|
1
|
Sandberg S, Røraas T and Aarsand AK:
Biological variation and analytical performance specifications
(Internet). In: Tietz textbook of Laboratory medicine. Rifai N,
Chiu RWK, Young I, Burnham CAD and Wittver CT (eds). 7th edition.
Elsevier, St Lous, MO, pp335-356, 2022.
|
|
2
|
Sandberg S, Carobene A, Bartlett B, Coşkun
A, Fernandez-Calle P, Jonker N, Díaz-Garzón J and Aarsand AK:
Biological variation: Recent development and future challenges.
Clin Chem Lab Med. 61:741–750. 2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fraser C and Sandberg S: Biological
variation. In: Tietz textbook of clinical chemistry and molecular
biology. Rifai N, Horvath A and Wittwer C (eds). 6th edition.
Elsevier, St Lous, MO, pp157-170, 2017.
|
|
4
|
Fraser CG: Biological Variation: From
Principles to Practice. AACC Press, Washington, DC, 2001.
|
|
5
|
Carobene A, Aarsand AK, Bartlett WA,
Coşkun A, Diaz-Garzon J, Fernandez-Calle P, Guerra E, Jonker N,
Locatelli M, Plebani M, et al: The European Biological Variation
Study (EuBIVAS): A summary report. Clin Chem Lab Med. 60:505–517.
2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lacher DA, Hughes JP and Carroll MD:
Estimate of biological variation of laboratory analytes based on
the third national health and nutrition examination survey. Clin
Chem. 51:450–452. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Carlsen S, Petersen PH, Skeie S, Skadberg
O and Sandberg S: Within subject biological variation of glucose
and HbA1c in healthy persons and in type 1 diabetes patients. Clin
Chem Lab Med. 49:1501–1507. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kristoffersen AH, Petersen PH, Røraas T
and Sandberg S: Estimates of within-subject biological variation of
protein C, antithrombin, protein S free, protein S activity, and
activated protein C resistance in pregnant women. Clin Chem.
63:898–907. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nunes LA, Brenzikofer R and de Macedo DV:
Reference change values of blood analytes from physically active
subjects. Eur J Appl Physiol. 110:191–198. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Coşkun A, Sandberg S, Unsal I, Cavusoglu
C, Serteser M, Kilercik M and Aarsand AK: Personalized reference
intervals in laboratory medicine: A new model based on
within-subject biological variation. Clin Chem. 67:374–384.
2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Johnson PR, Shahangian S and Astles JR:
Managing biological variation data: Modern approaches for study
design and clinical application. Crit Rev Clin Lab Sci. 58:493–512.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ricos C, Cava F, García-Lario JV,
Hernández A, Iglesias N, Jiménez CV, Minchinela J, Perich C, Simón
M, Domenech MV and Alvarez V: The reference change value: A
proposal to interpret laboratory reports in serial testing based on
biological variation. Scand J Clin Lab Invest. 64:175–184.
2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Braga F and Panteghini M: Generation of
data on within-subject biological variation in laboratory medicine:
An update. Crit Rev Clin Lab Sci. 53:313–325. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Aarsand AK, Fernandez-Calle P, Webster C,
Coskun A, Gonzales-Lao E, Diaz-Garzon J, Jonker N, Simon M, Braga
F, Perich C, et al: The EFLM Biological Variation Database.
European Federation of Clinical Chemistry and Laboratory Medicine,
2023. https://biologicalvariation.eu/.
|
|
15
|
D'orazzio P, Burnett RW, Fogh-Andersen N,
Jacobs E, Kuwa K, Külpmann WR, Larsson L, Lewenstam A, Maas AH,
Mager G, et al: Approved IFCC recommendation on reporting results
for blood glucose. International Federation of Clinical Chemistry
and Laboratory Medicine Scientific Division, Working Group on
Selective Electrodes and Point-of-Care Testing (IFCC-SD-WG-SEPOCT).
