Introduction
Honey and stingless bees (Apidae Meliponini),
called ‘lukut’ in Philippines, and ‘damar’ in India (1), generate propolis, a naturally
occurring resinous substance, by gathering exudates and materials
from plant parts, including flower buds, tree bark and leaf buds,
and combining them with beeswax and enzymes (2-4).
The active constituents of propolis are determined by the local
flora (5). Bees use propolis to
build and maintain their hive to seal holes and fissures and smooth
the internal walls because of its waxy structure and mechanical
characteristics. Propolis has been an essential part of apitherapy.
More recently, it has also been used as a food additive or
supplement in alternative and traditional medicine (6). Natural products produced by stingless
bees, such as propolis, honey, beehives and pollen exert
pharmacological effects and used as traditional medicine by many
Asian cultures (4). Propolis exerts
antibacterial, antioxidant, antiseptic, anti-inflammatory,
antifungal, hepatoprotective, and immunomodulatory effects
(7). The present systematic review
aimed to analyse the possible usefulness of propolis as a
preventive and therapeutic method based on in vitro and
in vivo research.
Materials and methods
Google Scholar (8),
Science Direct (7), and Web of
Science (WoS) (6), were searched
for studies assessing and reporting the biological activities of
Malaysian propolis. The search strategy was ‘propolis’ AND
‘Malaysian’ for Science Direct and WoS. For Google Scholar, the
‘Advance Search’ option was used to discover papers that contained
the words ‘propolis, Malaysian,’ ‘Malaysian,’ or ‘Malaysia,’ but
not ‘systematic review, meta-analysis, or review.’ Studies on the
biological activities of Malaysian propolis conducted in
vitro and in vivo were included. PRISMA 2020 flow
diagram for new systematic reviews which included searchers of
databases and registers was used (7). Only full-text studies published in
English between January 2012 and June, 2023 were included. Review
papers and research that included meta-analyses were not included
in. The present review did not include any studies on non-Malaysian
propolis. Two authors completed the data extraction, which included
the main author, year, bee species, location, propolis preparation,
study type and biological activities.
Results and Discussion
Database search
WOS is considered the global leading platform for
scientific citation search and analytical information (9). To ascertain whether Google Scholar can
be utilized as a reliable source of scientific information and data
for scientific evaluation, a previous study (10), reviewed 91 comparative articles from
2005 to 2016 that compared Google Scholar with various databases,
particularly WOS, and revealed that Google Scholar is a powerful
database of scholarly literature, having broadened its scope over
the years. PubMed is the most commonly searched database for
systematic reviews (11). However,
Pubmed resulted in a very small number of articles (n=19) in the
present preliminary search and most of the articles were identical
to with the results of Google Scholar, ScienceDirect and Web of
Science. Similar to PubMed Single Citation Matcher, the
ScienceDirect advanced search function allows user to search by
author, title, volume, issue, and page (12). Therefore, the present review
utilized three databases for literature search, namely Google
Scholar, ScienceDirect and Web of Science.
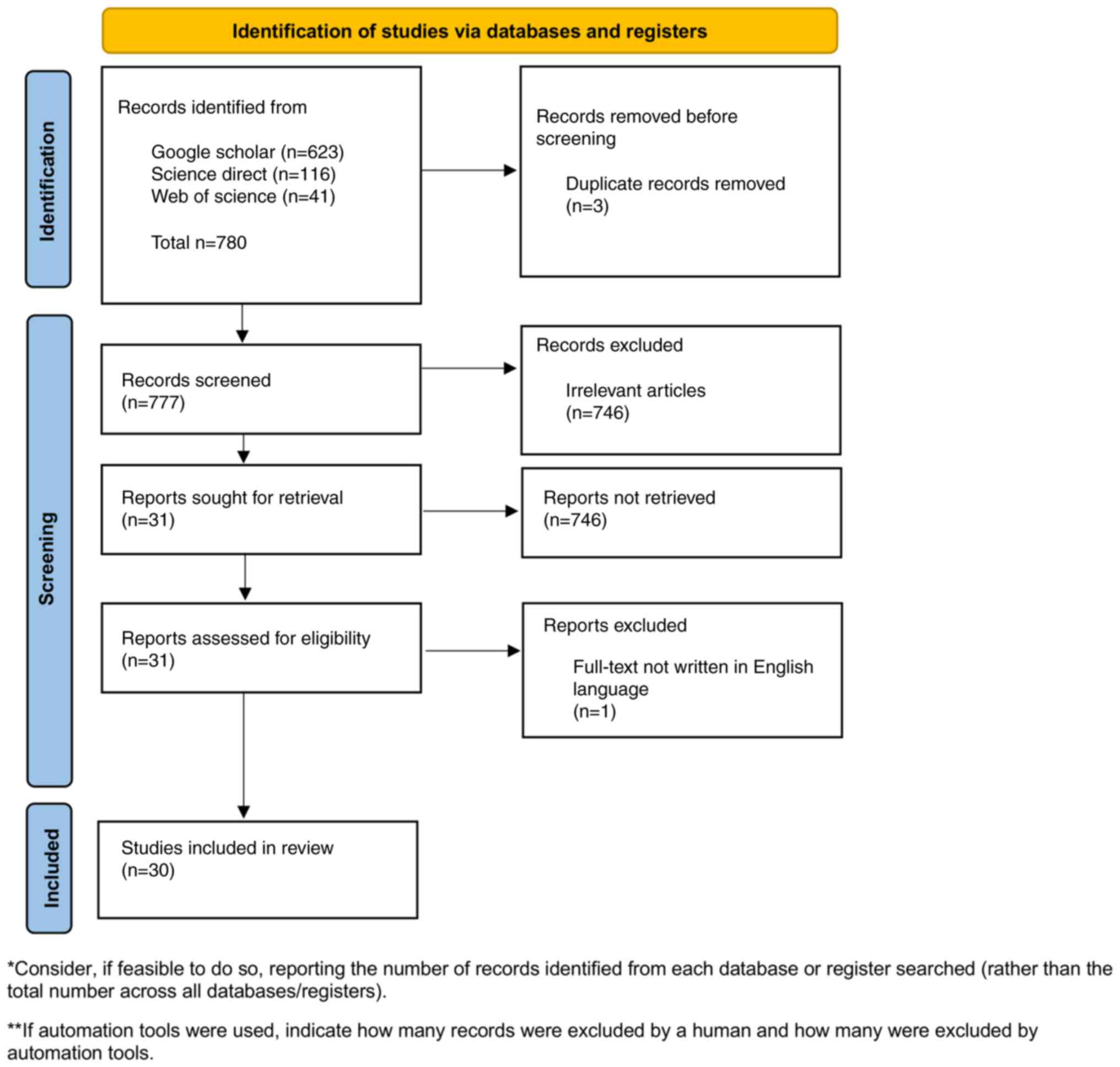
In total, 780 records were identified from the first
primary database search, of which 777 remained after duplicates
were eliminated (Fig. 1). After the
777 titles were filtered, 746 were removed (irrelevant article
title); 31 selected for further inspection and the full texts were
then obtained. Then, 30 entries were chosen from a full-text review
to be included in the review, and one was omitted (non-English
language article). Of these, 23 studies were conducted in
vitro and seven were conducted in vivo or on animals
(Table I).
 | Table IIn vitro and in vivo
biological activity of Malaysian propolis. |
Table I
In vitro and in vivo
biological activity of Malaysian propolis.
