL, Du J, Zheng YD, Dai JJ, Lin SW,
Zhang BY, Zhong FB, Lin ZG, Jiang SQ, Wei W and Fang ZY: CXCL1
clone evolution induced by the HDAC inhibitor belinostat might be a
favorable prognostic indicator in triple-negative breast cancer.
Biomed Res Int. 2021(5089371)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tuncer Z, Kurar E and Duran T:
Investigation of the effect of belinostat on MCF-7 breast cancer
stem cells via the Wnt, Notch, and Hedgehog signaling pathway.
Saudi Med J. 45:121–127. 2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tuncer Z, Duran T, Gunes C and Kurar E:
Apoptotic effect of belinostat (PXD101) on MCF-7 cancer cells. Ann
Med Res. 28:941–945. 2021.
|
|
15
|
Lu P, Gu Y, Li L, Wang F, Yang X and Yang
Y: Belinostat suppresses cell proliferation by inactivating
Wnt/β-catenin pathway and promotes apoptosis through regulating PKC
pathway in breast cancer. Artif Cells Nanomed Biotechnol.
47:3955–3960. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Stefanachi A, Leonetti F, Pisani L, Catto
M and Carotti A: Coumarin: A natural, privileged and versatile
scaffold for bioactive compounds. Molecules. 23(250)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Flores-Morales V, Villasana-Ruíz AP,
Garza-Veloz I, González-Delgado S and Martinez-Fierro ML:
Therapeutic effects of coumarins with different substitution
patterns. Molecules. 28(2413)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yang F, Zhao N, Song J, Zhu K, Jiang CS,
Shan P and Zhang H: Design, synthesis and biological evaluation of
novel coumarin-based hydroxamate derivatives as histone deacetylase
(Hdac) inhibitors with antitumor activities. Molecules.
24(2569)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Balbuena-Rebolledo I, Rivera-Antonio AM,
Sixto-López Y, Basurto J, Rosales-Hernández MC, Mendieta-Wejebe JE,
Martínez-Martínez FJ, Olivares-Corichi IM, García-Sánchez JR,
Guevara-Salazar JA, et al: Dihydropyrazole-carbohydrazide
derivatives with dual activity as antioxidant and
anti-proliferative drugs on breast cancer targeting the HDAC6.
Pharmaceuticals (Basel). 15(690)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Calderón-Segura ME, Gómez-Arroyo S,
Villalobos-Pietrini R, Martínez-Valenzuela C, Carbajal-López Y,
Calderón-Ezquerro Mdel C, Cortés-Eslava J, García-Martínez R,
Flores-Ramírez D, Rodríguez-Romero MI, et al: Evaluation of
genotoxic and cytotoxic effects in human peripheral blood
lymphocytes exposed in vitro to neonicotinoid insecticides news. J
Toxicol. 2012(612647)2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hernández-Fuentes GA, Delgado-Enciso I,
Enríquez-Maldonado IG, Delgado-Machuca JJ, Zaizar-Fregoso S,
Hernandez-Rangel AE, Garcia-Casillas AC, Guzman-Esquivel J,
Rodriguez-Sanchez IP, Martinez-Fierro ML, et al: Antitumor effects
of annopurpuricin A, an acetogenin from the roots of annona
purpurea. Rev Bras Farmacogn. 34:111–121. 2024.
|
|
22
|
Haji Agha Bozorgi A and Zarghi A: Search
for the pharmacophore of histone deacetylase inhibitors using
pharmacophore query and docking study. Iran J Pharm Res.
13:1165–1172. 2014.PubMed/NCBI
|
|
23
|
Zagni C, Citarella A, Oussama M, Rescifina
A, Maugeri A, Navarra M, Scala A, Piperno A and Micale N:
Hydroxamic acid-based histone deacetylase (HDAC) inhibitors bearing
a pyrazole scaffold and a cinnamoyl linker. Int J Mol Sci.
20(945)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang JH, Mottamal M, Jin HS, Guo S, Gu Y,
Wang G and Zhao LM: Design, synthesis and evaluation of belinostat
analogs as histone deacetylase inhibitors. Future Med Chem.
