Introduction
Deep venous thrombosis (DVT) can develop into
pulmonary thromboembolism (PTE), which may even be
life-threatening. DVT and PTE, collectively known as venous
thromboembolism (VTE), after acute coronary syndrome and stroke, is
the third most common clinical cardiovascular disease, and is also
one of the preventable causes of death in hospitalized patients
(1-3).
The incidence of DVT is high in surgical patients, with
hospital-acquired DVT occurring in up to 60% after major orthopedic
surgery (4). Therefore, the
prevention of DVT becomes essential. Prevention with drugs is one
of the most effective measures to reduce the risk of DVT formation.
Widely used anticoagulants include warfarin, heparin,
low-molecular-weight heparin (LMWH) and novel oral anticoagulants
(5). However, even with the use of
anticoagulant drugs, the incidence of DVT in patients with surgery
and trauma cannot be ignored (6,7). In
addition, the adverse reactions of anticoagulant drugs should be
noted, such as hemorrhage, hematoma and thrombocytopenia (8,9). New
treatments are urgently needed to prevent DVT.
Traditional Chinese medicine (TCM) and herbal
medicines have been widely used for thousands of years to treat
various diseases, including thrombosis. TCM is mainly derived from
natural plants and has the advantages of good efficacy, less
toxicity and side effects, and low cost (10). Panax notoginseng is a
classical TCM rich in >70 kinds of Panax notoginseng
saponins (PNS), mainly including notoginsenoside R1, ginsenoside
Rg1, ginsenoside Re, ginsenoside Rb1 and ginsenoside Rd qt
(7,11,12).
PNS have been found to have positive effects on various diseases,
including coronary heart disease, ischemic stroke, gastrointestinal
injury and Alzheimer's disease (11-14).
The mechanisms involved in these effects include anti-inflammation,
anti-oxidation, inhibition of platelet aggregation, anti-apoptosis,
promotion of blood circulation, improvement of vascular endothelial
function and regulation of blood lipids (12,15,16).
TCM indicates that Panax notoginseng may be used for the
prevention and treatment of thrombosis. However, the mechanisms
underlying the effect of PNS against DVT have remained to be fully
elucidated.
In recent years, an increasing number of studies
have been devoted to exploring the mechanisms of action of TCM in
the treatment of various diseases based on network pharmacology
(10). Network pharmacology
combines pharmacology and bioinformatics to reveal the specific
targets of drug interventions in the processes of disease, which is
helpful to promote the development of precision medicine (11,14).
In the present study, several public databases were used to predict
PNS-DVT targets and establish pharmacological networks, from which
key drug components and hub targets were screened. Subsequently,
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes
(KEGG) functional enrichment analysis combined with molecular
docking verification were performed to investigate the complex
effects of PNS in the prevention of DVT. Finally, clinical data
were used to confirm the efficacy and safety of PNS in preventing
DVT.
Materials and methods
Prediction of the targets of PNS
The main active components of PNS were identified
from the TCM Systems Pharmacology Database and Analysis Platform
(TCMSP; http://lsp.nwu.edu.cn/tcmsp.php), which is a unique
systems pharmacology platform of Chinese herbal medicines (17). Symmap v2 (http://www.symmap.org/) was used to identify targets
related to these main active components in the study. Symmap
provides massive information on herbs/ingredients, targets, the
clinical symptoms and diseases, as well as the associations among
them (18).
Collection of PNS-DVT common
targets
The Online Mendelian Inheritance in Man (OMIM;
https://omim.org/) (19), DrugBank (https://go.drugbank.com/) (20), GeneCards (https://www.genecards.org/) (21) and DisGeNET (https://www.disgenet.org/) (22) databases were searched for genes
associated with DVT using ‘deep venous thrombosis’ as the keyword.
Common PNS-DVT targets were obtained by drawing a Venn diagram of
DVT-related genes and targets related to the active components of
PNS using an online drawing tool (http://bioinformatics.psb.ugent.be/webtools/Venn/).
Cytoscape software (v3.9.0) was used to visualize the
herb-component-target gene network (23). The Degree value of each active
compound was calculated using Cytoscape's application named
Cytohubba (v0.1) (24).
Construction of protein-protein
interaction (PPI) network
The PNS-DVT common targets were uploaded to the
Search Tool for the Retrieval of Interacting Genes (STRING; v.11.5;
https://cn.string-db.org/) database to obtain the
PPIs (25). ‘Homo sapiens’
and ‘interaction score ≥0.7’ were used as the screening criteria.
Cytoscape software (v.3.9.0) was used to visualize the PPI network
(23). The degree value of each
node was also calculated using Cytohubba for screening hub genes
(24).
Enrichment analysis
To determine the functions and signaling pathways
involved in the PNS-DVT common targets, GO and KEGG pathway
enrichment analyses were performed using the Metascape (https://metascape.org) platform (26). After uploading PNS-DVT common
targets to Metascape, GO terms in the categories biological process
(BP), cellular component (CC) and molecular function (MF), and KEGG
pathways were obtained from the enrichment analysis. The conditions
for the analysis were set as min overlap=3, P-value cutoff=0.01 and
min enrichment=1.5. The results of the enrichment analysis were
visualized using GraphPad Prism 9.0.0 (Dotmatics).
Molecular docking
Molecular docking was used to examine
receptor-ligand interactions and affinities. The PDB files of the
3D structure of the proteins expressed by the hub genes were
downloaded from the Protein Data Bank (PDB) database (https://www.rcsb.org/) (27), and PyMol (v.2.6) software was used
to remove water molecules and unrelated ligands from the 3D
structure. The PDB file of the protein was then imported into
AutodockTools (v.1.5.7) (28)
software for hydrogenating and saved in PDBQT format. Mol2 files of
hub active compounds were downloaded from the TCMSP database and
then saved in PDBQT format after hydrogenating and rotatable bonds
setting by AutodockTools (v.1.5.7) software. Finally, by setting
the maximum ‘Gird box’, i.e., the blind docking method, the
molecular docking was verified in AutodockTools (v.1.5.7) software,
and the results with the lowest binding energy were visualized by
PyMol (v2.6) software (29). A
binding energy <0 kcal/mol indicates that the ligand can
spontaneously bind to the protein.
Validation of clinical data
To verify the efficacy of PNS in preventing DVT,
patients undergoing orthopedic surgery at the Department of Surgery
of Xuanwu Hospital, Capital Medical University (Beijing, China)
from January 2016 to December 2018 were screened. The inclusion
criteria were as follows: Age ≥18 years; Caprini scale scores
suggest ≥ moderate VTE risk; the anticoagulant therapy received
during the perioperative period was LMWH (hypodermic injection;
4,000-8,000 AxaIU once daily; 100 AxaIU/kg) or LMWH plus PNS (drug
named Xue-Shuan-Tong oral tablets; 100 mg; 3 times daily) (7); deep vein ultrasound of the lower
extremities on admission did not reveal DVT, and the lower
extremity deep vein ultrasound was reexamined before discharge.