| First author/s,
year | Bee species | Location | Propolis
extract | Study type | Biological
activity | (Refs.) |
|---|
| Chew et al,
2014 | N/A | N/A | 2.50% ethanol | In
vitro | Increases stem cell
proliferation | (32) |
| Jacob et al,
2015 | Trigona
spp | Gurun, Kedah | 80.00% ethanol | In
vitro | Enhance the Wound
healing activity against normal human fibroblast cell line
CRL-7522 | (33) |
| Ibrahim et
al, 2015 |
Heterotrigona itama;
Geniotrigona thoracica | Besut,
Terengganu | 100.00%
methanol | In
vitro | Possess
antibacterial activity | (34) |
| Akhir et al,
2017 |
Heterotrigona itama | Parit Botak,
Johor | 100.00% hexane and
70.00% ethanol | In
vitro | Heterotrigona
itama propolis from southern Malaysia have antimicrobial and
antioxidant activity | (35) |
| Yusoff et
al, 2016 | Trigona
spp | N/A | 100.00% water | In
vitro | Weaker antifungal
activity | (29) |
| Rosli et al,
2016 | Trigona
apicalis | N/A | 100.00%
Ethanol | In
vitro | Possess high
antioxidant activity with higher phenolic and flavonoids
contents | (18) |
| Usman et al,
2016 | N/A | Kota Bharu,
Kelantan | 100.00% Water and
ethanol | In
vitro | Exhibited higher
antioxidant activity | (20) |
| Ahmed et al,
2017 | Tetratrigona
spp | Kuband Kerian,
Kelantan | 70.00% ethanol | In vivo | Direct
cytotoxicradical- scavenging activity, which provides
cardioprotective action against ISO-induced oxidative stress. | (15) |
| Azemin et
al, 2017 |
Heterotrigona itama | Terengganu | 100.00%
Ethanol | In
vitro | Unprocessed
propolis has considerably higher antioxidant activity, regardless
of extraction method | (36) |
| Ong et al,
2017 | N/A | Pahang | 100.00% Ethanol and
ethyl acetate | In
vitro | Chitosan-propolis
nano formulation might be considered a possible anti-biofilm agent
in fighting infections | (37) |
| Salim et al,
2018 | Geniotrigona
horacica | Kuala Kangsar,
Perak | 80% ethanol | In
vitro | Active components
in propolis contribute to its antioxidant properties | (16) |
| Lim et al,
2022 |
Heterotrigona itama | Besut, Dungun,
Terengganu; Tanah Merah, Gua Musang, Kelantan | 95% ethanol | In
vitro | Propolis and
metformin combination in reducing histological features of diabetic
cardiomyopathy | (38) |
| Nna et al,
2018 |
Heterotrigona itama | Kelantan | 70% ethanol | In vivo | Reduces hepatic
lesion and has a synergistic protective effect | (39) |
| Usman et al,
2018 |
Heterotrigona itama | Kelantan | 70% ethanol | In vivo | Improvement in
preg- nancy outcomes and placental oxidative stress | (31) |
| Asem et al,
2020 | N/A | Kuala Kangsar,
Perak | 80% ethanol | In
vitro | Demonstrated
positive antioxidant activity | (40) |
| Annisava et
al, 2019 |
Heterotrigona itama | Kelantan;
Terengganu | 100.00%
Ethanol | In
vitro | Contains the
highest total phenolic, flavonoid content and antioxidant
activity. | (41) |
| Nafi et al,
2019 |
Heterotrigona itama,
Geniotrigona thoracica, Lepidotrigona
terminate; Tretrigona apicalis. | N/A | 95% ethanol | In
vitro | Heterotrigona
itama possessed the highest antioxidant activity compared to
other species | (42) |
| Badiazaman et
al, 2019 | Geniotrigona
thoracica | Besut; Dungun,
Terengganu; Tanah Merah, Kelantan; Gua Musang, Kelantan | 100.00%
Methanol | In
vitro | Possessed the
highest total flavonoid content and antioxidant activity | (13) |
| Yusop et al,
2019 | Trigona
itama | Beladin,
Sarawak | 100.00% Hexane,
ethyl acetate and methanol | In
vitro | Antioxidant and
anti- bacterial effects against both gram-positive and
gram-negative bacteria | (30) |
| Nna et al,
2019 |
Heterotrigona itama | Kelantan | 70% ethanol | In vivo | Reduced testicular
oxidative stress, inflam- mation, and apoptosis in diabetic
rats | (43) |
| Mohamed et
al, 2020 | Tetrigona
apicalis | Tanjung Malim,
Perak | 80% ethanol | In
vitro | Demonstrated
antioxi- dant activity and suppressed the prolifera- tion of MCF7
cells | (28) |
| Mohd Suib et
al, 2021 | Geniotrigona
thoracica | Kuala Kangsar,
Perak | 80% ethanol | In
vitro | Inhibition of the
forma- tion of THP-1 derived macrophage foam cells | (26) |
| Zainal et
al, 2022 | Tetrigona
apicalis | Kuantan,
Pahang | 100.00% Water and
70 and 80% ethanol | In
vitro | Exhibited strong
anti- oxidant activity | (44) |
| Syed Salleh et
al, 2021 | Tetrigona
apicalis, Tetrigona binghami;
Homotrigona fimbriata | Selangor | 100.00% Water | In
vitro | Demonstrated
greater antioxidant potential, with higher phenolic and flavonoid
levels | (19) |
| Maroof et
al, 2023 | Geniotrigona
thoracica | Negeri
Sembilan | 100.00%
Ethanol | In
vitro | Increased
antioxidant activity and antibacterial efficacy against gram-
positive bacteria | (22) |
Phytochemicals properties of
propolis
Generally, propolis is rich in various bioactive
compounds, such as fatty, aliphatic and aromatic acids, flavonoids,
terpenoids, sugars, alcohol and esters. The chemical composition of
propolis is affected by its geographical location as well its
botanical origin as the resin from different plant species may
contain various compounds (13). In
Malaysia, 78 stingless bee species have been discovered, including
Geniotrigona thoracica, Heterotrigona itama,
Tetrigona apicalis and Tetragonilla atripes (14). H. itama is the most common
stingless bee species in Malaysia (15). H. itama favours Averrhoa
carambola and Antigonon leptopus because both flowers
produce notable amounts of nectar, and their morphology is
compatible with H. itama tongue morphology (16). Hydrocarbons and oxygenated
sesquiterpenes derivatives, such as β-caryophyllene, copaene,
cyclohexane, 1H-cycloprop[e]azulen-7-ol and β-caryophyllene oxide,
are detected in T. apicalis propolis from Malaysia by gas
chromatography-mass spectrometry (GC-MS) analysis. T.