Clin Chem Lab Med. 44:1486–1490. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sagkal Midilli T, Ergın E, Baysal E and
Arı Z: Comparison of glucose values of blood samples taken in three
different ways. Clin Nurs Res. 28:436–455. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Davis GM, Spanakis EK, Migdal AL, Singh
LG, Albury B, Urrutia MA, Zamudio-Coronado KW, Scott WH, Doerfler
R, Lizama S, et al: Accuracy of Dexcom G6 continuous glucose
monitoring in non-critically ill hospitalized patients with
diabetes. Diabetes Care. 44:1641–1646. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gallardo-Rincon H, Lomelin-Gascon J,
Martinez-Juarez LA, Montoya A, Ortega-Montiel J, Galicia-Hernandez
V, Álvarez-Hernández DA, Ávila-Domínguez R, Reyes-Muñoz E,
Illescas-Correa LM, et al: Diagnostic accuracy of aapillary blood
glucometer testing for gestational diabetes. Diabetes Metab Syndr
Obes. 15:3855–3870. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Topping J, Reardon M, Coleman J, Hunter B,
Shojima-Perera H, Thyer L and Simpson P: A Comparison of venous
versus capillary blood samples when measuring blood glucose using a
point-of-care, capillary-based glucometer. Prehosp Disaster Med.
34:506–509. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
American Diabetes Association. 6. Glycemic
targets: Standards of medical care in diabetes - 2021. Diabetes
Care. 44 (Suppl_1):S73–S84. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