Pregnant women, patients with coagulopathy and/or contraindications
to anticoagulation, and patients already on anticoagulants prior to
hospitalization, were not included. This study was approved by the
Institutional Review Board of Xuanwu Hospital, Capital Medical
University (Beijing, China; approval no. [2017]088). The primary
endpoint was the incidence of DVT in a lower extremity. Other
endpoints included the incidence of major bleeding (hemoglobin lost
≥2 g/l), pulmonary embolism and pulmonary embolism-related
death.
Statistical analysis
Descriptions of statistical methods relevant to
networks pharmacology were presented in preceding each part of the
Methods section. In the validation of clinical data,
normally distributed continuous variables were analyzed using a
two-sided unpaired t-test, while non-normally distributed variables
were analyzed using the Wilcoxon rank-sum test. Dichotomous data
were compared using Fisher's exact test or chi-square test.
Significance was defined as P<0.05.
Results
Predicted targets of PNS
Notoginsenoside R1, ginsenoside Rg1, ginsenoside Re,
ginsenoside Rb1 and ginsenoside Rd qt were the 5 main active
components in PNS (11,12), and their details are described in
Table I. Among them, ginsenoside Rd
qt has the best oral bioavailability and drug-likeness quality, so
it may be the main bioactive component in oral management. The
results obtained from the Symmap v2 databases were integrated to
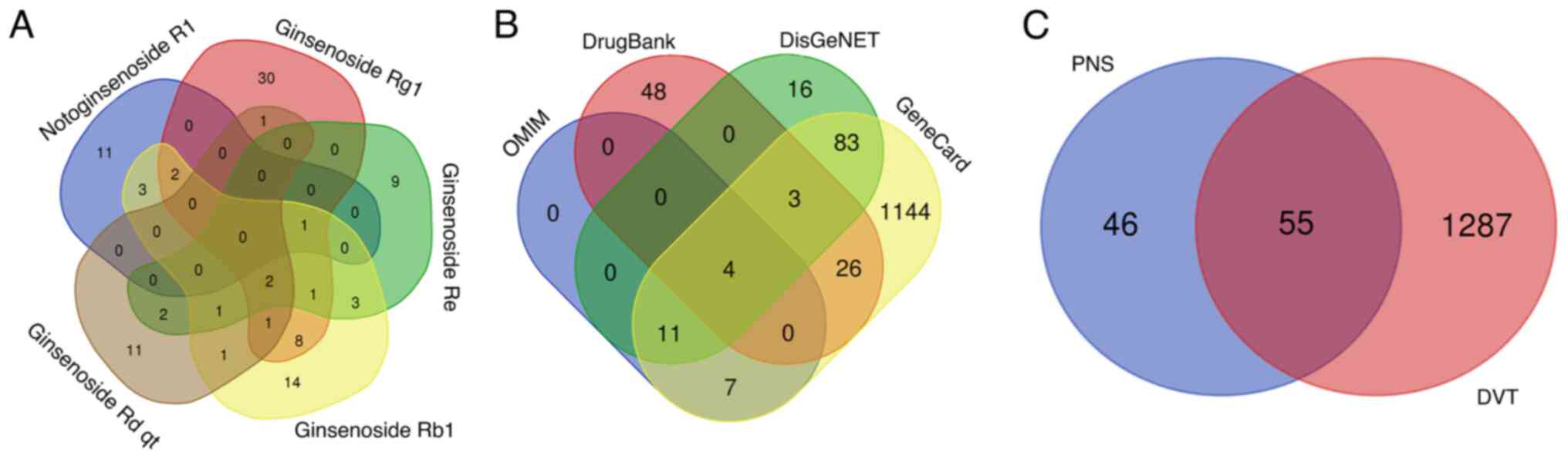
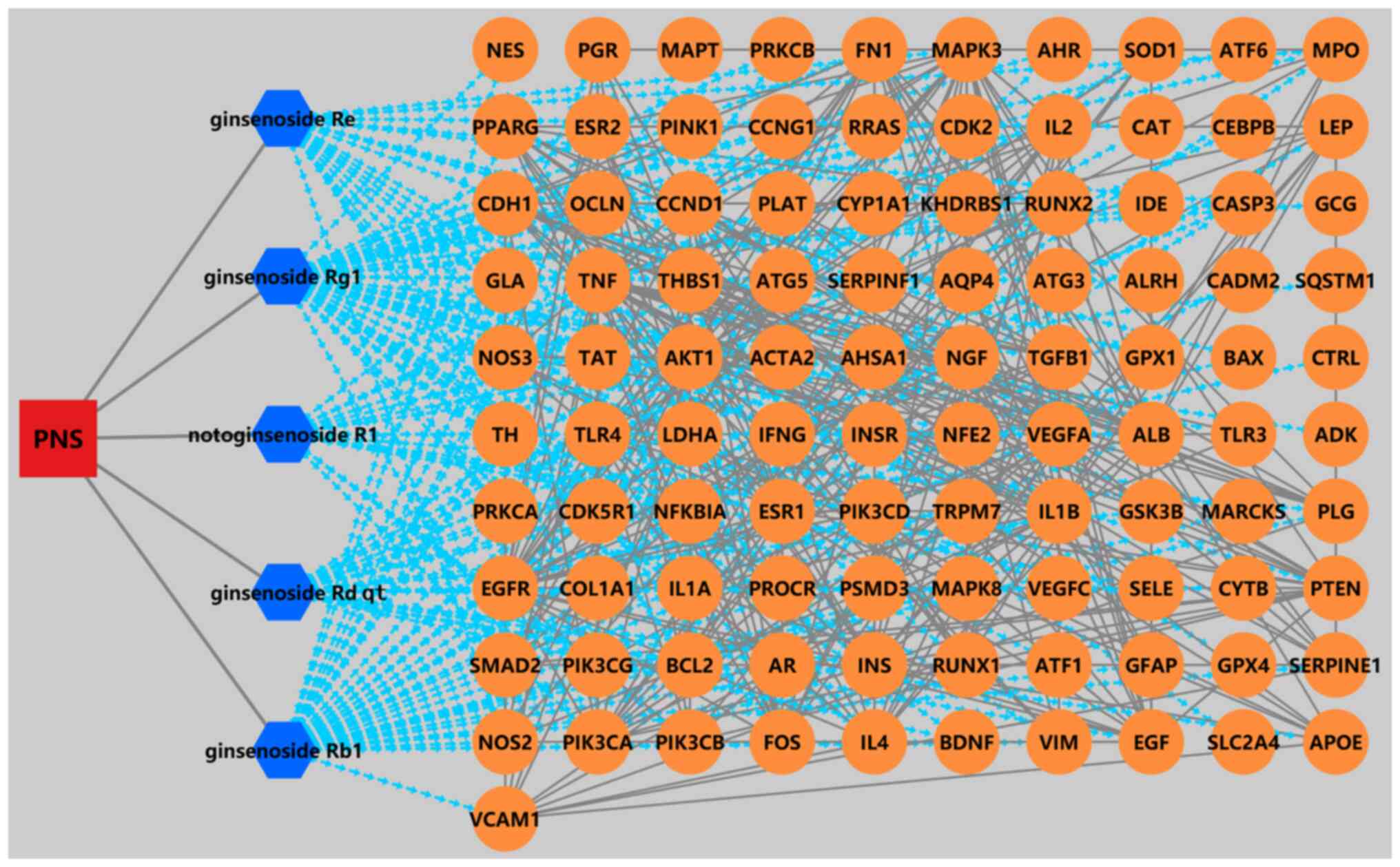
obtain the 101 non-repetitive targets with the five active PNS
compounds (Fig. 1A). As shown in
Fig. 2, PNS mainly acts through
multiple components corresponding to multiple targets.
