Introduction
Bacillus cereus is a flagellated,
spore-forming, gram-positive Bacillus that is widely
distributed in the environment, including soil, dust and sewage.
Except for cases of food poisoning caused by enterotoxin-producing
strains, it rarely causes infections in humans. Moreover, when
B. cereus is detected in blood cultures, it is often assumed
to be a contaminant (1). However,
in the field of clinical microbiology, B. cereus is
considered a pathogen (2).
According to the Japan Nosocomial Infections Surveillance in 2022,
the detection rate of B. cereus in blood culture-positive
samples is 1.2% (3). B.
cereus is an opportunistic pathogen and nosocomial infections
of neonates and immunocompromised patients are problematic as they
can result in the development of severe sepsis (4,5). The
main routes of B. cereus infection are inappropriate
handling of intravascular indwelling catheters and contamination
from the environment, including hospital bed linens (6,7).
In 2019, the Ministry of Health, Labor and Welfare
of Japan notified medical institutions of cases of nosocomial
infections caused by B. cereus and introduced a requirement
that medical institutions implement control measures (8). In Japan, amino acid infusions have
been associated with peripheral venous catheter-associated
bloodstream infection (PVC-BSI) caused by B. cereus
(9,10). Patients receiving peripheral
parenteral nutrition (PPN) containing amino acids have a higher
risk of developing B. cereus PVC-BSI compared with patients
receiving other types of parenteral nutrition solution (9,10).
Additionally, long-term infusion of a large volume of fluid is
associated with the development of BSIs caused by Bacillus
spp (6).
A previous case-control study conducted in a large
teaching hospital identified risk factors for B. cereus
PVC-BSI (10). In this previous
study, unadjusted logistic regression analysis identified the
following risk factors: Increased age, male sex, steroid therapy
and the use of a solution containing 7.5% glucose, amino acids and
electrolytes for PPN. A subsequent multivariable analysis using a
conditional logistic regression model identified steroid therapy
and use of PPN solution with 7.5% glucose, amino acids and
electrolytes as factors significantly associated with B.
cereus PVC-BSI. The most probable mechanism for the increased
risk of developing B. cereus PVC-BSI in patients receiving
PPN solutions is that B. cereus can contaminate and rapidly
proliferate in PPN solutions. An in vitro study reported
that B. cereus proliferates rapidly in PPN solutions
containing 3% amino acids, 7.5% glucose and electrolytes (11,12).
Another possible mechanism underlying this association is that the
chemical characteristics of the solutions [an acidic pH (6.4) and a
high solution-to-serum osmolality ratio of ~3] can cause peripheral
thrombophlebitis and large vessel damage (10). An additional possible mechanism is
that B. cereus can easily form a biofilm in artificial
tubing (13). Possible infection
routes include bacterial contamination through the skin of patients
or healthcare providers and bacterial contamination in venous lines
or 3-way stopcocks. Specifically, in peripheral venous catheters,
3-way stopcocks are frequently left open. Additionally, B.
cereus, which is alcohol-resistant and exhibits rapid
proliferation, can contaminate the lumens of the venous line hubs
(6).
In vitro studies have reported that, to
prevent B. cereus PVC-BSI, the infusion time for PPN
containing amino acids should not exceed 6 or 8 h (14,15).
However, clinical evidence regarding the infusion time limit for
preventing catheter-related infection to support current guidelines
is lacking. Accordingly, the present study aimed to conduct a
quantitative analysis to provide evidence regarding the optimal
infusion time. The primary objective of the present study was to
investigate the effect of the time taken to administer BFLUID PPN
solution on the risk of BSI caused by B. cereus.
Materials and methods
Study design and patients
The present study conducted a single-center,
retrospective cohort study. Clinical information was extracted from
the electronic medical records of patients aged ≥18 years who
received BFLUID (Otsuka Pharmaceutical Factory Co., Ltd.) infusions
via a peripheral line between April 2017 and October 2020 at
Saitama Red Cross Hospital (Saitama, Japan). BSI was determined
based on cases isolated from ³2 sets of blood cultures within 48 h
(Fig. S1), in which there was no
evidence of a source of infection other than the catheter, the
clinical presentation was fever and antimicrobial therapy was
initiated. B. cereus was cultured on Trypticase Soy Agar M
with 5% Sheep Blood (BD Biosciences). The identity of the organism
was assessed according to the manufacturer's protocols using the
VITEK 2 System (bioMérieux Japan, Ltd.), an automated bacterial
identification and susceptibility testing system. Propensity score
matching was used to minimize biases resulting from differences in
background factors for infusions administered within 6 vs. >6 h
and within 8 vs. >8 h.
