Introduction
General anesthesia with tracheal intubation is a key
procedure in surgical settings that necessitate effective sedation
and muscle relaxation (1). General
anesthesia, which induces a reversible state of unconsciousness,
analgesia and muscle relaxation, has a rich history dating back to
the 19th century when ether and chloroform were first used. Since
then, numerous advancements have been made, with propofol emerging
as a widely used intravenous anesthetic agent for induction and
maintenance of anesthesia (2,3).
Despite its widespread use, propofol is associated with certain
limitations, including injection pain and dose-dependent
cardiorespiratory depression (4,5). Other
commonly used sedatives, such as midazolam (6), etomidate (7) and dexmedetomidine (8), also demonstrate similar drawbacks.
Remimazolam is an ultra-short-acting intravenous
benzodiazepine derivative and has been approved for sedation during
gastroscopy and colonoscopy (9-11).
It acts on γ-aminobutyric acid type A receptors and is rapidly
metabolized by tissue esterase enzymes into an inactive form
(12). Due to its rapid onset and
offset of action, short recovery time, rare accumulation following
long-term infusion and fewer serious side effects, remimazolam has
been approved by regulatory authorities for general anesthesia as
an alternative to other currently commonly used sedatives (first
approved in January 2020 in Japan and the European Commission in
April 2023) (13,14). Despite these promising attributes,
to the best of our knowledge, there is a lack of comprehensive
evidence comparing remimazolam directly with propofol for general
anesthesia with tracheal intubation. The present meta-analysis
aimed to pool data from eligible studies to provide a comprehensive
evaluation of the comparative efficacy and safety profiles of
remimazolam and propofol for general anesthesia with tracheal
intubation.
Materials and methods
Literature search
A comprehensive search was conducted in databases,
including PubMed (ncbi.nlm.nih.gov/pubmed/), Embase (Embase.com), Cochrane Library (https://www.cochranelibrary.com/), and Web of Science
(https://www.webofscience.com/wos/),
to identify relevant studies. The search period was from the
inception of these databases until February 2024. The search
strategy employed specific keywords such as ‘remimazolam’,
‘propofol’, ‘general anesthesia’, ‘tracheal intubation’,
‘endotracheal intubation’ and ‘control’ (Table I).
 | Table ILiterature search strategy. |
Table I
Literature search strategy.
| A, PubMed |
|---|
| Search number | Query |
|---|
| #1 | Search ‘tracheal
intubation’ [Mesh] |
| #2 | Search ((tracheal
intubation [Title/Abstract]) OR endotracheal intubation
[Title/Abstract]) |
| #3 | #1 OR #2 |
| #4 | Search
(((remimazolam [Title/Abstract]) AND propofol [Title/Abstract]) AND
control [Title/Abstract]) |
| #5 | Search ((anesthesia
[Title/Abstract]) OR general anesthesia [Title/Abstract]) |
| #6 | #3 AND #4 AND
#5 |
| B, Cochrane |
| # 1 | MeSH descriptor:
(tracheal intubation) explode all trees |
| # 2 | ((tracheal
intubation*) OR (endotracheal intubation*)): ti, ab, kw |
| # 3 | #1 OR #2 |
| # 4 | MeSH descriptor:
(remimazolam) explode all trees |
| # 5 | MeSH descriptor:
(propofol) explode all trees |
| # 6 | MeSH descriptor:
(control) explode all trees |
| # 7 | MeSH descriptor:
(anesthesia) explode all trees |
| # 8 | ((anesthesia*) OR
(general anesthesia*)): ti, ab, kw |
| # 9 | #7 OR #8 |
| #10 | #3 AND #4 AND #5
AND #6 AND #9 |
| C, Embase |
| #1 | ‘tracheal
intubation’/exp OR ‘endotracheal intubation’: ti, ab |
| #2 | ‘remimazolam’: ti,
ab AND ‘propofol’: ti,ab AND ‘control’: ti, ab |
| #3 | ‘anesthesia’: ti,
ab OR ‘general anesthesia’: ti,ab |
| #4 | #1 AND #2 AND
#3 |
| D, Web of
science |
| #1 | TS=(tracheal
intubation OR endotracheal intubation) |
| #2 | TI=(remimazolam AND
propofol AND control AND anesthesia) |
| #3 | #1 AND #2 |
Inclusion criteria
Only randomized controlled trials (RCTs) were
considered for inclusion, regardless of whether they implemented
allocation concealment or blinding methods. The intervention group
received remimazolam (group R) as an intravenous anesthetic, either
alone or in combination with analgesics such as sufentanil and
rocuronium, as well as muscle relaxants. The comparison group
received propofol (group P) as intravenous anesthetic and the same
combination of drugs as the remimazolam group. The selected
literature needed to report ≥1 of the following outcome measures:
Time to loss of consciousness (LOC), time to recovery of
consciousness (ROC), time to extubation and occurrence of adverse
reactions such as hypotension, injection pain, bradycardia, nausea
or vomiting and hypoxemia.
Exclusion criteria
Patients who did not undergo endotracheal intubation
were excluded from the study. Additionally, studies without control
groups, literature types such as reviews, case reports or
abstracts, those without full-text availability, lacking relevant
outcome measures or written in languages other than English were
also excluded.
Data extraction and quality
assessment
Two independent investigators performed the
literature screening based on the aforementioned inclusion and
exclusion criteria. Once the eligible studies were identified, data
extraction was conducted using a standardized form. The extracted
information included the first author, publication year, age and
sex distribution of the study population, sample size and details
regarding the anesthesia induction and maintenance protocols in the
experimental and control groups. Any discrepancies were resolved
through discussion.
In accordance with the Cochrane Handbook for
Systematic Reviews of Interventions (Version 5), the Cochrane Risk
of Bias Tool was employed to assess the methodological quality of
the included studies (15). The
risk of bias assessment encompassed random sequence generation,
allocation concealment, blinding, intention-to-treat analysis,
completeness of data, selecting outcome reporting and other
potential biases associated with baseline comparability. The
evaluation was performed independently by two researchers using
Review Manager (Version 5.4; Cochrane Collaboration, 2020.
The present systematic review was conducted
following the Preferred Reporting Items for Systematic Reviews and
Meta-Analyses guidelines (16) and
the protocol was registered in the PROSPERO database (ID no.
CRD42024520840; crd.york.ac.uk/prospero/#myprospero).
