Introduction
Biological variation (BV) refers to changes in the
levels of biochemical constituents in blood or other biological
fluids, which reflect the body's regulation through homeostatic
processes (1). In an equilibrium
state, most measurands exhibit random variations around a
homeostatic set point, while others may also be influenced by
factors such as different life stages or predictable cyclic
variation (2). Moreover, BV has two
main components: Coefficient of intra-individual BV
(CVI) and coefficient of interindividual BV, also called
group coefficient BV (CVG); the former refers to the
random fluctuation around the homeostatic set point of an analyte
within an individual, while the latter represents the variation
between the homeostatic set points of an analyte among different
individuals (3).
Intra-individual variation can occur in either
cyclical or random patterns. Cyclical variation occurs predictably
(e.g., variations in diurnal cortisol levels, monthly hormonal
changes during the female reproductive cycle and seasonal
fluctuations in vitamin D levels). Conversely, random variation is
unpredictable and occurs naturally around a subject's homeostatic
set point (4).
Currently, BV data are estimated by repeatedly
measuring biomarkers or analytes in a healthy population under
normal physiological conditions (5). Population-based studies have revealed
that healthy individuals' BV estimates remain constant (6). The impact of a specific disease on the
BV of an analyte can vary, and it may or may not remain unchanged.
Therefore, although a disease may alter the homeostatic set point
for an analyte, the intra-individual variability may not
necessarily change (4).
Data on BV are also available for populations with
different diagnoses, including diabetes or chronic kidney disease,
as individuals may be stable despite their pathological condition
(7). Research has also revealed
that pregnancy and prolonged high-intensity physical exercise can
influence individuals' BV (8,9).
Moreover, these data are extensively utilized in laboratory
medicine for various purposes. These include establishing
analytical performance specifications for measurement systems
(3,10), calculating the reference change
value (RCV) to assess the significance of changes between
consecutive measurements, using the individuality index (II) to
evaluate the utility of population-based reference intervals and
determining personalized reference intervals (2,10). In
general, utilizing BV estimates in an individual's test results can
provide evidence of pathological conditions or response to therapy
(11).
According to Fraser (2001) (4), once the pre-analytical phase is
properly managed, the total variation of laboratory results, also
known as the total coefficient of variation (CVT), can
be calculated by adding the analytical coefficient of variation
(CVA) and the CVI. The CVA can be
determined by analyzing control samples with known results
(12,13).
At present, the most comprehensive BV database for a
wide range of analytes only provides the CVI and
CVG for plasma (4.5 and 5.8%, respectively) and serum
glucose (5.6 and 7.5%, respectively) (14). However, this database does not
include the intra- and interindividual BV of capillary blood
glucose.
Blood glucose levels can be measured in plasma,
serum, whole blood, capillary blood and more recently in
interstitial fluid, using laboratory equipment and portable meters
called glucometers (15-17).
Plasma glucose testing is used as the gold standard
for screening diabetes; however, numerous challenges exist with
regard to its widespread use, including low availability in
low-resource settings, where capillary glucose testing is suggested
as an alternative screening method (18).
Glucometers were first designed to analyze blood
samples from capillary sources and are capable of reporting blood
glucose levels within seconds (19). These devices are used for monitoring
blood glucose (MBG) and can be used by patients and healthcare
teams (20).
MBG is recommended for insulin-treated individuals
with diabetes in any age group. MBG brings great benefits, by
reducing the risk of acute complications, such as ketoacidosis and
hypoglycemia, and by allowing the patient to understand the
determinants of their blood glucose levels by correlating real-time
glycemic results with food intake or physical activity, for example
(21,22).
How often an individual with diabetes should check
their glucose levels each day can vary from 1 to 10 times,
depending on their type of diabetes, treatment plan and individual
needs. In hospitalized diabetic patients who can eat, point-of-care
(POC) MBG should be performed prior to meals, while in those not
eating, MBG is recommended every 4-6 h. POC MBG occurring at a more
frequent interval, ranging from every 30 min to every 2 h, is the
required standard for safe intravenous insulin therapy (23).
Given the significance of capillary blood glucose
monitoring (22), it is crucial to
ascertain the intra- and interindividual BV data for this
parameter, which is the primary objective of the present study.
Materials and methods
Study aim
The present study aimed to ascertain the coefficient
of BV in capillary blood glucose levels, as measured by a
glucometer. The Preferred Reporting Items for Systematic Reviews
and Meta-Analyses (PRISMA) 2009 criteria were adhered in order to
maintain methodological rigor. This included utilizing the related
checklist and flowchart, as provided by Galvao et al
(24).
