Introduction
Cancer encompasses a range of conditions marked by
an unregulated cell proliferation that infiltrates into nearby
tissues, and potentially metastasizes to distant areas of the body
(1). Despite progress in cancer
treatment, it remains a worldwide major health issue. In 2023, the
United States reported 1,958,310 new cases of cancer and 609,820
mortalities (2). Jordan also faces
a similar challenge, and reported 11,559 new cases and 6,190
mortalities in 2023. Furthermore, patients are mainly diagnosed
with breast (20%), colorectal (11.6%) and lung cancer (7.4%)
(2).
Cancer treatment predominantly relies on
conventional chemotherapy, which uses a combination of drugs (such
as 5-fluorouracil, leucovorin, oxaliplatin and irinotecan)
alongside surgical procedures and radiation therapy (3). The development of resistance towards
these conventional chemotherapy agents reduces their effectiveness,
resulting in only minimal and temporary advantages (4). However, the process of developing
novel drugs has considerable challenges, including high financial
burdens, extensive clinical trials and rigorous regulatory demands
(5).
Nanomedicine is an emerging discipline and focuses
on using nano-sized materials as biomedical tools for both
diagnosis and treatment purposes, especially within oncology
(6). This approach is gaining
interest as a preferred method over chemotherapy drugs due to its
enhanced safety and efficacy (7).
It facilitates the amalgamation of drugs with synergistic effects,
potentially enhancing treatment efficacy, reducing adverse effects
and mitigating the emergence of drug resistance (8,9). These
multifaceted benefits render the use of nano-scaled carriers and
drugs a progressively favored option over conventional therapies,
particularly in addressing diseases such as cancer (10-17).
Silver nanoparticles (AgNPs) have gained attention
within pharmaceutical and medical settings due to having
antimicrobial properties and inducing cytotoxicity in diverse
cancer cell lines such as colorectal and breast cancer cell lines
(18,19). AgNPs exhibit multifaceted
applications in the research and therapy of cancer. They are
utilized primarily as effective drug delivery systems,
encapsulating anticancer agents (such as methotrexate, imatinib,
doxorubicin and gemcitabine) to enhance their targeting and
efficacy against tumors while minimizing systematic side effects
(20). AgNPs themselves possess
intrinsic anticancer properties through mechanisms such as the
induction of apoptosis and disruption of cellular signaling
pathways in cancer cells (21).
Furthermore, AgNPs are used in diagnostics by functionalizing their
surfaces with specific biomolecules (such as aptamer or specific
antibodies) that bind to cancer biomarkers [such as
platelet-derived growth factors, human epidermal growth factor
receptor (EGFR) 2 and prostate-specific antigen], resulting in
sensitive detection methods (22).
Additionally, AgNPs are radiosensitizers and increase the
effectiveness of radiotherapy by enhancing the DNA damage in cancer
cells (23). These diverse
applications highlight the potential for AgNPs to advance cancer
treatment modalities through targeted drug delivery, diagnostic and
therapeutic interventions.
Bioactive components sourced from a variety of
organisms, including plants and animals, offer anticancer and
various other biological effects, presenting opportunities for the
development of pharmaceutical products (24-27).
Phytoestrogens and Rhizoma polygonati exhibit anticancer and
antiaging properties (28,29). Tribulus terrestris indicates
a potential for neuroprotection, and may be a treatment option for
cognitive disorders such as Parkinson's and Alzheimer's disease
(30), while camel milk whey
protein hydrolysates provide anti-inflammatory and anticancer
benefits (31). Furthermore,
actinobacterial agents (such as indole-3-acetic acid, polyamines
and 1-aminocyclopropane-1-carboxylic acid deaminase) from
Salicornia bigelovii indicate a potential for enhancing
agricultural productivity, such as enhancing the root biomass,
increasing the yield of the seeds and increasing the tolerance of
plants to saline soil (32).
Additionally, sea sponges in particular have shown antioxidant and
anti-inflammatory properties, which reduce the risk of cancer
(33).
Stylissa carteri and Amphimedon
chloros, marine sponges that inhabit the exclusive economic
zone of Indonesia, have gained attention for their therapeutic
properties, particularly their potential in the treatment of cancer
(34,35). The present study aimed to
investigate the cytotoxic effects of AgNPs with extracts of
Stylissa carteri or Amphimedon chloros, collected
from the Red Sea coastline, against various types of cancer and
bacterial strains. To the best of our knowledge, the present study
was the first to conduct an evaluation of the cytotoxicity of
Stylissa carteri and Amphimedon chloros.
Materials and methods
Materials
L-glutamine-containing Dulbecco's Modified Eagle
Medium (DMEM), penicillin/streptomycin and fetal bovine serum (FBS)
were purchased from EuroClone SpA. Both phosphate buffer saline and
trypsin were purchased from Thermo Fisher Scientific, Inc. Ethyl
acetate was purchased from Sigma-Aldrich; Merck KGaA. The MTT
reagent the stop solution [dimethyl sulfoxide (DMSO)] and trypan
blue dye were provided by Promega Corporation. Flow tubes (BD
Biosciences) were used for centrifugation. BD FACSDiva™ software
(version 8.0; BD Biosciences) and StemPro™ Accutase™ Cell
Dissociation Reagent (Gibco; Thermo Fisher Scientific, Inc.) were
used in the present study.
Biosynthesis of AgNPs using
Aspergillus flavus
In the present study, AgNPs were synthesized using
Aspergillus flavus strain MG973280, which was obtain from
American Type Culture Collection (ATCC). The fungal culture was
prepared as previously described (36). Briefly, Aspergillus flavus
spores were adjusted to a concentration of 2.0x106 and
cultivated in a complex broth medium that included 10 g/l glucose,
10 g/l yeast extract and 5 g/l NaCl (pH 7). Subsequently, the
culture was incubated for 72 h at 33˚C and cells were agitated
using an orbital shaker at 0.10 x g. Whatman® grade 1
filter paper was used to filter the culture at the end of the
incubation period. The resultant mycelia, or biomass, were then
collected and washed with deionized distilled water.
To produce AgNPs using the fungal biomass, the
method reported in the study by Jadidev and Narasimha (37) was followed with minor adjustments.
Briefly, 10 g of the first crude biomass were agitated using an
orbital shaker at 0.10 x g for 72 h while submerged in 100 ml of
sterile deionized water at 33˚C and pH 7.0. After the crude biomass
was filtered using Whatman® grade 1 filter paper, the
resulting suspension containing the fungal filtrate without biomass
was collected. Subsequently, 100 ml of this biomass-free fungal
filtrate was combined with 1 mM of silver nitrate to produce AgNPs.
