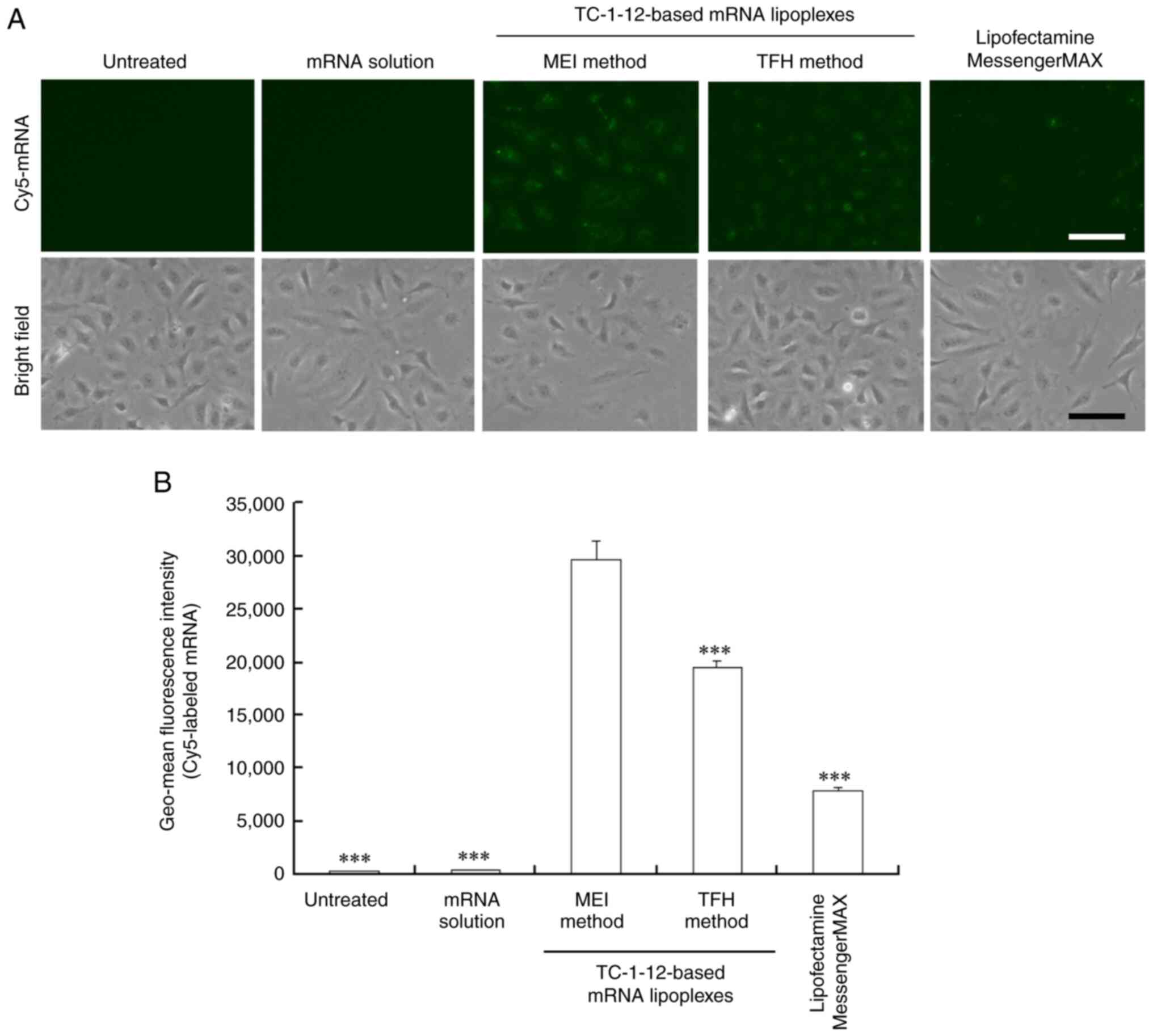
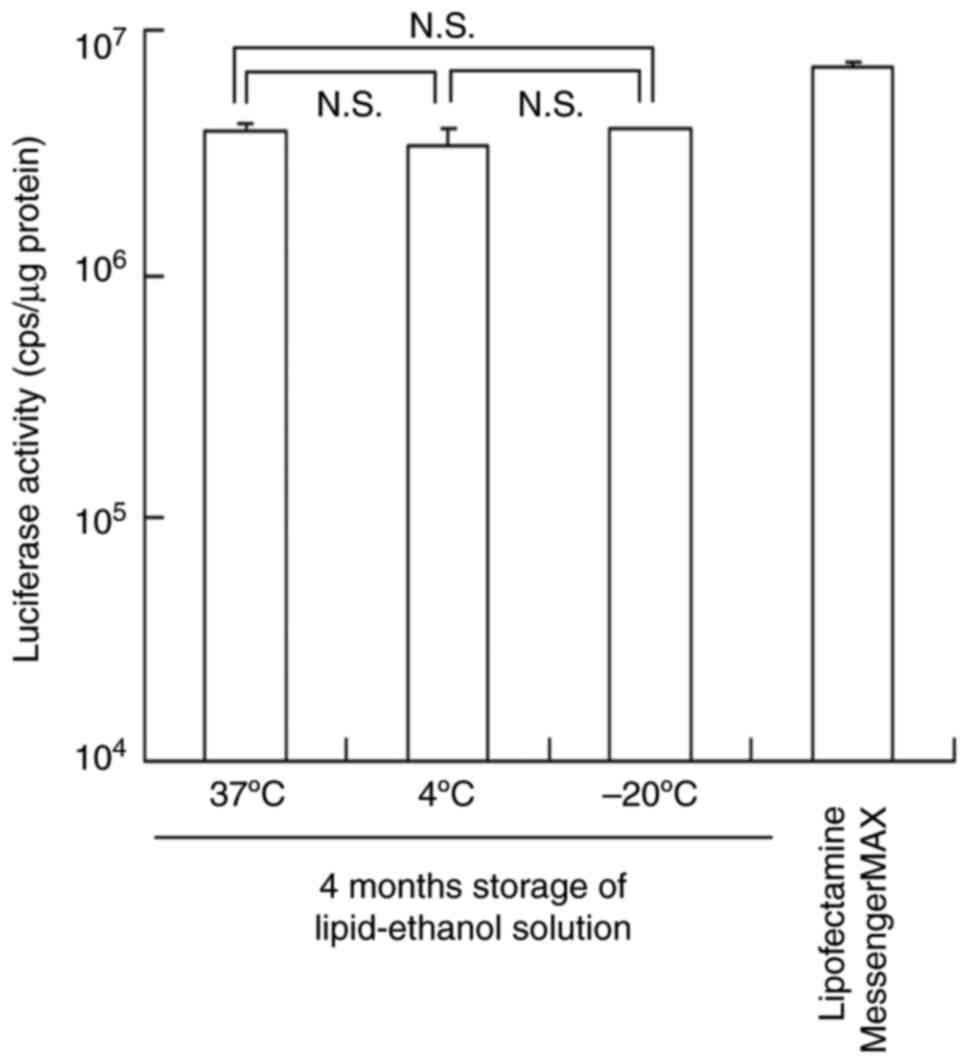
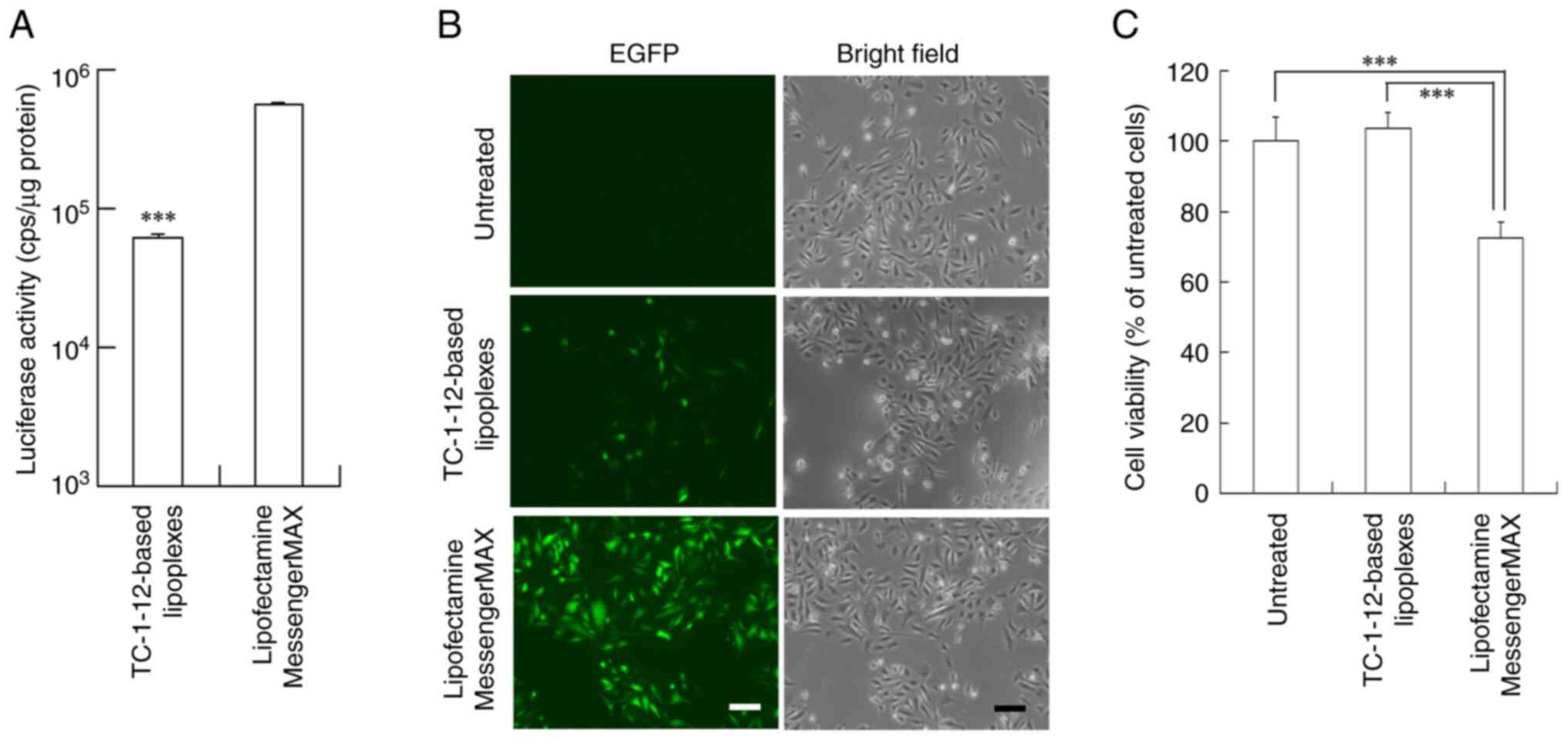
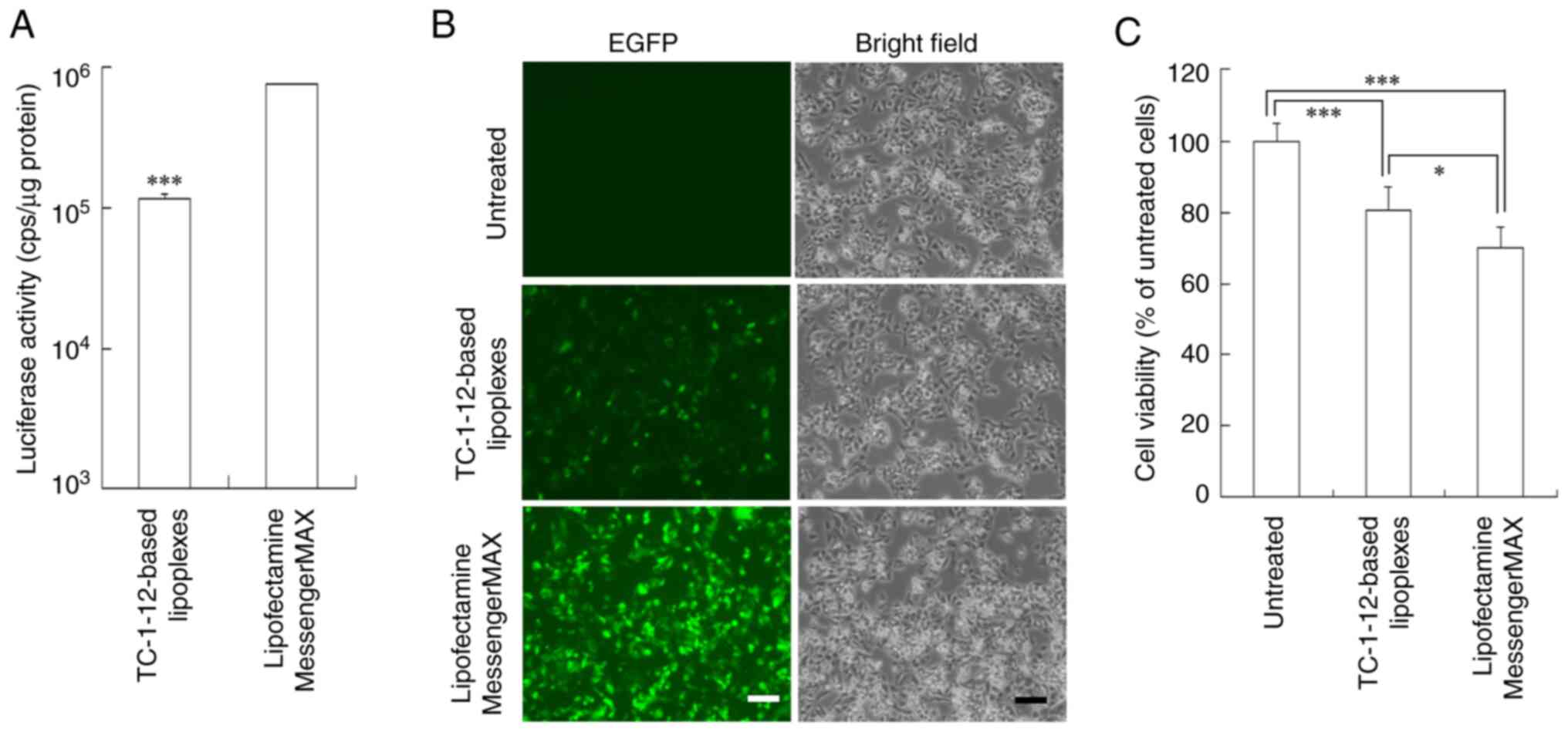
|
1
|
Sahin U, Karikó K and Türeci Ö: mRNA-based
therapeutics-developing a new class of drugs. Nat Rev Drug Discov.
13:759–780. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Liu T, Liang Y and Huang L: Development
and delivery systems of mRNA vaccines. Front Bioeng Biotechnol.