Data sources
The search was conducted between January and March
2023 through the PubMed (https://pubmed.ncbi.nlm.nih.gov/), Scielo (https://scielo.org/en/), Scopus (https://www.scopus.com/) and Google Scholar
(https://scholar.google.com/) databases
for articles without a defined publication date range. PubMed was
selected as it is the largest indexer of medical journals globally.
Scopus was chosen for its expansive database of article abstracts
and citations. Scielo, an open-access electronic library featuring
scientific periodicals in various languages from countries such as
those in Latin America, South Africa, Spain, India and Portugal,
was also utilized. Lastly, Google Scholar, which is a freely
accessible virtual search engine that indexes full-text academic
literature in a diverse range of publication formats, was included
in the search. Studies were retrieved using a combination of
keywords in English, utilizing the AND operator to pair the
following descriptors: ‘glucometer AND capillary glucose’,
‘capillary glucose AND biological variation’ and ‘within subject
AND biological variation AND capillary glucose’.
To select appropriate studies, the inclusion
criteria of the present study were original articles presenting the
object of the present study either in the title, abstract or text,
which contained the coefficient of intra-individual or BVs of
capillary blood glucose. The exclusion criteria were literature
review articles, dissertations, case studies, book chapters and
editorials. Articles that simply reiterated the BV of capillary
glycemia found in other studies were also omitted. No limitations
were imposed regarding publication dates, participant
characteristics or funding in order to maximize the potential for
data recovery. A single reviewer conducted the research up to this
point. The literature review and article selection processes are
illustrated in Fig. 1.
BV data critical appraisal checklist
(BIVAC)
The quality of the studies and the BV data generated
by the articles incorporated in the present review were evaluated
using the BIVAC. The BIVAC tool is composed of 14 quality items
(QI) (25), which were assessed in
the present study in terms of whether articles met the criteria or
not, without evaluating the degree of compliance. The quality items
of the BIVAC tool, as described by Aarsand et al (25) are listed as follows:
QI 1: Ratio scale. This item explores whether
the measurand is reported on a ratio scale. The importance of this
rests on the fact that only ratio scales possess a meaningful zero.
Therefore, any estimation of the coefficient of intra-individual
variation for measurands on non-ratio scales demands careful
consideration.
QI 2, 3 and 4: Subjects, samples and
measurands. These items pertain to the subjects (QI 2) and
samples (QI 3), and are critical for ensuring the reliability of
the assessed BV results. Full characterization and precise
reporting of the population attributes wherein the BV was assessed
are vital for BV studies. The measurand and analytical method (QI
4) are also essential as earlier-generation analytical procedures
might produce different estimations of the measurand.
QI 5, 6 and 7: Pre-analytical procedures,
estimation of analytical variation and steady state.
Standardized and appropriate pre-analytical procedures are
necessary for obtaining reliable BV data (QI 5). A lack of
compliance with this requirement may result in an overestimation of
the coefficient of intra-individual variation and the coefficient
of glucose variation. The accurate estimation of the coefficient of
analytical variation (QI 6) should be conducted through replicate
analyses within the same analytical run. In addition, there should
be no systematic fluctuation in the concentration of the measurand
throughout the study period (QI 7: steady state). If such
modifications are detected, the data should be properly
adjusted.
QI 8, 9 and 10: Outliers, normality, and
homogeneity of variance. Outliers must be recognized and
excluded from the replicates, each individual's samples and the
individuals themselves (QI 8). Any failure to address outliers
could lead to an overestimation or underestimation of the
CVI. Each individual's data distribution must be
examined for normality, and should a departure from normality be
observed, the data must undergo transformation (QI 9). Assessment
of variance homogeneity is also necessary, for any variance
heterogeneity would render the estimates inapplicable to a broader
population (QI 10).
QI 11 and 12: Statistical method and confidence
intervals (CIs). The statistical method deployed for BV
estimation must be explicitly stated and suitable for the research
(QI 11). Until recently, BV estimates rarely included reports of
measurement uncertainty (QI 12). In the context of the BIVAC, if
the study does not report the IC, at least the required data for
its calculation must be present.
QI 13 and 14: Number of results and
concentrations studied. It is imperative to reveal the number
of results included (QI 13) and the average concentrations of the
studied analytes (QI 14) in evaluating the correlation between the
CVI and concentration. Importantly, this requirement
does not affect the reliability of BV estimates.