11:2765–2778. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shen S and Kozikowski AP: Why hydroxamates
may not be the best histone deacetylase inhibitors-what some may
have forgotten or would rather forget? ChemMedChem. 11:15–21.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang L, Zhang J, Jiang Q, Zhang L and
Song W: Zinc binding groups for histone deacetylase inhibitors. J
Enzyme Inhib Med Chem. 33:714–721. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Vickers CJ, Olsen CA, Leman LJ and Ghadiri
MR: Discovery of HDAC inhibitors that lack an active site
Zn(2+)-binding functional group. ACS Med Chem Lett. 3:505–508.
2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Traoré MDM, Zwick V, SimoÌes-Pires CA,
Nurisso A, Issa M, Cuendet M, Maynadier M, Wein S, Vial H, Jamet H
and Wong YS: Hydroxyl ketone-based histone deacetylase inhibitors
to gain insight into class I HDAC selectivity versus that of HDAC6.
ACS Omega. 2:1550–1562. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yu W, Liu J, Clausen D, Yu Y, Duffy JL,
Wang M, Xu S, Deng L, Suzuki T, Chung CC, et al: Discovery of zthyl
ketone-based highly selective HDACs 1, 2, 3 inhibitors for HIV
latency reactivation with minimum cellular potency serum shift and
reduced hERG activity. J Med Chem. 64:4709–4729. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Osko JD and Christianson DW: Structural
basis of catalysis and inhibition of HDAC6 CD1, the enigmatic
catalytic domain of histone deacetylase 6. Biochemistry.
58:4912–4924. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lobera M, Madauss KP, Pohlhaus DT, Wright
QG, Trocha M, Schmidt DR, Baloglu E, Trump RP, Head MS, Hofmann GA,
et al: Selective class IIa histone deacetylase inhibition via a
nonchelating zinc-binding group. Nat Chem Biol. 9:319–325.
2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Butler KV, Kalin J, Brochier C, Vistoli G,
Langley B and Kozikowski AP: Rational design and simple chemistry
yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A. J
Am Chem Soc. 132:10842–10846. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sixto-López Y, Gómez-Vidal JA, de Pedro N,
Bello M, Rosales-Hernández MC and Correa-Basurto J: Hydroxamic acid
derivatives as HDAC1, HDAC6 and HDAC8 inhibitors with
antiproliferative activity in cancer cell lines. Sci Rep.
10(10462)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Osko JD and Christianson DW: Structural
determinants of affinity and selectivity in the binding of
inhibitors to histone deacetylase 6. Bioorg Med Chem Lett.
30(127023)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Villa-Martínez CA, Magaña-Vergara NE,
Rodríguez M, Mojica-Sánchez JP, Ramos-Organillo ÁA, Barroso-Flores
J, Padilla-Martínez II and Martínez-Martínez FJ: Synthesis, optical
characterization in solution and solid-state, and DFT calculations
of 3-acetyl and
3-(1'-(2'-phenylhydrazono)ethyl)-coumarin-(7)-substituted
derivatives. Molecules. 27(3677)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Martínez-Martínez FJ, Padilla-Martínez II
and Trujillo-Ferrara J: 1H and 13C NMR
assignments of 2-oxo-2H-1-benzopyran-3-acyl and -3-amide
derivatives. Magn Reson Chem. 39:765–767. 2001.
|
|
37
|
García-Báez EV, Martínez-Martínez FJ,
Höpfl H and Padilla-Martínez II: π-Stacking Interactions and CH···X
(X=O, Aryl) hydrogen bonding as directing features of the
supramolecular self-association in 3-carboxy and 3-amido coumarin
derivatives. Cryst Growth Des. 3:35–45. 2003.
|
|
38
|
Shen S and Kozikowski AP: A patent review
of histone deacetylase 6 inhibitors in neurodegenerative diseases
(2014-2019). Expert Opin Ther Pat. 30:121–136. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Vögerl K, Ong N, Senger J, Herp D,
Schmidtkunz K, Marek M, Müller M, Bartel K, Shaik TB, Porter NJ, et
al: Synthesis and biological investigation of phenothiazine-based
benzhydroxamic acids as selective histone deacetylase 6 inhibitors.
