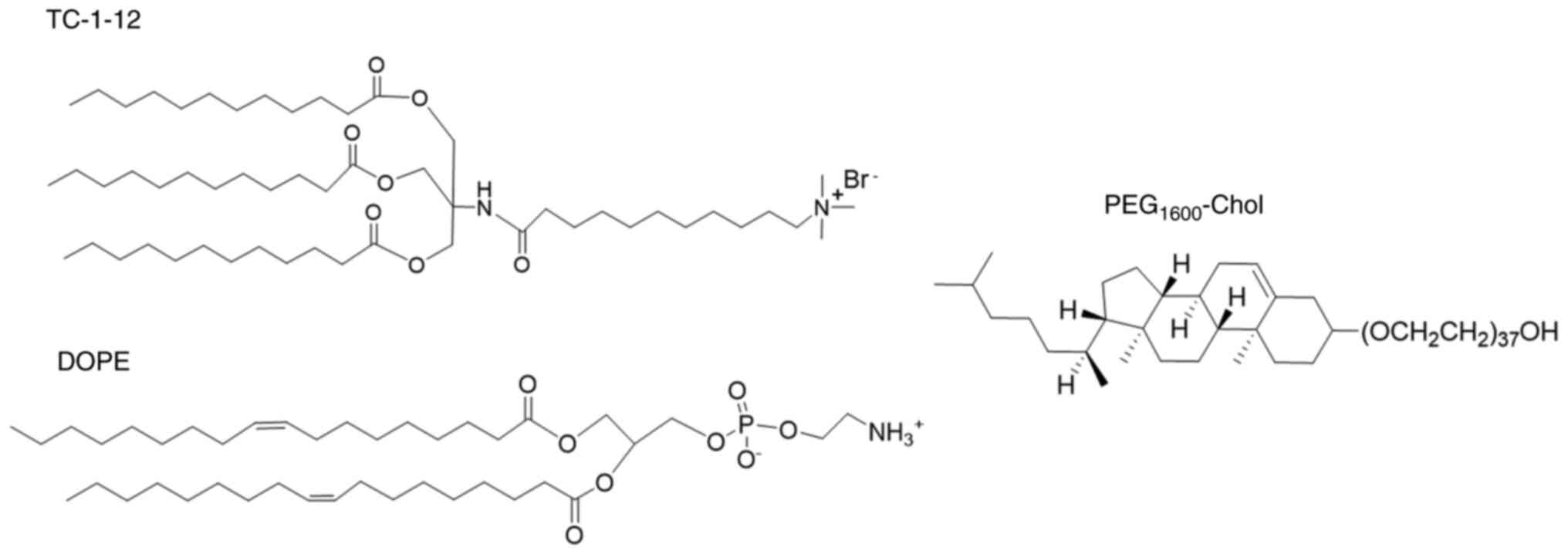
Introduction
mRNAs are single-stranded RNAs encoding protein
sequences that are translated into proteins by ribosomes in the
cytoplasm. mRNAs are designed and synthesized to immediately and
efficiently express therapeutic proteins in the target cells for
various therapeutic applications (1). For example, mRNAs are used as vaccines
to produce protein antigens to activate the host immune system
(2,3) and reprogram differentiated somatic
cells into pluripotent stem cells via the expression of
transcription factors associated with pluripotency (4). mRNA therapeutics must be delivered to
the cytoplasm of the target cells via the cell membrane (5). However, mRNA is unstable and has
limited permeability across cell membranes (6), necessitating the development of
effective mRNA delivery systems. Among the various mRNA carriers
for cells, cationic liposomes have attracted considerable attention
(7-9).
Cationic liposomes are often prepared via thin-film
hydration (TFH) (10,11). This method involves making a thin
lipid film containing cationic and neutral lipids on the surface of
a round-bottom flask by evaporating chloroform from the
lipids-containing chloroform. Cationic liposomes are prepared by
adding a heated aqueous solution to a thin lipid film, followed by
sonication and/or extrusion. For mRNA transfection, a cationic
liposome suspension is added to an mRNA solution to form
mRNA/cationic liposome complexes (mRNA lipoplexes) (12). Therefore, the TFH method requires
considerable time and equipment for the preparation of mRNA
lipoplexes. Recently, the authors developed a simple one-step
procedure to prepare mRNA lipoplexes using a modified ethanol
injection (MEI) method. In this method, mRNA-containing
phosphate-buffered saline (PBS) is rapidly poured into a small
volume of lipid-ethanol solution, resulting in the formation of
small and homogeneous mRNA lipoplexes without the need for special
equipment (13). The advantage of
this method relies on that it does not require the preparation of
cationic liposomes in advance, allowing the easy preparation of
mRNA lipoplexes by only using the lipid-ethanol solution.
Generally, cationic liposomes consist of cationic
and neutral lipids that increase the transfection efficiency and
stability (9,11). However, structures of cationic and
neutral lipids, such as head group size and charge, acyl chain
length, and saturation, affect the transfection efficiency of mRNA
lipoplexes into cells (9).
Therefore, the determination of the optimal combination of cationic
and neutral lipids in liposomal formulations is important for
efficient mRNA transfection. Previously, the authors demonstrated
that cationic liposomes composed of the cationic triacyl lipid,
11-((1,3-bis
(dodecanoyloxy)-2-((dodecanoyloxy)methyl)propan-2-yl)amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide (TC-1-12), with
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) as a
neutral lipid and poly (ethylene glycol) cholesteryl ether
(PEG-Chol) as a dispersant agent exhibited high mRNA transfection
efficiency in cells (9). However,
an optimal preparation method for mRNA lipoplexes is needed for
their effective transfection into cells. In the present study, in
order to determine the optimal preparation method for mRNA
lipoplexes, TC-1-12-based mRNA lipoplexes were prepared at various
charge ratios (+:-) using the MEI and TFH methods, and the protein
expression efficiency and cytotoxicity in cells transfected with
these lipoplexes were evaluated.
Materials and methods
Materials
TC-1-12 was obtained from Sogo Pharmaceutical Co.,
Ltd. DOPE and PEG-Chol (mean molecular weight of PEG =1,600 g/mol)
were purchased from NOF Co., Ltd. Firefly luciferase (FLuc) mRNA
(CleanCap FLuc mRNA; 1,922 nucleotides; cat. no. L-7602) and
enhanced green fluorescent protein (EGFP) mRNA (CleanCap EGFP mRNA;
997 nucleotides; cat. no. L-7601) were purchased from TriLink
Biotechnologies. Cyanine 5 (Cy5)-labeled mRNA [EZCap Cyanine 5 FLuc
mRNA (5 moUTP); 1,921 nucleotides; cat. no. R1010] was purchased
from APeXBIO Technology LLC.
Cell culture
Human cervical carcinoma cell line, HeLa (cat. no.
93021013; CVCL_0030) and human liver cancer cell line, HepG2 (cat.
no. 85011430; CVCL_0027) were purchased from the European
Collection of Authenticated Cell Cultures. Human prostate carcinoma
cell line, PC-3 (cat. no. TKG 0600; CVCL_0035) was obtained from
the Cell Resource Center for Biomedical Research, Tohoku
University, Miyagi, Japan.
HeLa cells were maintained in the Eagle's minimum
essential medium (Wako Pure Chemical Industries, Ltd.) with 10%
heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific,
Inc.) and 100 µg/ml kanamycin (KM) in a humidified incubator at
37˚C with 5% CO2. HepG2 cells were maintained in
Dulbecco's modified Eagle's medium (Wako Pure Chemical Industries,
Ltd.) with 10% FBS and 100 µg/ml KM under the same conditions. PC-3
cells were maintained in the Roswell Park Memorial Institute-1640
medium (Wako Pure Chemical Industries, Ltd.) with 10% FBS and 100
µg/ml KM under the same conditions.
Preparation of mRNA lipoplexes for
transfection
To prepare mRNA lipoplexes using the MEI method, 2
mg TC-1-12, 1.53 mg DOPE and 0.08 mg PEG-Chol (molar ratio of
49.5:49.5:1) were dissolved in 1 ml ethanol, as previously
described (9). Briefly, 0.5 µl of a
1 mg/ml mRNA solution (0.5 µg mRNA) was transferred to a tube
containing 100 µl of PBS (pH 7.4). The resulting solution was
rapidly added to 0.76, 1.52, 2.27, 3.03, 3.79 and 4.55 µl of the
lipid-ethanol solution in another tube at charge ratios of 1:1,
2:1, 3:1, 4:1, 5:1 and 6:1, respectively. The charge ratio was
calculated as the molar ratio of the quaternary amine in TC-1-12 to
the mRNA phosphate.