The mixtures were then continuously stirred in the dark at 27˚C for
72 h using a magnetic stirrer.
Morphology and particle size
analysis
NPs were lyophilized at -54˚C and 0.2 mbar for 24 h
using a Lyovapor L-200 freeze dryer (BUCHI UK Ltd.). Scanning
electron microscope (SEM) images were obtained at 200 kV using a
JEM-2010 microscope (JEOL, Ltd.) in order to analyze the surface
morphology of the AgNPs. The powdered samples were coated with gold
using sputter coater and a carbon thread coater (Leica Biosystems).
The resulting particle size was measured using the Zetasizer Nano
ZSP (Malvern Panalytical, Ltd.).
Sponge collection and
identification
Sponge samples were gathered from several locations
in the Gulf of Aqaba (29˚27' N, 34˚58' E) by specialists from the
Marine Science Station at the University of Jordan (Aqaba, Jordan).
The sponges were found by divers between the depths of 1-18 meters.
After being cleared of debris, the samples were frozen at -80˚C,
and then sent in sealed sterile polyethylene containers (which were
submerged in seawater in order to maintain a moist environment
during transport) to Aqaba International Laboratories-BEN HAYYAN
for freeze-drying and extraction. Using specified morphological
traits listed in the Systema Porifera and World Porifera Database
(35,38-42),
the sponges, Stylissa carteri and Amphimedon chloros,
were identified.
Extraction of sponge extracts
Sponge fragments were weighed, freeze-dried for 48 h
at -40˚C and then ground into a powder. The powder (weighing
45.0-350.0 g) was soaked at room temperature for 48 h in a 1:1
mixture of methanol and dichloromethane. Subsequently, a
lyophilizer was used to filter and dehydrate the solution. The
final pure extract was divided into non-polar, semi-polar and polar
components by dispersing it in distilled water and using n-hexane
and ethyl acetate as partitioning agents. The pure extract from
each solvent was labeled and kept frozen at -20˚C (43,44).
Cancer cell lines culture
In the present study, to investigate the anticancer
activity of the candidate mixtures, four cancer cell lines were
used: i) A human lung cancer cell line (A549; cat. no. CCL-185;
ATCC); ii) a human colorectal cancer cell line (HT-29; cat. no.
HTB-38; ATCC); iii) a human breast cancer cell line (MCF7; cat. no.
HTB-22; ATCC); and iv) a pancreatic cancer cell line (PANC-1; cat.
no. CRL-1469; ATCC). For selective purposes, a normal human
umbilical vein endothelial cell line (HUVEC; cat. no. CRL-1730;
ATCC) was used. All of the cell lines, were cultured in DMEM
containing 10% FBS, 10 mM HEPES buffer, 100 µg/ml L-glutamine, 50
µg/ml gentamicin, 100 µg/ml penicillin and 100 mg/ml streptomycin.
ATCC provided authenticated cell lines, which ensured that the cell
lines were correctly identified and contamination-free.
Additionally, mycoplasma testing was performed on all of the cell
lines used in the present study, which confirmed the absence of
mycoplasma contamination.
Colorimetric MTT assay
A colorimetric MTT assay was used to evaluate the
viability of the cells. Each type of cell line was seeded in
96-well plates at a density of 1x104 cells per well and
cultured for 24 h at 37˚C (45).
Subsequently, the cells were treated with: i) The extracts of each
of the sponge species, Stylissa carteri and Amphimedon
chloros, separately at concentrations ranging from 6-200 µg/ml;
ii) AgNPs at concentrations ranging from 0.75-200 µg/ml; or iii)
mixtures of 0.75 µg/ml AgNPs with extracts of Stylissa
carteri or Amphimedon chloros at concentrations ranging
from 6-200 µg/ml. Following a 72-h incubation period at 37˚C
(46), 15 µl of MTT solution was
added to each well, and then the plates were incubated at 37˚C for
4 h. Subsequently, 100 µl of DMSO was added to each well to
dissolve the formed formazan crystal. The BioTek ELx800™ microplate
reader was then used to measure the optical density at 590/630 nm
to assess cell growth.
Screening and identifying compounds
from the sponge extracts using liquid chromatography-mass
spectrometry (LC-MS)
LC-MS is an analytical method that combines MS and
LC. LC separates components of a mixture by passing them through a
chromatographic column. Even when LC is unable to positively
identify these separated components, MS can be used to identify
both known and unknown chemicals and provide information on their
structures (47).
A mobile phase comprising solvents A and B in a
gradient was used in the LC-MS analysis. Solvent A consisted of
formic acid dissolved in water at a concentration of 0.1% (v/v),
and solvent B consisted of formic acid dissolved in acetonitrile at
the same concentration. The experimental parameters used included
an Agilent Zorbax Eclipse XDB-C18 column (Agilent Technologies,
Inc.) measuring 2.1x150 mm x3.5 µm, a temperature maintained at
25˚C, the sponge extract dissolved in methanol at a concentration
of 18 mg/ml and an injection volume of 1 µl. The sample was
injectedinto the LC-MS system, which included the following
components: A mass detector using a SIL-30AC autosampler with a
cooler, a Shimadzu CBM-20A system controller, an LC-30AD pump, a
CTO-30 column oven and an electrospray ion-mass spectrometer with a
skimmer voltage of 65 V and a fragmentor voltage of 125 V, all
components were of the Shimadzu LC-MS 8030 (Shimadzu Corporation).
Nitrogen gas with 99.99% purity and a flow rate of 10 l/min served
as the drying gas during operation in positive ion mode.
Additionally, a nebulizer operating at a pressure of 45 psi and a
capillary temperature of 350˚C were used. Subsequently, the
mass/number of ions of the eluent was scanned from positions
100-1,000. Authentic standard substances were used for result
validation.
Molecular docking
Molecular docking simulations were performed using
tyrosine kinase receptor A [TrkA; Protein Data Bank (PDB) ID, 7VKO;
https://www.rcsb.org/structure/7VKO]
(48) and an EGFR kinase domain
(PDB ID, 4I23; https://www.rcsb.org/structure/4i23) (49) in order to investigate the binding
affinities of the major components in Stylissa carteri or
Amphimedon chloros. AutoDock (version 4.2.6) (50,51)
was used according the methods described in the studies by Saqallah
et al (52) and Shtaiwi
et al (53) with slight
modifications. Briefly, all protein structures were prepared using
BIOVIA® Discovery Studio® (version 16.1)
(54) by removing water molecules
(if applicable) and complexed co-structures. Complexed inhibitors
(dacomitinib and repotrectinib in the EGFR kinase domain and TrkA
structures, respectively) were separated from the crystal
structures to be used as control ligands. Using AutoDockTools
(version 1.5.6) (50), Kollman
charges and polar hydrogen atoms were assigned to the proteins.