Results
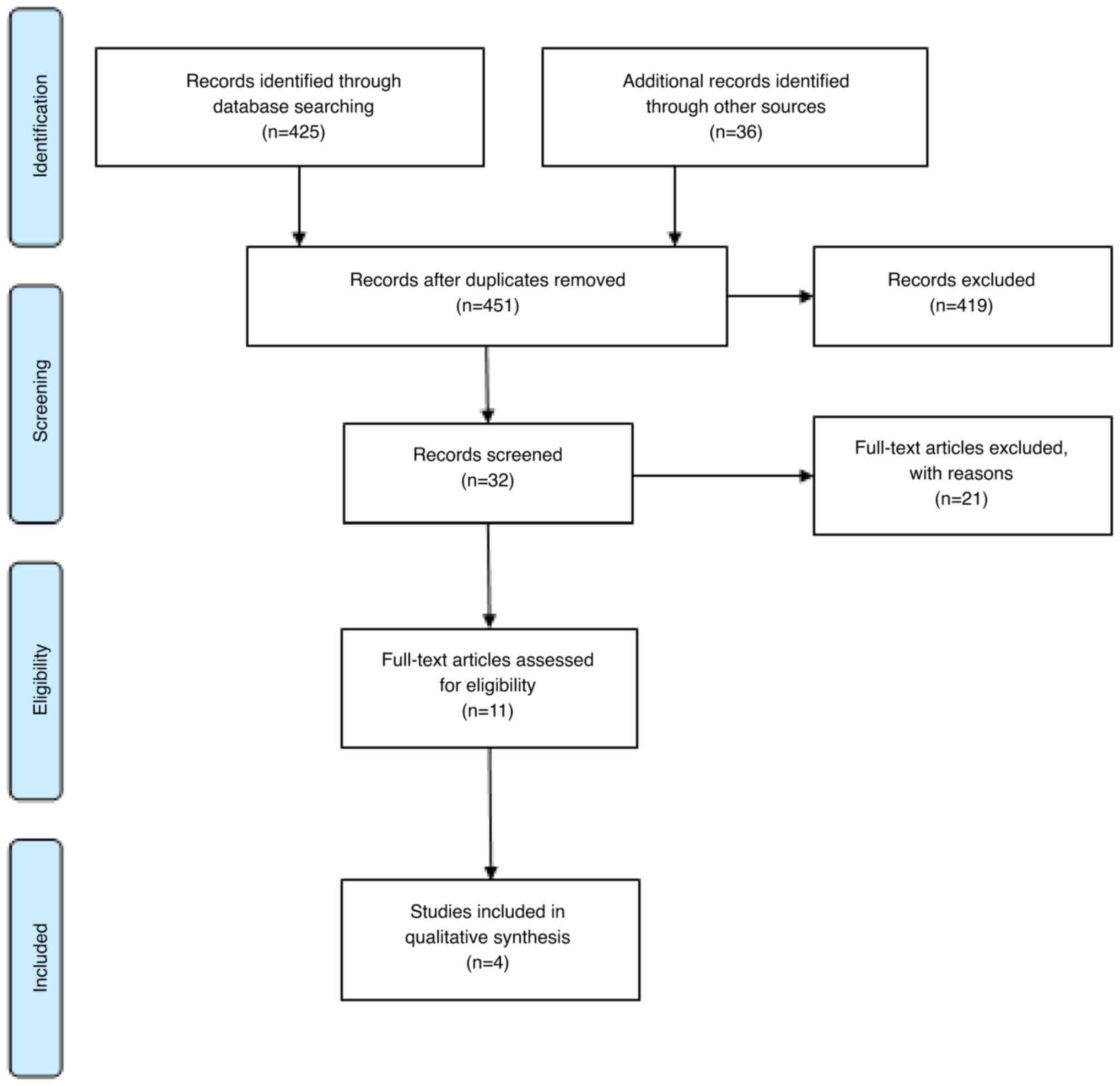
A review of the four databases produced 461 articles
for consideration. The articles were selected based on specific
inclusion and exclusion criteria, which eliminated 419 studies
after scrutinizing their titles, and with 10 articles dismissed due
to duplication. The abstracts of the remaining 32 articles were
then read. A further 21 articles were excluded for failing to meet
the inclusion criteria. In the end, 11 articles were selected for a
full reading, of which only 4 were included in the final review
(7,26-28).
Fig. 2 presents the article
selection process structured according to the PRISMA protocol.