To prepare mRNA lipoplexes using the TFH method, 10
mg TC-1-12, 7.64 mg DOPE and 0.38 mg PEG-Chol (molar ratio of
49.5:49.5:1) were dissolved in chloroform, as previously described
(14). Briefly, chloroform was
evaporated under vacuum using a rotary evaporator at 60˚C to form a
thin film. The film was then hydrated with 10 ml of sterilized
water at 60˚C via vortexing, followed by 10 min of sonication at
room temperature in a bath-type sonicator (Bransonic 2510 JMTH; 42
kHz, 100 W; Branson Ultrasonics Co.). To prepare the mRNA
lipoplexes, cationic liposome suspensions (1.52, 3.03, 4.55, 6.06,
7.58 and 9.09 µl at charge ratios of 1:1, 2:1, 3:1, 4:1, 5:1 and
6:1, respectively) were mixed with 0.5 µl of 1 mg/ml mRNA solution
(0.5 µg mRNA).
Size measurement of mRNA lipoplexes. To
prepare mRNA lipoplexes using the MEI method, 5 µg of FLuc mRNA (5
µl of a 1 mg/ml mRNA) was transferred to a tube containing 1,000 µl
of PBS (pH 7.4), and the obtained solution was quickly added to the
lipid-ethanol solution (7.6, 15.2, 22.7, 30.3, 37.9 and 45.5 µl at
charge ratios of 1:1, 2:1, 3:1, 4;1, 5:1 and 6:1, respectively) in
another tube. The mRNA lipoplex suspension was diluted three times
with water before measurement of particle size and ζ-potential.
To prepare mRNA lipoplexes using the TFH method,
cationic liposome suspensions (15.2, 30.3, 45.5, 60.6, 75.8, and
90.9 µl at charge ratios of 1:1, 2:1, 3:1, 4;1, 5:1, and 6:1,
respectively) were added to FLuc mRNA solution (5 µg mRNA). The
mRNA lipoplex suspension was diluted with the appropriate volume of
water before measurement of particle size and ζ-potential.
Next, particle size distribution, polydispersity
index (PDI), and ζ-potential of mRNA lipoplexes were determined
using a light-scattering photometer (ELS-Z2; Otsuka Electronics
Co., Ltd.), as previously described (14).
Luciferase activity in cells
transfected with the FLuc mRNA lipoplexes
HeLa, PC-3 and HepG2 cells were seeded in 12-well
culture plates at a density of 1x105 cells per well.
Following 24 h of incubation at 37˚C, FLuc mRNA lipoplexes (0.5 µg
mRNA) were prepared by employing the MEI and TFH methods, then they
were diluted with the culture medium containing 10% FBS (final mRNA
concentration: 0.5 µg/ml) and lastly, they were added to the cells.
Transfection of FLuc mRNA using Lipofectamine MessengerMAX (Thermo
Fisher Scientific, Inc.) was performed according to the
manufacturer's instructions. Briefly, 0.75 µl of Lipofectamine
MessengerMAX transfection reagent was diluted in 25 µl of Opti-MEM
medium (Thermo Fisher Scientific, Inc.) and incubated for 10 min at
room temperature. FLuc mRNA (0.5 µg) diluted in 25 µl of Opti-MEM
medium was added to the reagent solution and incubated for 5 min at
room temperature. The mixture was diluted with culture medium
containing 10% FBS (final mRNA concentration: 0.5 µg/ml) and
introduced into the cells.
A total of 24 h post-transfection, the cells were
lysed with 125 µl of cell lysis buffer (Pierce™ Luciferase Cell
Lysis Buffer; Thermo Fisher Scientific Inc.), and subjected to one
cycle of freezing (-80˚C) and thawing at 37˚C, followed by
centrifugation at 15,000 g for 10 sec at 4˚C. A total of 10 µl of
supernatant were mixed with 50 µl of PicaGene MelioraStar-LT
Luminescence Reagent (Toyo Ink Mfg. Co. Ltd.), and luminescence
[counts per sec (cps)] was measured using a chemo-luminometer (ARVO
X2; PerkinElmer, Inc.). Protein concentration in the supernatant
was measured by BCA assay (Pierce BCA Protein Assay Kit; Pierce;
Thermo Fisher Scientific, Inc.), and luciferase (Luc) activity
(cps/µg protein) was calculated.
EGFP expression in cells transfected
with the EGFP mRNA lipoplexes
HeLa, PC-3 and HepG2 cells were seeded in a 12-well
culture plate at a density of 1x105 cells per well.
Following 24 h of incubation at 37˚C, EGFP mRNA lipoplexes (0.5 µg
mRNA) were prepared using the MEI and TFH methods at charge ratios
of 2:1 to 5:1. Next, they were diluted with the culture medium,
which contained 10% FBS (final mRNA concentration: 0.5 µg/ml), and
were subsequently introduced into the cells. Transfection of EGFP
mRNA using Lipofectamine® MessengerMAX was performed as
aforementioned. At 24 h post-transfection, the cells were washed
two times with PBS and were fixed with 10% neutral buffered
formalin (Mildform 10N; Wako Pure Chemical Industries, Ltd.) for 10
min at room temperature. EGFP expression in cells was detected
using a fluorescence microscope (Eclipse TS100-F; Nikon
Corporation) equipped with an optical filter (excitation, 480/30
nm; dichroic mirror, 505 nm; emission, 535/45 nm; Nikon
Corporation).
To calculate the percentage of EGFP-expressing cells
using flow cytometry, EGFP mRNA lipoplexes (0.5 µg mRNA) were
prepared using the MEI and TFH methods at charge ratios of 2:1 to
5:1. These lipoplexes were diluted in culture medium containing 10%
FBS (final mRNA concentration: 0.5 µg/ml) and were subsequently
added to the cells. After 24 h of transfection, the cells were
detached with TrypLE™ Express Enzyme (Gibco; Thermo Fisher
Scientific, Inc.) and resuspended in PBS containing 0.1% bovine
serum albumin (BSA) and 1 mM ethylenediaminetetraacetic acid
(EDTA). EGFP expression levels were evaluated by measuring
EGFP-expressing cells with flow cytometry (BD
FACSVerseTM; BD Biosciences) using a 488-nm laser and
analyzed with BD FACSuite software ver. 1.0.3 (BD Biosciences).
Data for 10,000 fluorescence events were collected, including
forward scatter, side scatter, and 527/32 nm fluorescence.
Cytotoxicity in cells transfected with
the FLuc mRNA lipoplexes
HeLa, PC-3 and HepG2 cells were seeded in 96-well
culture plates at a density of 1x104 cells per well.
Following 24 h of incubation at 37˚C, FLuc mRNA lipoplexes (0.05 µg
mRNA) were prepared using the MEI and TFH methods at charge ratios
of 1:1 to 6:1, were diluted with the culture medium containing 10%
FBS (final mRNA concentration: 0.5 µg/ml) and were subsequently
added to the cells. Cytotoxicity was assessed 24 h
post-transfection using Cell Counting Kit-8 (CCK-8; cat. no. CK04;
Dojindo Laboratories, Inc.). Briefly, 10 µl of CCK-8 solution was
added to each well and incubated for 1 h at 37˚C. Cell viability
was calculated as a percentage of the absorbance of untreated cells
at 450 nm using an iMark microplate reader (Bio-Rad Laboratories,
Inc).
Cellular uptake of mRNA
lipoplexes
HeLa cells were seeded in a 12-well culture plate at
a density of 1x105 cells per well. After 24 h of
incubation at 37˚C, mRNA lipoplexes containing 0.5 µg of
Cy5-labeled mRNA in 1 ml of culture medium were transferred to the
cells (final mRNA concentration: 0.5 µg/ml). A total of 3 h
post-transfection, the cells were washed two times with PBS and
fixed with Mildform 10N for 10 min at room temperature. Cy5-labeled
mRNAs in the cells were detected using a fluorescence microscope
equipped with optical filter Cy5 HQ (excitation, 620/60 nm;
dichroic mirror, 660 nm; emission, 700/75 nm; Nikon
Corporation).
To quantify intracellular of Cy5-labeled mRNA in
HeLa cells using flow cytometry, mRNA lipoplexes containing 0.5 µg
of Cy5-labeled mRNA in 1 ml of culture medium were added to the
cells (final mRNA concentration: 0.5 µg/ml). After a 3 h
transfection period, the cells were detached with TrypLE™ Express
Enzyme, followed by suspension in PBS containing 0.1% BSA and 1 mM
EDTA. Cy5-labeled mRNA levels were assessed by flow cytometry (BD
FACSVerse™) using a 640-nm laser as aforementioned. Data for 10,000
fluorescence events were obtained by recording the forward scatter,
side scatter, and 660/10 nm fluorescence. Geo-mean fluorescence
intensity in Cy5-labeled mRNA-transfected cells was calculated.