Additionally, the 3D conformers of the compounds identified in the
sponge extracts were downloaded from the NCBI PubChem database
(pubchem.ncbi.nlm.nih.gov) and Gasteiger
charges were assigned accordingly. A grid box with the size of 153
Å was set with the coordinates of -0.697, -52.750, -23.233 as x, y,
z, respectively, for the EGFR kinase domain protein, and with the
same size at -18.081, -43.125, -13.177 as x, y, z, respectively,
for the TrkA protein. Simulations were carried out using 100
Lamarckian Genetic Algorithm runs with default parameters.
Conformations with the lowest free energy of binding and the most
populated cluster were selected for further analysis. Analyses of
the interactions were carried out using BIOVIA®
Discovery Studio® (version 16.1).
Isolation of the bacterial strains
utilized in antibacterial studies
To study the antibacterial activity of the
sponge-AgNPs mixtures, bacterial cells were obtained from hydatid
cyst fluid sourced from various affected anatomical sites, such as
the liver and lung, and five different isolated bacterial species
were obtained in a previous study by Al Qaisi et al
(55). The bacterial species used
in the present study were Staphylococcus xylosus,
Klebsiella oxytoca, Enterobacter aerogenes,
Micrococcus spp. and Escherichia coli. Pseudomonas
aeruginosa (cat. no. 27853; ATCC) was used as a control. The
isolates were preserved on nutrient agar slants at 4˚C for up to 1
month.
Antibacterial agar disc diffusion
assay
Isolated bacteria were first cultured in Muller
Hinton Broth at 37˚C for 24 h for activation before carrying out
the antibacterial tests. The antibacterial activity of the sponge
extracts and AgNPs were measured using Muller Hinton agar and the
disc diffusion method. Inhibitory zone diameters were calculated in
mm (56). Briefly, 20 µg of sponge
extract (either from Stylissa carteri or Amphimedon
chloros) or AgNPs were used to impregnate sterile filter paper
discs, which were then put on inoculated Petri dishes containing
0.1 ml of a bacterial solution containing 1.5x108 colony
forming units/ml. Discs pre-dosed with 50 g/ml chloramphenicol and
50 g/ml ampicillin were used as positive controls, while discs
containing 5% DMSO served as negative controls. After 24 h of
incubation at 37˚C, the diameters of the inhibition zones around
the extract-impregnated discs were measured and compared with those
of the controls. To identify active and inactive sponge
preparations, the inhibition zone diameter was used as a metric.
Each sample was tested three times. To test for sponge extract-AgNP
synergy, two different concentrations were used, which were 10 µg
sponge extract and 10 µg AgNPs/disc, and 10 µg sponge extract and 5
µg AgNPs/disc (57).
Statistical analysis
Results from three or four independent experiments
are presented as the means ± standard deviation. Statistical
variances between the control group and various treatment groups
were evaluated using GraphPad Prism version 10 (Dotmatics). Results
were analyzed using one-way ANOVA followed by the Dunnett's test,
or using an unpaired t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
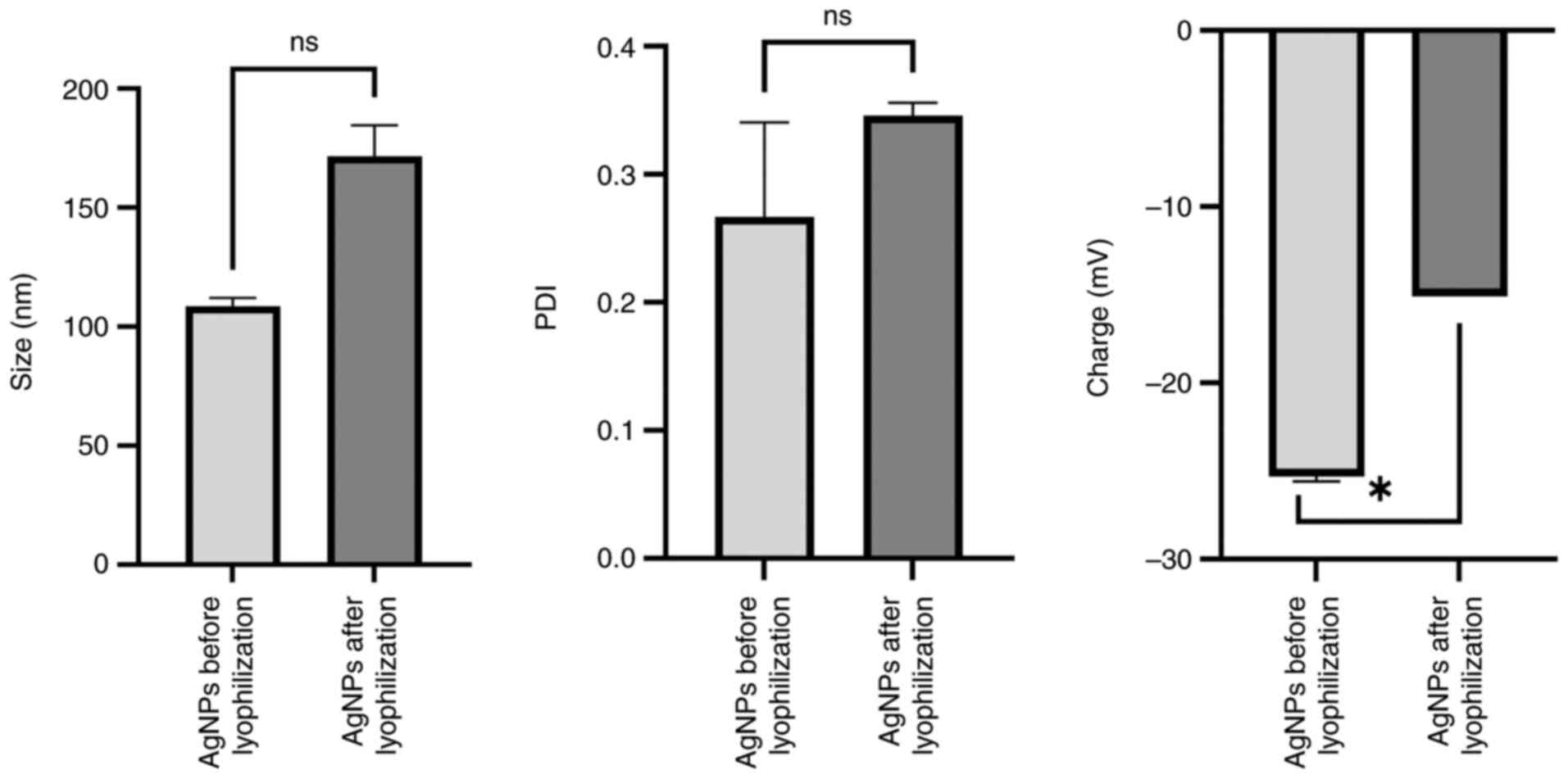
Lyophilization alters the size, charge
and stability of AgNPs
A significant difference in charge, but not in size
and polydispersity index (PDI) between the pre-and
post-lyophilization states are presented in Fig. 1. Before lyophilization, the mean
charge was-25.30±0.28 mV, the mean size was 108.65±3.45 nm and the
PDI was 0.27±0.07. After the process of lyophilization, the mean
size increased to 171.60±13.01 nm, the mean charge increased to
-15.05±0.01 mV and the PDI increased to 0.34±0.01. Although all
measurements for nanoparticle size (ideal range, 50-200 nm), charge
(ideal range, -10 to -30 mV) and PDI (ideal range, ≤0.5) remained
within the ideal ranges, these differences indicated that the
lyophilization process did not significantly affect the properties
of the AgNPs.