 | Table IActive components of Panax
notoginseng saponins. |
Table I
Active components of Panax
notoginseng saponins.
| Molecule ID | Compound name | OB% | DL |
|---|
| MOL007487 | Notoginsenoside
R1 | 5.43 | 0.13 |
| MOL005341 | Ginsenoside
Rg1 | 10.04 | 0.28 |
| MOL005338 | Ginsenoside Re | 4.27 | 0.12 |
| MOL007476 | Ginsenoside
Rb1 | 6.29 | 0.04 |
| MOL007480 | Ginsenoside Rd
qt | 12.23 | 0.77 |
Collected common targets of
PNS-DVT
After deduplication, a total of 1,342 DVT-related
targets were obtained from the OMIM, DrugBank, GeneCards and
DisGeNET databases (Fig. 1B). There
were 55 common targets shared by PNS active components and DVT
(Fig. 1C).
Construction of the PPI network
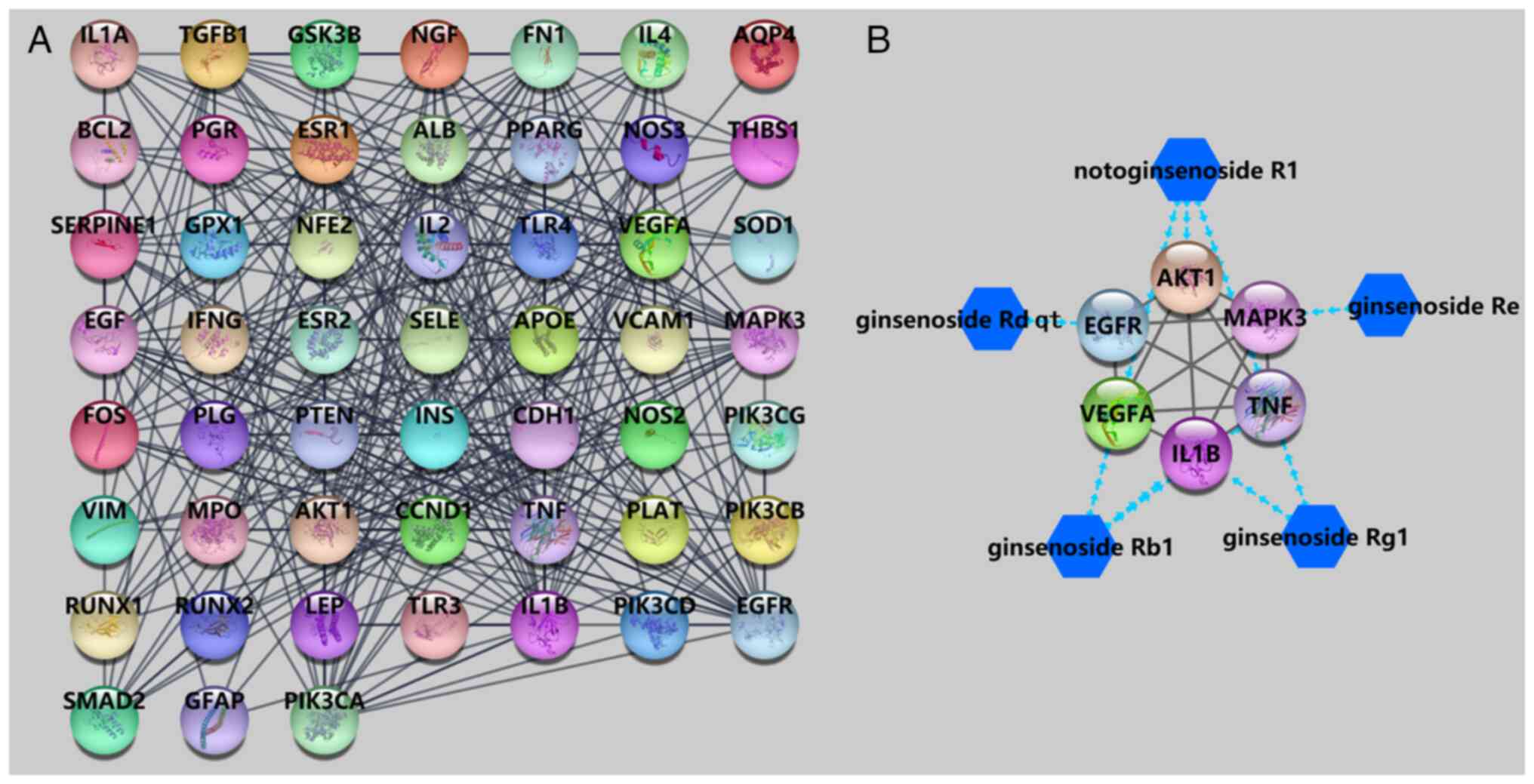
A total of 55 common targets of PNS and DVT were
introduced into the STRING database for PPI network analysis. As
presented in Fig. 3A, there were 52
nodes (genes) with 301 edges (interactions) in the PPI network, and
those nodes with higher degree values were regarded as hub target
genes. AKT1, TNF, IL1B, EGFR, VEGFA and MAPK3 were the top 6
targets, with degree values of 32, 28, 25, 25, 25 and 23,
respectively. The network of the 5 main active compounds and hub
target genes is shown in Fig.
3B.