Out of the 11 articles selected for full reading,
six were excluded as they solely focused on evaluating the
performance and accuracy of glucometers, while 1 article was
excluded as one of its objectives was to calculate glycemic
variability after consuming certain foods. These studies used data
on BV and CVI from other articles but did not perform an
analysis of them.
Table I summarizes
the information obtained from the four reviewed articles (7,26-28).
All articles had as one of their objectives the investigation of BV
or the assessment of glycemic variability, which indirectly
provides data on BV. However, various methodologies were employed
to determine BV or glycemic variability, directly impacting the
results. The articles under review had a range of 3 to 11 authors
and were published in English between 2010 and 2019.
 | Table IStudies, objectives and methods of
the selected articles. |
Table I
Studies, objectives and methods of
the selected articles.
| First author,
year | Title | BV CBG-related
research objective | Equipment and
manufacturer | Formula used to
calculate the CV | (Refs.) |
|---|
| Mu et al,
2011 | Comparison of
fasting capillary glucose variability between insulin glargine and
NPH | Investigate
glycemic variability in individuals using insulin glargine and
NPH | Glucometer,
OneTouch Ultra 1 (Lifescan Inc.) | CV=SD/mean | (26) |
| Carlsen et
al, 2011 | Within-subject BV
of glucose and HbA1c in healthy persons and DM1 patients | Estimate the BV of
capillary glucose and HbA1c in healthy individuals and patients
with DM1 | HK Gluco-quant
Glucose Modular Analyzer (Roche Diagnostics) | CV=SD/mean;
analytical and inter- subject CVs were estimated separately via
analysis of variance | (7) |
| Allsop et
al, 2016 | The between-day
reproducibility of fasting, satiety- related analytes, in 8- to
11-year-old boys | To evaluate the
reproducibility, between days, of plasma GLP-1, glucagon, leptin,
insulin and capillary glucose in boys aged 8 to 11 years, who were
fasting, lean and overweight/obese | Automated point-of-
care glucose (glucose oxidase) analyzer (BiosenC_line, EKF
Diagnostics Holdings plc.) | NA | (27) |
| Colomo et
al, 2019 | Relationship
between glucose control, glycemic variability and oxidative stress
in children with DM1 | To evaluate the
relationship between glycemic control, glycemic variability and
oxidative stress in children with DM1 | One Touch Verio iQ,
(Lifescan Inc.) | CV=SD/mean | (28) |
Among the four studies, only one aimed specifically
to assess individuals' between-visit BV of capillary blood glucose
levels (7). This was the study that
most complied with the BIVAC criteria, and reported the
CVI values of 4.5% for healthy individuals and 31.1% for
diabetic patients (7) (Table II). On the other hand, all four
articles presented the CVI of fasting capillary blood
glucose. However, as the studies were conducted on different
populations, and the analyses were performed differently among the
four articles, it was not possible to estimate an average
CVI.
 | Table IIResearch characteristics of selected
articles. |
Table II
Research characteristics of selected
articles.
| First author,
year | Participants
(age) | Inclusion/exclusion
criteria | Samples and
collection frequency | | CV (%) | Method analytical
performance | Does it answer the
guiding question of the systematic review? | (Refs.) |
|---|
| Mu et al,
2011 | 130 diabetic
individuals using insulin glargine (31-49 years) | Participants with
diabetes using oral antidiabetics, without changing medication for
at least 3 months; fasting CBG >7.0 mmol/l and HbA1c >7.5%;
no serious chronic or acute diabetic complications, nor serious
intercurrent illness; female patients were not pregnant and did not
plan to become pregnant within 6 months. | Fasting capillary
blood (daily collections for 3 months) | Pre- treatment:
CVI BG fasting = 13.4% (±3.6) | Post- treatment:
CVI BG fasting = 10.2% (±4.2) | NA | Yes | (26) |
| | 130 diabetic
individuals using NPH insulin (32-49 years) | | | Pre- treatment:
CVI BG fasting = 12.9% (±4.0) | Post- treatment:
CVI BG fasting = 19.6% (±6.1) | NA | | |
| Carlsen et
al, 2011 | 15 diabetic
individuals (26-61 years) | Stable diabetic
patients with HbA1c between 6-8% and ≤1% change in HbA1c
concentration in the last 18 months, no changes in basal insulin
dose in the last 2 months and stable body weight (≤10% change in