SEM analysis reveals uniform distribution and
nanoscale size of the AgNPs with minimal aggregation. To
investigate the produced AgNPs, SEM was used (Fig. 2). The AgNPs had a uniform
distribution and an approximately spherical shape, with negligible
aggregation, which indicated a stable production process. Based on
the 200 nm scale bar, the majority of the particles had a diameter
of <100 nm, which indicated that the size distribution was in
the nanoscale zone. Particle aggregation occurs frequently in
nanoparticle samples (58);
however, in the present study, only minimal aggregation was
observed, which indicated high dispersion stability. The AgNPs
appeared to have a smooth surface, which is consistent with the
nature of nanoparticles made using chemical reduction techniques
(59,60). These features implied that these
AgNPs were suitable for various applications including anticancer
and antimicrobial activity.
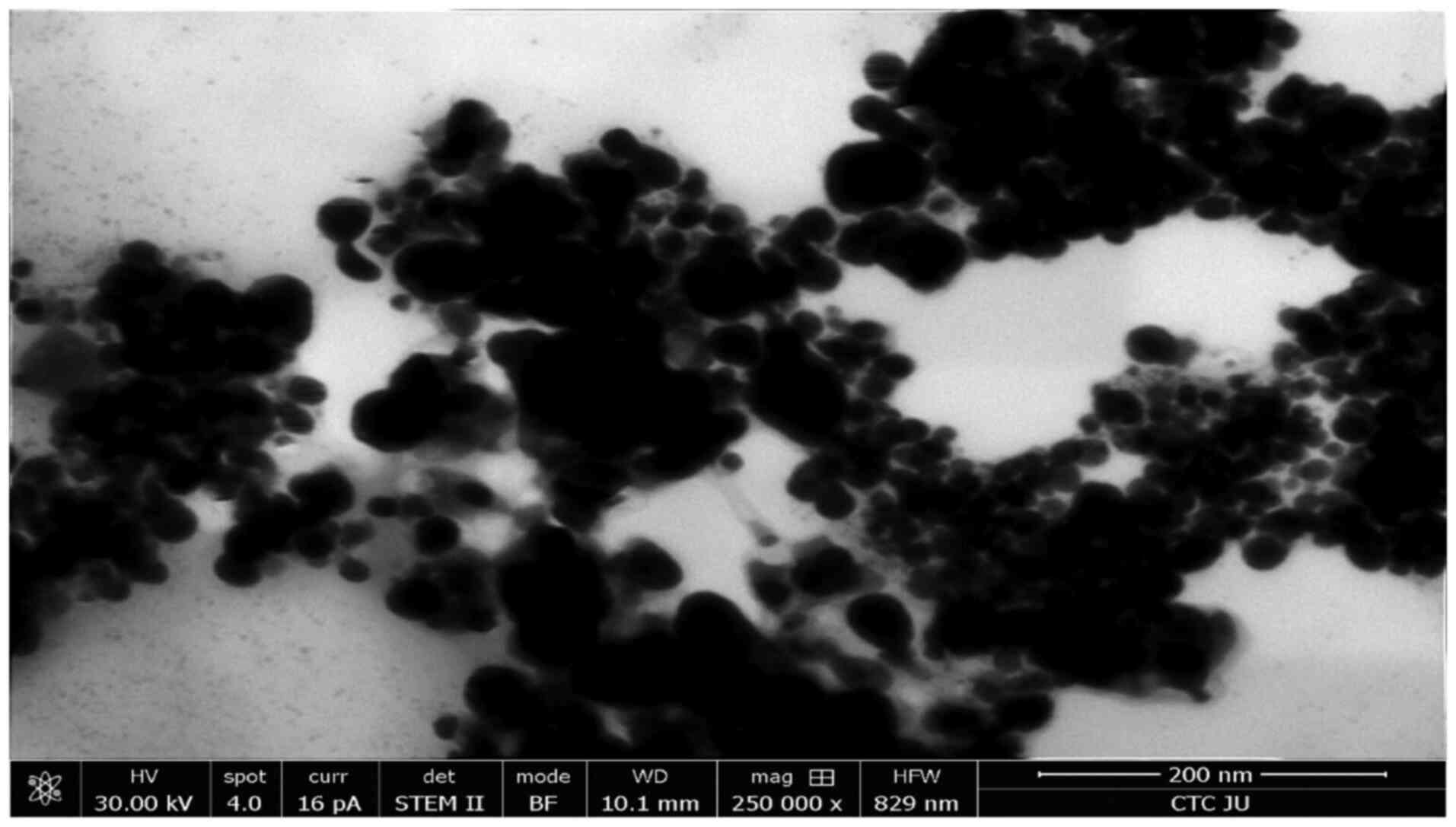 | Figure 2SEM micrograph of AgNPs. A
high-resolution SEM image of the morphology and distribution of