GO and KEGG pathway enrichment
analyses
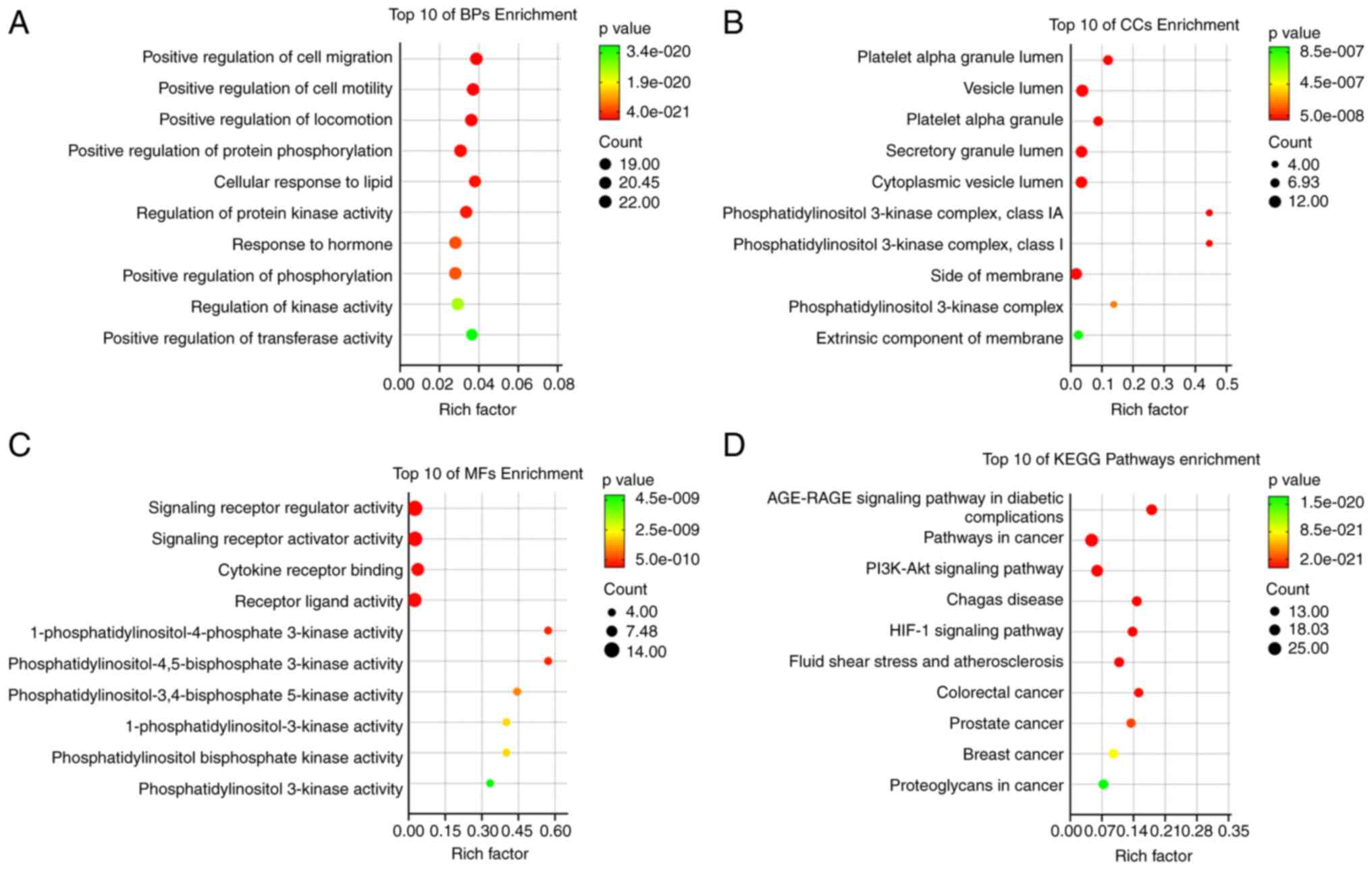
The Metascape platform was used for the GO and KEGG
pathway analyses, and the top 10 terms in each category were
visualized (Fig. 4). The top 3
enriched terms in GO BP were ‘positive regulation of cell
migration’, ‘positive regulation of cell motility’ and ‘positive
regulation of locomotion’. The top 3 enriched terms in GO CC were
‘platelet alpha granule lumen’, ‘vesicle lumen’ and ‘platelet alpha
granule’. The top 3 enriched terms in GO MF were ‘signaling
receptor regulator activity’, ‘signaling receptor activator
activity’ and ‘cytokine receptor binding’. The ‘advanced glycation
end products (AGE)/receptors for AGE (RAGE) signaling pathway in
diabetic complications’, ‘pathways in cancer’ and the ‘PI3K-Akt
signaling pathway’ were the most significant KEGG signaling
pathways.
Molecular docking
According to the targeting relationship shown in
Fig. 3B, the 6 hub target genes
from the PPI network were docked to the 5 active compounds of PNS.
Table II shows the PDB ID of the
hub target genes used in molecular docking. The results presented a
total of 10 binding interactions, with all molecules showing a
binding energy <-6 kcal/mol with the targets. In other words,
these five active PNS compounds have good binding ability with
PNS-DVT common hub target genes. Both notoginsenoside R1 and
ginsenoside Rb1 had 3 targets. The visual docking results are shown
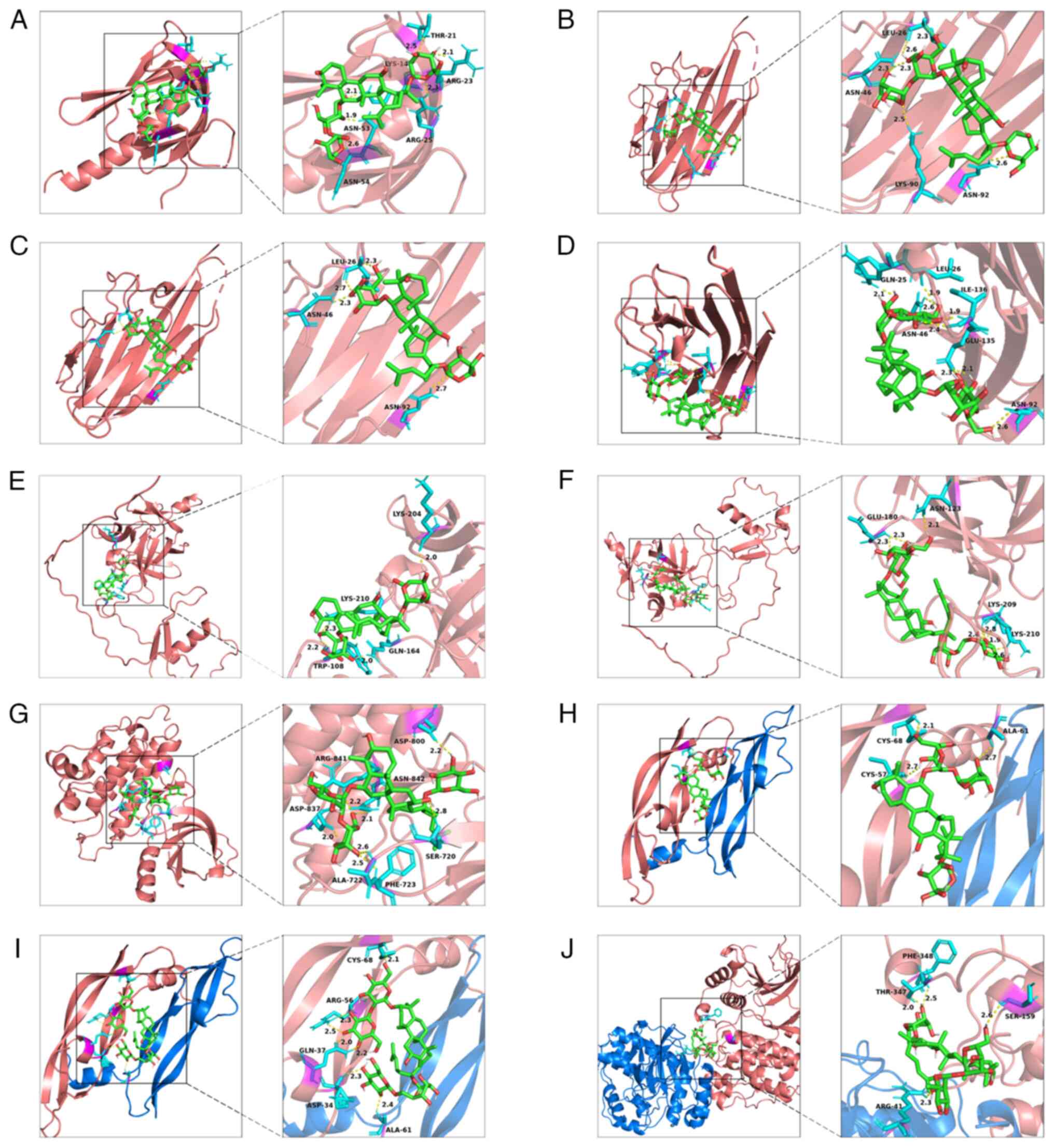
in Fig. 5.
 | Table IIMolecular docking results between the
hub targets and main compounds. |
Table II
Molecular docking results between the
hub targets and main compounds.
| Hub gene | PDB ID | Compound name | Binding energy,
kcal/mol |
|---|
| AKT1 | 7myx | Notoginsenoside
R1 | -6.7 |
| TNF | 5uui | Notoginsenoside
R1 | -7 |
| TNF | 5uui | Ginsenoside
Rg1 | -6.4 |
| TNF | 5uui | Ginsenoside
Rb1 | -6.7 |
| IL1B | AF_P01584_F1 | Ginsenoside
Rg1 | -7.1 |
| IL1B | AF_P01584_F1 | Ginsenoside
Rb1 | -8.2 |
| EGFR | 5ug9 | Ginsenoside Rd
qt | -9.1 |
| VEGFA | 1mkk | Notoginsenoside
R1 | -7.3 |
| VEGFA | 1mkk | Ginsenoside
Rb1 | -7.2 |
| MAPK3 | 6ges | Ginsenoside Re | -8.7 |
Clinical results of PNS in preventing
DVT
A total of 194 patients were screened for this
clinical validation, and 99 and 95 patients were in the LMWH group
and PNS + LMWH group, respectively. Female patients account for