total body weight in the last year) | Venous blood and
fasting capillary blood plasma (weekly collections for 10
weeks) | CVI
Fasting capillary blood plasma glucose =31.1% (27.3-36.3)
CVI venous Blood = 30.5% (26.7-35.5) | CVG
plasma glycemia of capillary blood fast = 16.3% (7.4-29.2)
CVG venous blood = 16.8% (8.2-29.8) | CVA
fasting capillary blood plasma glucose = 0.9% (0.8-1.0)
CVA venous blood = 1.0% (0.9-1.0) | No | (7) |
| | 15 healthy
individuals (27-59 years) | Self-declared
healthy non-obese individuals | | CVI
Fasting capillary blood plasma glucose = 4.5% (3.9-5.1)
CVI venous blood = 5.4% (4.7-6.0) | CVG
Fasting capillary blood plasma glucose = 5.8% (4.1-9.3)
CVG venous blood = 5.6% (3.9-9.0) | CVA
fasting capillary blood plasma glucose = 1.4% (1.2-1.6)
CVAvenous blood = 1.4% (1.3-1.6) | | |
| Allsop et
al, 2016 | 23 healthy
individuals (8-11 years) | Exclusion:
Diabetics or taking any medication known to affect taste, smell or
appetite | Fasting capillary
blood plasma (2 collections with an interval of 1 week between
collections) | Skinny boys
CVG = 5.2% | Overweight boys
CVG = 4.7% | Not shown | No | (27) |
| Colomo et
al, 2019 | 25 diabetic
individuals (8-15 years) | Inclusion: Children
and adolescents with type 1 diabetes mellitus | Capillary blood
without fasting [6 daily collections (before meals and 2 h after
meals) for 5 days in summer camp and in the home routine] | 1st phase (holiday
camp for children with diabetes) CV = 0.41% (+/- 0.10) | CVG
plasma glycemia of capillary blood fast = 16.3% (7.4-29.2) | Not shown | No | (28) |
As demonstrated in Table II, three articles analyzed
capillary blood glucose during fasting (7,26,27),
while one examined capillary blood glucose throughout the day or
after food intake (28). Table III evaluates the four studies
based on the 14 BIVAC quality criteria.
 | Table IIIAssessment of articles regarding
whether or not they fulfilled each of the 14 QI of BIVAC. |
Table III
Assessment of articles regarding
whether or not they fulfilled each of the 14 QI of BIVAC.
| BIVAC QI | Panwei et
al, 2010 | Carlsen et
al, 2011 | Allsop et
al, 2016 | Colomo et
al, 2019 |
|---|
| QI 1 - ratio
scale | Yes | Yes | Yes | Yes |
| QI 2 -
participants | Yes | Yes | Yes | Yes |
| QI 3 - samples | Yes | Yes | Yes | Yes |
| QI 4 -
measuring | Yes | Yes | Yes | Yes |
| QI 5 -
pre-analytic | Yes | Yes | Yes | No |
| QI 6 - estimation
of analytical variation | No | Yes | Yes | No |
| QI 7 - steady
state | No | Yes | Yes | Yes |
| QI 8 -
outliers | No | Yes | No | No |
| QI 9 -
normality | Yes | No | No | No |
| QI 10 - homogeneity
of variance | Yes | Yes | Yes | NA |
| QI 11 - statistical
method | Yes | Yes | Yes | Yes |
| QI 12 - confidence
interval | No | Yes | Yes | Yes |
| QI 13 - number of
results | Yes | Yes | Yes | Yes |
| QI 14 -
concentrations studied | Yes | Yes | Yes | Yes |
Discussion
The primary objective of the present systematic
review was to comprehensively analyze the current state of
knowledge regarding the BV of capillary glycemia, as measured by
glucometers. The analysis of the present study aimed to establish
specifications for analytical quality, II and RCV. Specific
protocols were employed to guarantee the reliability of the BV
studies (29,30).
Mu et al (26) conducted a study where fasting
capillary blood glucose was measured daily for three months.
However, the study lacked standardization in terms of fasting time
and the food consumed in the previous meal. Additionally, the
present study did not provide data on the CVA of the
glucometer used, indicating that it was not considered when
calculating the CVI.
Carlsen et al (7) estimated the BV of capillary blood
plasma glucose and venous hemoglobin A1C in healthy individuals and
those with diabetes, as presented in Table II. Weekly collections were
performed over 10 weeks, with participants required to fast
overnight before each collection. However, the specific fasting
time was not disclosed. Duplicate samples were analyzed to assess
the CVA, and two-level control samples were utilized to
monitor the analytical accuracy of the equipment. The
CVI and CVG results obtained for plasma
glycemia from capillary blood were included in the mean estimate
published by the European Federation of Clinical Chemistry and
Laboratory Medicine (2023) (14),
although they do not represent the capillary glycemia normally
analyzed by a glucometer.