The present study was conducted in accordance with
the ethical standards of Saitama Red Cross Hospital and the
Declaration of Helsinki. The present study protocol was approved by
the ethics committee of the Saitama Red Cross Hospital (approval
no. 20-T; Saitama, Japan) prior to study initiation.
Clinical data collection
The following clinical data were collected:
Demographic characteristics of patients (sex, age, body weight and
height), laboratory values [serum albumin (ALB), hemoglobin A1c
(HbA1c) and estimated glomerular filtration rate (eGFR)], clinical
characteristics (dialysis, cancer, chemotherapy, steroid therapy
and immunosuppressant use), dosage regimen, time to administer
BFLUID and duration of BFLUID use.
Statistical analysis
Patient characteristics were expressed as the mean ±
SD or count (percentage), as appropriate. Between-group comparisons
were performed using the unpaired Student's t-test for continuous
variables and the χ2 test for categorical variables. A
multivariable logistic regression analysis was performed to
determine the propensity score using the following demographic
characteristics and baseline laboratory values: Sex, age, body
weight, height, ALB and HbA1c levels, eGFR and duration of BFLUID
use. The 1:1 propensity score matching was performed using
nearest-neighbor matching, with a maximum caliper width of 0.2 SDs
of the propensity scores. The ability of the model to discriminate
the cut-off time was assessed using Harrell's C index and the
standardized mean difference. P<0.05 was considered to indicate
a statistically significant difference.
A multivariable logistic regression analysis was
performed to identify independent predictors of B. cereus
BSI. Odds ratios (ORs) and 95% CIs were calculated for variables
included in the model. Statistical analyses were performed using
JMP pro statistical software (version 17.2; SAS Institute, Inc.)
and SPSS (version 27; IBM Corp.).
Model development
All clinically relevant parameters were included in
the multivariable logistic regression model for identifying
significant predictors of B. cereus PVC-BSI. Backward
elimination was performed based on the P-value of each predictor
and the overall predictive performance of the model, which was
assessed using the area under the receiver operating characteristic
curve (AUROC). First, non-contributing factors with large P-values
(P>0.1) and the lowest magnitude of effect (ORs closest to 1.00)
were eliminated from the regression model. After each predictor was
removed, the performance of the model was checked using the AUROC.
The predictor was re-entered into the model if its removal caused a
substantial decrease in the AUROC. These steps were performed
iteratively until all the remaining predictors within the model had