American Diabetes Association Professional
Practice Committee. 6. Glycemic goals and hypoglycemia: Standards
of care in diabetes - 2024. Diabetes Care. 47 (Suppl_1):S111–S125.
2024.PubMed/NCBI View Article : Google Scholar
|
|
22
|
American Diabetes Association Professional
Practice Committee. 16. Diabetes Care in the Hospital: Standards of
Care in Diabetes-2024. Diabetes Care. 47 (Suppl 1):S295–S306.
2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Brazilian Diabetes Societ (SBD): Brazilian
Diabetes Society Guidelines 2019-2020. SBD, Brasília City, p491,
2019.
|
|
24
|
Galvão TF, Pansani TS and Harrad D:
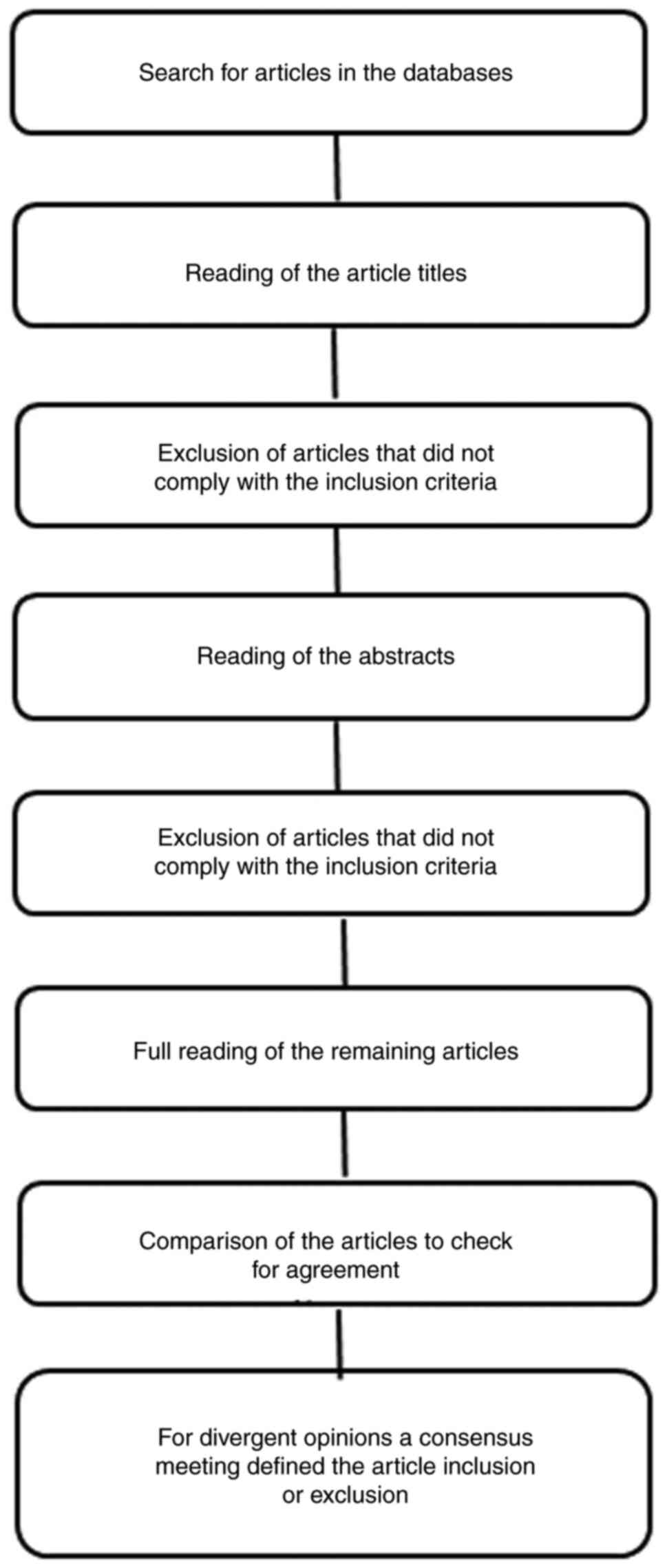
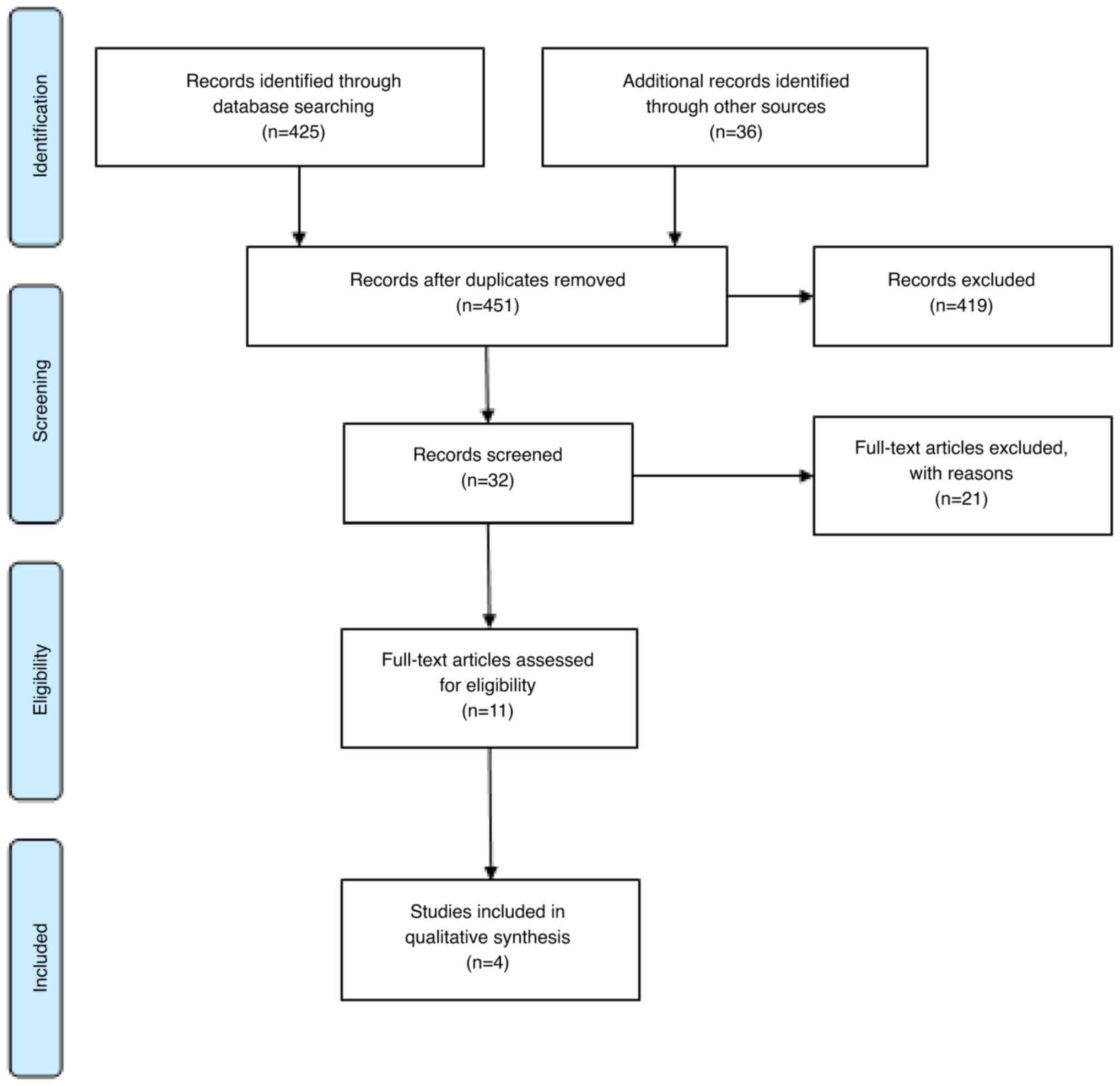
Reporting items for systematic reviews and meta-analyses: The
PRISMA 2009 Statement. Epidemiol Serv Saúde. 24:335–342. 2015.
|
|
25
|
Aarsand AK, Røraas T, Fernandez-Calle P,
Ricos C, Diaz-Garzo J, Jonker N, Perich C, González-Lao E, Carobene
A, Minchinela J, et al: The biological variation data critical
appraisal checklist: A standard for evaluating studies on
biological variation. Clin Chem. 64:501–514. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mu P, Lu H, Zhang G, Chen Y, Fu J, Wang M,
Shu J and Zeng L: Comparison of fasting capillary glucose
variability between insulin glargine and NPH. Diabetes Res Clin
Pract. 91:e4–e7. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Allsop S, Rumbold PLS and Green BP: The
between-day reproducibility of fasting, satiety-related analytes,