AgNPs, which were synthesized using 1.0 mM silver nitrate and the
fungus Aspergillus flavus. SEM, scanning electron
microscopy; AgNPs, silver nanoparticles; HV, high voltage
(accelerating voltage in kV applied to the microscope); spot, spot
size (beam diameter for optimal resolution); pA, picoamperes; curr,
current (electron beam current in pA); det, detector (type of
detector used to capture the image); STEM II, scanning transmission
electron microscopy secondary image; BF, bright field (imaging mode
providing high-contrast details in transmission images); WD,
working distance (distance between sample surface and objective
lens); mag, magnification; HFW, horizontal field width; CTC JU,
Cells Therapy Center, Jordan University. |
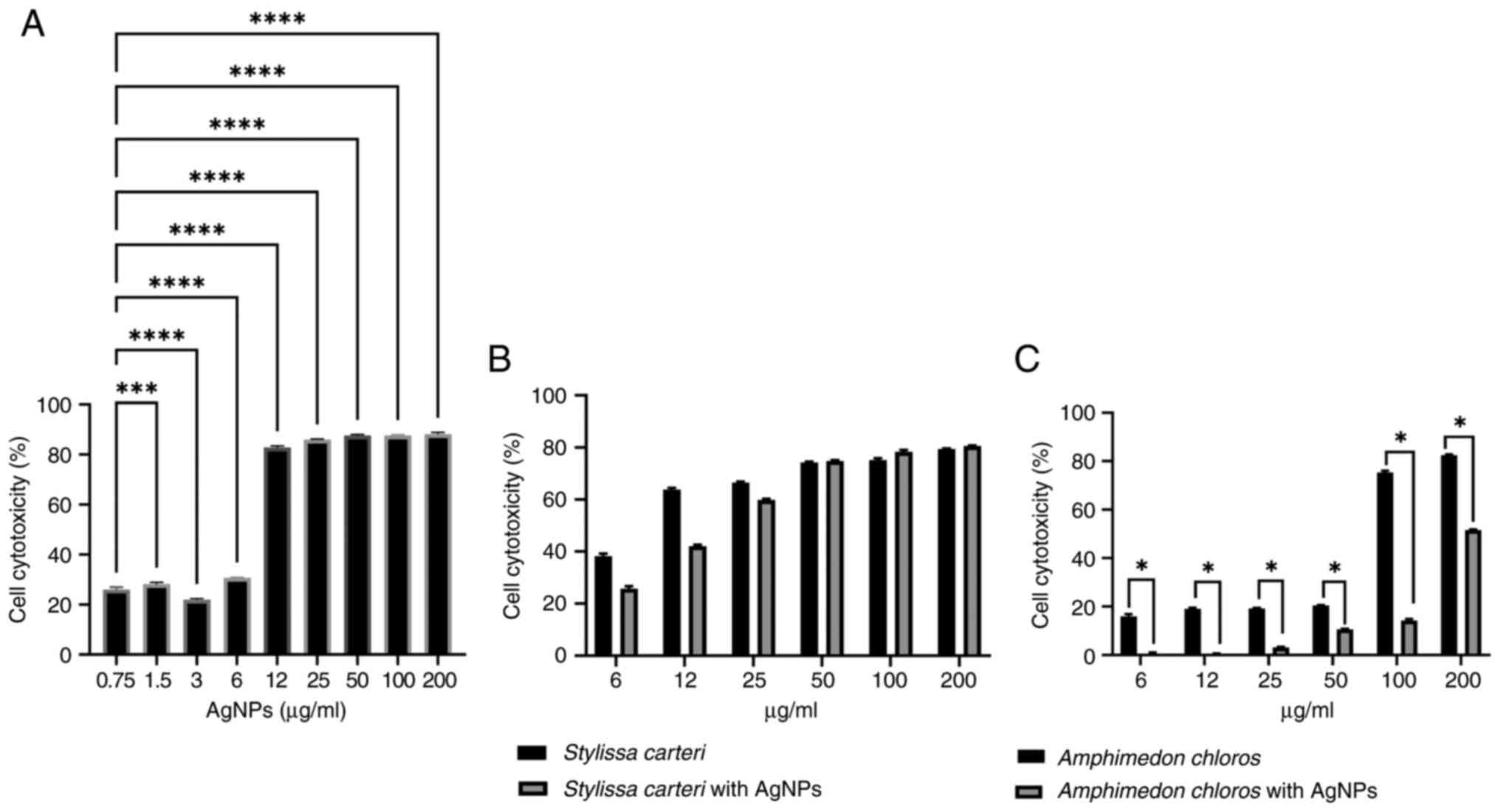
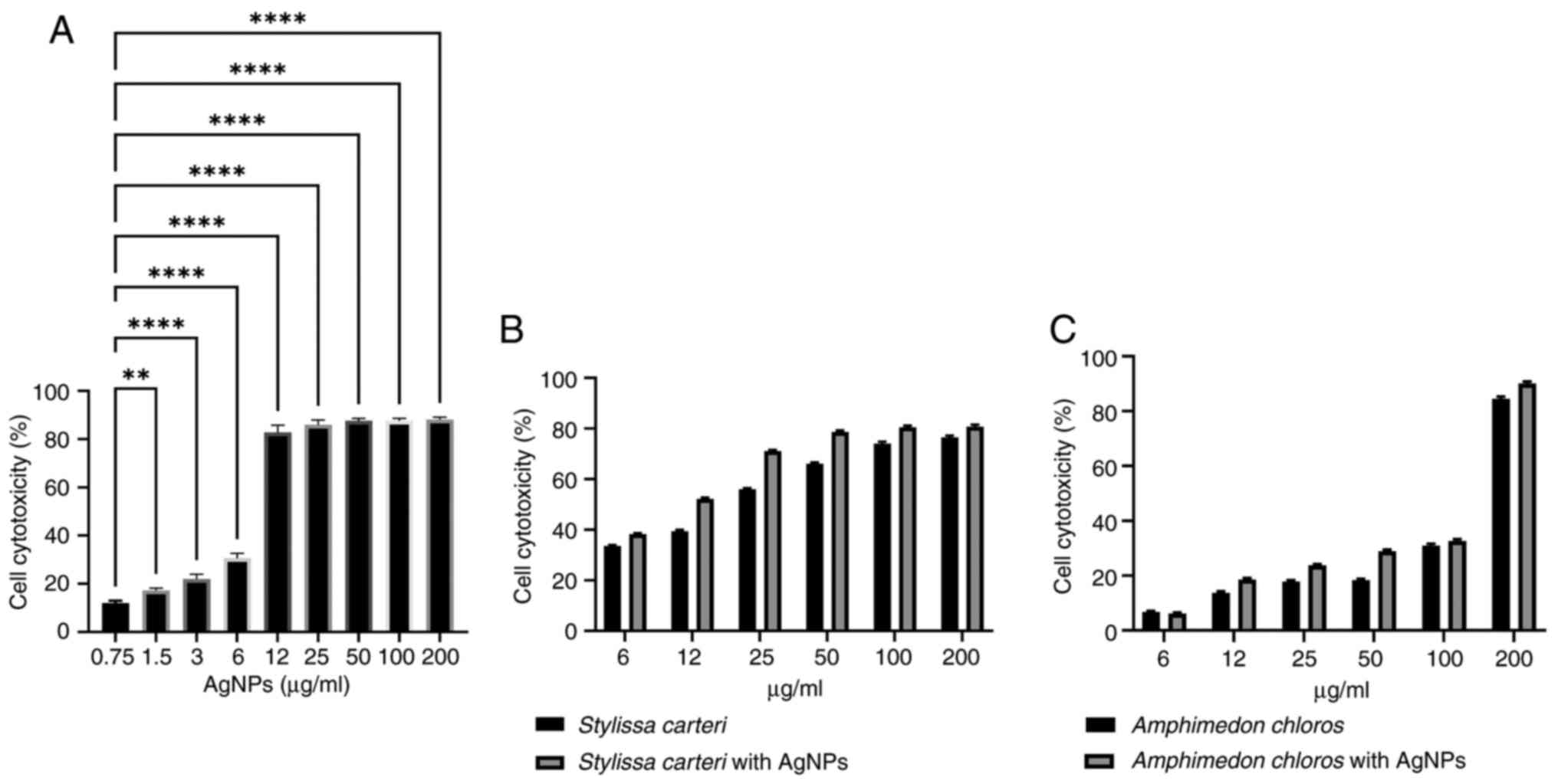
AgNPs with sponge extracts reveal
selective cytotoxicity when using the HUVEC normal cell line
To investigate the selective cytotoxicity of AgNPs
on the HUVEC normal cell line, an MTT assay was carried out.