a P<0.10 on the condition that the AUROC of the reduced model
was preserved. The discrimination and calibration of the final
reduced model was assessed using the AUROC curve.
Results
Patient characteristics
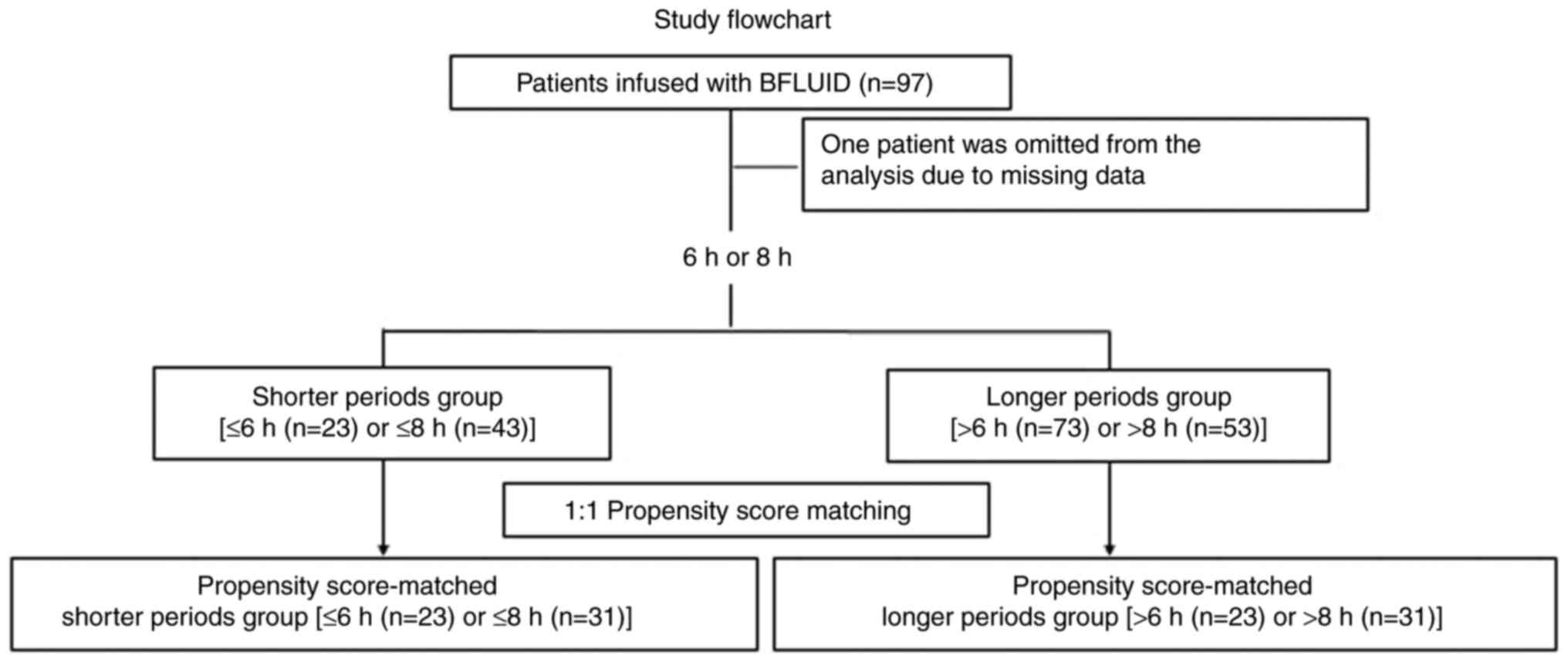
During the study period, 97 patients were enrolled;
among them, 32 developed B. cereus infection, excluding 1
patient who was omitted from the analysis due to missing data
(Fig. 1). The clinical
characteristics of patients with and without B. cereus
infection were analyzed (Table I).
After propensity score matching, 23 and 31 matched patient pairs
were identified for infusions administered within 6 h (Table II) and 8 h (Table III), respectively. Before the
propensity score-matched analysis, patient sex significantly
differed between patients with longer and shorter infusion times
for infusions administered within 6 and 8 h and the eGFR
significantly differed between patients with longer and shorter
infusion times for infusions administered within 8 h (Tables II and III).
 | Table IDemographic and clinical
characteristics of patients with and without Bacillus cereus
peripheral venous catheter-related bloodstream infections. |
Table I
Demographic and clinical
characteristics of patients with and without Bacillus cereus
peripheral venous catheter-related bloodstream infections.
| Clinical
parameter | With B. cereus
infection (n=32) | Without B.
cereus infection (n=64) | P-value |
|---|
| Sex, n (%) | | | 0.516 |
|
Male | 13 (41%) | 32 (50%) | |
|
Female | 19 (59%) | 32 (50%) | |
| Mean age, years ±
SD | 68.2±2.1 | 70.9±1.4 | 0.284 |
| Mean body weight, kg
± SD | 51.9±2.9 | 53.7±1.5 | 0.537 |
| Mean height, cm ±
SD | 158.9±1.8 | 159.1±1.5 | 0.924 |
| Mean serum albumin
level, mg/dl ± SD | 2.58±0.14 | 3.30±0.10 | 0.001 |
| HbA1c, n (%) | | | 0.207 |
|
With HbA1c
value data | 15 (47%) | 19 (30%) | |
|
Without
HbA1c value data | 17 (53%) | 45 (70%) | |
| Mean HbA1c level, %
± SD | 6.16±0.2 | 5.87±0.13 | 0.207 |
| Mean eGFR,
ml/min/1.73 m2 ± SD | 84.5±8.0 | 63.4±3.0 | 0.030 |
| Dialysis, n
(%) | 1 (3%) | 2 (7%) | >0.999 |
| Cancer, n (%) | 18 (56%) | 31 (48%) | >0.999 |
| Chemotherapy, n
(%) | 5 (16%) | 9 (14%) | 0.837 |
| Steroid therapy, n
(%) | 4 (16%) | 5 (11%) | 0.458 |
| Immunosuppressant
use, n (%) | 2 (6%) | 1 (2%) | 0.213 |
| Number of
doses/day, n (%) | 25 (78%) | 54 (81%) | 0.493 |
| Mean time to
administer BF, h/infusion ± SD | 21.0±2.24 | 8.9±0.64 | 0.001 |
| Mean duration of BF
use, days ± SD | 12.6±2.19 | 5.63±0.67 | 0.001 |
 | Table IIComparison of characteristics of
patients who received BFLUID infusions via a peripheral line within
>6 vs. within ≤6 h. |
Table II
Comparison of characteristics of
patients who received BFLUID infusions via a peripheral line within
>6 vs. within ≤6 h.