Statistical analysis
Data analysis was conducted using R software (4.1.2)
(17). Categorical variables were
assessed using odds ratio (OR) and their corresponding 95%
confidence interval (CI), while continuous variables were analyzed
using mean difference (MD) and their corresponding 95% CI. The
included studies were considered heterogenous based on the guidance
of Cochrane Handbook and a random effect model was used for the
meta-analysis. Publication bias among the included studies was
assessed using funnel plots and Egger's test (18). P<0.05 was considered to indicate
a statistically significant difference.
Results
Literature search
A comprehensive search yielded 638 relevant
articles. After eliminating duplicates, 484 unique articles
remained. Assessment of titles and abstracts led to the exclusion
of 353 studies that were irrelevant to the research question.
Following this initial screening, the full texts of the remaining
131 articles were obtained and subjected to evaluation based on the
predetermined inclusion and exclusion criteria. Consequently, 117
studies were excluded from the final analysis due to reasons such
as being review articles, not adhering to the specified study
design or lacking the requisite outcome variables. Ultimately, 14
articles fulfilled the inclusion criteria and were included in the
present study (Fig. 1).
General characteristics of included
studies
Included studies were published from 2021 to 2023.
The sample sizes of the studies ranged between 40 and 190
participants, aggregating to a total of 1,275 individuals. Among
the included articles, group R comprised 686 cases, while group P
encompassed 598 cases. A comprehensive summary of included studies
is shown in Table II.
 | Table IIGeneral characteristics of
studies. |
Table II
General characteristics of
studies.
| | Sample size | Age, years | Sex, M/F | | Induction
dosage | Maintenance
dosage | |
|---|
| First author,
year | Country | R | P | R | P | R | P | Surgery | R | P | R | P | (Refs.) |
|---|
| Li et al,
2021 | China | 52 | 52 |
48.6±13.8a |
46.8±11.3a | 24/28 | 25/27 | Elective
endotracheal intubation | 0.2 mg/kg | 2.5 mg/kg | 0.2-0.6
mg/kg/h | 4.0-10.0
mg/kg/h | (19) |
| Xu et al,
2023 | China | 30 | 30 |
69.9±4.3a |
68.6±3.3a | 14/16 | 13/17 | Orthopedic | 0.2 mg kg | 1.5 mg/kg | Remifentanil
10.0-15.0 µg/kg/h/1.02.0% sevoflurane | Remifentanil
10.0-15.0 µg/kg/h & 1.0%-2.0% sevoflurane | (20) |
| Gao et al,
2023 | China | 20 | 20 |
67.22±4.4a |
67.2±4.4a | 16/4 | 15/5 | Carotid artery
stenosis | 0.3 mg/kg | 1.5-2.0 mg/kg | Sevoflurane at
adjusted concentration | Sevoflurane at
adjusted concentration | (21) |
| Qiu et al,
2022 | China | 28 | 28 |
62.8±7.1a |
64.7±8.9a | 21/7 | 19/9 | Endoscopic
submucosal dissection | 0.3 mg/kg | 2.0 mg/kg | NR | NR | (22) |
| Tang et al,
2021 | China | 40 | 40 |
54.9±8.5a |
52.7±7.0a | 25/15 | 20/20 | Cardiac | 0.3 mg/kg | 1.5 mg/kg | NR | NR | (23) |