apicalis propolis also contains triterpenoids, such as α-amyrin
and β-amyrin (17). The GC-MS
chromatographic analysis of G. thoracica propolis from
Malaysia demonstrates presence of phenol, benzoic acid,
trimethylsilyl ester, hydroginkgol, resorcinol, Δ-cadinene,
nootkatone, β-amyrenol, friedelany-al, cycloeucalenol and myristic,
palmitic linoleic and octadecanoic acid (18). A recent study by Syed Salleh et
al (19) revealed the
prevalence of some classes of compounds in T. apicalis,
T. binghami and Homotrigona fimbriata propolis from
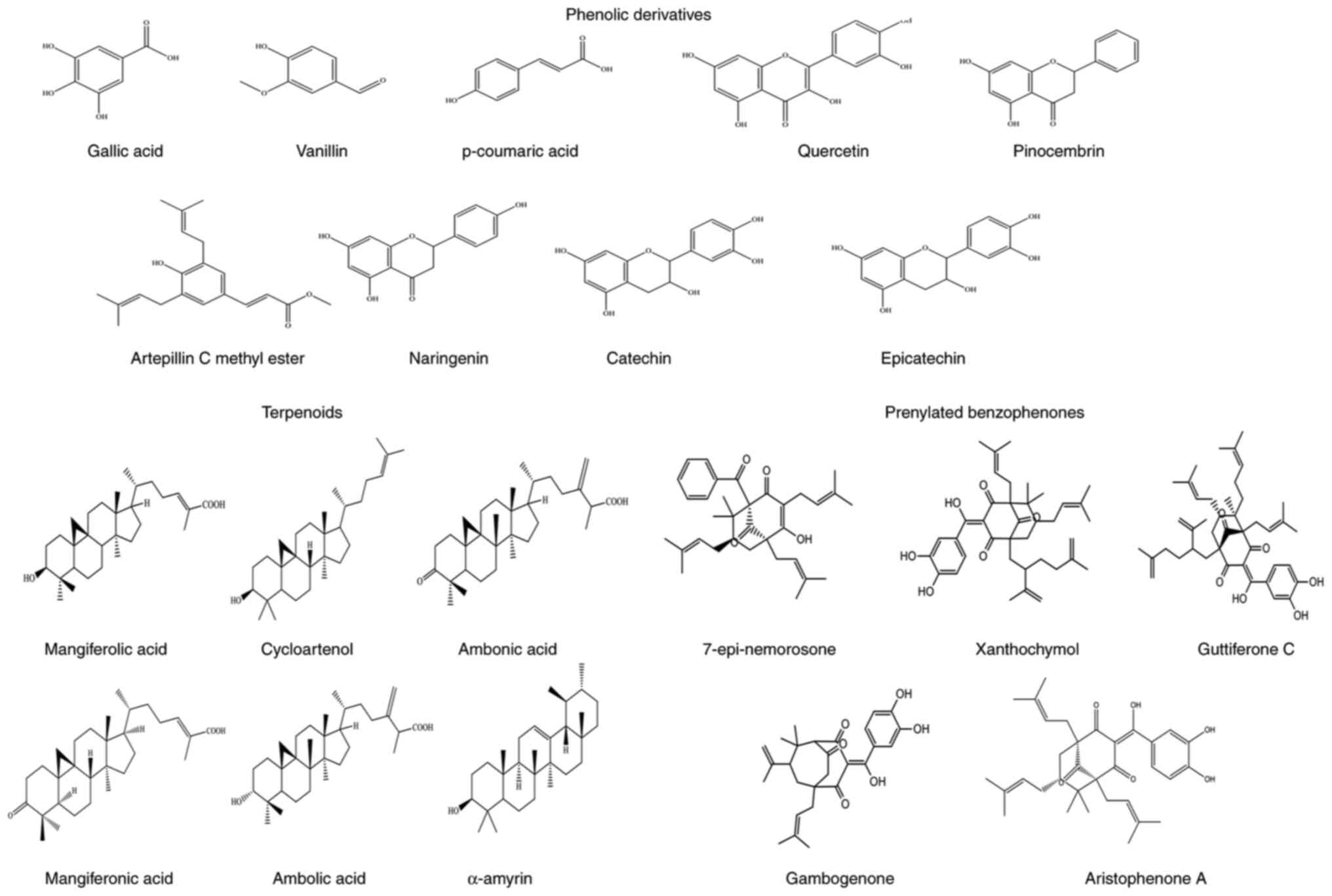
Malaysia. The identified components included phenolic (Gallic acid,
Vanillin, p-coumaric acid, Quercetin, Pinocembrin Artepillin C
methyl ester, Naringenin, Catechin, Epicatechin), terpenoids
(mangiferolic acid, Cycloartenol, Ambonic acid, Mangiferonic acid,
Ambolic acid, amyrin), Prenylated benzophenones (7-epi-nemorosone,
Xanthochymol, Guttiferone C, Gambogenone and Aristophenone A),
carboxylic acids, sugar alcohols, hydrocarbon, aldehydes and amino
acids when analysed using GC-MS. Therefore, Malaysian propolis is
considered as terpenoid-type propolis. The chemical structures of
bioactive compounds in propolis are shown in Fig. 2.
Pharmacological activity and mechanism
of action of propolis. Cytotoxic activity
Cytotoxicity refers to the capacity of a molecule or
compound to result in cellular damage, which may involve damage to
specific cell structures or the essential functions that keep cells
alive, such as cell division, survival and normal physiology
(20). Extracts of H. itama
propolis from different locations possess low to moderate cytotoxic
effects against HeLa cells with half-maximal inhibitory
concentration (IC50) value ranging from 14 to 60 µg/µl
(21). Propolis extract induces
apoptosis in HeLa cells in a dose-dependent manner. Cytotoxic
activities of propolis extract are also species-dependent. Propolis
produced by H. itama, G. thoracica, L.
terminate and T. apicalis have been extracted and
evaluated for their cytotoxicity against three cancer cell lines
(22). H. itama propolis
extract demonstrates the highest cytotoxic activity against
MDA-MB-231, SK-UT-1 and HeLa cells, with IC50 values of
5, 4 and 8 µg/ml, respectively (22). It is proposed that the capacity of
terpenoid compounds to impede proliferation, induce apoptosis and
inhibit metastasis makes them useful against tumours and
inflammation (22). Another study
demonstrated that propolis extract from T. apicalis exerts
cytotoxic activity against breast cancer cell lines in a dose- and
time-dependent manner (17).
IC50 values of T. apicalis propolis extract were
reduced with longer incubation time in MCF7 cells. However, in MCF
10A cells, longer incubation time increased the IC50
values of the propolis extract (17). MCF7 is an Estrogen Receptor,
Progesterone Receptor (PR)-positive, and Human Epidermal Growth
Factor Receptor 2 (HER2)-negative breast cancer cell line while MCF
10A is originally non-tumorigenic cells. The effects of propolis on
cell proliferation of MCF7 and MCF10A were cell type-dependant,
thus the activity is reduced in MCF7 cells and increased in MCF 10A
cells. At incubation up to 72 h, T. apicalis propolis
extract demonstrated selectivity, with a high selectivity index
(SI) of 2.20(17). SI is an
important indicator to evaluate the toxicity of a compound or
extract against normal cells, and to predict their therapeutic
potential on cancer cells (23,24).
FITC Annexin V with flow cytometry is one of the most powerful
tools for quantitative determination of the percentage of cells
that are actively undergoing apoptosis within a population
(25). A previous study utilized
this method to evaluate apoptosis induction by T. apicalis
propolis extract in MCF7 cells (26). At IC50 of 32.70 µg/ml and
72 h incubation with MCF7 cells, the percentage of apoptosis
induction by propolis extract in viable, early and late apoptotic
and necrotic or dead cells corresponded to 48.39±2.06, 14.02±0.98,
35.25±1.16 and 2.34±0.14%, respectively (26). It was suggested that the antioxidant
properties of propolis extract are partly responsible for apoptosis
induction in cancer cells (26).
Apart from cytotoxicity study in mammalian cells, there was also a
study that tested H. itama propolis extracts using the Brine
shrimp lethality test; extracts showed a low level of toxicity
(15).
Antimicrobial activity. An antimicrobial
substance generally eliminates or prevents the growth of bacteria.
Antimicrobial substances can be microbiostatic, which prevents
microbial development, antibacterial, which fights bacteria, or
antifungal, which fights fungi (27). Propolis extract from Trigona
spp. exerts antifungal activities against oral Candida
albicans, C. tropicalis and C. glabrata with
minimal inhibitory concentration (MIC) of 500 mg/ml (28). Limited studies have reported the
antifungal activities of propolis extracts (28,29).