| | All patients | Propensity
score-matched patients |
|---|
| Clinical
parameter | >6 h (n=73) | ≤6 h (n=23) | P-value | SMD | >6 h (n=23) | ≤6 h (n=23) | P-value | SMD |
|---|
| Sex, n (%) | | | 0.017 | 0.628 | | | >0.999 | 0.093 |
|
Male | 44 (60%) | 7 (30%) | | | 8 (35%) | 7 (30%) | | |
|
Female | 29 (40%) | 16 (70%) | | | 15 (65%) | 16 (70%) | | |
| Mean age, years ±
SD | 70.2±11.4 | 69.3±12.1 | 0.722 | 0.077 | 65.7±13.8 | 69.3±12.1 | 0.356 | 0.277 |
| Mean body weight,
kg ± SD | 52.0±14.1 | 56.5±12.6 | 0.172 | 0.337 | 57.2±13.3 | 56.5±12.9 | 0.867 | 0.053 |
| Mean height, cm ±
SD | 157.8±11.5 | 163.1±8.6 | 0.044 | 0.521 | 162.9±10.2 | 163.1±8.6 | 0.915 | 0.021 |
| Mean serum albumin
level, mg/dl ± SD | 3.02±0.89 | 3.18±0.90 | 0.451 | 0.179 | 3.22±0.86 | 3.18±0.86 | 0.895 | 0.047 |
| HbA1c, n (%) | 29 (40%) | 5 (22%) | 0.139 | 0.400 | 9 (39%) | 5 (22%) | 0.337 | 0.385 |
| Mean HbA1c level, %
± SD | 6.01±0.65 | 5.64±0.65 | 0.200 | 0.569 | 6.40±0.67 | 5.64±0.69 | 0.066 | 1.12 |
| Mean eGFR,
ml/min/1.73 m2 ± SD | | | | | | | | |
| Mean duration of
BFLUID use, days ± SD | 8.58±9.8 | 6.00±5.4 | 0.231 | 0.326 | 3.52±3.46 | 6.00±5.38 | 0.070 | 0.548 |
| Dialysis, n
(%) | 3 (4%) | 0 (0%) | >0.999 | 0.293 | 1 (4%) | 0 (0%) | >0.999 | 0.302 |
| Cancer, n (%) | 36 (49%) | 13 (57%) | 0.635 | 0.145 | 10 (43%) | 13 (57%) | 0.556 | 0.263 |
| Chemotherapy, n
(%) | 10 (14%) | 4 (17%) | 0.737 | 0.102 | 3 (13%) | 4 (17%) | >0.999 | 0.121 |
| Steroid therapy, n
(%) | 6 (8%) | 2 (9%) | >0.999 | 0.017 | 0 (0%) | 2 (9%) | 0.489 | 0.436 |
| Immunosuppressant
use, n (%) | 2 (3%) | 1 (4%) | 0.565 | 0.087 | 0 (0%) | 1 (4%) | >0.999 | 0.302 |
| Number of
doses/day, n (%) | 59 (81%) | 20 (87%) | 0.755 | 0.167 | 18 (78%) | 20 (87%) | 0.699 | 0.231 |
 | Table IIIComparison of characteristics of
patients who received BFLUID infusions via a peripheral line within
>8 vs. within ≤8 h. |
Table III
Comparison of characteristics of
patients who received BFLUID infusions via a peripheral line within
>8 vs. within ≤8 h.