The study by Allsop et al (27) implemented a standardized 12-h
overnight fast, with each participant recording the food consumed
the night before the first collection, which they were then
instructed to repeat before the second collection. Nevertheless,
the participants did not standardize food intake or quantity
consumed. In terms of sample collection, the study only conducted
two collections with a 1-week interval between them. According to
current literature protocols, this number is considered low, and a
minimum of five collections is recommended (31). Although a glucometer was employed,
the capillary blood sample was placed in a heparinized tube and
mixed with hemolysis solution prior to glucose analysis. This
sample treatment does not reflect the routine use of glucometers
for capillary blood glucose analysis. Furthermore, the method did
not consider the analytical variation when calculating VB.
In the study by Colomo et al (28), there was no report on the
standardization of food intake before sample collection.
Additionally, fasting samples were not collected in the study.
Furthermore, the analysis did not include information on the
accuracy and precision of the equipment used in the tests (i.e.,
the CVA was not provided). Considering that this study
was conducted after the publication of BV verification protocols,
such as BIVAC, the analysis of glycemic BV should have followed the
criteria established by these protocols.
The reviewed articles presented varying
CVI values of capillary blood glucose. This discrepancy
could be attributed to differences in verification protocols and
the evaluation of different populations, reflecting varying study
objectives. Specifically, one study focused on CVI in
healthy individuals (27), while
two other studies examined it solely in people with diabetes
(26,28). Lastly, one study explored the
variation between healthy individuals and those with diabetes
(7). Two studies were conducted
with children (27,28), while the remaining two involved
adult populations (7,26).
The number of data collections varied across
studies, and the samples analyzed also differed. Carlsen et
al (7) and Allsop et al
(27) assessed glycemia in
capillary blood plasma. There were also variations in the timing of
blood glucose sample collection, with three studies monitoring
fasting blood glucose levels and one study evaluating glucose
levels before meals, without specifying the fasting duration, and 2
h after meals (7,26-28).
In the study conducted by Allsop et al (27), food standardization prior to fasting
was performed.
Specifically, in capillary blood glucose testing,
the variation in glycemic index from food consumed immediately
before sample collection could introduce bias into the results,
thereby necessitating standardization (22). The choice of blood glucose
measurement equipment also varied across studies, with Carlsen
et al (7) and Allsop et
al (27) utilizing biochemical
analyzers while the others used glucometers. Moreover, concerning
BV, the study by Allsop et al (27) solely evaluated CVG, while
the study by Colomo et al (28) did not present either CVI
or CVG, instead solely calculating the coefficient of
variation for capillary blood glucose between fasting and post-meal
collections.
The difference in study protocols can be justified
by the fact that three of the analyzed articles predate the
initiative to harmonize the generation and reporting of BV data
with the publication of BIVAC (7,13,25-27),
which brings weaknesses and uncertainties in the results found.
However, even the subsequent article did not use the BIVAC
protocol, demonstrating the need for reliable studies to estimate
the BV of capillary blood glucose measured by glucometers (28). Moreover, BIVAC allows for the
critically evaluation and classification of BV studies concerning
study design, pre-analytical handling, analytical methods and
statistical analysis (24). The
data from applying BIVAC indicate that studies either omitted or
did not address essential details related to BIVAC quality
indicators. Currently, BIVAC allows for a retrospective assessment
of published studies and serves as a guide for future studies.
The evaluated studies found that Mu et al
(26) did not meet BIVAC QI 7,
which refers to the steady state of the sample. The research
evaluated the results both with and without the use of insulin.
Both Mu et al (26) and
Colomo et al (28) did not
meet QI 6, which requires estimating the analytical variation of
the method for estimating BV.
Allsop et al (27) and Colomo et al (28) did not meet QI 14 regarding the
presentation of the concentration of the measurand among
participants. Corroborating the theory of systematic reviews
described by Gurevitch et al (32), the review results allowed the
identification of future research priorities that would otherwise
not have been noticed. For example, the reviewed studies were
conducted primarily in Europe and Asia, suggesting the need to
conduct them in other regions to consider each continent's eating
habits and lifestyle.