70.71 and 70.53% in the two groups, with average ages of 69.83 and
68.61 years, respectively. As shown as in Table III, there were no significant
differences in baseline characteristics between the two groups. The
incidence of postoperative DVT in the LMWH group was 25.25%, which
was significantly higher than the 12.63% in the LMWH + PNS group
(P=0.025), while there were no significant differences in
coagulation function indexes except D-dimer (P=0.044) after surgery
between the two groups. No major bleeding, pulmonary embolism or
pulmonary embolism-related death occurred in either group.
 | Table IIIDemographic and clinical
characteristics of participants. |
Table III
Demographic and clinical
characteristics of participants.
| Variable | LMWH alone
(n=99) | LMWH + PNS
(n=95) | P-value |
|---|
| Female sex | 70 (70.71) | 67 (70.53) | 0.978 |
| Age, years | 69.83±9.23 | 68.61±11.12 | 0.578 |
| Body mass index,
kg/m2 | 25.72±3.98 | 25.64±4.12 | 0.887 |
| Hypertension | 55 (55.56) | 46 (48.42) | 0.320 |
| Diabetes | 33 (33.33) | 26 (27.37) | 0.367 |
| Location of
surgery | | | 0.811 |
|
Knee | 62 (62.63) | 54 (56.84) | |
|
Hip | 35 (35.35) | 38 (40.00) | |
|
Calcaneus | 1 (1.01) | 1 (1.05) | |
|
Ankle | 1 (1.01) | 2 (2.11) | |
| Pre-operation | | | |
|
PT, sec | 13.20±0.72 | 13.32±0.83 | 0.282 |
|
TT, sec | 16.12±1.11 | 16.77±7.90 | 0.419 |
|
APTT,
sec | 37.33±5.09 | 36.91±4.63 | 0.553 |
|
INR | 1.01±0.07 | 1.02±0.08 | 0.441 |
|
Fibrinogen,
g/l | 3.63±0.96 | 3.56±0.79 | 0.546 |
|
D-dimer,
µg/ml | 0.61 (0.35,
1.70) | 0.69 (0.34,
1.93) | 0.999 |
| Post-operation | | | |
|
PT, sec | 13.82±0.86 | 13.66±0.85 | 0.214 |
|
TT, sec | 15.04±1.65 | 14.76±0.92 | 0.176 |
|
APTT,
sec | 42.88±6.86 | 42.39±6.40 | 0.638 |
|
INR | 1.07±0.09 | 1.05±0.08 | 0.109 |
|
Fibrinogen,
g/l | 4.64±1.32 | 4.95±1.31 | 0.140 |
|
D-dimer,
µg/ml | 1.78 (1.05,
2.76) | 1.34 (0.87,
2.32) | 0.044 |
|
DVT | 25 (25.25) | 12 (12.63) | 0.025 |
Discussion
The clinical data showed that the combined use of
PNS significantly improved the effect of LMWH in preventing DVT
after orthopedic surgery. The molecular mechanism of this effect
was explored by network pharmacology analyses. In this study, it
was indicated that PNS and DVT have 55 common gene targets. Further
analysis showed that PNS may use AKT1, TNF, IL1B, EGFR, VEGFA and
MAPK3 as hub targets to prevent DVT.