| | All patients | Propensity
score-matched patients |
|---|
| Clinical
parameter | >8 h (n=53) | ≤8 h (n=43) | P-value | SMD | >8 h (n=31) | ≤8 h (n=31) | P-value | SMD |
|---|
| Sex | | | 0.016 | 0.508 | | | 0.611 | 0.129 |
|
Male | 34 (64%) | 17 (40%) | | | 17 (55%) | 15 (48%) | | |
|
Female | 19 (36%) | 26 (60%) | | | 14 (45%) | 16 (52%) | | |
| Mean age, years ±
SD | 68.8±11.7 | 71.5±11.2 | 0.252 | 0.236 | 68.5±11.4 | 70.7±11.5 | 0.469 | 0.192 |
| Mean body weight,
kg ± SD | 51.2±14.2 | 55.5±13.0 | 0.134 | 0.316 | 55.0±13.6 | 55.4±13.0 | 0.909 | 0.030 |
| Mean height, cm ±
SD | 158.1±10.6 | 160.2±11.8 | 0.375 | 0.187 | 158.4±13.0 | 159.7±10.6 | 0.672 | 0.110 |
| Mean serum albumin
level, mg/dl ± SD | 2.91±0.90 | 3.24±0.85 | 0.064 | 0.377 | 3.27±0.86 | 3.21±0.88 | 0.772 | 0.069 |
| HbA1c, n (%) | 22 (42%) | 12 (28%) | 0.201 | 0.289 | 11 (35%) | 9 (29%) | 0.786 | 0.138 |
| Mean HbA1c level, %
± SD | 6.00±0.70 | 5.99±0.61 | 0.973 | 0.015 | 6.15±0.25 | 6.02±0.27 | 0.973 | 0.247 |
| Mean eGFR,
ml/min/1.73 m2 ± SD | | | | | | | | |
| Mean duration of
BFLUID use, days ± SD | 9.51±10.6 | 6.05±6.0 | 0.060 | 0.402 | 6.06±1.13 | 6.39±1.13 | 0.650 | 0.079 |
| Dialysis, n
(%) | 1 (2%) | 2 (5%) | 0.585 | 0.156 | 0 (0%) | 2 (6%) | 0.492 | 0.371 |
| Cancer, n (%) | 31 (58%) | 18 (42%) | 0.150 | 0.337 | 18 (58%) | 15 (52%) | 0.611 | 0.195 |
| Chemotherapy, n
(%) | 8 (15%) | 6 (14%) | >0.999 | 0.032 | 5 (16%) | 6 (19%) | >0.999 | 0.084 |
| Steroid therapy, n
(%) | 4 (8%) | 4 (11%) | >0.999 | 0.063 | 1 (3%) | 3 (10%) | 0.612 | 0.265 |
| Immunosuppressant
use, n (%) | 2 (4%) | 1 (2%) | 0.585 | 0.084 | 0 (0%) | 2 (6%) | 0.492 | 0.371 |
| Number of
doses/day, n (%) | 41 (77%) | 38 (88%) | 0.183 | 0.295 | 29 (94%) | 24 (77%) | 0.147 | 0.408 |
Propensity score allocation
The propensity score was calculated using patient
characteristics. The 6 and 8 h models had high discriminative power
with Harrell's C index values of 0.69 and 0.75, respectively.
Propensity score-matched analyses with matching performed on
infusion times >6 or >8 h showed smaller standardized mean
differences compared with those before matching for all variables
except age and HbA1c level (Tables
II and III). No patients who
received BFLUID infusions within 6 h developed B. cereus
BSI. However, 8/23 patients who received BFLUID infusions
administered over >6 h developed B. cereus BSIs. The
proportion of patients with B. cereus BSI was significantly
higher among those who received infusions administered over >8 h
compared with those who received infusions administered over <8
h. B. cereus BSIs occurred in 2/31 patients who received
BFLUID infusions administered within 8 h and in 14/31 patients who
received BFLUID infusions administered over >8 h. The relative
risk was 7.0 (95% CI, 1.73-28.3) (Tables SI and SII). This indicated that patients with an
infusion time of BFLUID >8 h had a 7-fold increased risk of BSI
compared with those with an infusion time of ≤8 h. However, due to
the wide CI, cautious interpretation of this estimate is
necessary.
B. cereus PVC-BSI score
In the multivariable analysis (Table SIII), the BFLUID infusion time
[adjusted OR (aOR), 1.22; 95% CI, 1.11-1.34; P=0.001], duration of
BFLUID use (aOR, 1.11; 95% CI, 1.03-1.21) and ALB level (aOR, 0.35;
95% CI, 0.21-0.58) were associated with a significantly increased
risk of B. cereus BSI.