J Med Chem. 62:1138–1166. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Campiani G, Cavella C, Osko JD, Brindisi
M, Relitti N, Brogi S, Saraswati AP, Federico S, Chemi G, Maramai
S, et al: Harnessing the role of HDAC6 in idiopathic pulmonary
fibrosis: Design, synthesis, structural analysis, and biological
evaluation of potent inhibitors. J Med Chem. 64:9960–9988.
2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Saraswati AP, Relitti N, Brindisi M, Osko
JD, Chemi G, Federico S, Grillo A, Brogi S, McCabe NH, Turkington
RC, et al: Spiroindoline-capped selective HDAC6 inhibitors: Design,
synthesis, structural analysis, and biological evaluation. ACS Med
Chem Lett. 11:2268–2276. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Xu Y, Tang H, Xu Y, Guo J, Zhao X, Meng Q
and Xiao J: Design, synthesis, bioactivity evaluation, crystal
structures, and in silico studies of new α-amino amide derivatives
as potential histone deacetylase 6 inhibitors. Molecules.
27(3335)2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Takla FN, Bayoumi WA, El-Messery SM and
Nasr MNA: Developing multitarget coumarin based anti-breast cancer
agents: Synthesis and molecular modeling study. Sci Rep.
13(13370)2023.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sodji QH, Kornacki JR, McDonald JF,
Mrksich M and Oyelere AK: Design and structure activity
relationship of tumor-homing histone deacetylase inhibitors
conjugated to folic and pteroic acids. Eur J Med Chem. 96:340–359.
2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhang Z, Yamashita H, Toyama T, Sugiura H,
Omoto Y, Ando Y, Mita K, Hamaguchi M, Hayashi S and Iwase H: HDAC6
expression is correlated with better survival in breast cancer.
Clin Cancer Res. 10:6962–6968. 2004.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Rey M, Irondelle M, Waharte F, Lizarraga F
and Chavrier P: HDAC6 is required for invadopodia activity and
invasion by breast tumor cells. Eur J Cell Biol. 90:128–135.
2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Park SY, Jun JA, Jeong KJ, Heo HJ, Sohn
JS, Lee HY, Park CG and Kang J: Histone deacetylases 1, 6 and 8 are
critical for invasion in breast cancer. Oncol Rep. 25:1677–1681.
2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Vale N, Silva S, Duarte D, Crista DMA,
Pinto da Silva L and Esteves da Silva JCG: Normal breast epithelial
MCF-10A cells to evaluate the safety of carbon dots. RSC Med Chem.
12:245–253. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Paine TM, Soule HD, Pauley RJ and Dawson
PJ: Characterization of epithelial phenotypes in mortal and
immortal human breast cells. Int J Cancer. 50:463–473.
1992.PubMed/NCBI View Article : Google Scholar
|
|
50
|
United Mexican States and General Health
Council: 2018 Edition of the Basic Framework and Catalogue of
Medicines. Official Journal of the Federation, Mexico, 2018 (In
Spanish). https://www.dof.gob.mx/nota_detalle.php?codigo=5544613&fecha=23/11/2018#gsc.tab=0.
|
|
51
|
Jasso L, Lifshitz A, Arrieta O, Burgos R,
Campillo C, Celis MÁ, de la Llata M, Domínguez J, Halabe J, Islas
S, et al: Importance of the list of essential medicines in medical
prescription. Gac Med Mex. 156:598–599. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Romani AMP: Cisplatin in cancer treatment.
Biochem Pharmacol. 206(115323)2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Nauta IH, Klausch T, van de Ven PM,
Hoebers FJP, Licitra L, Poli T, Scheckenbach K, Brakenhoff RH,
Berkhof J and René Leemans C: The important role of cisplatin in
the treatment of HPV-positive oropharyngeal cancer assessed by
real-world data analysis. Oral Oncol. 121(105454)2021.PubMed/NCBI View Article : Google Scholar
|