| Song et al,
2023 | South Korea | 40 | 41 |
58.6±6.4a |
60.1±5.2a | 25/15 | 28/13 | NR | 6.0 mg/kg/h | 2.0 mg/kg | 1.0 mg/kg/h | 1.0 mg/kg/h | (24) |
| Choi et al,
2022 | South Korea | 70 | 69 | 39.5
(33.0-48.0)b | 41.0
(37.0-47.0)b | 0/70 | 0/69 | Open
thyroidectomy | 6.0 mg/kg/h | 5.0 µg/ml | 1.0-2.0
mg/kg/h | 2.0-6.0 µg/ml | (25) |
| Shi et al,
2022 | China | 38 | 38 |
52.7±4.9a |
51.6±5.55a | 16/22 | 18/20 | Endoscopic variceal
ligation | 0.2 mg/kg | 2.0 mg/kg | 1.0-2.0
mg/kg/h | NR | (26) |
| So et al,
2023 | South Korea | 42 | 39 | 74.5
(70.0-78.3)b | 76.0
(70.0-81.0)b | 22/20 | 20/19 | Elective
laparoscopic cholecystectomy | 6.0 mg/kg/h | 1.5-2.0 mg/kg | 1.0-2.0
mg/kg/h | 100.0
µg/kg/min | (27) |
| Choi et al,
2023 | South Korea | 48 | 48 |
52.3±9.1a |
52.8±8.2a | 26/22 | 22/26 | NR | 6.0 mg/kg/h | 1.5-2.0 mg/kg | 1.0 mg/kg/h | 3.0-6.0
mg/kg/h | (28) |
| Dai et al,
2021 | China | 142 | 48 |
47.9±13.6a |
52.0±13.7a | 86/56 | 26/22 | Elective | 0.2-0.4 mg/kg | 2.0 mg/kg |
Sufentanil/remifentanil 6.0-8.0 mg/kg |
Sufentanil/remifentanil 6.0-8.0 mg/kg | (29) |
| Kuang et al,
2023 | China | 42 | 42 |
65.4±3.9a |
65.2±4.4a | 19/23 | 20/22 | Lobectomy | 0.3 mg/kg | 2.0 mg/kg | 0.6-1.2
mg/kg/h | 2.0-10.0
mg/kg/h | (30) |
| Liu et al,
2021 | China | 30 | 30 |
54.9±11.3a |
50.6±10.5a | 14/16 | 17/13 | Valve
replacement | 1.8 mg/kg/h | 2.5 µg/ml | Sufentanil 0.2
µg/kg/h/dexmedetomidine 0.5 µg/kg/h | Sufentanil 0.2
µg/kg/h/dexmedetomidine 0.5 µg/kg/h | (31) |
| Mao et al,
2022 | China | 64 | 64 |
52.5±17.5a |
50.0±25.8a | 41/23 | 45/19 | Urological | 0.2-0.3 mg/kg | 2.0-3.0 mg/kg | 1.0-2.0
mg/kg/h | 4.0-10.0
mg/kg/h | (32) |
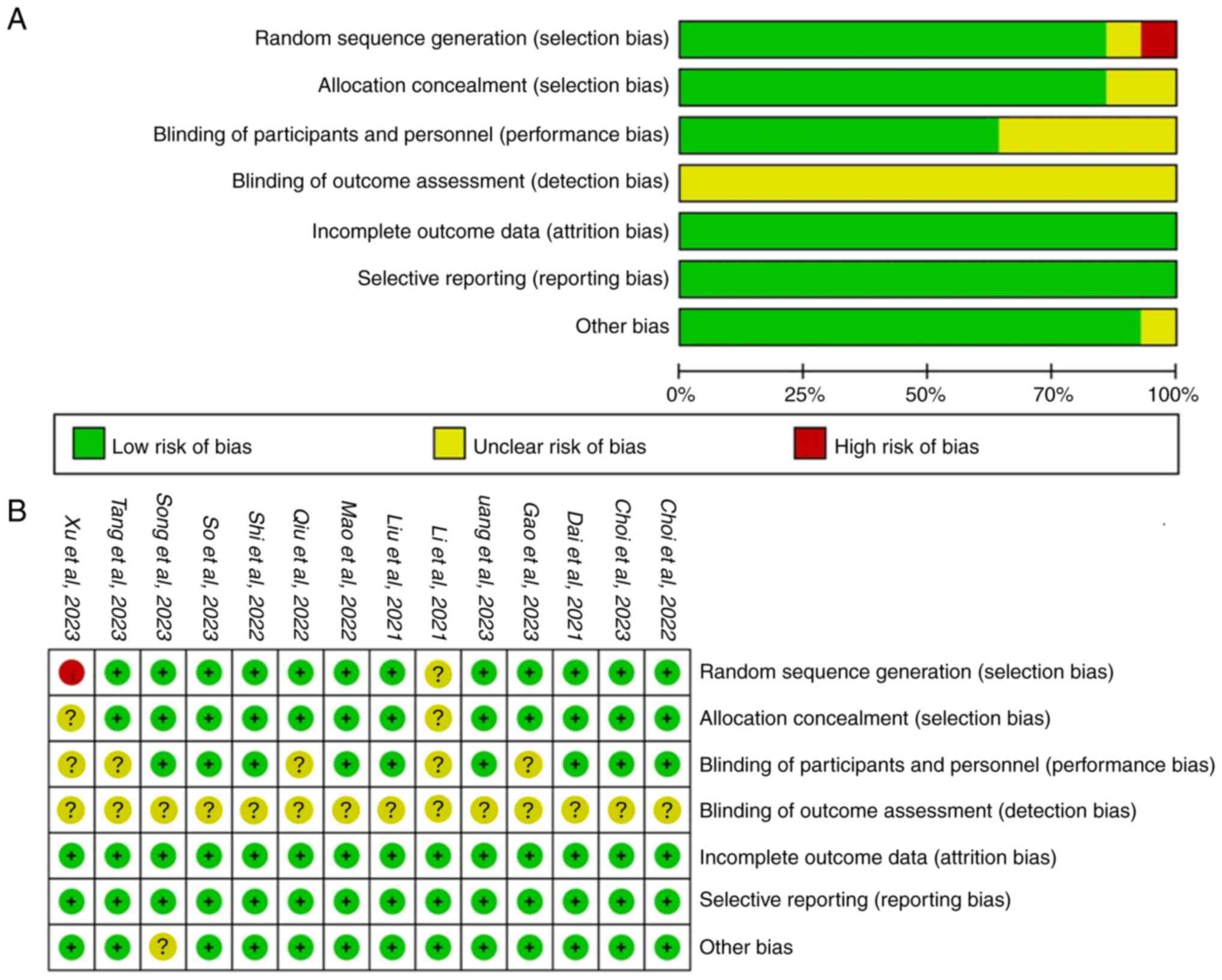
Quality assessment of included
studies
All 14 included articles were RCTs (Fig. 2). However, there were instances of
unclear and high risk of bias. One study had unclear randomization
(19) and another used admission
order for random allocation (20),
posing a high risk of bias. Two studies had unclear allocation
concealment (19,20), while five did not report blinding,
resulting in an unclear risk (19-23).
None of the studies mentioned intention-to-treat analysis and one
study did not address baseline comparability (24), both of which were categorized as
unclear risk. Overall, all included studies had some degree of
bias, with uncertain impact on the reliability and stability of the
combined results.
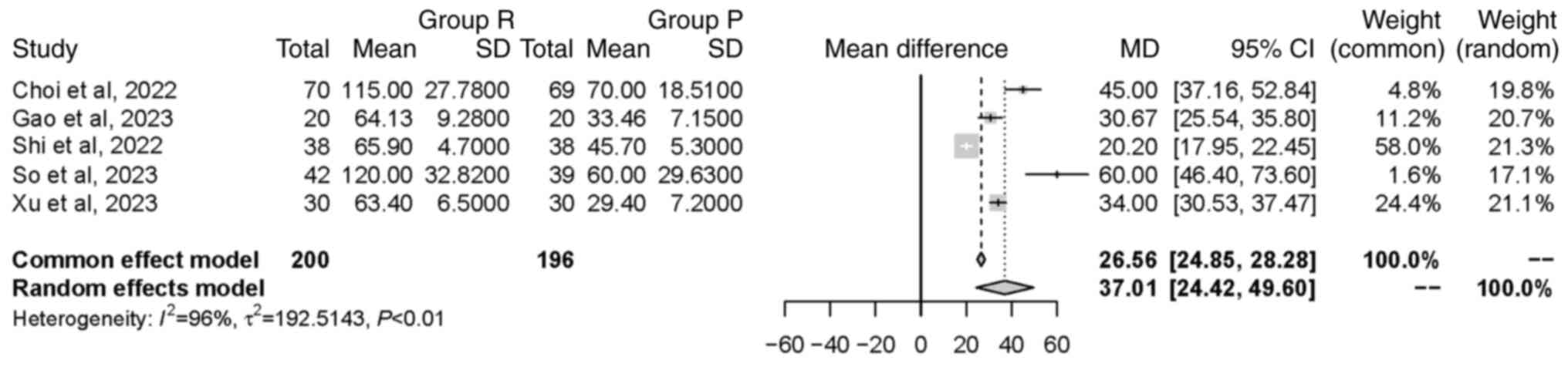
Meta-analysis. Time to LOC
A total of five studies (20,21,25-27),
involving a total of 396 patients, investigated the time to LOC.
Significant heterogeneity was observed between the included studies
(P<0.01; I2=96%). The random effects model revealed
that group R had a significantly longer time to LOC compared with
group P [MD=37.01 sec, 95% CI (24.42, 49.60 sec), P<0.0001].
This indicated that the intervention in group R led to delayed
onset of unconsciousness (Fig.
3).