Cytotoxicity was observed across concentrations ranging from
0.75-200 µg/ml of AgNPs (Fig. 3A).
There was a significant difference in the cell cytotoxicity between
the 0.75 and 3 µg/ml AgNPs (Fig.
3A), which indicated that 3 µg/ml AgNPs had a lower
cytotoxicity compared with 0.75 µg/ml AgNPs in the HUVEC normal
cell line. However, 0.75 µg/ml AgNPs were used in the subsequent
experiments based on several factors, such as to prevent drug
resistance. An advantage of combination therapy is the prevention
of drug resistance, which often occurs when high doses of a single
agent are used repeatedly (61);
therefore, using a lower concentration (0.75 µg/ml) of AgNPs
avoided a high-dose exposure, which could otherwise lead to
resistance. Additionally, lower doses of nanoparticles may be
preferable for long-term use in order to minimize potential side
effects while still retaining efficacy (62). Therefore, the subsequent combination
experiments used Stylissa carteri or Amphimedon
chloros extracts with 0.75 µg/ml AgNPs to minimize the
cytotoxic effect of the AgNPs on the HUVEC normal cell line while
maximizing the cytotoxic potential against various cancer cell
lines. Fig. 3B presents the
cytotoxic effects of Stylissa carteri extract alone and in
combination with 0.75 µg/ml AgNPs. Fig.
3C presents the cytotoxicity of Amphimedon chloros
extract alone and in combination with 0.75 µg/ml AgNPs. The results
indicated a significant reduction in HUVEC cytotoxicity when the
Amphimedon chloros extract was combined with AgNPs compared
with the Amphimedon chloros extract alone.
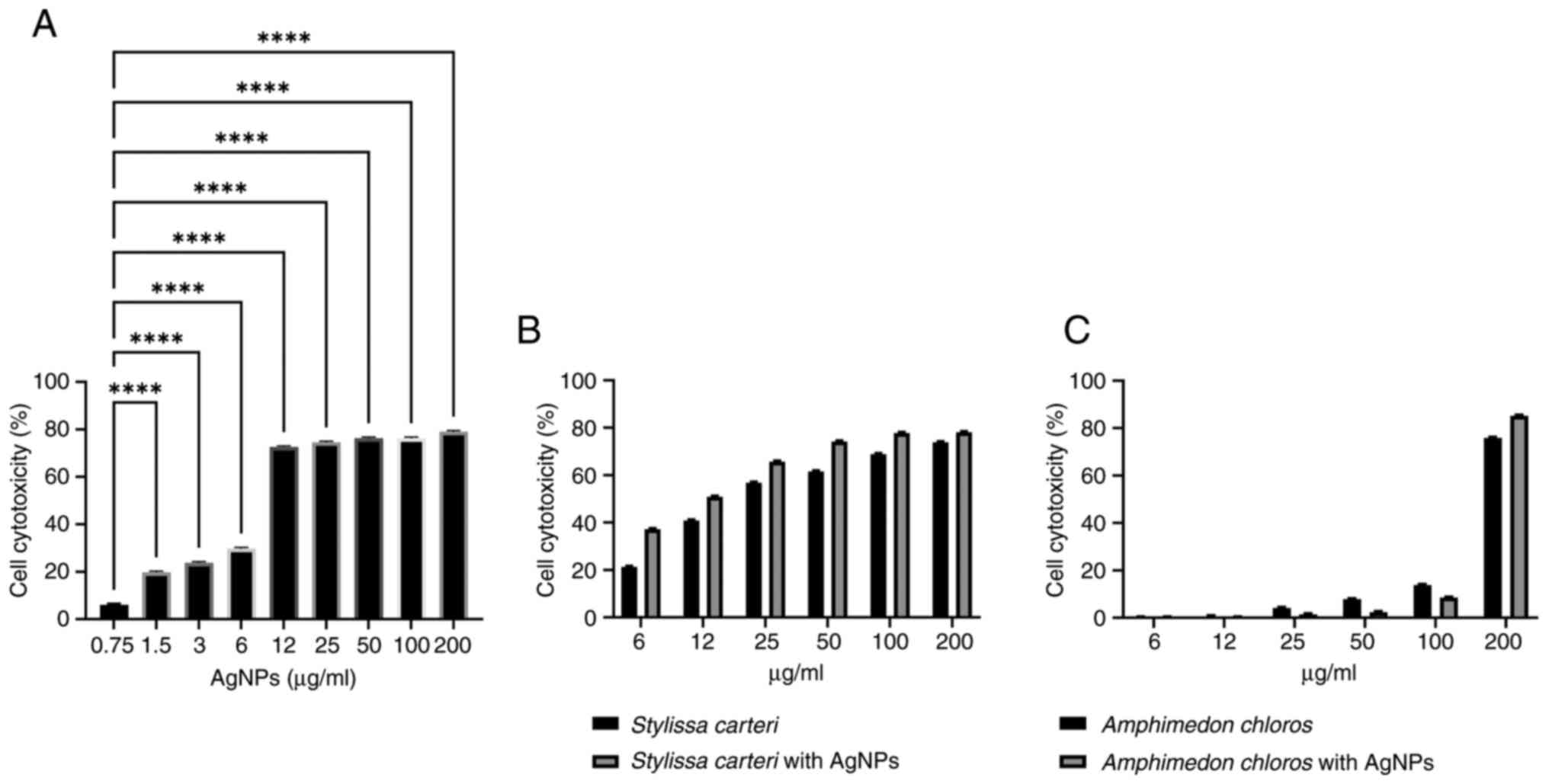
Enhancement of the cytotoxicity of
AgNPs with sponge extracts when using the A549 cell line
Fig. 4A demonstrates
the cytotoxic impact of the AgNPs on the A549 cell line. The
results revealed a significant increase in the cytotoxicity within
the concentration range of 1.5-200 µg/ml AgNPs compared with the
concentration of 0.75 µg/ml AgNPs. Fig.