in 8 to 11 year old boys. Physiol Behav. 164(Pt A):207–213.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Colomo N, López-Siguero JP, Leiva I,
Fuentes N, Rubio-Martín E, Omiste A, Guerrero M, Tapia MJ,
Martín-Tejedor B, Ruiz de Adana MS and Olveira G: Relationship
between glucose control, glycemic variability, and oxidative stress
in children with type 1 diabetes. Endocrinol Diabetes Nutr (Engl
Ed). 66:540–549. 2019.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
29
|
Aarsand AK, Røraas T, Bartlett WA, Coşkun
A, Carobene A, Fernandez-Calle P, Jonker N, Díaz-Garzón J, Braga F
and Sandberg S: European Federation of Clinical Chemistry and
Laboratory Medicine (EFLM) Working Group on Biological Variation.
Harmonization initiatives in the generation, reporting and
application of biological variation data. Clin Chem Lab Med.
56:1629–1636. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Badrick T: Biological variation:
Understanding why it is so important? Pract Lab Med.
23(e00199)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pasqualetti S, Braga F and Panteghini M:
Pre-analytical and analytical aspects affecting clinical
reliability of plasma glucose results. Clin Biochem. 50:587–594.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gurevitch J, Koricheva J, Nakagawa S and
Stewart G: Metanalysis and the science of research synthesis.
Nature. 555:175–182. 2018.PubMed/NCBI View Article : Google Scholar
|