Scores of 0-3, 0-8 and 0-5 points were assigned to
ALB, time to administer BFLUID (h/infusion) and duration of BFLUID
use (days), respectively. The scores for the three predictors were
added to calculate the total B. cereus PVC-BSI score, on a
scale of 0-16 points (16)
(Table SIV). The probability of
B. cereus PVC-BSI in patients with a score of 18 points was
98% (Table SV). Further, the
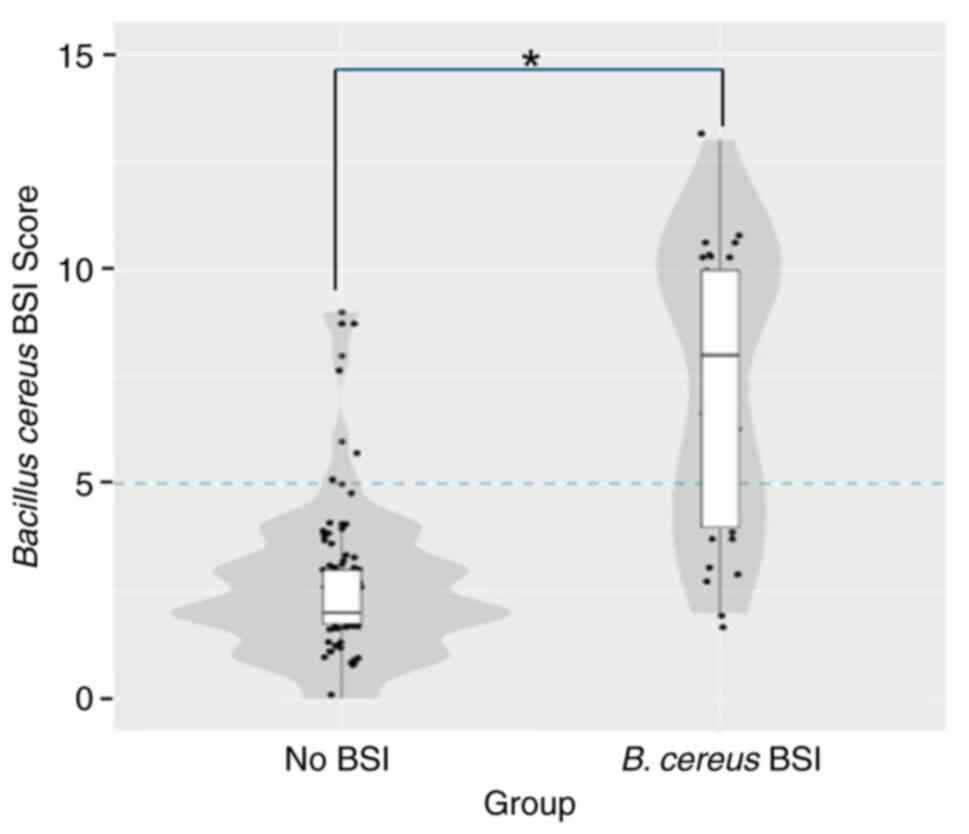
scores were higher in patients with B. cereus infection
compared with those without infection (Fig. 2). The AUROC of the B. cereus
PVC-BSI score was 0.90 (95% CI, 0.83-0.96) (Fig. S2), indicating satisfactory
diagnostic performance. The results of the AUROC analysis for the
diagnostic performance of the B. cereus BSI score indicated
a cut-off value of 5. Therefore, the PVC-BSI score should be
<5.
Discussion
An in vitro study suggested that BFLUID
administration is associated with an increased risk of B.
cereus PVC-BSI and that one of the causes of this infection was
the growth of B. cereus in the infusion bag during prolonged
infusion (14,15). To the best of our knowledge, the
present study was the first to report risk factors for B.
cereus PVC-BSI in patients receiving amino acid infusions via a
peripheral venous catheter. It was demonstrated that the BFLUID
infusion time, duration of use and low serum ALB level were
significantly associated with the incidence of B. cereus
PVC-BSI. Consistent with the findings of the present study,
previous studies have reported risk factors for BSI in patients
receiving PPN therapy, such as a longer average daily infusion time
of PPN and the administration of intravenous fluids (17). The serum ALB level is an indicator
of nutritional status and low serum ALB levels are associated with
an increased susceptibility to infectious diseases (18). Moreover, the present study conducted
a propensity score-matched analysis to investigate whether the time
within which BFLUID was administered affected the risk of B.
cereus PVC-BSI. The incidence of B. cereus PVC-BSI
increased with increasing BFLUID administration time. Based on the
diagnostic score, AUROC analysis indicated a cut-off value of 5 h
for B. cereus PVC-BSI. Further studies are warranted to
investigate whether the B. cereus PVC-BSI score developed in
the present study has utility in the prevention of B. cereus
PVC-BSI in clinical practice.