Evaluation of the stability of the
lipid-ethanol solution
TC-1-12, DOPE and PEG-Chol were dissolved in 1 ml
ethanol at a molar ratio of 49.5:49.5:1, as aforementioned. The
lipid-ethanol solution was stored at -20, 4 and 37˚C. After storage
for a total of 4 months, FLuc mRNA lipoplexes were prepared using
the lipid-ethanol solution, and the lipoplex size and Luc
expression in HeLa cells were measured as aforementioned.
Statistical analysis
Statistical analyses were conducted using an
unpaired Student's t-test to compare two groups or one-way analysis
of variance, followed by Tukey's post-hoc test to compare multiple
groups using the GraphPad Prism software (v.4.0; Dotmatics). The
data are presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Size and ζ-potential of mRNA
lipoplexes
In the present study, TC-1-12 was used as a cationic
lipid, DOPE as a neutral lipid, and PEG-Chol as a dispersing agent
(Fig. 1). mRNA lipoplexes were
prepared using the MEI and TFH methods. It was previously
demonstrated that incorporating 1 mol% PEG1600-Chol as a
dispersion agent into liposomal formulations effectively reduced
lipoplex size after preparation using the MEI method (13). In the MEI method, FLuc mRNA
lipoplexes were formed by rapidly mixing FLuc mRNA-containing PBS
with a lipid-ethanol solution at various charge ratios. FLuc mRNA
lipoplexes prepared at a charge ratio of 2:1 were 238 nm in size
(PDI: 0.11). However, an increase in the charge ratio from 2:1 to
6:1 in the mRNA lipoplexes decreased the size to 80 nm (PDI: 0.17;
Table I). By contrast, ζ-potential
increased from -24.5 to 9.9 mV with an increase in charge ratio
from 1:1 to 6:1.
 | Table ISize of TC-1-12-based mRNA lipoplexes
prepared using MEI and TFH methods. |
Table I
Size of TC-1-12-based mRNA lipoplexes
prepared using MEI and TFH methods.
| Preparation
method | Charge ratio | Sizea (nm) | PDI |
ζ-potentiala (mV) |
|---|
| MEI | 1:1 | 139.1±2.1 | 0.18±0.02 | -24.5±1.0 |
| | 2:1 | 237.5±3.9 | 0.11±0.01 | 5.2±0.1 |
| | 3:1 | 115.8±1.1 | 0.11±0.00 | 1.7±3.1 |
| | 4:1 | 80.8±0.7 | 0.20±0.02 | 6.1±0.6 |
| | 5:1 | 86.3±0.1 | 0.19±0.02 | 8.4±0.2 |
| | 6:1 | 80.0±1.1 | 0.17±0.01 | 9.9±0.9 |
| TFH | Only liposome | 104.7±0.8 | 0.25±0.00 | 54.2±1.6 |
| | 1:1 | 275.4±9.6 | 0.24±0.01 | 11.8±0.7 |
| | 2:1 | 186.9±3.7 | 0.24±0.01 | 18.5±1.2 |
| | 3:1 | 183.9±12.3 | 0.21±0.05 | 27.0±1.9 |
| | 4:1 | 165.3±29.1 | 0.18±0.08 | 44.1±3.5 |
| | 5:1 | 216.4±21.1 | 0.11±0.00 | 44.0±0.8 |
| | 6:1 | 198.2±23.1 | 0.13±0.04 | 47.1±1.3 |
In the TFH method, a dry lipid film was formed on
the surface of the flask by the evaporation of chloroform
containing TC-1-12, DOPE and PEG-Chol, followed by hydration with
water and sonication. The cationic liposomes were 105 nm in size
(PDI: 0.25). Mixing the cationic liposome suspension with FLuc mRNA
at charge ratios of 1:1 to 6:1 increased the size to 165-275 nm
(PDI: 0.11-0.24) and ζ-potential from 11.8 to 47.1 mV.
The mRNA lipoplexes were prepared by using the TFH
and MEI methods and were suspended in water and 1/3 diluted PBS,
respectively, for particle size and ζ-potential measurements.
Adsorption of ions to the surface of mRNA lipoplexes causes change
of ζ-potential value (15).
Therefore, ζ-potential of mRNA lipoplexes that were prepared using
the MEI method showed lower values than those prepared using the
TFH method with only water. By contrast, the measurement of
particle size using dynamic light scattering was influenced by
viscosity. The viscosity of PBS is very similar to that of water
(dynamic viscosity at 25˚C is 0.8882 mPa·s for PBS and 0.890 mPa·s
for water). Therefore, it was appropriate to compare particle sizes
obtained from water and 1/3 diluted PBS. Commercially available
Lipofectamine MessengerMAX is widely used as a standard for in
vitro mRNA transfection (16).
In the present study, Lipofectamine MessengerMAX mRNA lipoplexes
were 606±35.5 nm in size with 0.32±0.01 in PDI (data not
shown).
Effects of the charge ratios of mRNA
lipoplexes on the protein expression efficiency and cytotoxicity of
mRNA lipoplexes
To examine the effect of the charge ratio of
TC-1-12-based mRNA lipoplexes on protein expression, FLuc mRNA
lipoplexes were prepared at charge ratios of 1:1 to 6:1 and
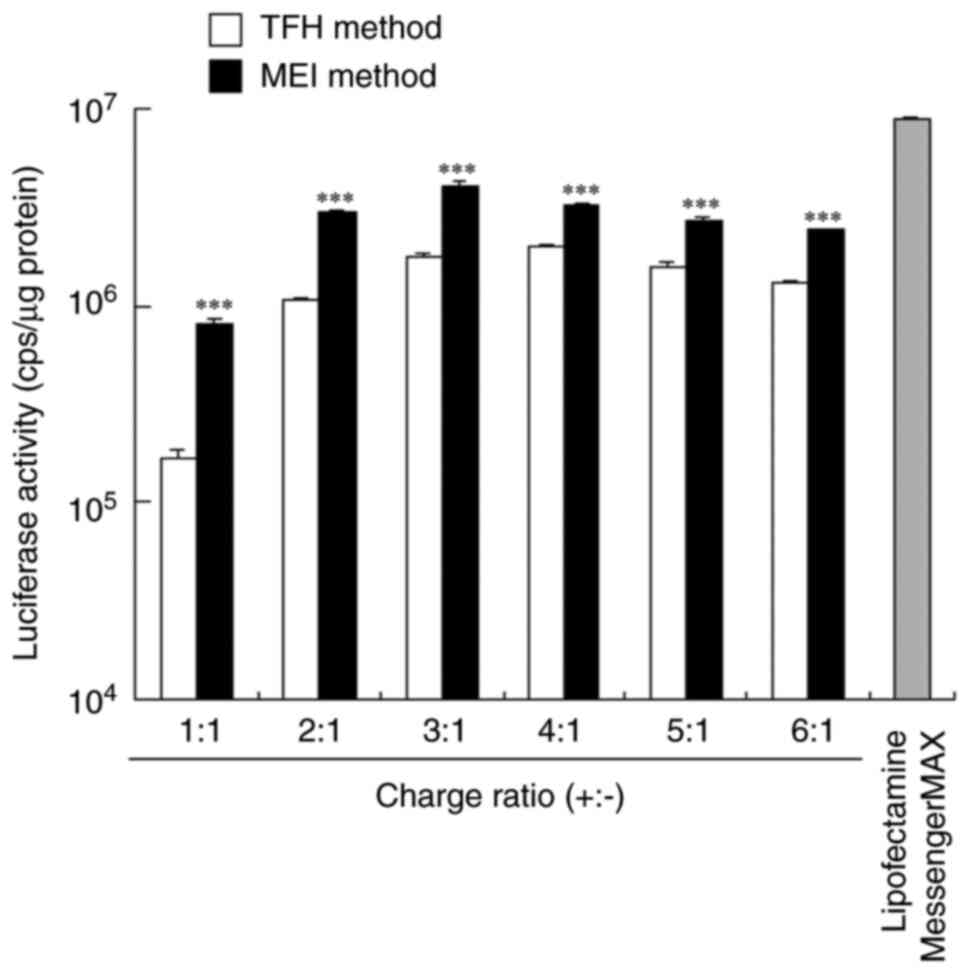
transfected into HeLa cells. Among the mRNA lipoplexes prepared
using the MEI method, FLuc mRNA lipoplexes prepared at a charge
ratio of 3:1 exhibited the highest Luc activity, which was 2-fold
lower than that of the Lipofectamine MessengerMAX mRNA lipoplexes
(Fig. 2). Among the mRNA lipoplexes
prepared using the TFH method, FLuc mRNA lipoplexes prepared at a
charge ratio of 4:1 exhibited the highest Luc activity, which was
2-fold lower than that of the lipoplexes prepared using the MEI
method at a charge ratio of 3:1. As for negative controls, cells
were transfected with cationic liposomes (mock transfection) that
were prepared using the MEI and TFH methods; however, these
liposomes did not induce Luc activity in HeLa cells (Fig. S1). These findings suggested that
the mRNA lipoplexes that were prepared using the MEI method induce
higher protein expression in HeLa cells than those that were
prepared using the TFH method.