However, there are numerous of studies that evaluated their
antibacterial effects (30,31).
A study utilized ethanol and water as solvents to
extract T. thoracica propolis, then evaluated the
antimicrobial activity of the extracts against Staphylococcus
aureus using broth microdilution method (31). Ethanolic extract of the propolis
exhibited the strongest antimicrobial effect due to its higher
content of phenolic compounds, such as quercetin (31). At 1 mg/ml, H. itama propolis
methanolic extract demonstrated the highest antimicrobial
activities against S. aureus and E. coli, both with
an inhibition zone of 10 mm, in comparison with hexane extract and
ethyl acetate extract (<10 mm) (15).
Antibacterial activity of H. itama propolis
and G. thoracica propolis extracts has been evaluated
against Gram-positive bacteria, such as S. aureus,
Bacillus subtilis, Enterococcus faecalis and
Listeria monocytogen, as well as Gram-negative bacteria,
such as Acinetobacter baumannii, Salmonella typhi and
E. coli (30). It was found
that the extract from H. itama propolis demonstrates better
inhibition against the strains (S. aureus, B.
subtilis, E. faecalis and L. monocytogen, A.
baumannii, S. thyphi and E. coli) with an
inhibition zone of 6-14 mm compared with G. thoracica
propolis extract (6-7 mm) (29).
Both propolis extracts exhibited greater inhibitory effect against
S. aureus (Gram-positive) than E. coli and S.
thyphi (Gram-negative) (30).
The aforementioned study suggested that the antibacterial
activities of propolis were species-dependent and affected by the
polar phenolic compounds of the extracts (30). These results were supported by
another study that reported better antibacterial activity shown by
propolis extract against Gram-positive bacteria (B. cereus
and S. aureus) in comparison with Gram-negative bacteria
(E. coli and Salmonella) (45). Extracts of Acacia mangium and
Garcinia mangostana propolis demonstrated promising
antibacterial effects against S. aureus with an inhibition
zone of 20.00±0.1 and 24.00±0.52 mm, respectively, compared with
erythromycin (24.80±0.72 mm) (35).
However, there are no antibacterial activities shown on
Gram-negative bacteria (E. coli and P. aeruginosa) by
both propolis extracts (35). It is
suggested that the lower susceptibility of Gram-negative bacteria
may be due to lipopolysaccharides of the outer membrane that hinder
the penetration of propolis antibacterial components into bacterial
cells (35).
Antioxidant activity. Oxidative stress is an
imbalance between the production and accumulation of reactive
oxygen species (ROS) and reactive nitrogen species (RNS), and the
capacity to neutralize and eliminate them. Moderate concentrations
of ROS and RNS are key for many physiological processes within the
human body. Key endogenous antioxidant enzymes are superoxide
dismutase (SOD), catalase (CAT) and glutathione peroxidase
(GSH-Px). SOD converts superoxide anion to
H2O2, a substrate for CAT and GSH-Px
(46). When reacting with GSH, CAT
metabolizes H2O2 in water and oxygen while
GSH-Px lowers H2O2 and organic hydroperoxide
levels (46). Several studies have
investigated the antioxidant activity of propolis in in
vitro and animal models (32,47,48).
Propolis from UniSZA Apiary, Besut (BST-1) has the highest total
phenolic and flavonoid content (TPC and TFC, respectively) and
antioxidant activity (35). BST-1
extract with the highest phenolic content had higher antioxidant
activity than the other localities (35). Another study compared ethanolic
extract of propolis samples was produced from three different
stingless bee species, T. apicalis, H. itama and
G. thoracica, collected from bee farms in Perak, Malaysia
(33). Among the species tested,
G. thoracica had the highest antioxidant activity, with
IC50 values of 206.27 for 2,2-diphenyl-1-picrylhydrazyl
(DPPH) and 64.98 mg/ml for
2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) assay,
respectively. This was consistent with another study on A.
mangium and G. mangostana-derived propolis (35). The is no significant difference in
the ABTS+ scavenging effect (A. mangium, 0.05±0.00; G.
mangostana, 0.05±0.00 mg/ml) and metal chelating activity
(A. mangium, 51.44±4.99; G. mangostana, 52.12±1.61
mg/ml) between the two propolis samples. However, SOD enzyme-like
activity is considerably higher in G. mangostana propolis
(0.17±0.01 mg/ml) compared with A. mangium (0.26±0.00 mg/ml)
(35). The ethanolic extracts from
propolis produced by H. itama in Terengganu, Malaysia, have
the strongest antioxidant activity with an IC50 of 30
µg/ml and the highest percentage of inhibition with 85.69%,
followed by G. thoracica with an IC50 of 40 µg/ml
and 82.22% inhibition at 150 µg/ml concentrations. The H.
itama scavenging activity is comparable to quercetin and
trolox, with IC50 values of 10 and 9 µg/ml,
respectively. On the other hand, Lepidotrigona terminate has
poor antioxidant activity with an IC50 of 128 µg/ml and
an inhibition percentage of 80.47% at a dosage of 500 µg/ml. T.
apicalis demonstrates antioxidant activity with IC50
values >500 µg/ml. The differences in antioxidant activity may
be attributed to the phenolic, flavonoid or other components of
propolis extracts, which have been associated with antioxidant
capabilities (22). This finding
was consistent with that of H. itama propolis from Besut,
which had the best antioxidant activity with the lowest
IC50 values (10.000±2.623), followed by Dungun
(84.000±2.623) and Gua Musang (151.000±2.623 µg/ml) (21). The aforementioned experiment
demonstrated that propolis from Besut had the highest antioxidant
activity while propolis from Tanah Merah had the lowest antioxidant
activity of DPPH scavenging radicals.
Soft propolis of H. itama (found inside the
beehive) contains more phytochemicals, specifically flavonoids,
phenols and terpenoids, than hard propolis (which forms part of the
wall of the hive) (47). In the
DPPH assay, the propolis samples demonstrate a dose-dependent
increase in radical scavenging activity, with soft propolis H.
itama exhibiting a significant effect (IC50,
79.90±11.75) compared with hard propolis H. itama
(180.00±16.67 µg/ml) (47). In
addition, at a higher concentration of 1,000 g/ml, soft propolis
H. itama demonstrated stronger H2O2
scavenging activity (53.94±1.88) than hard propolis H. itama
(43.34±0.51%) (47).
The impact of processing and extraction methods on
chemical profiles and antioxidant activity of propolis has been
studied. In comparison with processed propolis (maceration,
sonication, maceration-sonication), unprocessed propolis has more
potent antioxidant activity with the lowest IC50 value.
For the processed sample, raw propolis was heated at 1 h at 37˚C
(42). Meanwhile, the unprocessed
sample was retrieved fresh from the hives (21). Another study reported the
antioxidant activity of G. thoracica propolis from different
locations in Terengganu, Malaysia. It was demonstrated that
propolis from Besut has the lowest IC50 value of 53
µg/ml, whereas propolis from Dungun had the highest IC50
value of 190 µg/ml and propolis from Lundang was inactive. The
stronger the radical scavenging activity, the lower the
IC50 value (22). Thus,
propolis from Besut exerts the strongest antioxidant and radical
scavenging properties. These differences could be attributed to
changes in the chemical composition of the propolis extracts
(49). In addition, the hexane
extract of H. itama propolis from Johor, Malaysia, exhibits
the highest Ferric Reducing Antioxidant Power value of 6.64 mM
Ferrous Equivalent/g). These findings demonstrate that propolis is
a potent natural antioxidative agent (45). In another study, ethanolic extract
of T. apicalis inhibited ABTS+ radical with an
IC50 of 1.68 mg/ml while the positive control (Trolox)
used as a standard reference compound had a lower IC50
of 0.31 mg/ml (17). Another study
demonstrated that the ethanolic extract of G. thoracica
propolis in Perak, Malaysia has an IC50 value of
48.3±0.2 µg/ml using DPPH assay (50).