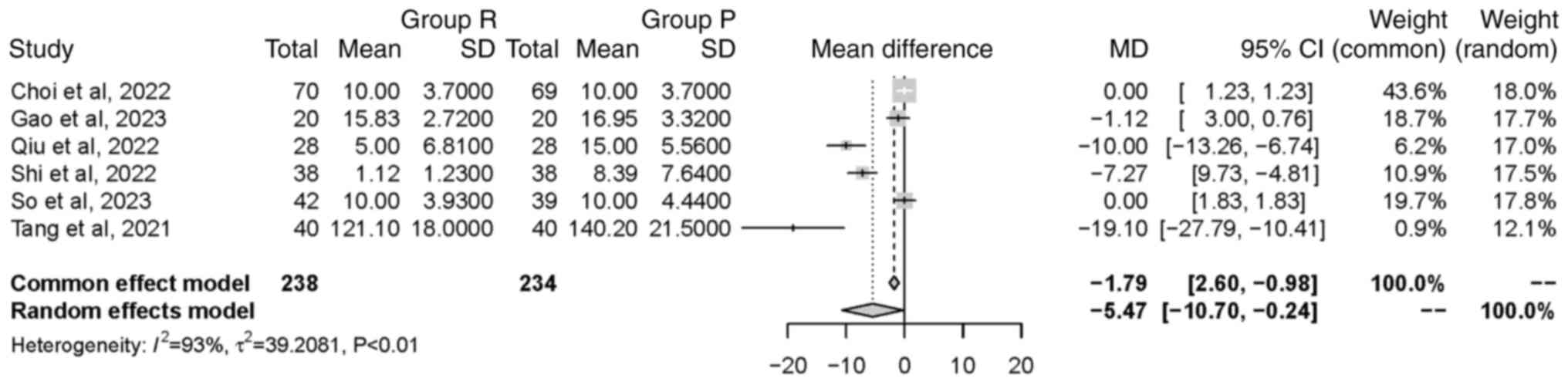
Time to ROC. A total of six studies (21-23,25-27),
involving a total of 472 patients, examined the time to ROC.
Significant heterogeneity was found between the included studies
(P<0.01; I2=93%). The random effects model revealed
that group R had a significantly shorter time to ROC compared with
group P [MD=-5.47 min, 95% CI (-10.70, -0.24 min), P=0.04]. This
indicated that the intervention in group R led to a faster ROC
(Fig. 4).
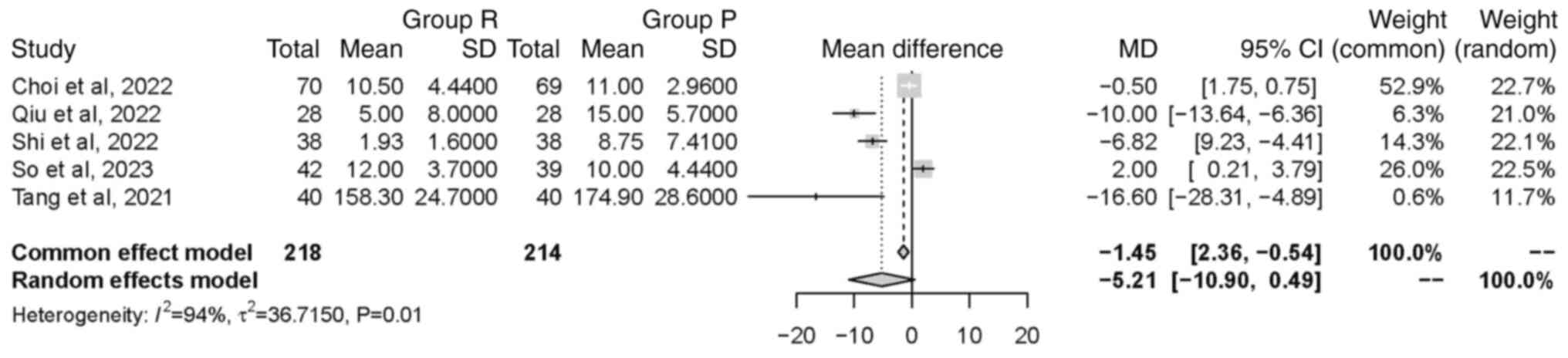
Time to extubation. A total of five studies
(22,23,25-27),
comprising a total of 432 patients, examined the time to
extubation. Significant heterogeneity was found between the
included studies (P<0.01; I2=94%). The random effects
model indicated no significant difference in time to extubation
between groups [MD=-5.21 min, 95% CI (-10.90, 0.49 min), P=0.07].
This finding suggested that the intervention in group R did not
have a significant impact on the duration of extubation (Fig. 5).
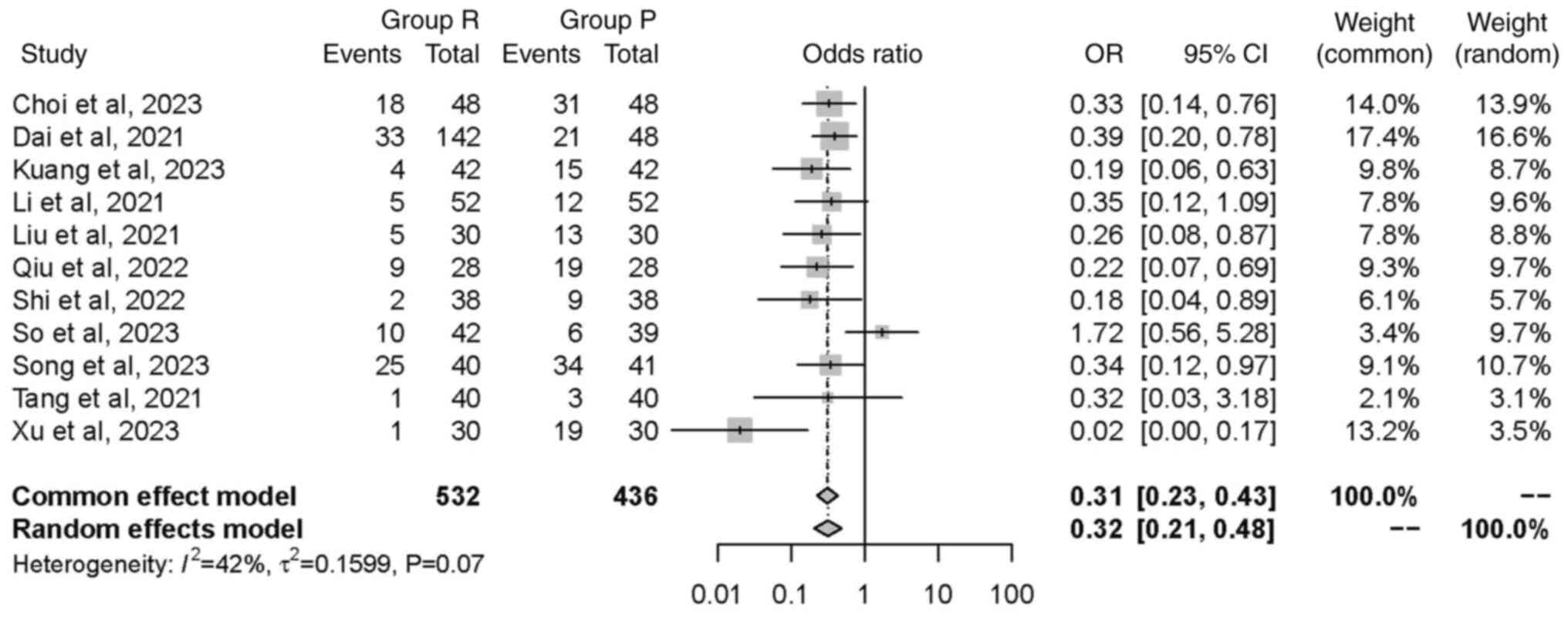
Hypotension. A total of 11 studies (19,20,22-24,27-32),
comprising a total of 968 patients, investigated the incidence of
hypotension. Although the studies showed relatively low
heterogeneity (P=0.07; I2=42%), a random effects model
was used and found a significant reduction in the incidence of
hypotension in group R compared with group P [OR=0.32, 95% CI
(0.21, 0.48), P<0.0001; Fig.