4B presents the cytotoxicity of the Stylissa carteri
extract alone and in combination with 0.75 µg/ml AgNPs. Fig. 4C presents the cytotoxic effects of
the Amphimedon chloros extract alone and in combination with
0.75 µg/ml AgNPs. The results revealed a significant augmentation
in the A549 cell cytotoxicity when the Stylissa carteri
extract was co-administered with 0.75 µg/ml AgNPs compared with the
cytotoxic effects of the Stylissa carteri extract alone.
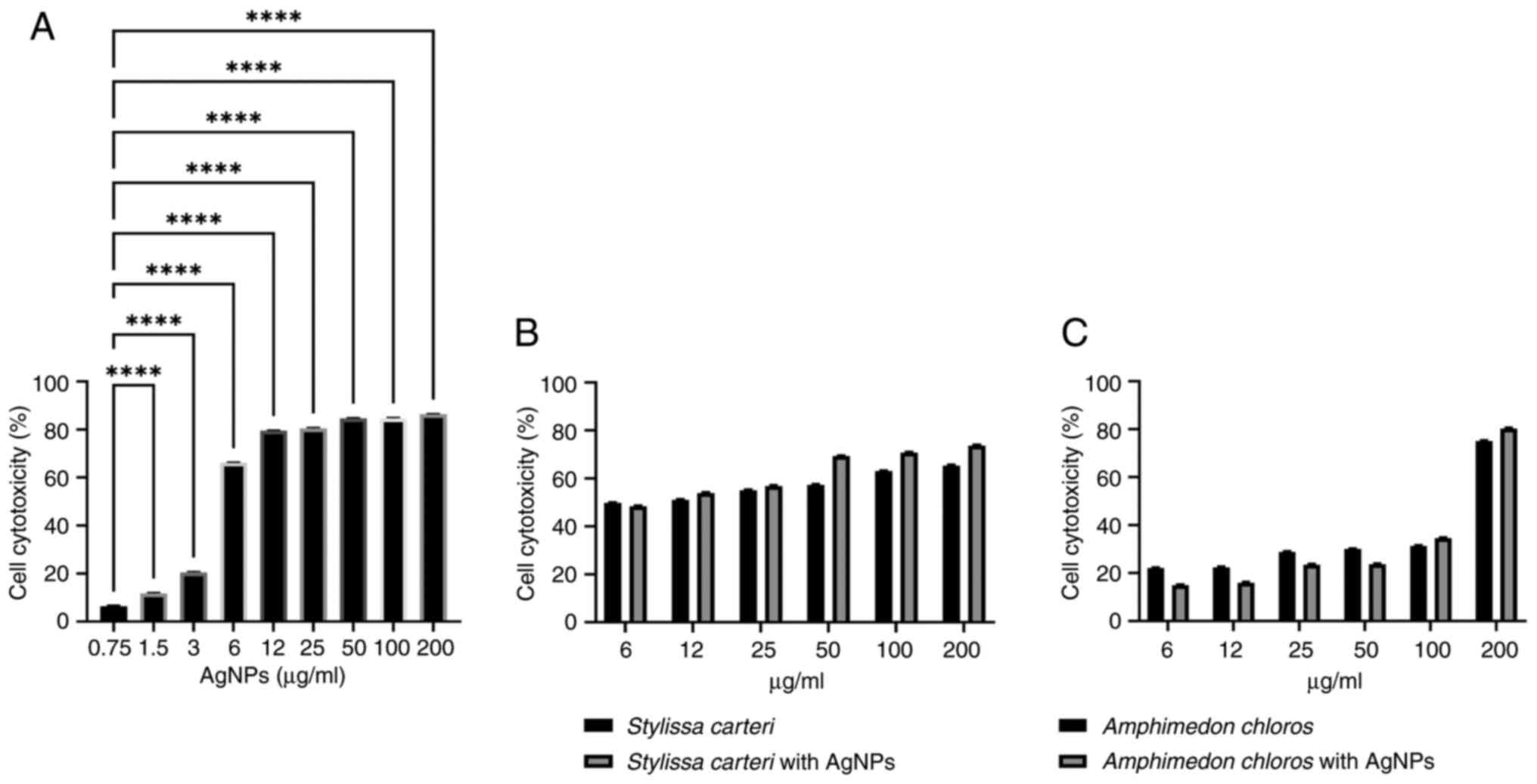
Neither of the sponge extracts
significantly increase the cytotoxicity of the AgNPs when using the
MCF7 cell line
Fig. 5A demonstrates
the cytotoxic effects of the AgNPs on the MCF7 cell line. The
results revealed a significant increase in the cytotoxicity within
the concentration range of 1.5-200 µg/ml AgNPs compared with the
concentration of 0.75 µg/ml AgNPs. Fig.
5B presents the cytotoxicity of the Stylissa carteri
extract alone and in combination with 0.75 µg/ml AgNPs, and
Fig. 5C presents the cytotoxicity
of the Amphimedon chloros extract alone and in combination
with 0.75 µg/ml AgNPs, when using the MCF7 cell line. However, the
cytotoxic effects of the Stylissa carteri or the
Amphimedon chloros extract with 0.75 µg/ml AgNPs were not
significantly different using the MCF7 cell line compared with the
cytotoxic effects of the Stylissa carteri or the
Amphimedon chloros extracts alone, respectively.
Neither of the sponge extracts
significantly increase the cytotoxicity of the AgNPs when using the
PANC-1 cell line
Using the PANC-1 cell line, there was a significant
increase in the cytotoxicity within the concentration range of
1.5-200 µg/ml AgNPs compared with the concentration of 0.75 µg/ml
AgNPs (Fig. 6A). Fig. 6B and C presents the cytotoxic effects of the
Stylissa carteri extract alone and in combination with 0.75
µg/ml AgNPs, along with the cytotoxicity of the Amphimedon
chloros extract alone and with 0.75 µg/ml AgNPs, when using the
PANC-1 cell line. However, the cytotoxic effects of the Stylissa
carteri or the Amphimedon chloros extract with 0.75
µg/ml AgNPs were not significantly different using the PANC-1 cell
line compared with the cytotoxic effects of the Stylissa
carteri or the Amphimedon chloros extracts alone,
respectively.