AKT1, also known as protein kinase B, is a potent
signal transducer of multiple signaling functions in platelets.
Studies have confirmed that AKT is involved in the positive
regulation of megakaryopoiesis and thrombopoiesis (30,31).
As the critical role of GPIb-IX-mediated early signals, AKT
mediates a variety of agonists-induced signaling cascades in
platelets. Under the stimulation by agonists, rapidly activating
AKT is involved in multiple signaling pathways, such as the
PI3K/Akt signaling pathway, contributing to integrin activation,
thromboxane synthesis and degranulation (32-34).
Inflammation plays an important role in the formation of DVT
(35). Inflammatory cells,
particularly macrophages, are the sources of the proinflammatory
cytokines, such as tumor necrosis factor-α (TNF-α) (36). However, the connection between the
TNF-α and thrombosis remains controversial. TNF-α can induce tissue
factor (TF), which is the key initiator of the physiological
coagulation process through activating the exogenous coagulation
pathway (37). As a strong
stimulator, TNF-α can activate the coagulation system, which is
manifested as the downregulation of physiological anticoagulant
mechanisms and the inhibition of fibrinolysis (38). However, it has also been proposed
that an altered inflammatory cytokine profile may be the result of
venous thrombosis rather than a cause of it (39). Of note, a study has found that TNF-α
interacting with TNF-Rp55 enhances the resolution of venous
thrombosis through the increased expression of fibrinolytic
mediators and enzymes linked to collagen remodeling by macrophages
(40).
IL-1β is produced by macrophages and monocytes and
is a marker of an early inflammatory response. IL-1β binds to the
IL-1β receptor on endothelial cells and activates the NF-κB pathway
to cause endothelial injury, and activates TF, coagulation and von
Willebrand factor to promote platelet adhesion and fibrin
deposition, thereby initiating and aggravating thrombosis (41-43).
Epidermal growth factor receptor (EGFR) is a widely used prognostic
marker for numerous cancers, and increasing studies have found an
association between EGFR and the occurrence of DVT and other
thromboembolic complications, particularly in cancer patients. EGFR
can stimulate the production of growth factors, including vascular
endothelial growth factor (VEGF), while VEGF is a chemokine factor
for cells that expresses TF, which is involved in thrombosis, as
mentioned above (44-46).
As a member of the MAPK family, MAPK3, also known as extracellular
signal-regulated kinase 1 (ERK1), is associated with
thrombin-activated platelet aggregation. Activated ERK is important
in GPIb-IX-mediated signaling, leading to integrin activation and,
thus, integrin-dependent stable platelet adhesion, aggregation and
thrombosis (33,47).
The mechanism of the above hub genes in thrombosis
was closely related to the results revealed by GO enrichment
analysis. KEGG enrichment analysis indicated that the common
targets were mainly enriched in the ‘AGE-RAGE signaling pathway in
diabetic complications’, ‘pathways in cancer’ and the ‘PI3K-Akt
signaling pathway’. AGEs and RAGEs were first studied in diabetes
(48). The AGE/RAGE pathway has
been substantiated to be involved in oxidative stress, inflammation
and a variety of diseases, including cardiovascular diseases and
thrombosis (49). RAGE inhibition
can suppress the release of proinflammatory cytokines IL-6, IL-1β
and TNF-α (48). In addition, RAGE
inhibition markedly suppressed malondialdehyde and reactive oxygen
species levels and increased the level of the antioxidant substance
superoxide dismutase, which effectively alleviated AGE-induced
oxidative stress. Inhibition of the AGE/RAGE axis also
significantly increased levels of nitric oxide-suppressed
endothenin-1 expression, which all helps prevent the occurrence of
thrombosis (48,50,51).
Inhibition of PI3K/AKT signaling reduced platelet aggregation and
thrombosis, while activation of PI3K/AKT signaling induced
endothelial damage, apoptosis and inflammation (52-54).
Integrins play a critical role in different phases of platelet
function during thrombosis, being involved in both platelet-matrix
interaction and platelet-platelet aggregation. The PI3K/Akt pathway
regulates both integrin inside-out and outside-in signaling
(55).
The five main active components of PNS are
notoginsenoside R1, ginsenoside Rg1, ginsenoside Re, ginsenoside
Rb1 and ginsenoside Rd qt. Network pharmacology analysis identified
the hub targets of these five main components in DVT prevention,
and the spontaneous binding among them was verified by molecular
docking. A binding energy <0 kcal/mol indicates the docking
molecule had spontaneous binding activity to the target, with a
smaller value of binding energy reflecting a higher binding
ability. All molecules showed a binding energy <-6 kcal/mol with
the targets, indicating excellent spontaneous binding of the
ligands to the receptors.
A limitation of the present study is that no
experiments were performed to validate the network pharmacology
results, particularly in vivo experiments. Although network
pharmacology and clinical data have provided preliminary insights
into the mechanisms and effects of PNS for DVT prevention, these
results may not fully reflect the complex conditions within a
living organism. The results of pure clinical data without in
vivo experiments limit the clinical generalization of the
present findings. Therefore, in vivo experiments may be
performed in future studies by our group to more comprehensively
evaluate the potential value of PNS in preventing DVT.
In conclusion, PNS can promote the effect of LMWH to
prevent DVT. We identified potential targets and pathways for PNS
in the prevention of DVT, and a basis for subsequent experimental
verification was provided. The hub targets of PNS in preventing DVT
were AKT1, TNF, IL1B, EGFR, VEGFA and MAPK3. Molecular docking
analysis showed that the main active components of PNS could
combine well with these hub targets. The AGE-RAGE signaling pathway
and the PI3K-Akt signaling pathway may be critical pathways for PNS
to prevent DVT.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by Capital's Funds for Health
Improvement and Research (grant no. SHOUFA 2022-2-2016) and the
Traditional Chinese Medicine Technology Development Fund Project
(grant no. JJ2015-09).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
BY and YN conceptualized and supervised the study,
validated and curated data and wrote, reviewed and edited the
manuscript. JG developed the methodology, and performed software
analysis and data validation, and reviewed and edited the
manuscript. YN and JG checked and confirmed the authenticity of the
raw data. LL conceptualized and supervised the study. CW performed
formal analysis and project administration and validated the data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board of Xuanwu Hospital, Capital Medical University (Beijing,
China; approval no. [2017]088). All patients provided their verbal
and written informed consent to participate in the present
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Giustozzi M, Franco L, Vedovati MC,
Becattini C and Agnelli G: Safety of direct oral anticoagulants
versus traditional anticoagulants in venous thromboembolism. J
Thromb Thrombolysis. 48:439–453. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shargall Y and Litle VR: European
perspectives in Thoracic Surgery, the ESTS venous thromboembolism
(VTE) working group. J Thorac Dis. 10 (Suppl 8):S963–S968.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fritz MK, Kincaid SE, Sargent CG, Green AH
and Davis GA: Venous thromboembolism (VTE) risk stratification in
general medical patients at an academic medical center. J Thromb
Thrombolysis. 51:67–73. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Saleh J, El-Othmani MM and Saleh KJ: Deep
vein thrombosis and pulmonary embolism considerations in orthopedic
surgery. Orthop Clin North Am. 48:127–135. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Anderson DR, Morgano GP, Bennett C,
Dentali F, Francis CW, Garcia DA, Kahn SR, Rahman M, Rajasekhar A,
Rogers FB, et al: American Society of Hematology 2019 guidelines
for management of venous thromboembolism: prevention of venous
thromboembolism in surgical hospitalized patients. Blood Adv.