9(718753)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Barbier AJ, Jiang AY, Zhang P, Wooster R
and Anderson DG: The clinical progress of mRNA vaccines and
immunotherapies. Nat Biotechnol. 40:840–854. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Warren L, Manos PD, Ahfeldt T, Loh YH, Li
H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, et al: Highly
efficient reprogramming to pluripotency and directed
differentiation of human cells with synthetic modified mRNA. Cell
Stem Cell. 7:618–630. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ramachandran S, Satapathy SR and Dutta T:
Delivery strategies for mRNA vaccines. Pharmaceut Med. 36:11–20.
2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yen A, Cheng Y, Sylvestre M, Gustafson HH,
Puri S and Pun SH: Serum nuclease susceptibility of mRNA cargo in
condensed polyplexes. Mol Pharm. 15:2268–2276. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen H, Ren X, Xu S, Zhang D and Han T:
Optimization of lipid nanoformulations for effective mRNA delivery.
Int J Nanomedicine. 17:2893–2905. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Malone RW, Felgner PL and Verma IM:
Cationic liposome- mediated RNA transfection. Proc Natl Acad Sci
USA. 86:6077–6081. 1989.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tang M, Sagawa A, Inoue N, Torii S, Tomita
K and Hattori Y: Efficient mRNA delivery with mRNA lipoplexes
prepared using a modified ethanol injection method. Pharmaceutics.