Furthermore, antioxidant properties of T.
apicalis propolis extract are dose-dependent, with
IC50 value for DPPH test of 4.27 mg/ml (51). The antioxidant properties of
propolis extract are regulated primarily by its phenolic and
flavonoid content. A similar pattern has been identified in which
TPC and TFC concentrations were related to the antioxidant activity
of T. apicalis propolis extract (14). For all extraction solvents, a
significantly high correlation between antioxidant activity and TPC
and TFC has been detected using maceration and ultrasound-assisted
extraction. Furthermore, propolis extracted with 70% ethanol gave
the highest extraction yield and had significantly higher radical
scavenging activity, TPC and TFC than water extract of propolis
(52). In addition, methanol
(IC50, 17.18 µg/ml) extract has the highest percentage
of antioxidants compared with hexane (32.10), ethyl acetate (21.05)
and ascorbic acid (30.63) (52).
In vivo, pre-treatment with propolis
significantly improved SOD, GRx, GPx, and GST enzyme levels in rats
(47). The effects of propolis
supplementation on antioxidant levels and its mode of action in the
aorta of diabetic rats have been examined; the propolis-treated
group showed lower SOD/(CAT + GPx-1) ratios than the control group,
indicating that the propolis has an antioxidative capability in
avoiding hydrogen peroxide accumulation (53). In another animal study, propolis
treatment in diabetic rats led to a significant decrease in the
antioxidant status of pancreatic tissue. Specifically, the
activities of superoxide dismutase (SOD), glutathione peroxidase
(GPx), glutathione S-transferase (GST), glutathione reductase (GR),
and catalase (CAT) were notably reduced compared to the control
group (47).
Anti-inflammatory activity. Inflammation
contributes significantly to the development of cardiovascular
illness and other comorbidities, such as hypertension,
hypercholesterolemia, type 2 diabetes, chronic renal disease and
obesity (34). Another study aimed
to investigate the anti-inflammatory effect and possible mechanisms
of propolis in Sprague Dawley rats models. In vivo,
proliferating cell nuclear antigen and IL-10 increased while
malondialdehyde, NF-κB, TNF-α, IL-1 and cleaved caspase-3 decreased
significantly in the propolis-treated diabetic groups compared with
the diabetic control group (48).
It has been reported that the elevated IL-17 levels are associated
with better outcomes in patients with myocardial infarction (MI)
caused by atherosclerosis (37).
In vitro study also confirmed anti-inflammatory effects;
cytokine secretion of TNF-α and IL-1b in supernatant of treated
THP-1-derived macrophages was measured using ELISA. TNF-α and IL-1b
secretion levels were significantly reduced in THP-1-derived
macrophages treated with both oxidized Low-Density Lipoprotein
(oxLDL) and ethanolic extract of propolis compared with
THP-1-derived macrophages treated with only oxLDL at 6, 24, and 48
h. Ethanol extract of ≤200 ug/ml was used to avoid toxic effect on
THP-1 derived macrophages cells. This finding indicates that
ethanol extract inhibited the release of both of these
pro-inflammatory cytokines (41).
Anti-hyperglycemia and MI activity. Type 2
diabetes mellitus (T2DM) is a type of diabetes characterized by
high blood glucose levels, insulin resistance and a poorer
insulin-stimulated response in the presence of high blood glucose
levels compared with other forms of diabetes (39). In a rat study where diabetes was
induced using intraperitoneal streptozotocin (60 mg/kg), the
effects of propolis extract were investigated. The rats were
administered either propolis alone (300 mg/kg/day), metformin alone
(standard diabetes medication), or a combination of both (DM + M +
P). The study aimed to assess their impact on blood sugar levels.
The results showed significant improvements in glycemic control.
Specifically, the fasting blood glucose (FBG) levels were reduced
to 8.9 (2.7) mM, 11.9 (0.5) mM, and 5.6 (0.8) mM in the
propolis-treated, metformin-treated, and combined treatment groups,
respectively. By contrast, the FBG value in the diabetic group was
substantially higher at 27.0 (5.8) mM (32). Combination treatment of metformin
and propolis results in the highest FBG improvement in comparison
with metformin or propolis treatment alone. In the aforementioned
study, all treatment arms significantly improved
acetylcholine-induced relaxation compared with the DM group.
Another study examined the effects of single oral dose of
metformin, soft propolis H. itama methanol extract (MP) and
their combination on the blood glucose levels of fasting rats over
9 h. MP had no discernible impact on blood sugar levels of normal
rats compared with the control (47). Furthermore, the aforementioned study
also reported that the most potent inhibitor of α-glucosidase is
soft H. itama with lower IC50 value (1.23±0.32
mg/ml) than acarbose (1.48±0.13 mg/ml) (positive control).
Therefore, one of the mechanisms used by soft H. itama to
lower blood sugar levels may involve restricting the digestion of
ingested carbohydrates to prevent glucose absorption. A previous
study examined the impact of propolis, both individually and in
combination with insulin treatment, on the maternal condition,
pregnancy outcomes, and placental oxidative stress in
streptozotocin-induced diabetic rats (29). The final FBG in the propolis-treated
diabetic rat group was comparable to the insulin-treated diabetic
rat group, indicating that propolis and insulin are equally
effective in producing an antihyperglycemic effect (29). Furthermore, the antihyperglycemic
effect was more pronounced in the combined group (propolis +
insulin)-treated diabetic rats compared with the propolis- and the
insulin-treated diabetic rat group groups, indicating that propolis
in combination with insulin produced a more significant
antihyperglycemic effect than propolis or insulin monotherapy
(29). This suggests propolis may
protect against DM-induced poor pregnancy outcomes and placental
oxidative stress, with more significant effects when supplemented
with insulin.
MI, also known as a heart attack, is caused by
disruption in the delivery of blood to heart tissue. Necrosis of
the myocardium occurs because of coronary artery blockage. The
primary cause of myocardium necrosis after MI is an imbalance
between coronary blood supply and myocardial demand (47). Pre-treatment with propolis
significantly decreases levels of creatine Kinase-MB, Lactate
Dehydrogenase, Aspartate Aminotransferase (AST), and Alanine
Aminotransferase (diagnostic markers of MI in rats), Total
Cholesterol (TC), Triglyceride and Very Low-Density Lipoprotein
Cholesterol while increasing the level of High-Density lipoprotein
Cholesterol. Furthermore, compared with control group, rats
receiving prior propolis treatment exhibit substantially decreased
serum cardiac troponin levels (47). Receptor for advanced glycation end
products (RAGE) exists as a full-length receptor that is attached
to the cell membrane. The soluble(s)RAGE isoform is produced by
either alternate splicing of the pre-mRNA (endogenous secretory
RAGE; esRAGE) or shedding of RAGE by sheddase (cleaved RAGE;
cRAGE). The cRAGE portion is large while the esRAGE portion is
minor. sRAGE (cRAGE and esRAGE) binds and sequesters RAGE ligands
or competes with RAGE binding, shielding the cell from the damaging
effects of AGE-RAGE signalling. Serum sRAGE levels were observed to
be greater or lower in people with T2DM (43). Another study reported that
propolis-treated diabetic rats have higher heart/serum esRAGE
levels than diabetic control groups, indicating a higher
concentration of protective decoy receptor esRAGE in the heart
compared with the serum. Because esRAGE binds to excess AGE and
eliminates it, the AGE/esRAGE ratio may be used as a biomarker in
diabetic cardiomyopathy. The combination of propolis and metformin
results in significant cardiac AGE/esRAGE ratio alterations,
implying a synergistic impact in preventing diabetic
cardiomyopathy. The cardioprotective activity of propolis requires
more research into whether propolis directly stimulates esRAGE
formation or indirectly increases esRAGE by lowering AGE, as in
hyperglycemia improvement (40).