Next, the effect of the charge ratio of
TC-1-12-based mRNA lipoplexes was investigated on the cytotoxicity
of HeLa cells 24 h after transfection with the FLuc mRNA
lipoplexes. In mRNA lipoplexes which were prepared using both the
MEI and TFL methods, cytotoxicity after transfection increased with
the increase in the charge ratio of mRNA lipoplexes (Fig. 3). Cytotoxicity of cationic liposomes
increases with the increase in ζ-potential (17). Similarly, in the present study,
ζ-potential of mRNA lipoplexes was associated with their
cytotoxicity in cells. Cell viabilities after transfection with the
FLuc mRNA lipoplexes that were prepared using the MEI method at a
charge ratio of 3:1 and the TFL method at a charge ratio of 4:1
were 46 and 57%, respectively. By contrast, cell viability after
transfection with the Lipofectamine MessengerMAX mRNA lipoplexes
was 22%, which is consistent with previously reported results
(18). These results suggested that
the Lipofectamine MessengerMAX mRNA lipoplexes induce higher
protein expression than the TC-1-12-based mRNA lipoplexes with
higher cytotoxicity in cells.
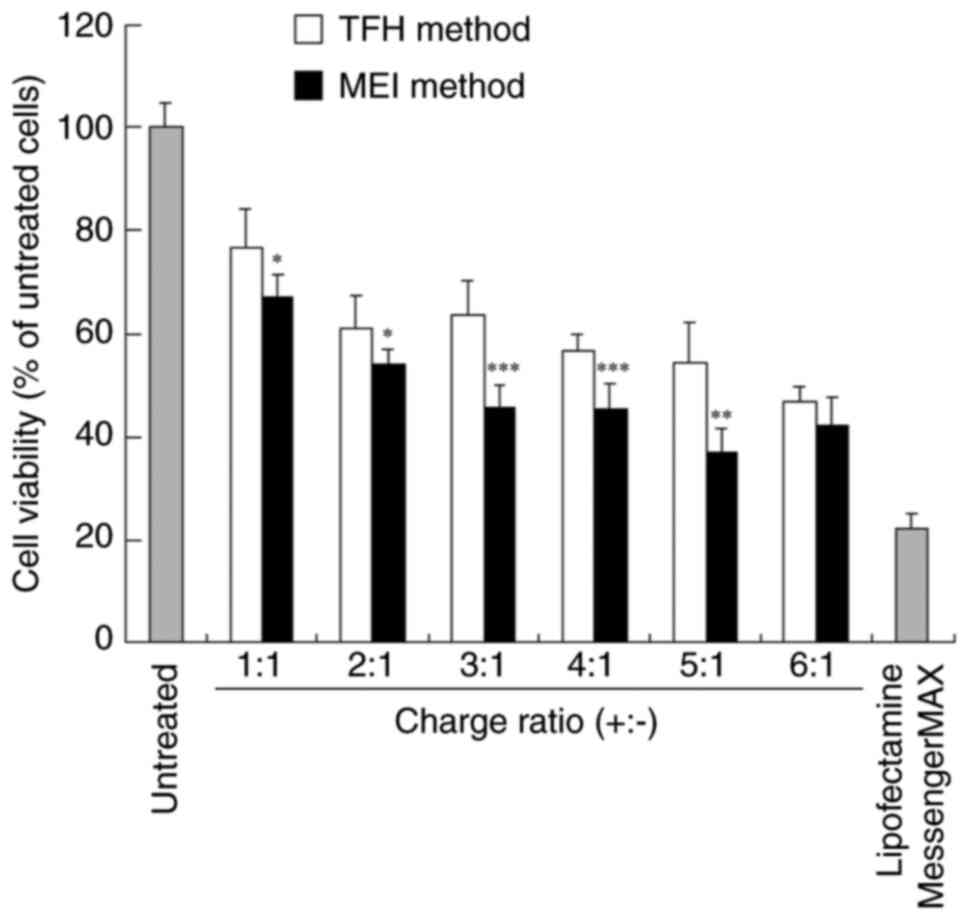 | Figure 3Effects of the charge ratios of
TC-1-12-based mRNA lipoplexes on cytotoxicity in HeLa cells
transfected with the FLuc mRNA lipoplexes. FLuc mRNA lipoplexes
were prepared using the MEI and TFH methods at charge ratios of 1:1
to 6:1, transfected into HeLa cells, and incubated for 24 h.
Lipofectamine MessengerMAX was used as a control. Each value is
represented as the mean ± standard deviation (n=5 for 5:1 (MEI),
1:1 (TFH), and 3:1 (TFH), and n=6 for other groups).
*P<0.05, **P<0.01 and
***P<0.001 vs. TFH method. TC-1-12,
11-((1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl)propan-2-yl)amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide; FLuc, firefly luciferase; MEI, modified ethanol injection;
TFH, thin-film hydration. |
EGFP expression in HeLa cells
transfected with the EGFP mRNA lipoplexes
To examine the effect of the charge ratio of
TC-1-12-based mRNA lipoplexes on protein expression efficiency,
EGFP mRNA lipoplexes were prepared at charge ratios of 2:1 to 5:1
and transfected into HeLa cells. EGFP expression was observed 24 h
after incubation (Figs. 4A and
B; S2). Transfection with EGFP mRNA lipoplexes
prepared using the MEI method resulted in high EGFP levels in most
cells (71-83% cells) at charge ratios of 2:1 to 4:1, which were
comparable to those observed after transfection with Lipofectamine
MessengerMAX mRNA lipoplexes (81% cells). By contrast, transfection
with EGFP mRNA lipoplexes that were prepared using the TFH method
resulted in lower EGFP levels (58-74% cells) at all charge ratios
compared with those observed after transfection with lipoplexes
prepared using the MEI method. These results suggested that mRNA
lipoplexes prepared using the MEI method effectively induce protein
expression in most cells.
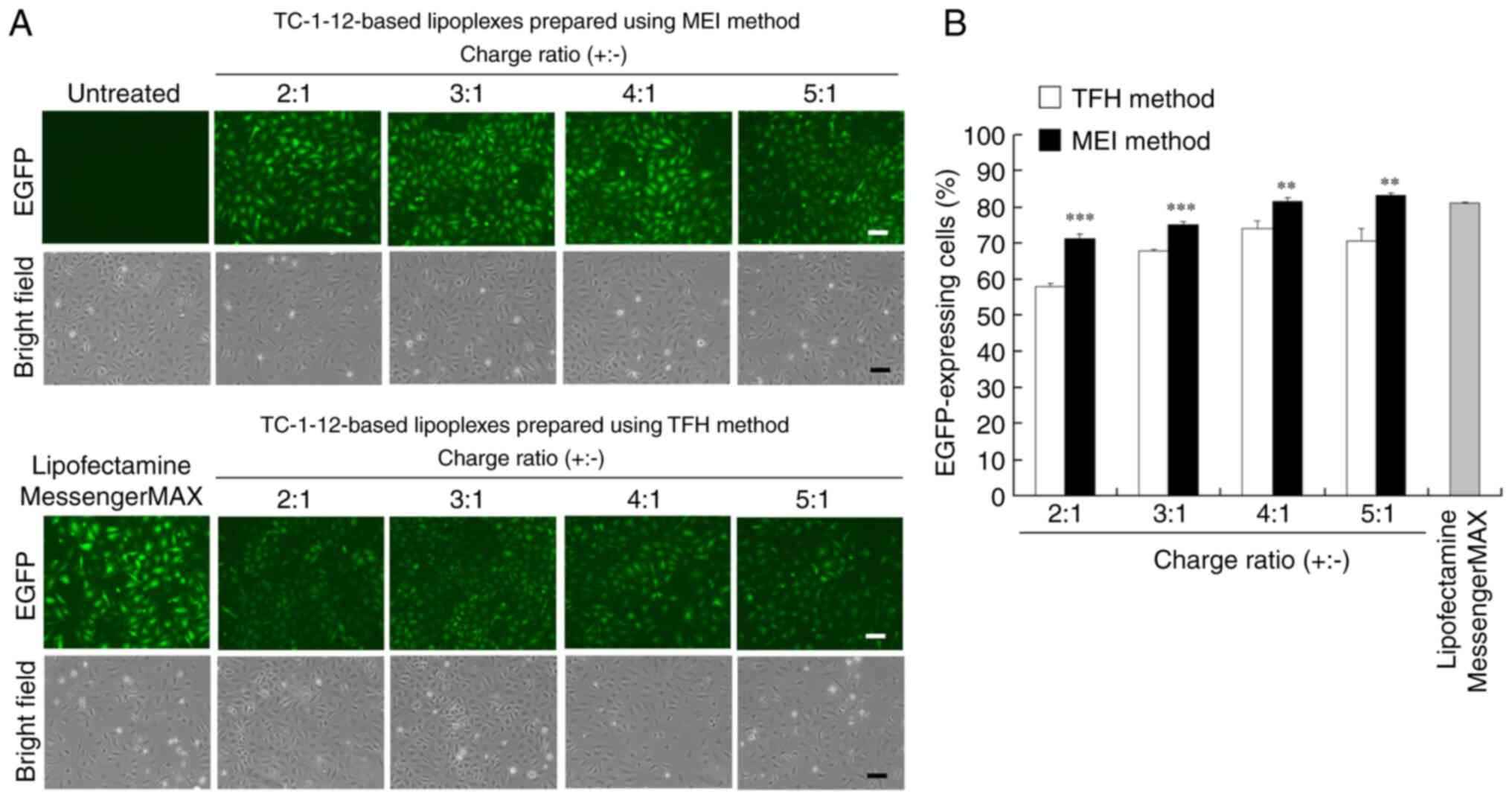 | Figure 4Effects of the charge ratios of
TC-1-12-based mRNA lipoplexes on EGFP levels in HeLa cells
transfected with the EGFP mRNA lipoplexes. EGFP mRNA lipoplexes
were prepared using the MEI and TFH methods at charge ratios of 2:1
to 5:1, transfected into HeLa cells, and incubated for 24 h.