3:3898–3944. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Louis SG, Van PY, Riha GM, Barton JS,
Kunio NR, Underwood SJ, Differding JA, Rick E, Ginzburg E and
Schreiber MA: Thromboelastogram-guided enoxaparin dosing does not
confer protection from deep venous thrombosis: A randomized
controlled pilot trial. J Trauma Acute Care Surg. 76:937–42;
discussion 942-3. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang CM, Guo XF, Liu LM, Huang Y, Meng L,
Song LP, Wu YF, Ning YC, Reilly KH and Wang HB: Prevention of deep
vein thrombosis by panax notoginseng saponins combined with
low-molecular-weight heparin in surgical patients. Chin J Integr
Med. 28:771–778. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
van Rein N, Biedermann JS, van der Meer
FJM, Cannegieter SC, Wiersma N, Vermaas HW, Reitsma PH, Kruip MJHA
and Lijfering WH: Major bleeding risks of different
low-molecular-weight heparin agents: A cohort study in 12 934
patients treated for acute venous thrombosis. J Thromb Haemost.
15:1386–1391. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hogan M and Berger JS: Heparin-induced
thrombocytopenia (HIT): Review of incidence, diagnosis, and
management. Vasc Med. 25:160–173. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang X, Wang M, Qiao Y, Shan Z, Yang M,
Li G, Xiao Y, Wei L, Bi H and Gao T: Exploring the mechanisms of
action of Cordyceps sinensis for the treatment of depression using
network pharmacology and molecular docking. Ann Transl Med.
10(282)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dong Y, Duan L, Chen HW, Liu YM, Zhang Y
and Wang J: Network pharmacology-based prediction and verification
of the targets and mechanism for panax notoginseng saponins against
coronary heart disease. Evid Based Complement Alternat Med.
2019(6503752)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang C, Chen H, Ma ST, Mao BB, Chen Y, Xu
HN and Yu H: A Network Pharmacology Approach for Exploring the
Mechanisms of Panax notoginseng Saponins in Ischaemic Stroke. Evid
Based Complement Alternat Med. 2021(5582782)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhu B, Zhang W, Lu Y, Hu S, Gao R, Sun Z,
Chen X, Ma J, Guo S, Du S and Li P: Network pharmacology-based
identification of protective mechanism of Panax Notoginseng
Saponins on aspirin induced gastrointestinal injury. Biomed
Pharmacother. 105:159–166. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jiang Y, Li S, Xie X, Li H, Huang P, Li B,
Huo L, Zhong J, Li Y and Xia X: Exploring the Mechanism of Panax
notoginseng Saponins against Alzheimer's Disease by Network
Pharmacology and Experimental Validation. Evid Based Complement
Alternat Med. 2021(5730812)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yu G and Wang J: Exploring mechanisms of
Panax notoginseng saponins in treating coronary heart disease by
integrating gene interaction network and functional enrichment
analysis. Chin J Integr Med. 22:589–596. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Han S, Chen Y, Wang J, Zhang Q, Han B, Ge
Y, Xiang Y, Liang R, Zhu X and Liao F: Anti-thrombosis Effects and
Mechanisms by Xueshuantong Capsule Under Different Flow Conditions.
Front Pharmacol. 10(35)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ru J, Li P, Wang J, Zhou W, Li B, Huang C,
Li P, Guo Z, Tao W, Yang Y, et al: TCMSP: A database of systems
pharmacology for drug discovery from herbal medicines. J
Cheminform. 6(13)2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wu Y, Zhang F, Yang K, Fang S, Bu D, Li H,
Sun L, Hu H, Gao K, Wang W, et al: SymMap: An integrative database
of traditional Chinese medicine enhanced by symptom mapping.
Nucleic Acids Res. 47:D1110–D1117. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Boyadjiev SA and Jabs EW: Online Mendelian
Inheritance in Man (OMIM) as a knowledgebase for human
developmental disorders. Clin Genet. 57:253–266. 2000.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wishart DS, Feunang YD, Guo AC, Lo EJ,
Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, et al:
DrugBank 5.0: A major update to the DrugBank database for 2018.
Nucleic Acids Res. 46:D1074–D1082. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Safran M, Chalifa-Caspi V, Shmueli O,
Olender T, Lapidot M, Rosen N, Shmoish M, Peter Y, Glusman G,
Feldmesser E, et al: Human Gene-Centric Databases at the Weizmann
Institute of Science: GeneCards, UDB, CroW 21 and HORDE. Nucleic
Acids Res. 31:142–146. 2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Piñero J, Queralt-Rosinach N, Bravo A,
Deu-Pons J, Bauer-Mehren A, Baron M, Sanz F and Furlong LI:
DisGeNET: A discovery platform for the dynamical exploration of
human diseases and their genes. Database (Oxford).
2015(bav028)2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chin CH, Chen SH, Wu HH, Ho CW, Ko MT and
Lin CY: CytoHubba: Identifying hub objects and sub-networks from
complex interactome. BMC Syst Biol. 8 (Suppl 4)(S11)2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Szklarczyk D, Gable AL, Lyon D, Junge A,
Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork
P, et al: STRING v11: Protein-protein association networks with
increased coverage, supporting functional discovery in genome-wide
experimental datasets. Nucleic Acids Res. 47:D607–D613.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhou Y, Zhou B, Pache L, Chang M,
Khodabakhshi AH, Tanaseichuk O, Benner C and Chanda SK: Metascape
provides a biologist-oriented resource for the analysis of
systems-level datasets. Nat Commun. 10(1523)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Burley SK, Berman HM, Kleywegt GJ, Markley
JL, Nakamura H and Velankar S: Protein Data Bank (PDB): The single
global macromolecular structure archive. Methods Mol Biol.
1607:627–641. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Forli S, Huey R, Pique ME, Sanner MF,
Goodsell DS and Olson AJ: Computational protein-ligand docking and
virtual drug screening with the AutoDock suite. Nat Protoc.