Furthermore, combination of propolis and metformin has synergistic
cardioprotective activity in the heart, as demonstrated by lower
cardiac AGE/esRAGE ratio (40).
Wound healing activity. Wound healing is a
dynamic system involving constant cell-cell and cell-matrix
interactions in a succession of overlapping phases, including
haemostasis (blood coagulation cascade), inflammatory,
proliferative, and remodelling (48). Several mediators and cell types
regulate this system, including platelets, inflammatory cells,
fibroblasts, keratinocytes, cytokines, growth factors and matrix
metalloproteinases (48). Propolis
at low concentrations (0.005, 0.125, 0.250 and 0.500 mg/ml)
maintains or increases stem cell proliferation considerably.
Propolis is bioactive and biocompatible at optimal concentrations
and may be used to boost stem cell proliferation in culture media
(44). In a proliferation assay
using propolis, the average number of cells increased, peaking at
500 µg/ml after 48 h and then falling significantly with 1,000
µg/ml (30). This
concentration-dependent pattern is the mechanism by which propolis
influences the proliferation of fibroblast cells. In addition,
propolis at concentrations of 1, 10 and 250 µg/ml results in
significantly faster wound closure than controls, but the other
concentrations have no notable effect. Nonetheless, only the 250
µg/ml concentration demonstrated a significantly higher migration
rate than the control at 30 h. Therefore, propolis showed a
generally positive effect on both assays compared with the control,
and it followed a concentration-dependent curve, with 250 µg/ml
being the most optimal concentration for cell migration and 500
µg/ml for cell proliferation. Doses >500 µg/ml may have toxic
effect on proliferation of fibroblast cells. The proposed modes of
action of propolis on pharmacological activities are illustrated in
Fig. 3. Moreover, to have a better
understanding of safe dosages and beneficial effects, it is crucial
that future research assess the toxicity effect of propolis.
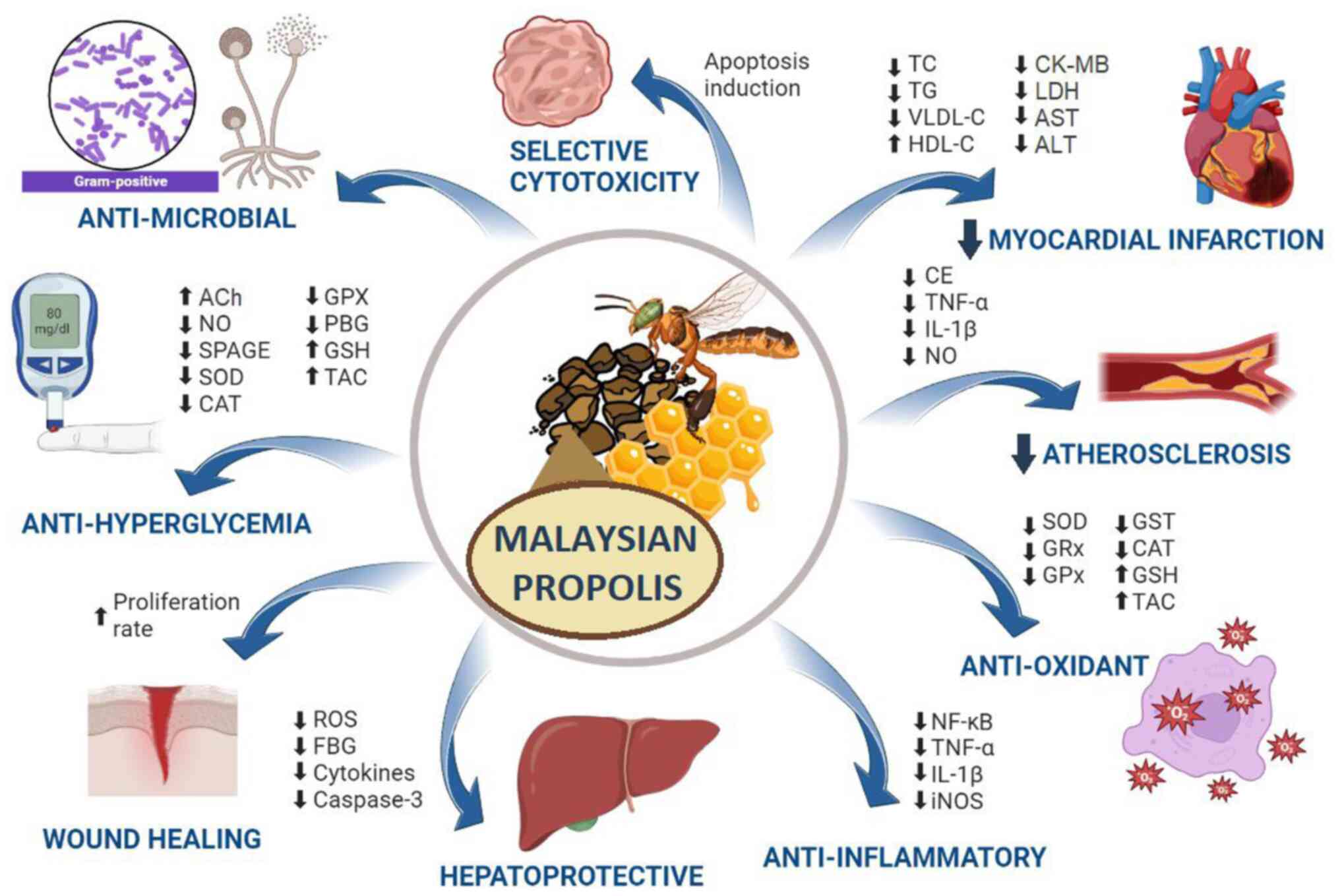 | Figure 3Summary of the proposed mechanism of
action of Malaysian propolis in pharmacological activities such as
antimicrobial, anti-hyperglycemia, wound healing, hepatoprotective,
anti-inflammatory, antioxidant, atherosclerosis, myocardial
infarction and cytotoxicity. ACh, acetylcholine; iNOS, inducible
nitric oxide synthase; SPAGE, spatial gene enhancement; SOD,
superoxide dismutase; CAT, catalase; GPX, glutathione peroxidase;
PBG, postprandial blood glucose; GSH, glutathione; TAC, total
antioxidant capacity; ROS, reactive oxygen species; FBG, fasting
blood glucose; TC, total cholesterol; TG, triglyceride; VLDL-C,
very low-density lipoprotein; HDL-C, high density lipoprotein;
CK-MB, creatine kinase-myocardial band; LDH, lactate dehydrogenase;
AST, aspartate aminotransferase; ALT, alanine aminotransferase; CE,
carcinoembryonic antigen; GRx, glutaredoxin; GPx, glutathione
peroxidase. |
In summary, the present review provided insight into
the therapeutic potential of Malaysian propolis based on in
vitro and in vivo studies. Apart from antioxidant
activity, propolis exhibits antimicrobial, proliferative,
anti-inflammatory, anti-hyperglycaemia, hepatoprotective, wound
healing effects and prevents MI and atherosclerosis. Nonetheless,
this review only summarised propolis activities based on the
limited number of studies available. In the future, more extensive
research and clinical studies, as well as meta-analyses, are
required.