Lastly, considering that the present review did not
limit the publication period of the studies, it is understood that
these are the current state-of-the-art findings. The reported
capillary glycemia BV results were intended to answer the specific
questions of their respective studies but cannot be used as a
reference for BV and CVI or CVG for
calculating analytical quality specifications, II or RCV. The
present systematic review revealed a lack of specific studies that
adhere to standardized criteria to assess the BV of
intra-individual and interindividual capillary blood glucose levels
as measured by glucometers. Consequently, as reported in the
current literature, the existing data on BV cannot be considered
reliable for establishing analytical quality specifications, II and
reference change values for capillary blood glucose measurement
using glucometers. Nevertheless, these findings have significant
implications for managing patients who monitor their capillary
blood glucose levels, as they can contribute to more effective
treatment strategies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
Data collection and writing was carried out by KDZ.
KDZ and FM were involved in the analysis of data and confirm the
authenticity of all the raw data. Supervision and reviewing was
carried out by FM. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sandberg S, Røraas T and Aarsand AK:
Biological variation and analytical performance specifications
(Internet). In: Tietz textbook of Laboratory medicine. Rifai N,
Chiu RWK, Young I, Burnham CAD and Wittver CT (eds). 7th edition.
Elsevier, St Lous, MO, pp335-356, 2022.
|
|
2
|
Sandberg S, Carobene A, Bartlett B, Coşkun
A, Fernandez-Calle P, Jonker N, Díaz-Garzón J and Aarsand AK:
Biological variation: Recent development and future challenges.
Clin Chem Lab Med. 61:741–750. 2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fraser C and Sandberg S: Biological
variation. In: Tietz textbook of clinical chemistry and molecular
biology. Rifai N, Horvath A and Wittwer C (eds). 6th edition.
Elsevier, St Lous, MO, pp157-170, 2017.
|
|
4
|
Fraser CG: Biological Variation: From
Principles to Practice. AACC Press, Washington, DC, 2001.
|
|
5
|
Carobene A, Aarsand AK, Bartlett WA,
Coşkun A, Diaz-Garzon J, Fernandez-Calle P, Guerra E, Jonker N,
Locatelli M, Plebani M, et al: The European Biological Variation
Study (EuBIVAS): A summary report. Clin Chem Lab Med. 60:505–517.
2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lacher DA, Hughes JP and Carroll MD:
Estimate of biological variation of laboratory analytes based on
the third national health and nutrition examination survey. Clin
Chem. 51:450–452. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Carlsen S, Petersen PH, Skeie S, Skadberg
O and Sandberg S: Within subject biological variation of glucose
and HbA1c in healthy persons and in type 1 diabetes patients. Clin
Chem Lab Med. 49:1501–1507. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kristoffersen AH, Petersen PH, Røraas T
and Sandberg S: Estimates of within-subject biological variation of
protein C, antithrombin, protein S free, protein S activity, and
activated protein C resistance in pregnant women. Clin Chem.
63:898–907. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nunes LA, Brenzikofer R and de Macedo DV:
Reference change values of blood analytes from physically active
subjects. Eur J Appl Physiol. 110:191–198. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Coşkun A, Sandberg S, Unsal I, Cavusoglu
C, Serteser M, Kilercik M and Aarsand AK: Personalized reference
intervals in laboratory medicine: A new model based on
within-subject biological variation. Clin Chem. 67:374–384.
2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Johnson PR, Shahangian S and Astles JR:
Managing biological variation data: Modern approaches for study
design and clinical application. Crit Rev Clin Lab Sci. 58:493–512.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ricos C, Cava F, García-Lario JV,
Hernández A, Iglesias N, Jiménez CV, Minchinela J, Perich C, Simón
M, Domenech MV and Alvarez V: The reference change value: A
proposal to interpret laboratory reports in serial testing based on
biological variation. Scand J Clin Lab Invest. 64:175–184.
2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Braga F and Panteghini M: Generation of
data on within-subject biological variation in laboratory medicine:
An update. Crit Rev Clin Lab Sci. 53:313–325. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Aarsand AK, Fernandez-Calle P, Webster C,
Coskun A, Gonzales-Lao E, Diaz-Garzon J, Jonker N, Simon M, Braga
F, Perich C, et al: The EFLM Biological Variation Database.
European Federation of Clinical Chemistry and Laboratory Medicine,
2023. https://biologicalvariation.eu/.
|
|
15
|
D'orazzio P, Burnett RW, Fogh-Andersen N,
Jacobs E, Kuwa K, Külpmann WR, Larsson L, Lewenstam A, Maas AH,
Mager G, et al: Approved IFCC recommendation on reporting results
for blood glucose. International Federation of Clinical Chemistry
and Laboratory Medicine Scientific Division, Working Group on
Selective Electrodes and Point-of-Care Testing (IFCC-SD-WG-SEPOCT).