6].
Injection pain. A total of four studies
(19,20,22,29),
comprising a total of 410 patients, investigated the incidence of
injection pain. Although the studies showed minimal heterogeneity
(P=0.78; I2=0%), a random effects model was used in the
meta-analysis and found a significant reduction in the incidence of
injection pain in group R compared with group P [OR=0.02, 95% CI
(0.01, 0.08), P<0.0001; Fig.
7].
Bradycardia. A total of four studies
(27,28,30,32),
involving a total of 321 patients, examined the incidence of
bradycardia. Although the studies showed minimal heterogeneity
(P=0.46; I2=0%), a random effects model was used in the
meta-analysis. The incidence of bradycardia in group R was
significantly lower compared with group P [OR=0.26, 95% CI (0.11,
0.58), P=0.001; Fig. 8].
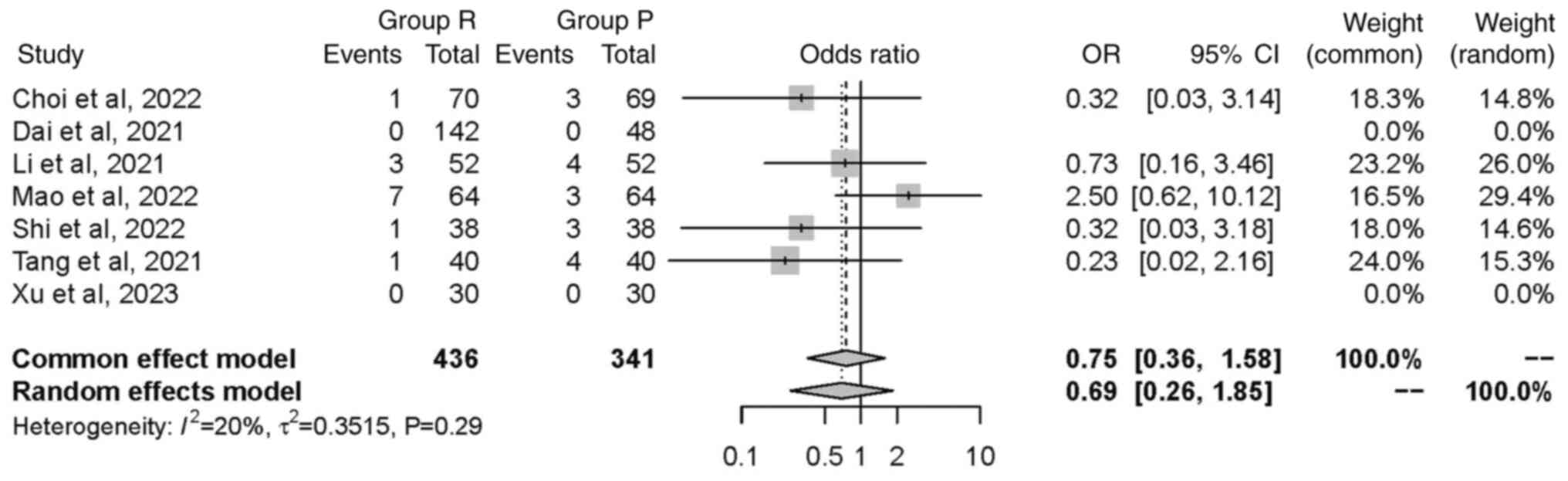
Nausea or vomiting. A total of seven studies
(19,20,23,25,26,29,32),
including a total of 777 patients, investigated the incidence of
nausea or vomiting. Although the studies demonstrated a low
heterogeneity (P=0.29; I2=20%), a random effects model
was used. No significant difference in the occurrence of nausea or
vomiting was found between groups [OR=0.69, 95% CI (0.26, 1.85),
P=0.46]. This suggested intervention in group R did not have a
significant effect on the incidence of nausea or vomiting (Fig. 9).