Lipofectamine MessengerMAX was used as a control. (A) EGFP
expression in cells was observed using a fluorescence microscope.
Scale bar, 100 µm. (B) EGFP transfection efficiency was determined
using flow cytometry. Each column represents the mean ± standard
deviation (n=3). **P<0.01 and
***P<0.001 vs. TFH method. TC-1-12,
11-((1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl)propan-2-yl)amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide; EGFP, enhanced green fluorescent protein; MEI, modified
ethanol injection; TFH, thin-film hydration. |
Cellular uptake following mRNA
lipoplex transfection
To understand the mechanism by which the
TC-1-12-based mRNA lipoplexes, which were prepared using the MEI
method, showed high protein expression levels, the cellular uptake
of Cy5-labeled mRNA 3 h post-transfection was investigated. For
this experiment, TC-1-12-based mRNA lipoplexes that were prepared
using the MEI method at a charge ratio of 3:1 and the TFH method at
a charge ratio of 4:1 were used; because these charge ratios
resulted in the highest protein expression (Fig. 2). Stronger fluorescent signals of
Cy5-labeled mRNA were detected in cells transfected with the mRNA
lipoplexes that were prepared using the MEI method than in those
transfected with the mRNA lipoplexes that were prepared using the
THF method (Fig. 5A and B; Fig.
S3). Fluorescent signals of Cy5-labeled mRNA in the cells
transfected with the Lipofectamine MessengerMAX mRNA lipoplexes
were weak, indicating that the size of lipoplexes (~600 nm) affects
the cellular uptake of mRNA lipoplexes. As a negative control,
Cy5-labeled mRNA solution did not introduce mRNA into the cells.
Regarding the TC-1-12-based mRNA lipoplexes, fluorescence levels of
Cy5-labeled mRNA in the cells closely corresponded to the efficacy
of Luc expression following cell transfection with FLuc mRNA
lipoplexes. Therefore, TC-1-12-based mRNA lipoplexes which were
prepared using the MEI method at a charge ratio of 3:1 were used in
subsequent experiments.
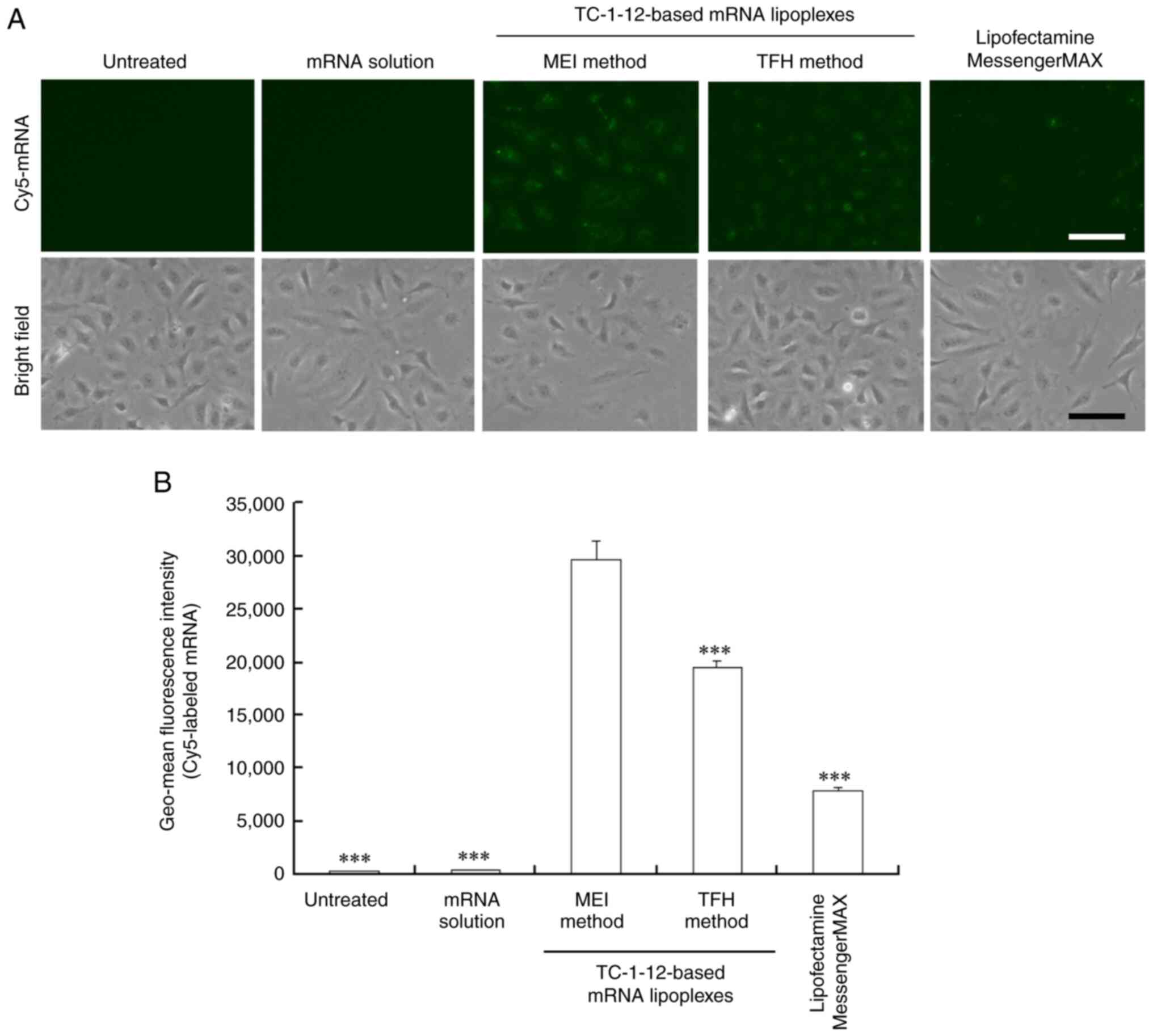 | Figure 5Cellular uptake by HeLa cells after
transfection with the TC-1-12-based Cy5-labeled mRNA lipoplexes.
Cy5-labeled mRNA lipoplexes were prepared at charge ratios of 3:1
and 4:1 using the MEI and TFH methods, respectively, and added to
HeLa cells at 0.5 μg/mL mRNA. As controls, Cy5-labeled mRNA
solution (free mRNA) and Lipofectamine MessengerMAX mRNA lipoplexes
were added to the HeLa cells. In (A), localization of Cy5-labeled
mRNA (green) was observed 3 h after incubation. Scale bar, 100 μm.
In (B), geo-mean fluorescence intensity of Cy5-labeled mRNA was
measured using flow cytometry 3 h after incubation. Each column
represents the mean ± standard deviation (n=3).