PPN was introduced in Japan in 1996 after a study
demonstrated the efficacy of nutritional support using peripherally
inserted venous catheters (19).
Compared with parenteral nutrition solutions administered via a
central line, administration via peripheral lines increases the
risk of catheter-associated BSIs (9). Total parenteral nutrition, which is
administered by a central venous line, is prepared under aseptic
conditions based on guidelines for preventing bacterial
contamination. By contrast, PPN does not require special techniques
and PPN solutions are prepared in the ward, which is a non-sterile
environment. This may lead to B. cereus PVC-BSI.
The present study had some limitations. First, it
was a single-center retrospective study; therefore, the findings
may lack generalizability. Half of the study participants (49
patients) had cancer; therefore, these findings might only be
generalizable to patients with cancer. The sample size was
insufficient to stratify the analysis according to cancer status.
The electronic health records of 11/49 patients with cancer did not
specify whether they were receiving cancer treatment at the time of
BFLUID administration; therefore, further detailed studies are
warranted. Second, the eGFR at baseline significantly differed
between patients with and without B. cereus BSIs, which may
have resulted in residual confounding. A total of 6 patients were
recorded as having a BMI <14 kg/m2 and an eGFR
>200 ml/min/1.73 m2. Serum creatinine levels tend to
be higher in people with increased muscle mass and lower in those
with lower muscle mass, and eGFR can also be lower or higher
accordingly. In addition, considering that the eGFR was calculated
and not directly measured and some results were judged to be
spuriously high, eGFR could not be adjusted for in the
multivariable analysis.
The present study investigated the incidence of and
risk factors for developing B. cereus BSI in patients
receiving PPN therapy via BFLUID administration. The identified
risk factors included the BFLUID infusion time, duration of use and
low serum ALB level. To reduce the risk of PVC-BSI, it could be
recommended that BFLUID infusion is administered within 6 h and
that PPN be performed to keep the score established in this study
as low as possible. Further multicenter studies are warranted to
validate the findings of the present study.
Supplementary Material
Appearance of Bacillus cereus
colonies cultured on Trypticase Soy Agar M with 5% sheep
blood.
Receiver operating. characteristic
curve for the diagnostic performance of the Bacillus cereus
BSI score. The B. cereus BSI score had a sensitivity of
71.9% and specificity of 90.6% using a cut-off value of 5. BSI,
bloodstream infection.
Number of Bacillus cereus BSIs
with a 6 h BFLUID infusion time cut-off period.
Number of Bacillus cereus BSIs
with an 8 h BFLUID infusion time cut-off period.
Factors associated with Bacillus
cereus bloodstream infection in the multivariable
analysis.
Diagnostic performance of the
Bacillus cereus peripheral venous catheter-associated
bloodstream infection score.
Probability of a Bacillus
cereus PVC-BSI according to the total PVC-BSI score.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by a Nihon
Pharmaceutical University Research Grant (grant no. NPU-2).
Availability of data and materials
The data generated in the present study are not
publicly available due to patient confidentiality but may be
requested from the corresponding author.
Authors' contributions
AtT, AsT, MI, MM, TK, AI, HN, YM and YF collected
the patient data. YN, KS and TM performed data analysis and wrote
the manuscript. TM and YM confirm the authenticity of all the raw
data. All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the ethical standards of Saitama Red Cross Hospital and the
Declaration of Helsinki Declaration. The study protocol was
approved by the ethics committee of the Saitama Red Cross Hospital
(approval no. 20-T) before study initiation. The requirement for
informed consent was waived due to the retrospective nature of the
study. Patient consent was obtained though the opt-out method.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bottone EJ: Bacillus cereus, a volatile
human pathogen. Clin Microbiol Rev. 23:382–398. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pearson HE: Human infections caused by
organisms of the Bacillus species. Am J Clin Pathol. 53:506–515.
1970.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Japan Nosocomial Infections Surveillance
in 2022: Ministry of Health, Labour and Welfare, 2023. https://janis.mhlw.go.jp/report/open_report/2022/3/1/ken_Open_Report_202200.pdf.
|
|
4
|
Kusama Y, Honma I, Masuda M, Goto H and
Onodera S: Bacillus cereus outbreak in normal neonates at our
hospital. Kankyo kansen-shi [Environmental Infection Magazine].