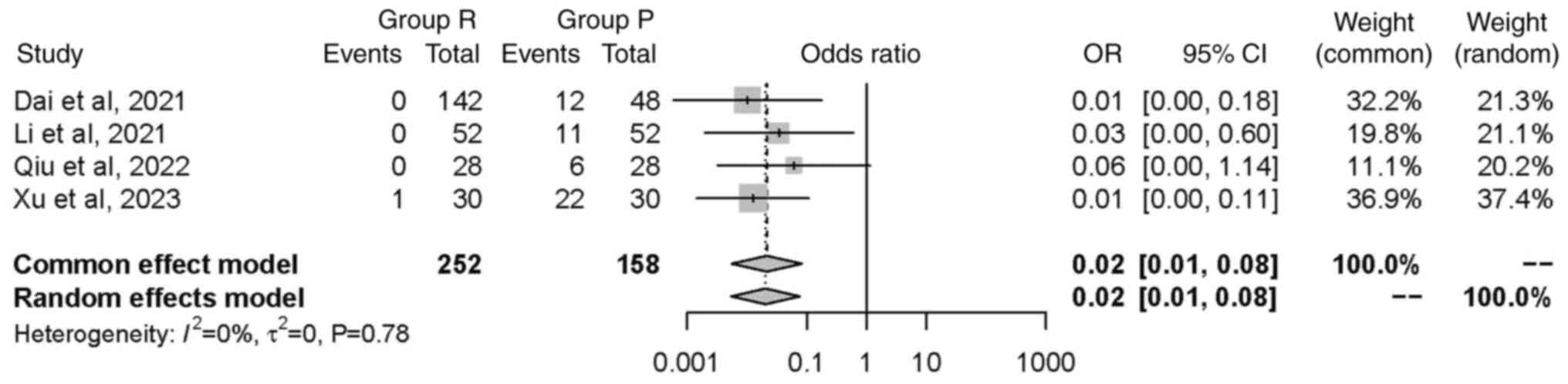
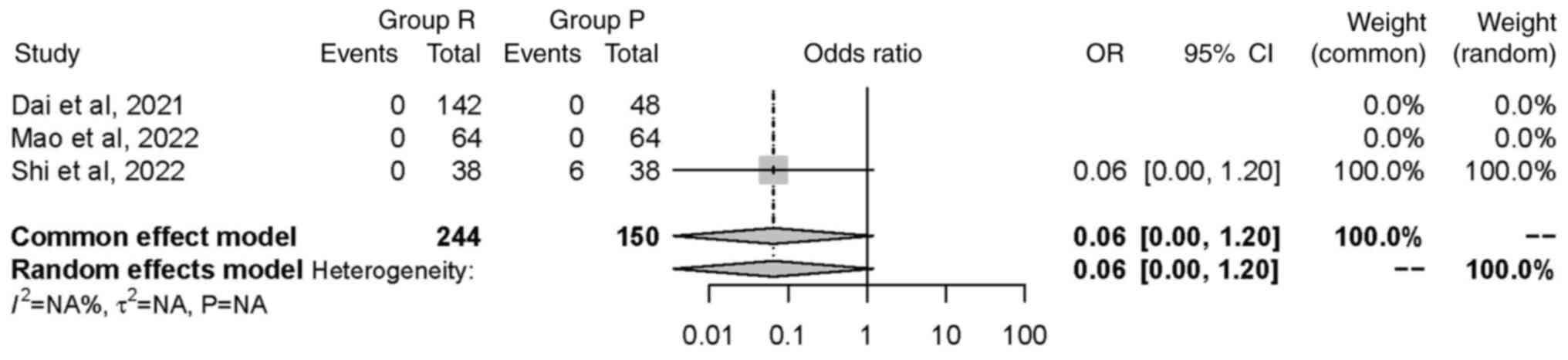
Hypoxemia. A total of three studies (26,29,32),
including a total of 394 patients, investigated the incidence of
hypoxemia; hypoxemia was reported in one study. The random effects
model was used and did not find any significant difference in
occurrence of hypoxemia between groups [OR=0.06, 95% CI (0.00,
1.20), P=0.07]. This suggested that the intervention in group R did
not have a statistically significant impact on the incidence of
hypoxemia (Fig. 10).
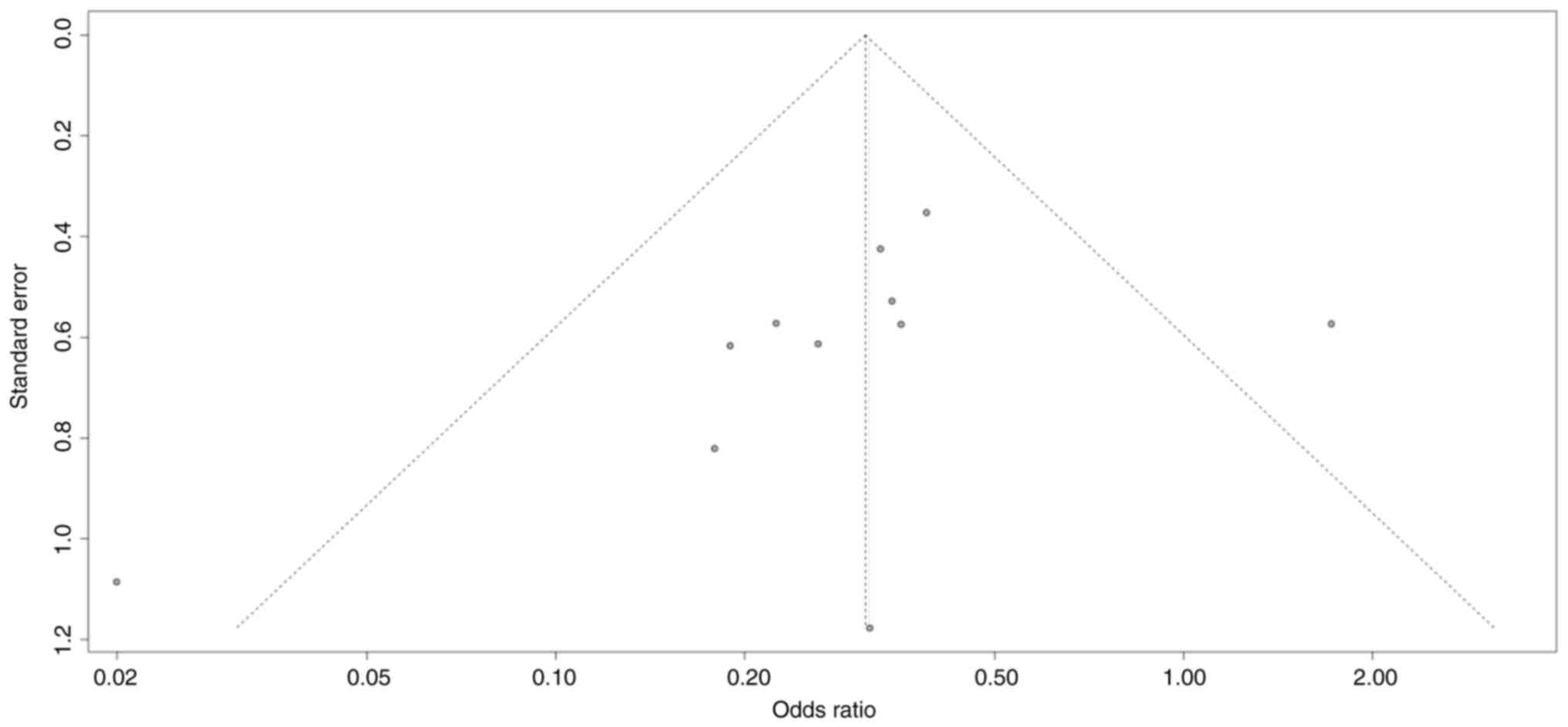
Publication bias
Hypotension was reported in 11 studies as the most
frequently studied outcome (19,20,22-24,27-32).