***P<0.001 vs. MEI method. TC-1-12,
11-((1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl)propan-2-yl)amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide; Cy5, cyanine 5; MEI, modified ethanol injection; TFH,
thin-film hydration. |
Effect of lipid-ethanol solution
storage on the size and protein expression efficiency of mRNA
lipoplexes
To examine the stability of lipids in the ethanol
solution, the lipid-ethanol solution was stored at -20, 4, and 37˚C
for 4 months. Subsequently, TC-1-12-based mRNA lipoplexes were
prepared by mixing the lipid-ethanol solution with a PBS solution
of mRNA. The size of mRNA lipoplexes increased to 191, 151 and 221
nm (PDI: 0.10, 0.08 and 0.11, respectively) after 4 months of
storage at -20, 4, and 37˚C, respectively (Table II). However, Luc expression levels
after transfection of the FLuc mRNA lipoplexes were not affected by
the 4-month storage at -20, 4 and 37˚C (Fig. 6). These results suggested that the
lipid-ethanol solution used for the preparation of mRNA lipoplexes
in the MEI method can be stored at 37˚C for at least 4 months.
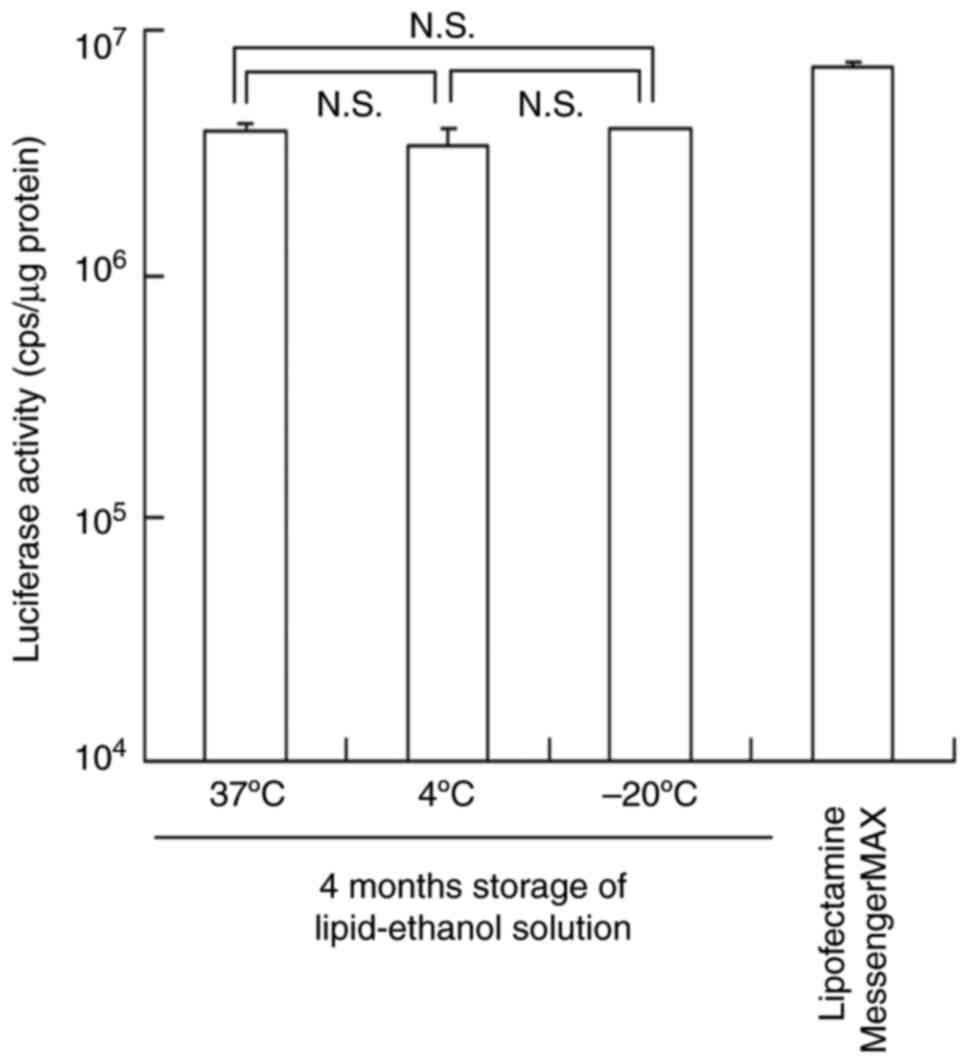 | Figure 6Effect of the storage of the
lipid-ethanol solution on Luc expression in HeLa cells transfected
with the TC-1-12-based FLuc mRNA lipoplexes. Lipid-ethanol solution
was stored at -20, 4, and 37˚C for 4 months. FLuc mRNA lipoplexes
were prepared at a charge ratio of 3:1 using the MEI method,
transfected into HeLa cells, and incubated for 24 h. Lipofectamine
MessengerMAX was used as a control. Each column represents the mean
± standard deviation (n=3). TC-1-12,
11-((1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl)propan-2-yl)amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide; N.S., not significant; FLuc, firefly luciferase; MEI,
modified ethanol injection. |
 | Table IISize of mRNA lipoplexes prepared
using modified ethanol injection method with a lipid-ethanol
solution stored at -20, 4 and 37˚C. |
Table II
Size of mRNA lipoplexes prepared
using modified ethanol injection method with a lipid-ethanol
solution stored at -20, 4 and 37˚C.
| | 4-month
storage |
|---|
| Temperature
(˚C) | Lipoplex
sizea (nm) | Polydispersity
index |
|---|
| -20 | 191.6±4.3 | 0.10±0.01 |
| 4 | 151.5±2.0 | 0.08±0.02 |
| 37 | 221.5±2.5 | 0.11±0.02 |
Effects of cell type on the protein
expression efficiency and cytotoxicity of mRNA lipoplexes
To examine the effect of cell type on the protein
expression efficiency of TC-1-12-based mRNA lipoplexes that were
prepared using the MEI method, FLuc and EGFP mRNA lipoplexes formed
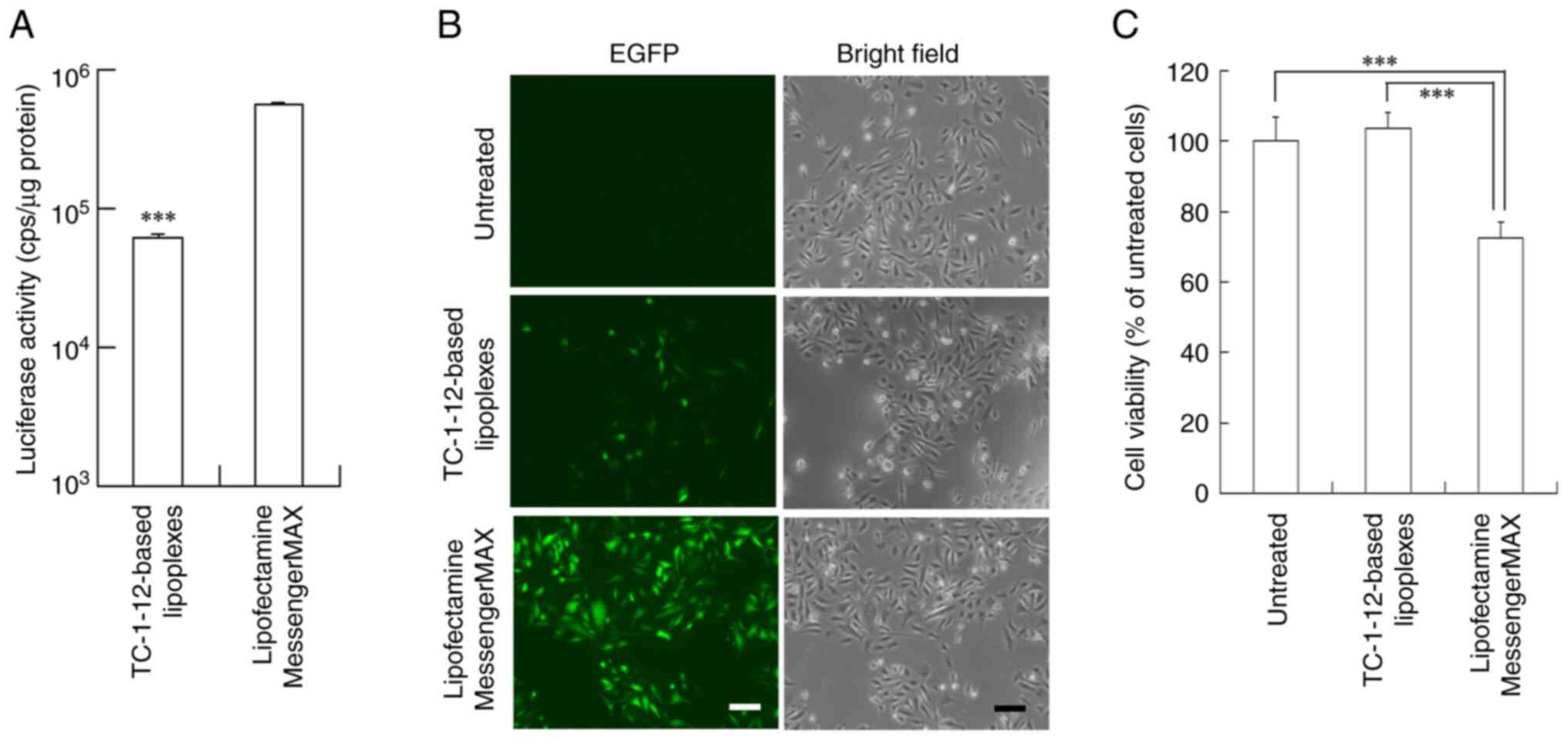
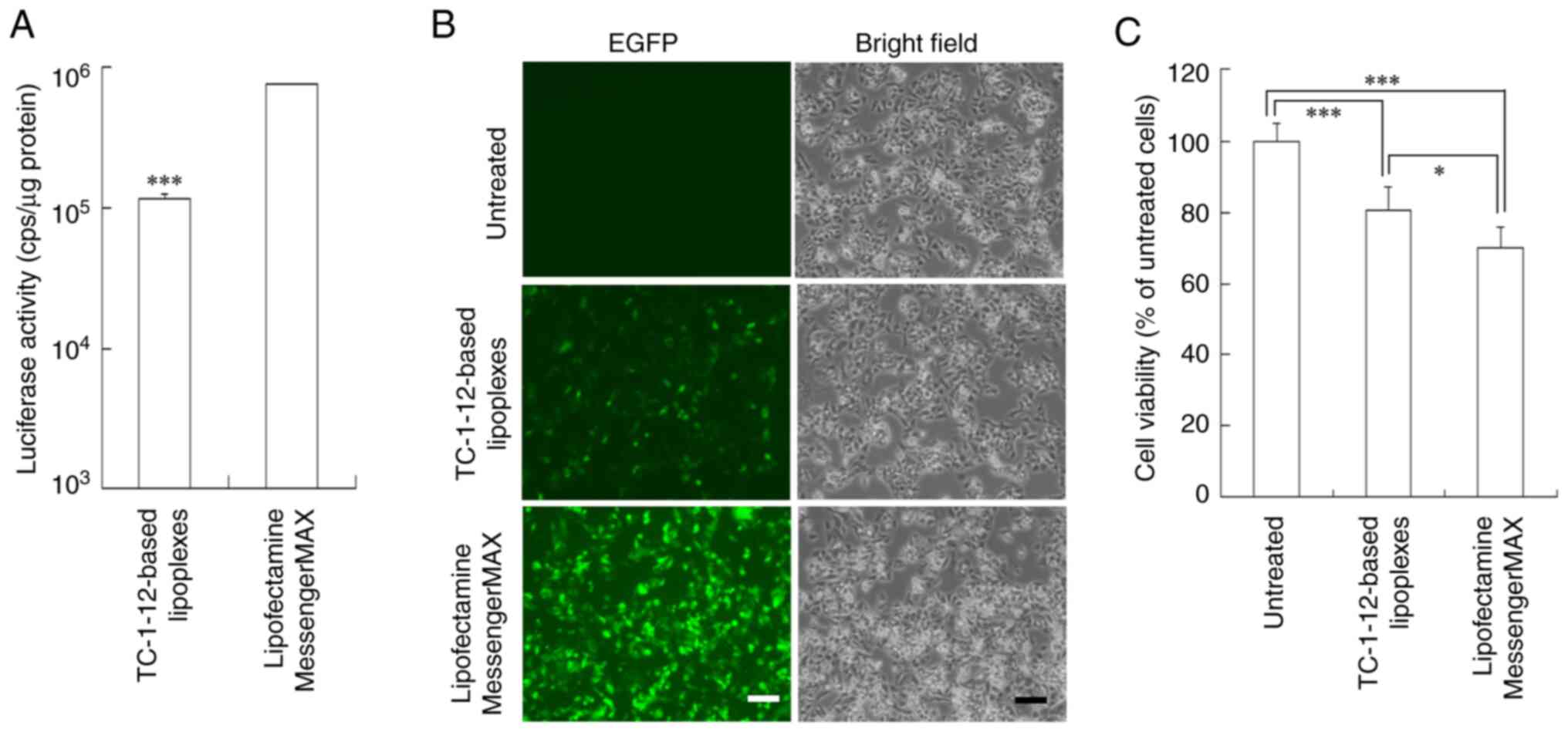
at a charge ratio of 3:1 were transfected into PC-3 (Fig. 7) and HepG2 cells (Fig. 8). HepG2 cells are known as a
hard-to-transfect-cell line (19).
TC-1-12-based FLuc mRNA lipoplexes exhibited 9- and 6.6-fold lower
Luc expression in PC-3 and HepG2 cells, respectively, than the
Lipofectamine MessengerMAX mRNA lipoplexes (Figs. 7A and 8A). However, TC-1-12-based EGFP mRNA
lipoplexes induced moderate EGFP expression in both cell lines
(Figs. 7B and 8B) with lower cytotoxicity (103 and 81%
cell viability, respectively) compared with the Lipofectamine
MessengerMAX mRNA lipoplexes (72 and 70% cell viability,
respectively; Figs. 7C and 8C).
Discussion
It was previously reported that mRNA lipoplexes