30:385–390. 2015.
|
|
5
|
Matsumoto S, Suenaga H, Naito K, Sawazaki
M, Hiramatsu T and Agata N: Management of suspected nosocomial
infection: an audit of 19 hospitalized patients with septicemia
caused by Bacillus species. Jpn J Infect Dis. 53:196–202.
2000.PubMed/NCBI
|
|
6
|
Aso Y, Nagatomi M, Nakazawa T, Sasaki S
and Ishi K: Examination of infusion fluid type and environmental
factors involved in increased Bacillus cereus bloodstream
infection. Jpn J Environ Infect. 27:81–90. 2012.
|
|
7
|
Nakamura K, Yarimizu A, Ihara T, Sawayama
Y and Ishimaru T: An analysis of a case in which Bacillus cereus
bacteremia developed from the use of carry-on towels which resulted
in the horizontal transmission of this organism in the same ward.
Kansenshogaku zasshi. 92:80–85. 2018.(In Japanese).
|
|
8
|
Hino C, Ozaki M, Kitahara T, Kouda K,
Shikichi K, Nakamura I, Kawai S and Oie S: Peripheral parenteral
nutrition solutions and bed bath towels as risk factors for
nosocomial peripheral venous catheter-related bloodstream infection
by bacillus cereus. Int J Med Sci. 20:566–571. 2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kutsuna S, Hayakawa K, Kita K, Katanami Y,
Imakita N, Kasahara K, Seto M, Akazawa K, Shimizu M, Kano T, et al:
Risk factors of catheter-related bloodstream infection caused by
Bacillus cereus: Case-control study in 8 teaching hospitals in
Japan. Am J Infect Control. 45:1281–1283. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sakihama T and Tokuda Y: Use of peripheral
parenteral nutrition solutions as a risk factor for Bacillus cereus
peripheral venous catheter-associated bloodstream infection at a
Japanese tertiary care hospital: A case-control study. Jpn J Infect
Dis. 69:531–533. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kuwahara T, Kaneda S, Shimono K and Inoue
Y: Effects of lipid emulsion and multivitamins on the growth of
microorganisms in peripheral parenteral nutrition solutions. Int J
Med Sci. 10:1079–1084. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sasahara T, Hayashi S, Hosoda K, Inagawa
H, Morisawa Y and Hirai Y: Bacterial growth in intravenous fluid
products. In: Proceedings in the 11th East Asian Conference on
Infection Control and Prevention (EACIC) [abstract], Tokyo, Japan.
EACIC, p24 2012.
|
|
13
|
Kuroki R, Kawakami K, Qin L, Kaji C,
Watanabe K, Kimura Y, Ishiguro C, Tanimura S, Tsuchiya Y, Hamaguchi
I, et al: Nosocomial bacteremia caused by biofilm-forming Bacillus
cereus and Bacillus thuringiensis. Intern Med. 48:791–796.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shiota A, Asai N, Koizumi Y, Watanabe H,
Sakata M, Kurumiya A, Takahashi T, Muramatsu Y, Hagihara M,
Suematsu H, et al: Extended drip infusion of peripheral parental
nutrition containing amino acids might be associated with Bacillus
cereus bloodstream infection. Am J Infect Control. 47:1154–1156.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sakai Y, Konishi T, Obayashi Y, Honda K,
Akae S and Ishihara K: Study of growth level of Bacillus cereus in
various infusion fluids. Shimane J Med Technol. 40:19–23. 2012.
|
|
16
|
Bonnett LJ, Snell KIE, Collins GS and
Riley RD: Guide to presenting clinical prediction models for use in
clinical settings. BMJ. 365(l737)2019.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Shimoda M, Tanaka Y, Morimoto K, Yoshimori
K and Ohta K: Risk factors for bloodstream infection in patients
receiving peripheral parenteral nutrition. Intern Med 2024 (Epub
ahead of print).
|
|
18
|
Minatoguchi S, Nomura A, Imaizumi T,
Sasaki S, Ozeki T, Uchida D, Kawarazaki H, Sasai F, Tomita K,
Shimizu H and Fujita Y: Low serum albumin as a risk factor for
infection-related in-hospital death among hemodialysis patients
hospitalized on suspicion of infectious disease: A Japanese
multicenter retrospective cohort study. Ren Replace Ther.
4(30)2018.
|
|
19
|
Payne-James JJ and Khawaja HT: First
choice for total parenteral nutrition: The peripheral route. JPEN J
Parenter Enteral Nutr. 17:468–478. 1993.PubMed/NCBI View Article : Google Scholar
|