15(1141)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bangham AD, Standish MM and Watkins JC:
Diffusion of univalent ions across the lamellae of swollen
phospholipids. J Mol Biol. 13:238–252. 1965.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hattori Y, Nakamura M, Takeuchi N, Tamaki
K, Shimizu S, Yoshiike Y, Taguchi M, Ohno H, Ozaki KI and Onishi H:
Effect of cationic lipid in cationic liposomes on siRNA delivery
into the lung by intravenous injection of cationic lipoplex. J Drug
Target. 27:217–227. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Barichello JM, Ishida T and Kiwada H:
Complexation of siRNA and pDNA with cationic liposomes: The
important aspects in lipoplex preparation. Methods Mol Biol.
605:461–472. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hattori Y, Saito H, Nakamura K, Yamanaka
A, Tang M and Ozaki KI: In vitro and in vivo transfections using
siRNA lipoplexes prepared by mixing siRNAs with a lipid-ethanol
solution. J Drug Deliv Sci Technol. 75(103635)2022.
|
|
14
|
Hattori Y, Tamaki K, Sakasai S, Ozaki KI
and Onishi H: Effects of PEG anchors in pegylated siRNA lipoplexes
on in vitro genesilencing effects and siRNA biodistribution
in mice. Mol Med Rep. 22:4183–4196. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Smith MC, Crist RM, Clogston JD and McNeil
SE: Zeta potential: A case study of cationic, anionic, and neutral
liposomes. Anal Bioanal Chem. 409:5779–5787. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jarzebska NT, Frei J, Lauchli S, French
LE, Guenova E, Gouttefangeas C, Kündig TM, Mellett M and Pascolo S:
Lipofection with synthetic mRNA as a simple method for T-cell
immunomonitoring. Viruses. 13(1232)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wei X, Shao B, He Z, Ye T, Luo M, Sang Y,
Liang X, Wang W, Luo S, Yang S, et al: Cationic nanocarriers induce
cell necrosis through impairment of Na(+)/K(+)-ATPase and cause
subsequent inflammatory response. Cell Res. 25:237–253.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Opsomer L, Jana S, Mertens I, Cui X,
Hoogenboom R and Sanders NN: Efficient in vitro and in vivo
transfection of self-amplifying mRNA with linear
poly(propylenimine) and poly(ethylenimine-propylenimine) random
copolymers as non-viral carriers. J Mater Chem B. 12:3927–3946.
2024.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ahlemeyer B, Vogt JF, Michel V,
Hahn-Kohlberger P and Baumgart-Vogt E: Microporation is an
efficient method for siRNA-induced knockdown of PEX5 in HepG2
cells: Evaluation of the transfection efficiency, the PEX5 mRNA and
protein levels and induction of peroxisomal deficiency. Histochem
Cell Biol. 142:577–591. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hattori Y, Yoshizawa T, Koga K and Maitani
Y: NaCl induced high cationic hydroxyethylated cholesterol-based
nanoparticle-mediated synthetic small interfering RNA transfer into
prostate carcinoma PC-3 cells. Biol Pharm Bull. 31:2294–2301.
2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hattori Y, Hagiwara A, Ding W and Maitani
Y: NaCl improves siRNA delivery mediated by nanoparticles of
hydroxyethylated cationic cholesterol with amido-linker. Bioorg Med
Chem Lett. 18:5228–5232. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hattori Y, Ding WX and Maitani Y: Highly
efficient cationic hydroxyethylated cholesterol-based
nanoparticle-mediated gene transfer in vivo and in vitro in
prostate carcinoma PC-3 cells. J Control Release. 120:122–130.
2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Koulov AV, Vares L, Jain M and Smith BD:
Cationic triple-chain amphiphiles facilitate vesicle fusion
compared to double-chain or single-chain analogues. Biochim Biophys
Acta. 1564:459–465. 2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ma X, Wu F, Peng C, Chen H, Zhang D and
Han T: Exploration of mRNA nanoparticles based on DOTAP through
optimization of the helper lipids. Biotechnol J.
18(e2300123)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Anderluzzi G, Lou G, Gallorini S, Brazzoli
M, Johnson R, O'Hagan DT, Baudner BC and Perrie Y: Investigating
the impact of delivery system design on the efficacy of
self-amplifying RNA vaccines. Vaccines (Basel).
8(212)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hattori Y and Tang M: Effect of cationic
and neutral lipids in cationic liposomes on antibody production
induced by systemic administration of mRNA lipoplexes into mice. J
Drug Deliv Sci Technol. 100(106034)2024.
|