containing the cationic triacyl lipid, TC-1-12, exhibit high mRNA
transfection efficiency in cells (9). In the present study, the preparation
method for mRNA lipoplexes was further optimized to ensure their
efficient delivery to cells. FLuc and EGFP mRNA lipoplexes prepared
using the MEI method exhibited higher Luc activity and EGFP
expression, respectively, than those prepared using the TFH method.
Moreover, mRNA lipoplexes prepared using the MEI method exhibited
higher cellular uptake than those prepared using the TFH method. It
was previously reported that the presence of sodium chloride in the
formation of cationic lipoplexes of plasmid DNA or small
interfering RNA enhances the transfection of cells (20-22).
Moderate neutralization of cationic charges on the surface of mRNA
lipoplexes using sodium chloride in PBS might stabilize the
TC-1-12-based mRNA lipoplexes and increase their cellular
association and transfection efficiency.
TC-1-12 is a cationic lipid with short triacyl
chains (C12) and long head groups. Cationic lipids with short
dialkyl chains (C14-C16) efficiently internalize mRNA lipoplexes
into cells compared with those with long dialkyl chains (C18)
(9). Koulov et al (23) reported that vesicles containing
cationic trialkyl chains promote greater membrane fusion than those
with structurally related cationic dialkyl and monoalkyl chains.
These studies indicate that TC-1-12-based mRNA lipoplexes are
effective mRNA carriers with fusogenic activity. Numerous studies
have developed the diacyl cationic lipids
1,2-dioleoyl-3-trimethylammonium-propane (DOTAP)- or dialkyl
cationic lipid dimethyl-dioctadecyl-ammonium bromide (DDAB)-based
cationic liposomes, which are combined with helper lipids for
efficient mRNA delivery (24,25).
It was previously revealed that TC-1-12-based mRNA lipoplexes
induce higher protein expression than DOTAP- or DDAB-based
lipoplexes (9). These findings
suggest TC-1-12-based mRNA lipoplexes as useful transfection
reagents for cultured cells.
In the present study, commercially available
Lipofectamine MessengerMAX mRNA lipoplexes exhibited higher
transfection efficiencies in HeLa, PC-3 and HepG2 cells than the
TC-1-12-based mRNA lipoplexes; however, they demonstrated high
cytotoxicity in these cell lines. Moreover, Lipofectamine
MessengerMAX mRNA lipoplexes are not suitable for in vivo
transfection. It was previously reported that intravenous injection
of TC-1-12-based ovalbumin mRNA lipoplexes prepared using the MEI
method induces ovalbumin-specific IgG1 antibodies in the serum
(26). Therefore, TC-1-12-based
mRNA lipoplexes can be used for both in vitro and in
vivo transfection.
The MEI method only requires the preparation of an
ethanol solution containing lipids to form mRNA lipoplexes, thus
eliminating the need for cationic liposome preparation before mRNA
transfection. Additionally, the lipid-ethanol solution used for
preparing mRNA lipoplexes in the MEI method can be stored at 37˚C
for at least 4 months. In the present study, mRNA lipoplexes
prepared using the MEI method at a charge ratio of 3:1 contained
0.23% (v/v) ethanol in the culture medium, and this ethanol
concentration was well-tolerated by the cultured cells (Fig. S4). Overall, the present findings
highlight the potential of TC-1-12-based mRNA lipoplexes prepared
using a lipid-ethanol solution for effective mRNA transfection.