Acknowledgements
The authors acknowledge the financial and technical
support for this Fundamental Research Grant Scheme (FRGS) project
provided by the Ministry of Higher Education of Malaysia
(MOHE).
Funding
Funding: The present study was funded by the Fundamental
Research Grant Scheme (FRGS) provided by the Ministry of Higher
Education of Malaysia (MOHE) (grant no.
FRGS/1/2022/STG02/UNISZA/02/1/).
Availability of data and materials
Not applicable.
Authors' contributions
NAJ, NAM and KSM contributed to the conception of
the study and critically reviewed the article. NAJ wrote and edited
the manuscript and constructed figures. NAJ, NAM and AAMB
contributed to the data acquisition and analysis and drafted the
manuscript. Data authentication is not applicable. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hamzah SA, Zawawi N and Sabri S, Amir
Hamzah AS and Sabri S: A review on the association of bacteria with
stingless bees. Sains Malays. 49:1853–1863. 2020.
|
|
2
|
Syahariza ZA and Kee LS: Antioxidant
activity of stingless bee propolis using different extraction
method. Int J Eng Adv Res. 4:1–15. 2022.
|
|
3
|
Ibrahim N, Niza NFSM, Rodi MM, Zakaria AJ,
Ismail Z and Mohd KS: Chemical and biological analyses of Malaysian
stingless bee propolis extracts. Malays J Anal Sci. 20:413–422.
2016.
|
|
4
|
Guler HI, Tatar G, Yildiz O, Belduz AO and
Kolayli S: Investigation of potential inhibitor properties of
ethanolic propolis extracts against ACE-II receptors for COVID-19
treatment by molecular docking study. Arch Microbiol.
203:3557–3564. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Abdullah NA, Ja'afar F, Yasin HM, Taha H,
Petalcorin MIR, Mamit MH, Kusrini E and Usman A: Physicochemical
analyses, antioxidant, antibacterial, and toxicity of propolis
particles produced by stingless bee Heterotrigona itama
found in Brunei Darussalam. Heliyon. 5(e02476)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Abd Mutalib N, Syed Mohamad SA, Jusril NA,
Hasbullah NI, Mohd Amin MCI and Ismail NH: Lactic acid bacteria
(LAB) and neuroprotection, what is new? An up-to-date systematic
review. Pharmaceuticals (Basel). 16(712)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Justesen T, Freyberg J and Schultz ANØ:
Database selection and data gathering methods in systematic reviews
of qualitative research regarding diabetes mellitus-an explorative
study. BMC Med Res Methodol. 21(94)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li K, Rollins J and Yan E: Web of Science
use in published research and review papers 1997-2017: A selective,
dynamic, cross-domain, content-based analysis. Scientometrics.
115:1–20. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Halevi G, Moed H and Bar-Ilan J:
Suitability of google scholar as a source of scientific information
and as a source of data for scientific evaluation-review of the
literature. J Informetr. 11:823–834. 2017.
|
|
11
|
Colvin J: Electronic resources reviews.
Music Ref Serv Q. 13:122–124. 2010.
|
|
12
|
Bankova V, Popova M and Trusheva B: The
phytochemistry of the honeybee. Phytochemistry. 155:1–11.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Badiazaman AAM, Zin NBM, Annisava AR, Nafi
NEM and Mohd KS: Phytochemical screening and antioxidant properties
of stingless bee Geniotrigona thoracica propolis. Malays J
Fundam Appl Sci. 15:330–335. 2019.
|
|
14
|
Sari DM, Anwar E and Arifianti AE:
Antioxidant and tyrosinase inhibitor activities of ethanol extracts
of brown seaweed (Turbinaria conoides) as lightening ingredient.
Pharmacogn J. 11:379–382. 2019.
|
|
15
|
Ahmed R, Tanvir EM, Hossen MS, Afroz R,
Ahmmed I, Rumpa NE, Paul S, Gan SH, Sulaiman SA and Khalil MI:
Antioxidant properties and cardioprotective mechanism of Malaysian
propolis in rats. Evid Based Complement Alternat Med.
2017(5370545)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Salim NHM, Omar EA, Omar WAW and Mohamed
R: Chemical constituents and antioxidant activity of ethanolic
extract of propolis from Malaysian stingless bee Geniotrigona
thoracica species. Res J Pharm Biol Chem Sci. 9:646–651.
2018.
|
|
17
|
Nna VU, Abu Bakar AB, Md Lazin MRML and
Mohamed M: Antioxidant, anti-inflammatory and synergistic
anti-hyperglycemic effects of Malaysian propolis and metformin in
streptozotocin-induced diabetic rats. Food Chem Toxicol.
120:305–320. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rosli NL, Roslan H, Omar EA, Mokhtar N,
Hapit NHA and Asem N: Phytochemical analysis and antioxidant
activities of Trigona Apicalis propolis extract. AIP Conf Proc.
1791(020018)2016.
|
|
19
|
Syed Salleh SNA, Mohd Hanapiah NA, Ahmad
H, Wan Johari WL, Osman NH and Mamat MR: Determination of total
phenolics, flavonoids, and antioxidant activity and GC-MS analysis
of Malaysian stingless bee propolis water extracts. Scientifica
(Cairo). 2021(3789351)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Usman UZ, Bakar ABA and Mohamed M:
Phytochemical screening and comparison of antioxidant activity of
water and ethanol extract propolis from Malaysia. Int J Pharm Pharm
Sci. 8:413–415. 2016.
|
|
21
|
Mohamed WAS, Ismail NZ, Muhamad M, Omar
EA, Abdul Samad N, Ooi JP and Mohamad S: Q-TOF LC-MS compounds
evaluation of propolis extract derived from Malaysian stingless
bees, Tetrigona apicalis, and their bioactivities in breast
cancer cell, MCF7. Saudi J Biol Sci. 29(103403)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Maroof K, Chen KF, Lee RF, Goh BH,
Mahendra CK, Siow LF and Gan SH: A preliminary study on phenolics,
antioxidant and antibacterial activities of Acacia mangium
and Garcinia mangostana propolis collected by
Geniotrigona thoracica. Food Chem Adv. 2(100255)2023.
|
|
23
|
Nagaraju P, Reddy PN, Padmaja P and Ugale
VG: Synthesis, antiproliferative activity and molecular docking
studies of novel benzo[a]pyrano-[2,3-c]phenazine derivatives.
Chemical Data Collections. 30(100541)2020.
|
|
24
|
Pereira CPM, Souza ACR, Vasconcelos AR,
Prado PS and Name JJ: Antioxidant and anti-inflammatory mechanisms
of action of astaxanthin in cardiovascular diseases (Review). Int J
Mol Med. 47:37–48. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Balica G, Vostinaru O, Stefanescu C,
Mogosan C, Iaru I, Cristina A and Pop CE: Potential role of
propolis in the prevention and treatment of metabolic diseases.
Plants (Basel). 10(883)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mohd Suib MS, Wan Omar WA, Omar EA and
Mohamed R: Ethanolic extract of propolis from the Malaysian
stingless bee Geniotrigona thoracica inhibits formation of
THP-1 derived macrophage foam cells. J Apic Res. 60:478–490.