11:905–919. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Seeliger D and de Groot BL: Ligand docking
and binding site analysis with PyMOL and Autodock/Vina. J Comput
Aided Mol Des. 24:417–422. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yang X, Wang L, Zeng J, Wu A, Qin M, Wen
M, Zhang T, Chen W, Mei Q, Qin D, et al: Caulis Polygoni Multiflori
Accelerates Megakaryopoiesis and Thrombopoiesis via Activating
PI3K/Akt and MEK/ERK Signaling Pathways. Pharmaceuticals (Basel).
15(1204)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fritz DI, Ding Y, Merrill-Skoloff G,
Flaumenhaft R, Hanada T and Chishti AH: Dematin regulates calcium
mobilization, thrombosis, and early akt activation in platelets.
Mol Cell Biol. 43:283–299. 2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Woulfe DS: Akt signaling in platelets and
thrombosis. Expert Rev Hematol. 3:81–91. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yin H, Stojanovic A, Hay N and Du X: The
role of Akt in the signaling pathway of the glycoprotein Ib-IX
induced platelet activation. Blood. 111:658–665. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen S, Tian CB, Bai LY, He XC, Lu QY,
Zhao YL and Luo XD: Thrombosis inhibited by Corydalis decumbens
through regulating PI3K-Akt pathway. J Ethnopharmacol.
329(118177)2024.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang T, Li Q, Wang L and Li G: Expression
variations and clinical significance of MMP-1, MMP-2 and
inflammatory factors in serum of patients with deep venous
thrombosis of lower extremity. Exp Ther Med. 17:181–186.
2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Saha P and Smith A: TNF-α (Tumor Necrosis
Factor-α). Arterioscler Thromb Vasc Biol. 38:2542–2543.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lopes-Bezerra LM and Filler SG:
Endothelial cells, tissue factor and infectious diseases. Braz J
Med Biol Res. 36:987–991. 2003.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Nagareddy P and Smyth SS: Inflammation and
thrombosis in cardiovascular disease. Curr Opin Hematol.
20:457–463. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Christiansen SC, Naess IA, Cannegieter SC,
Hammerstrøm J, Rosendaal FR and Reitsma PH: Inflammatory cytokines
as risk factors for a first venous thrombosis: A prospective
population-based study. PLoS Med. 3(e334)2006.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nosaka M, Ishida Y, Kimura A, Kuninaka Y,
Taruya A, Furuta M, Mukaida N and Kondo T: Contribution of the
TNF-α (Tumor Necrosis Factor-α)-TNF-Rp55 (Tumor Necrosis Factor
Receptor p55) Axis in the Resolution of Venous Thrombus.
Arterioscler Thromb Vasc Biol. 38:2638–2650. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Pai RZ, Fang Q, Tian G, Zhu B and Ge X:
Expression and role of interleukin-1β and associated biomarkers in
deep vein thrombosis. Exp Ther Med. 22(1366)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Fan LM, Douglas G, Bendall JK, McNeill E,
Crabtree MJ, Hale AB, Mai A, Li JM, McAteer MA, Schneider JE, et
al: Endothelial cell-specific reactive oxygen species production
increases susceptibility to aortic dissection. Circulation.
129:2661–2672. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kanaji N, Sato T, Nelson A, Wang X, Li Y,
Kim M, Nakanishi M, Basma H, Michalski J, Farid M, et al:
Inflammatory cytokines regulate endothelial cell survival and
tissue repair functions via NF-κB signaling. J Inflamm Res.
4:127–138. 2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zepeda-Orozco D, Wen HM, Hamilton BA,
Raikwar NS and Thomas CP: . EGF regulation of proximal tubule cell
proliferation and VEGF-A secretion. Physiol Rep.
5(e13453)2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kaye B, Ali A, Correa Bastianon Santiago
RA, Ibrahim B, Isidor J, Awad H, Sabahi M, Obrzut M, Adada B,
Ranjan S and Borghei-Razavi H: The Role of EGFR amplification in
deep venous thrombosis occurrence in IDH Wild-Type Glioblastoma.
Curr Oncol. 30:4946–4956. 2023.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Roopkumar J, Poudel SK, Gervaso L, Reddy
CA, Velcheti V, Pennell NA, McCrae KR and Khorana AA: Risk of
thromboembolism in patients with ALK- and EGFR-mutant lung cancer:
A cohort study. J Thromb Haemost. 19:822–829. 2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Li Q, Chen Y, Zhao D, Yang S, Zhang S, Wei
Z, Wang Y, Qian K, Zhao B, Zhu Y, et al: LongShengZhi Capsule
reduces carrageenan-induced thrombosis by reducing activation of
platelets and endothelial cells. Pharmacol Res. 144:167–180.
2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Zhang Y, Liu J, Jia W, Tian X, Jiang P,
Cheng Z and Li J: AGEs/RAGE blockade downregulates Endothenin-1
(ET-1), mitigating Human Umbilical Vein Endothelial Cells (HUVEC)
injury in deep vein thrombosis (DVT). Bioengineered. 12:1360–1368.
2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Yamagishi SI: Role of advanced glycation
end products (AGEs) and receptor for AGEs (RAGE) in vascular damage
in diabetes. Exp Gerontol. 46:217–224. 2011.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Cyr AR, Huckaby LV, Shiva SS and
Zuckerbraun BS: Nitric oxide and endothelial dysfunction. Crit Care
Clin. 36:307–321. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yang S, Zheng Y and Hou X: Lipoxin A4
restores oxidative stress-induced vascular endothelial cell injury
and thrombosis-related factor expression by its receptor-mediated
activation of Nrf2-HO-1 axis. Cell Signal. 60:146–153.
2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Lyu X, Yi Z, He Y, Zhang C, Zhu P and Liu
C: Astragaloside IV induces endothelial progenitor cell
angiogenesis in deep venous thrombosis through inactivation of
PI3K/AKT signaling. Histol Histopathol. 39:1149–1157.
2024.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Chang MC, Chen YJ, Liou EJ, Tseng WY, Chan
CP, Lin HJ, Liao WC, Chang YC, Jeng PY and Jeng JH:
7-Ketocholesterol induces ATM/ATR, Chk1/Chk2, PI3K/Akt signalings,
cytotoxicity and IL-8 production in endothelial cells. Oncotarget.
7:74473–74483. 2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Su XL, Su W, Wang Y, Wang YH, Ming X and
Kong Y: The pyrrolidinoindoline alkaloid Psm2 inhibits platelet
aggregation and thrombus formation by affecting PI3K/Akt signaling.
Acta Pharmacol Sin. 37:1208–1217. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Guidetti GF, Canobbio I and Torti M:
PI3K/Akt in platelet integrin signaling and implications in
thrombosis. Adv Biol Regul. 59:36–52. 2015.PubMed/NCBI View Article : Google Scholar
|