A funnel plot was used to assess publication bias for hypotension
and showed an asymmetrical scatter distribution (Fig. 11). Utilizing Egger's test for
quantitative analysis, the results yielded a non-significant P
value of 0.19, indicating no significant publication bias in the
studies.
Discussion
The present meta-analysis assessed the efficacy and
safety of remimazolam over propofol as an anesthetic agent in
general anesthesia with tracheal intubation and supported the
superiority of remimazolam. Compared with propofol, general
anesthesia with tracheal intubation on remimazolam led to a
reduction in time to ROC by 5.47 min and an increase in time to LOC
by 37 sec. Although no significant difference was found in time to
extubation, remimazolam still demonstrated a trend towards a
shorter time to extubation. These outcomes suggested that
remimazolam may facilitate a more favorable recovery profile, which
is key in clinical settings where rapid patient recovery is
essential.
Prior to the emergence of remimazolam, both
intravenous and volatile anesthetic agents used in general
anesthesia exerted cardiovascular depressant effects, which could
result in severe low cardiac output and bradycardia in patients
with impaired cardiac function. Here, remimazolam was associated
with a significantly lower incidence of hypotension, bradycardia
and injection pain. Specifically, the incidence of hypotension was
reduced by 69% and bradycardia by 75% in patients administered
remimazolam compared with those given propofol. Additionally, the
incidence of injection pain was markedly lower in the remimazolam
group, indicating an advantage for patients sensitive to injection
discomfort.
The present findings align with previous studies
that highlighted the stable hemodynamic properties of remimazolam
(13,33). For example, Nakayama et al
(34) emphasized the minimal
cardiovascular depression associated with remimazolam, which is
particularly beneficial for elderly and critically ill patients.
Furthermore, its metabolism independent of organ function renders
it suitable for patients with hepatic or renal impairment (12,33).
It also decreases surgical stress response and respiratory
depression without significant myocardial depression, resulting in
fewer anesthesia-associated adverse reactions (23,31,35).
However, in patients routinely taking long-term
angiotensin-converting enzyme inhibitors or angiotensin receptor
blockers, remimazolam has high incidence of hypotensive events (up
to 62.5%, compared with 82.9% for propofol) during induction and
maintenance of general anesthesia. Therefore, further evaluation of
the potential adverse events of remimazolam in specific patient
populations is still needed.
Rapid recovery time and superior safety profile of
remimazolam could enhance patient throughput and decrease
postoperative monitoring requirements, thereby improving surgical
workflow and patient outcomes. Unlike propofol which does not have
a specific antagonist and may lead to unpredictable delayed
recovery following general anesthesia, the effects of remimazolam,
as a benzodiazepine, can be completely reversed by flumazenil. The
availability of flumazenil as a specific antagonist for remimazolam
provides an added safety measure, although its routine use should
be approached with caution due to potential re-sedation risks. This
is because flumazenil only competitively antagonizes
GABAA receptors, and as the plasma concentration of
flumazenil decreases, the sedative effect of remimazolam may
reappear (36,37). It is important to continue
monitoring patients for a sufficient duration after administering
flumazenil.
The present study has limitations that should be
acknowledged. The visible differences in remimazolam and propofol
make it challenging to conduct a double-blind clinical trial, which
may introduce potential bias. Variability in surgical procedures
and dosages between studies may also contribute to heterogeneity.
While Egger's test did not indicate significant bias, this does not
entirely rule out the possibility of bias affecting the results.
The asymmetry in the funnel plot should be interpreted cautiously.
Further sensitivity analyses on age, sex and dosage and stricter
requirements on study quality can provide additional insights into
the robustness of the conclusions drawn from the present
meta-analysis. In addition, the studies included in the present
meta-analysis were conducted in Asian countries. This may limit the
generalizability of the findings to other populations.
In conclusion, remimazolam is a safer and more
effective alternative to propofol for general anesthesia with
tracheal intubation, with lower risk of adverse events such as
hypotension, bradycardia and injection pain and a shorter time to
ROC.
Acknowledgements
The authors would like to thank Dr Mengru Zhang
(Hull York Medical School, Shanghai, China) for assistance with
protocol registration in the PROSPERO database and Mr Sam Morice
(Castle Hill Hospital, Hull, United Kingdom) for language
editing.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HL and ZT conceived and designed the study,
performed the literature review and analyzed and interpreted data.
HL wrote the manuscript. HL and ZT confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wojciechowski PJ: 17-General Anesthesia.
In: Stehr W, editor. The mont reid surgical handbook (sixth
edition). Philadelphia: W.B. Saunders 181-191, 2008.
|
|
2
|
Chau PL: New insights into the molecular
mechanisms of general anaesthetics. Br J Pharmacol. 161:288–307.
2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mahmoud M and Mason KP: Recent advances in
intravenous anesthesia and anesthetics. F1000Res.
7(F1000)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Propofol. In: Aronson JK, editor. Meyler's
side effects of drugs (sixteenth edition). Oxford: Elsevier;
988-1016, 2016.
|
|
5
|
Sahinovic MM, Struys MMRF and Absalom AR:
Clinical pharmacokinetics and pharmacodynamics of propofol. Clin
Pharmacokinet. 57:1539–1558. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Conway A, Chang K, Mafeld S and Sutherland
J: Midazolam for sedation before procedures in adults and children:
A systematic review update. Syst Rev. 10(69)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kotani Y, Piersanti G, Maiucci G, Fresilli
S, Turi S, Montanaro G, Zangrillo A, Lee TC and Landoni G:
Etomidate as an induction agent for endotracheal intubation in
critically ill patients: A meta-analysis of randomized trials. J
Critical Care. 77(154317)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Khare A, Sharma SP, Deganwa ML, Sharma M
and Gill N: Effects of dexmedetomidine on intraoperative
hemodynamics and propofol requirement in patients undergoing
laparoscopic cholecystectomy. Anesth Essays Res. 11:1040–1045.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rex DK, Bhandari R, Desta T, DeMicco MP,
Schaeffer C, Etzkorn K, Barish CF, Pruitt R, Cash BD, Quirk D, et
al: A phase III study evaluating the efficacy and safety of
remimazolam (CNS 7056) compared with placebo and midazolam in
patients undergoing colonoscopy. Gastrointest Endosc.