2021.
|
|
27
|
Mandal BK: Scopes of green synthesized
metal and metal oxide nanomaterials in antimicrobial therapy. In:
Nanobiomaterials in Antimicrobial Therapy. Grumezescu AM (ed). Vol.
6. William Andrew Publishing, Norwich, NY, pp313-341, 2016.
|
|
28
|
Mohamed WAS, Ismail NZ, Omar EA, Abdul
Samad N, Adam SK and Mohamad S: GC-MS evaluation, antioxidant
content, and cytotoxic activity of propolis extract from peninsular
malaysian stingless bees, Tetrigona apicalis. Evid Based
Complement Alternat Med. 2020(8895262)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yusoff NYN, Mohamad S, Abdullah HN and
Rahman NA: Antifungal activity of Malaysian honey and propolis
extracts against pathogens implicated in denture stomatitis. AIP
Conf Proc. 1791(020006)2016.
|
|
30
|
Yusop SATW, Sukairi AH, Sabri WMAW and
Asaruddin MR: Antioxidant, antimicrobial and cytotoxicity
activities of propolis from Beladin, Sarawak stingless bees Trigona
itama extract. Mater Today Proc. 19:1752–1760. 2019.
|
|
31
|
Usman UZ, Bakar ABA and Mohamed M:
Propolis improves pregnancy outcomes and placental oxidative stress
status in streptozotocin-induced diabetic rats. BMC Complement
Altern Med. 18(324)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chew Shi Fung HM, Hashim SNM, Htun AT and
Ahmad A: Proliferative effect of Malaysian propolis on stem cells
from human exfoliated deciduous teeth: An in vitro study.
Methodology. 2014.
|
|
33
|
Jacob A, Parolia A, Pau A and Davamani
Amalraj F: The effects of Malaysian propolis and Brazilian red
propolis on connective tissue fibroblasts in the wound healing
process. BMC Complement Altern Med. 15(294)2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ibrahim N, Zakaria AJ, Ismail Z and Mohd
KS: Antibacterial and phenolic content of propolis produced by two
malaysian stingless bees, Heterotrigona itama and
Geniotrigona thoracica. Int J Pharmacogn Phytochem Res.
8:156–161. 2016.
|
|
35
|
Akhir RAM, Bakar MFA and Sanusi SB:
Antioxidant and antimicrobial activity of stingless bee bread and
propolis extracts. AIP Conf Proc. 1891(020090)2017.
|
|
36
|
Azemin A, Md-Zin NB, Mohd-Rodi MM,
Kim-Chee AS, Zakaria AJ and Mohd KS: Application of metabolite
profiling and antioxidant activity in assessing the quality of
processed and unprocessed stingless bee's propolis. J Fund Appl
Sci. 9:637–660. 2017.
|
|
37
|
Ong TH, Chitra E, Ramamurthy S,
Siddalingam RP, Yuen KH, Ambu SP and Davamani F: Chitosan-propolis
nanoparticle formulation demonstrates anti-bacterial activity
against Enterococcus faecalis biofilms. PLoS One.
12(e0174888)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lim OZ, Yeoh BS, Omar N, Mohamed M, Zin
AAM and Ahmad R: Stingless bee propolis, metformin, and their
combination alleviate diabetic cardiomyopathy. Braz J Pharm Sci.
58(e19652)2022.
|
|
39
|
Nna VU, Bakar ABA and Mohamed M: Malaysian
propolis, metformin and their combination, exert hepatoprotective
effect in streptozotocin-induced diabetic rats. Life Sci.
211:40–50. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Asem N, Abdul Gapar NA, Abd Hapit NH and
Omar EA: Correlation between total phenolic and flavonoid contents
with antioxidant activity of Malaysian stingless bee propolis
extract. J Apic Res. 59:437–442. 2020.
|
|
41
|
Annisava AR, Mohd KS, Nafi NEM, Khadar
ASA, Zin NBM, Pauzi N, Badiazaman AAM and Zakaria AJ: Chemical
profiling and antioxidant activity of Malaysian stingless bee
propolis from ten different locations. Biosci Res. 16:91–104.
2019.
|
|
42
|
Nafi NEM, Zin NBM, Pauzi N, Khadar ASA,
Anisava AR, Badiazaman AAM and Mohd KS: Cytotoxicity, antioxidant
and phytochemical screening of propolis extracts from four
different Malaysian stingless bee species. Malays J Fundam Appl
Sci. 15:307–312. 2019.
|
|
43
|
Nna VU, Abu Bakar AB, Ahmad A, Eleazu CO
and Mohamed M: Oxidative stress, NF-κB-mediated inflammation and
apoptosis in the testes of streptozotocin-induced diabetic rats:
Combined protective effects of Malaysian propolis and metformin.
Antioxidants (Basel). 8(465)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zainal WNHW, Azian NAAM, Albar SS and
Rusli AS: Effects of extraction method, solvent and time on the
bioactive compounds and antioxidant activity of Tetrigona
apicalis Malaysian propolis. J Apic Res. 61:264–270. 2022.
|
|
45
|
Basari N, Ramli SN, Abdul-Mutalid NA,
Shaipulah NFM and Hashim NA: Flowers morphology and nectar
concentration determine the preferred food source of stingless bee,
Heterotrigona itama. J Asia Pac Entomol. 24:232–236.
2021.
|
|
46
|
Ragavi R, Adole PS, Vinod KV and Pillai
AA: Altered expression of a disintegrin and metalloproteinase 10 in
peripheral blood mononuclear cells in type 2 diabetes mellitus
patients with the acute coronary syndrome: A pilot study.
Endocrine. 77:461–468. 2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Mohd-Yazid NA, Zin NBM, Pauzi N and Mohd
KS: Preliminary evaluation of antioxidant and cytotoxic activity of
ethanolic extract of stingless bees propolis from different
localities. J Agrobiotech. 9:132–141. 2018.
|
|
48
|
Ustuner O, Anlas C, Bakirel T, Ustun-Alkan
F, Diren Sigirci B, Ak S, Akpulat HA, Donmez C and Koca-Caliskan U:
In vitro evaluation of antioxidant, anti-inflammatory,
antimicrobial and wound healing potential of Thymus sipyleus
Boiss. subsp. rosulans (borbas) Jalas. Molecules.
24(3353)2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kong YT, Abdullah V, Mokhtar SU and Kutty
RV: Preliminary studies on extraction of propolis using vitamin E
d-α-tocopheryl polyethylene glycol succinate (vitamin E TPGS) and
compare their antimicrobial activities. Malays Journal of
Microbiol. 16:346–352. 2020.
|
|
50
|
Logue SE, Elgendy M and Martin SJ:
Expression, purification and use of recombinant annexin V for the
detection of apoptotic cells. Nature protocols. 4:1383–1395.
2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Misra TN, Singh RS, Upadhyay J and
Srivastava R: Chemical constituents of Vernonia cinerea. Isolation
and structure elucidation of a new pentacyclic triterpenoid. J Nat
Prod. 47:865–867. 1984.
|
|
52
|
León-Mejía G, Guevara AM, Moreno OF and
Cruz CU: Cytotoxicity as a fundamental response to xenobiotics. In:
Cytotoxicity-New Insights into Toxic Assessment. doi:
10.5772/intechopen.96239. Accessed Feb 17, 2021.
|
|
53
|
Yeoh BS, Omar N, Mohammad M, Mokhtar SS
and Ahmad R: Antioxidative propolis from stingless bees
(Heterotrigona itama) preserves endothelium-dependent aortic
relaxation of diabetic rats: The role of nitric oxide and cyclic
guanosine monophosphate. Braz J Pharm Sci. 57(e19187)2021.
|