Supplementary Material
Effects of cationic liposomes on Luc
activity in HeLa cells post-transfection. HeLa cells were
transfected only with cationic liposomes (mock transfection) at the
indicated charge ratios and incubated for 24 h. Each value
represents the mean ± standard deviation (n=3). FLuc, Firefly
luciferase.
Representative histograms of flow
cytometry after transfection with TC-1-12-based EGFP mRNA
lipoplexes to HeLa cells. EGFP mRNA lipoplexes were prepared using
the MEI and TFH methods at charge ratios of 2:1 to 5:1, transfected
into HeLa cells, and incubated for 24 h. Lipofectamine MessengerMAX
was used as a control. Percentage of EGFP-positive cells was
determined using flow cytometry. TC-1-12,
11-((1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl)propan-2-yl)amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide; EGFP, enhanced green fluorescent protein; MEI, modified
ethanol injection; TFH, thin-film hydration.
Representative histograms of flow
cytometry after transfection with the TC-1-12-based Cy5-labeled
mRNA lipoplexes to HeLa cells. Cy5-labeled mRNA lipoplexes were
prepared at charge ratios of 3:1 and 4:1 using the MEI and TFH
methods, respectively, and added to HeLa cells at 0.5 μg/ml
mRNA. As controls, Cy5-labeled mRNA solution (free mRNA) and
Lipofectamine MessengerMAX mRNA lipoplexes were added to the HeLa
cells. Fluorescence intensity in Cy5-labeled mRNA-transfected cells
was measured using flow cytometry 3 h after incubation. MEI method.
TC-1-12,
11-((1,3-bis(dodecanoyl-oxy)-2-((dodecanoyloxy)methyl)propan-2-yl)amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide; Cy5, cyanine 5; MEI, modified ethanol injection; TFH,
thin-film hydration.
Cytotoxicity in HeLa, PC-3 and HepG2
cells after treatment with the culture medium containing ethanol.
HeLa, HepG2, and PC-3 cells were incubated with the culture medium
containing 0.156-5% (v/v) ethanol for 24 h. Each value is
represented as the mean ± standard deviation (n=5 for 2.5% ethanol
in HeLa cells, n=8 for 0.313 and 0.156% ethanol in HeLa cells, n=7
for other groups in HeLa cells; n=3 for 5% ethanol in HepG2 cells
and n=4 for other groups in HepG2 cell; n=5 for all groups in PC-3
cells). **P<0.01 and ***P<0.001 vs.
untreated cells (0% ethanol).
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YH conceptualized the study, developed methodology,
conducted investigation, curated data, preformed formal analysis,
prepared the original draft, wrote, reviewed and edited the
manuscript. RS conducted investigation. YH and RS confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sahin U, Karikó K and Türeci Ö: mRNA-based
therapeutics-developing a new class of drugs. Nat Rev Drug Discov.
13:759–780. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Liu T, Liang Y and Huang L: Development
and delivery systems of mRNA vaccines. Front Bioeng Biotechnol.
9(718753)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Barbier AJ, Jiang AY, Zhang P, Wooster R
and Anderson DG: The clinical progress of mRNA vaccines and
immunotherapies. Nat Biotechnol. 40:840–854. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Warren L, Manos PD, Ahfeldt T, Loh YH, Li
H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, et al: Highly
efficient reprogramming to pluripotency and directed
differentiation of human cells with synthetic modified mRNA. Cell
Stem Cell. 7:618–630. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ramachandran S, Satapathy SR and Dutta T:
Delivery strategies for mRNA vaccines. Pharmaceut Med. 36:11–20.
2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yen A, Cheng Y, Sylvestre M, Gustafson HH,
Puri S and Pun SH: Serum nuclease susceptibility of mRNA cargo in
condensed polyplexes. Mol Pharm. 15:2268–2276. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen H, Ren X, Xu S, Zhang D and Han T:
Optimization of lipid nanoformulations for effective mRNA delivery.
Int J Nanomedicine. 17:2893–2905. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Malone RW, Felgner PL and Verma IM:
Cationic liposome- mediated RNA transfection. Proc Natl Acad Sci
USA. 86:6077–6081. 1989.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tang M, Sagawa A, Inoue N, Torii S, Tomita
K and Hattori Y: Efficient mRNA delivery with mRNA lipoplexes
prepared using a modified ethanol injection method. Pharmaceutics.
15(1141)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bangham AD, Standish MM and Watkins JC:
Diffusion of univalent ions across the lamellae of swollen
phospholipids. J Mol Biol. 13:238–252. 1965.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hattori Y, Nakamura M, Takeuchi N, Tamaki
K, Shimizu S, Yoshiike Y, Taguchi M, Ohno H, Ozaki KI and Onishi H:
Effect of cationic lipid in cationic liposomes on siRNA delivery
into the lung by intravenous injection of cationic lipoplex. J Drug
Target. 27:217–227. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Barichello JM, Ishida T and Kiwada H:
Complexation of siRNA and pDNA with cationic liposomes: The
important aspects in lipoplex preparation. Methods Mol Biol.
605:461–472. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hattori Y, Saito H, Nakamura K, Yamanaka
A, Tang M and Ozaki KI: In vitro and in vivo transfections using
siRNA lipoplexes prepared by mixing siRNAs with a lipid-ethanol
solution. J Drug Deliv Sci Technol. 75(103635)2022.
|
|
14
|
Hattori Y, Tamaki K, Sakasai S, Ozaki KI
and Onishi H: Effects of PEG anchors in pegylated siRNA lipoplexes
on in vitro genesilencing effects and siRNA biodistribution
in mice. Mol Med Rep. 22:4183–4196. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Smith MC, Crist RM, Clogston JD and McNeil
SE: Zeta potential: A case study of cationic, anionic, and neutral
liposomes. Anal Bioanal Chem. 409:5779–5787. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jarzebska NT, Frei J, Lauchli S, French
LE, Guenova E, Gouttefangeas C, Kündig TM, Mellett M and Pascolo S:
Lipofection with synthetic mRNA as a simple method for T-cell
immunomonitoring. Viruses. 13(1232)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wei X, Shao B, He Z, Ye T, Luo M, Sang Y,
Liang X, Wang W, Luo S, Yang S, et al: Cationic nanocarriers induce
cell necrosis through impairment of Na(+)/K(+)-ATPase and cause
subsequent inflammatory response. Cell Res. 25:237–253.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Opsomer L, Jana S, Mertens I, Cui X,
Hoogenboom R and Sanders NN: Efficient in vitro and in vivo
transfection of self-amplifying mRNA with linear
poly(propylenimine) and poly(ethylenimine-propylenimine) random
copolymers as non-viral carriers. J Mater Chem B. 12:3927–3946.
2024.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ahlemeyer B, Vogt JF, Michel V,
Hahn-Kohlberger P and Baumgart-Vogt E: Microporation is an
efficient method for siRNA-induced knockdown of PEX5 in HepG2
cells: Evaluation of the transfection efficiency, the PEX5 mRNA and
protein levels and induction of peroxisomal deficiency. Histochem
Cell Biol. 142:577–591. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hattori Y, Yoshizawa T, Koga K and Maitani
Y: NaCl induced high cationic hydroxyethylated cholesterol-based
nanoparticle-mediated synthetic small interfering RNA transfer into
prostate carcinoma PC-3 cells. Biol Pharm Bull. 31:2294–2301.
2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hattori Y, Hagiwara A, Ding W and Maitani
Y: NaCl improves siRNA delivery mediated by nanoparticles of
hydroxyethylated cationic cholesterol with amido-linker. Bioorg Med
Chem Lett. 18:5228–5232. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hattori Y, Ding WX and Maitani Y: Highly
efficient cationic hydroxyethylated cholesterol-based
nanoparticle-mediated gene transfer in vivo and in vitro in
prostate carcinoma PC-3 cells. J Control Release. 120:122–130.
2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Koulov AV, Vares L, Jain M and Smith BD:
Cationic triple-chain amphiphiles facilitate vesicle fusion
compared to double-chain or single-chain analogues. Biochim Biophys
Acta. 1564:459–465. 2002.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ma X, Wu F, Peng C, Chen H, Zhang D and
Han T: Exploration of mRNA nanoparticles based on DOTAP through
optimization of the helper lipids. Biotechnol J.
18(e2300123)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Anderluzzi G, Lou G, Gallorini S, Brazzoli
M, Johnson R, O'Hagan DT, Baudner BC and Perrie Y: Investigating
the impact of delivery system design on the efficacy of
self-amplifying RNA vaccines. Vaccines (Basel).
8(212)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hattori Y and Tang M: Effect of cationic
and neutral lipids in cationic liposomes on antibody production
induced by systemic administration of mRNA lipoplexes into mice. J
Drug Deliv Sci Technol. 100(106034)2024.
|