88:427–437.e6. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pambianco DJ, Borkett KM, Riff DS, Winkle
PJ, Schwartz HI, Melson TI and Wilhelm-Ogunbiyi K: A phase IIb
study comparing the safety and efficacy of remimazolam and
midazolam in patients undergoing colonoscopy. Gastrointest Endosc.
83:984–992. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Borkett KM, Riff DS, Schwartz HI, Winkle
PJ, Pambianco DJ, Lees JP and Wilhelm-Ogunbiyi K: A phase IIa,
randomized, double-blind study of remimazolam (CNS 7056) versus
midazolam for sedation in upper gastrointestinal endoscopy. Anesth
Analg. 120:771–780. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Goudra BG and Singh PM: . Remimazolam: The
future of its sedative potential. Saudi J Anaesth. 8:388–391.
2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kilpatrick GJ: Remimazolam: Non-clinical
and clinical profile of a new sedative/anesthetic agent. Front
Pharmacol. 12(690875)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hu Q, Liu X, Wen C, Li D and Lei X:
Remimazolam: An updated review of a new sedative and anaesthetic.
Drug Des Devel Ther. 16:3957–3974. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Doi M, Morita K, Takeda J, Sakamoto A,
Yamakage M and Suzuki T: Efficacy and safety of remimazolam versus
propofol for general anesthesia: A multicenter, single-blind,
randomized, parallel-group, phase IIb/III trial. J Anesth.
34:543–553. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
R Core Team. The R Project for Statistical
Computing [updated 14/06/2024. Available from: https://www.r-project.org/.
|
|
18
|
Lin L and Chu H: Quantifying publication
bias in meta-analysis. Biometrics. 74:785–794. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li J, Zhou D, Jin Y, Zhou HS, Fang CL, Zhu
ZQ and Xiong LL: Difference between remimazolam toluenesulfonic
acid and propofol in waking quality and conscious state after
general anesthesia. Ibrain. 7:171–180. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xu Q, Wu J, Shan W, Duan G and Lan H:
Effects of remimazolam combined with sufentanil on hemodynamics
during anesthetic induction in elderly patients with mild
hypertension undergoing orthopedic surgery of the lower limbs: A
randomized controlled trial. BMC Anesthesiol.
23(311)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gao J, Yang C, Ji Q and Li J: Effect of
remimazolam versus propofol for the induction of general anesthesia
on cerebral blood flow and oxygen saturation in elderly patients
undergoing carotid endarterectomy. BMC Anesthesiol.
23(153)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Qiu Y, Gu W, Zhao M, Zhang Y and Wu J: The
hemodynamic stability of remimazolam compared with propofol in
patients undergoing endoscopic submucosal dissection: A randomized
trial. Front Med. 9(938940)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tang F, Yi JM, Gong HY, Lu ZY, Chen J,
Fang B, Chen C and Liu ZY: Remimazolam benzenesulfonate anesthesia
effectiveness in cardiac surgery patients under general anesthesia.
World J Clin Cases. 9:10595–10603. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Song SW, Kim S, Park JH, Cho YH and Jeon
YG: Post-induction hypotension with remimazolam versus propofol in
patients routinely administered angiotensin axis blockades: A
randomized control trial. BMC Anesthesiol. 23(219)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Choi JY, Lee HS, Kim JY, Han DW, Yang JY,
Kim MJ and Song Y: Comparison of remimazolam-based and
propofol-based total intravenous anesthesia on postoperative
quality of recovery: A randomized non-inferiority trial. J Clin
Anesth. 82(110955)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shi F, Chen Y, Li H, Zhang Y and Zhao T:
Efficacy and safety of remimazolam tosilate versus propofol for
general anesthesia in cirrhotic patients undergoing endoscopic
variceal ligation. Int J Gen Med. 15:583–591. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
So KY, Park J and Kim SH: Safety and
efficacy of remimazolam for general anesthesia in elderly patients
undergoing laparoscopic cholecystectomy: A randomized controlled
trial. Front Med (Lausanne). 10(1265860)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Choi EK, Jang Y and Park SJ: Comparison of
remimazolam and propofol induction on hemodynamic response in
hypertensive patients. Medicine (Baltimore).
102(e34358)2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dai G, Pei L, Duan F, Liao M, Zhang Y, Zhu
M, Zhao Z and Zhang X: Safety and efficacy of remimazolam compared
with propofol in induction of general anesthesia. Minerva
Anestesiol. 87:1073–1079. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kuang Q, Zhong N, Ye C, Zhu X and Wei F:
Propofol versus remimazolam on cognitive function, hemodynamics,
and oxygenation during one-lung ventilation in older patients
undergoing pulmonary lobectomy: A randomized controlled trial. J
Cardiothorac Vasc Anesth. 37:1996–2005. 2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu T, Lai T, Chen J, Lu Y, He F, Chen Y
and Xie Y: Effect of remimazolam induction on hemodynamics in
patients undergoing valve replacement surgery: A randomized,
double-blind, controlled trial. Pharmacol Res Perspect.
9(e00851)2021.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Mao Y, Guo J, Yuan J, Zhao E and Yang J:
Quality of recovery after general anesthesia with remimazolam in
patients' undergoing urologic surgery: A randomized controlled
trial comparing remimazolam with propofol. Drug Des Devel Ther.
16:1199–209. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kim KM: Remimazolam: Pharmacological
characteristics and clinical applications in anesthesiology. Anesth
Pain Med. 17:1–11. 2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Nakayama J, Ogihara T, Yajima R, Innami Y
and Ouchi T: Anesthetic management of super-elderly patients with
remimazolam: A report of two cases. JA Clin Rep.
7(71)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Satoh T, Nishihara N, Sawashita Y, Ohno S,
Hirata N and Yamakage M: Remimazolam anesthesia for mitraclip
implantation in a patient with advanced heart failure. Case Rep
Anesthesiol. 2021(5536442)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hohjoh H, Horikawa I, Nakagawa K,
Segi-Nishida E and Hasegawa H: Induced mRNA expression of matrix
metalloproteinases Mmp-3, Mmp-12, and Mmp-13 in the infarct
cerebral cortex of photothrombosis model mice. Neurosci Lett.
739(135406)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wu Q, Xu F, Wang J and Jiang M: Comparison
of remimazolam-flumazenil versus propofol for recovery from general
anesthesia: A systematic review and meta-analysis. J Clin Med.
12(7316)2020.PubMed/NCBI View Article : Google Scholar
|