Clin Chem Lab Med. 44:1486–1490. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sagkal Midilli T, Ergın E, Baysal E and
Arı Z: Comparison of glucose values of blood samples taken in three
different ways. Clin Nurs Res. 28:436–455. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Davis GM, Spanakis EK, Migdal AL, Singh
LG, Albury B, Urrutia MA, Zamudio-Coronado KW, Scott WH, Doerfler
R, Lizama S, et al: Accuracy of Dexcom G6 continuous glucose
monitoring in non-critically ill hospitalized patients with
diabetes. Diabetes Care. 44:1641–1646. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gallardo-Rincon H, Lomelin-Gascon J,
Martinez-Juarez LA, Montoya A, Ortega-Montiel J, Galicia-Hernandez
V, Álvarez-Hernández DA, Ávila-Domínguez R, Reyes-Muñoz E,
Illescas-Correa LM, et al: Diagnostic accuracy of aapillary blood
glucometer testing for gestational diabetes. Diabetes Metab Syndr
Obes. 15:3855–3870. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Topping J, Reardon M, Coleman J, Hunter B,
Shojima-Perera H, Thyer L and Simpson P: A Comparison of venous
versus capillary blood samples when measuring blood glucose using a
point-of-care, capillary-based glucometer. Prehosp Disaster Med.
34:506–509. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
American Diabetes Association. 6. Glycemic
targets: Standards of medical care in diabetes - 2021. Diabetes
Care. 44 (Suppl_1):S73–S84. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
American Diabetes Association Professional
Practice Committee. 6. Glycemic goals and hypoglycemia: Standards
of care in diabetes - 2024. Diabetes Care. 47 (Suppl_1):S111–S125.
2024.PubMed/NCBI View Article : Google Scholar
|
|
22
|
American Diabetes Association Professional
Practice Committee. 16. Diabetes Care in the Hospital: Standards of
Care in Diabetes-2024. Diabetes Care. 47 (Suppl 1):S295–S306.
2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Brazilian Diabetes Societ (SBD): Brazilian
Diabetes Society Guidelines 2019-2020. SBD, Brasília City, p491,
2019.
|
|
24
|
Galvão TF, Pansani TS and Harrad D:
Reporting items for systematic reviews and meta-analyses: The
PRISMA 2009 Statement. Epidemiol Serv Saúde. 24:335–342. 2015.
|
|
25
|
Aarsand AK, Røraas T, Fernandez-Calle P,
Ricos C, Diaz-Garzo J, Jonker N, Perich C, González-Lao E, Carobene
A, Minchinela J, et al: The biological variation data critical
appraisal checklist: A standard for evaluating studies on
biological variation. Clin Chem. 64:501–514. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mu P, Lu H, Zhang G, Chen Y, Fu J, Wang M,
Shu J and Zeng L: Comparison of fasting capillary glucose
variability between insulin glargine and NPH. Diabetes Res Clin
Pract. 91:e4–e7. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Allsop S, Rumbold PLS and Green BP: The
between-day reproducibility of fasting, satiety-related analytes,
in 8 to 11 year old boys. Physiol Behav. 164(Pt A):207–213.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Colomo N, López-Siguero JP, Leiva I,
Fuentes N, Rubio-Martín E, Omiste A, Guerrero M, Tapia MJ,
Martín-Tejedor B, Ruiz de Adana MS and Olveira G: Relationship
between glucose control, glycemic variability, and oxidative stress
in children with type 1 diabetes. Endocrinol Diabetes Nutr (Engl
Ed). 66:540–549. 2019.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
29
|
Aarsand AK, Røraas T, Bartlett WA, Coşkun
A, Carobene A, Fernandez-Calle P, Jonker N, Díaz-Garzón J, Braga F
and Sandberg S: European Federation of Clinical Chemistry and
Laboratory Medicine (EFLM) Working Group on Biological Variation.
Harmonization initiatives in the generation, reporting and
application of biological variation data. Clin Chem Lab Med.
56:1629–1636. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Badrick T: Biological variation:
Understanding why it is so important? Pract Lab Med.
23(e00199)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pasqualetti S, Braga F and Panteghini M:
Pre-analytical and analytical aspects affecting clinical
reliability of plasma glucose results. Clin Biochem. 50:587–594.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gurevitch J, Koricheva J, Nakagawa S and
Stewart G: Metanalysis and the science of research synthesis.
Nature. 555:175–182. 2018.PubMed/NCBI View Article : Google Scholar
|