Neither of the sponge extracts
significantly increase the cytotoxicity of the AgNPs when using the
HT-29 cell line
Fig. 7A presents the
cytotoxic effects of the AgNPs on the HT-29 cell line across a
concentration range of 0.75-200 µg/ml. The results revealed a
significant increase in the cytotoxicity within the concentration
range of 1.5-200 µg/ml AgNPs compared with the concentration of
0.75 µg/ml AgNPs. Furthermore, a notable increase in the cell
cytotoxicity was observed at AgNP concentrations of 12-200 µg/ml.
Additionally, Fig. 7B presents the
cytotoxicity of the Stylissa carteri extract alone and in
combination with 0.75 µg/ml AgNPs, and Fig. 7C presents the cytotoxicity of the
Amphimedon chloros extract alone and in combination with
0.75 µg/ml AgNPs, when using the HT-29 cell line. However, the
cytotoxic effects of the Stylissa carteri or the
Amphimedon chloros extract with 0.75 µg/ml AgNPs were not
significantly different using the HT-29 cell line compared with the
cytotoxic effects of the Stylissa carteri or the
Amphimedon chloros extracts alone, respectively.
LC-MS reveals key bioactive compounds
in the sponge extracts, indicating their potential mechanisms of
action
LC-MS analysis revealed that there were different
chemical components contained in the Stylissa carteri and
Amphimedon chloros extracts (Tables I and II, respectively). Based on the LC-MS
analysis, manzacidine A, debromohymenialdisine and hymenialdisine
were identified as the major components in the Stylissa
carteri extract (Fig. 8).
Whereas in the Amphimedon chloros extract,
methoxyhexadecanoic acid, keramaphidin B and hydroxytricosanoic
acid were identified as the major components (Fig. 9). The chemical structures of the
predicated components contained in the Stylissa carteri and
the Amphimedon chloros extracts are presented in Fig. 10.
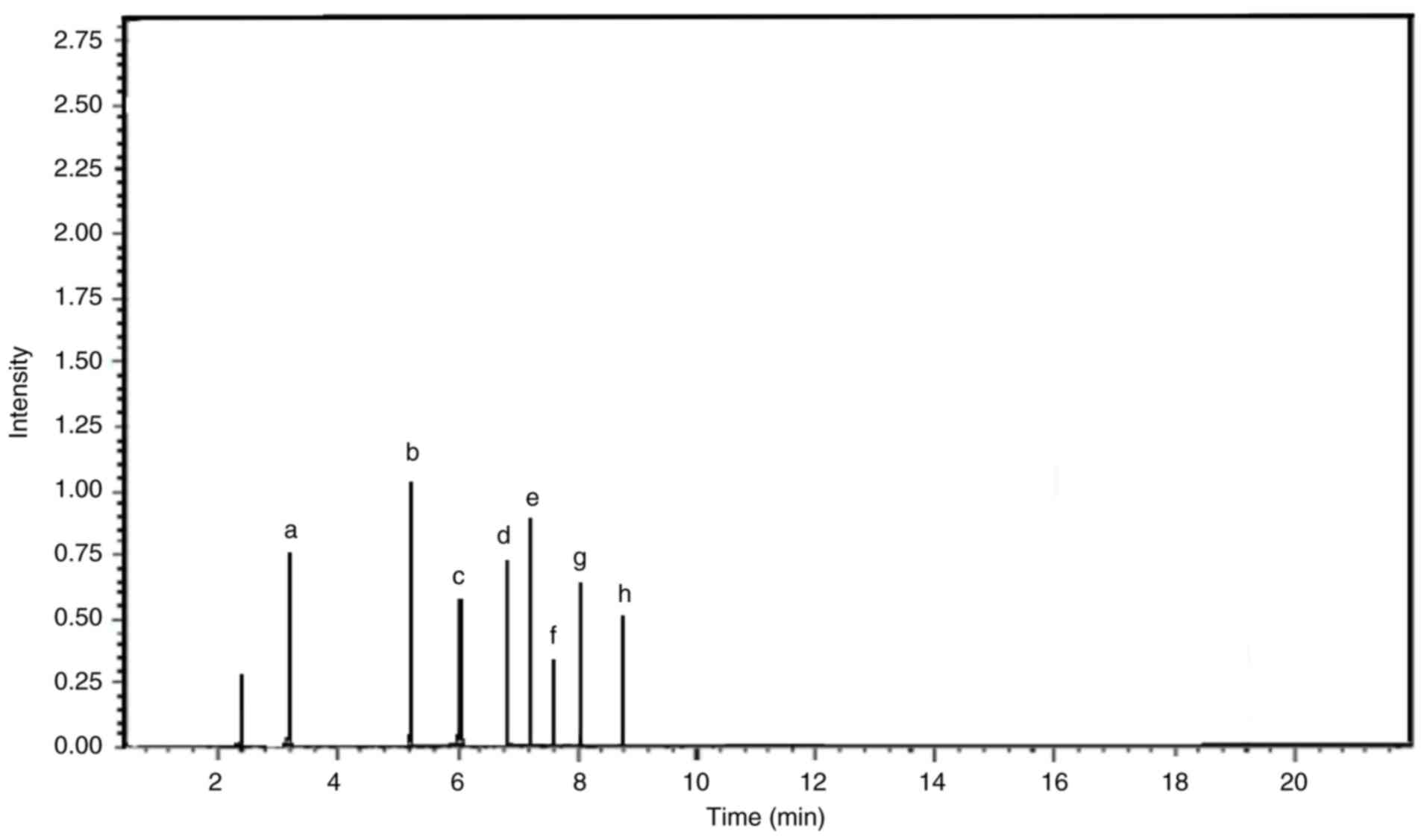 | Figure 8Liquid chromatography-mass
spectrometry chromatogram of the components in the Stylissa
carteri extract. Chromatogram of the Stylissa carteri
extract with peaks that correspond to individual compounds, which
were identified based on their mass-to-charge ratio. Each peak
provided detail on the chemical composition of the extract and the
relative abundance of its components. The chromatogram revealed the
molecular profile of Stylissa carteri and highlighted key
compounds such as (a) debromohymenialdisine, (b) hymenialdisine,
(c) agelongine, (d) calthramide, (e) manzacidine A, (f)
3-bromohymenialdisine, (g) spongiacidine and (h) ageliferin. The
chromatogram corresponds to ions with an m/z of 193.00, which have
a relative intensity of 81.82% compared with the total ion
intensity across all detected m/z values. The maximum intensity
range or full-scale limit of the detector was 1,000,000,000. m/z,
mass-to-charge ratio. |
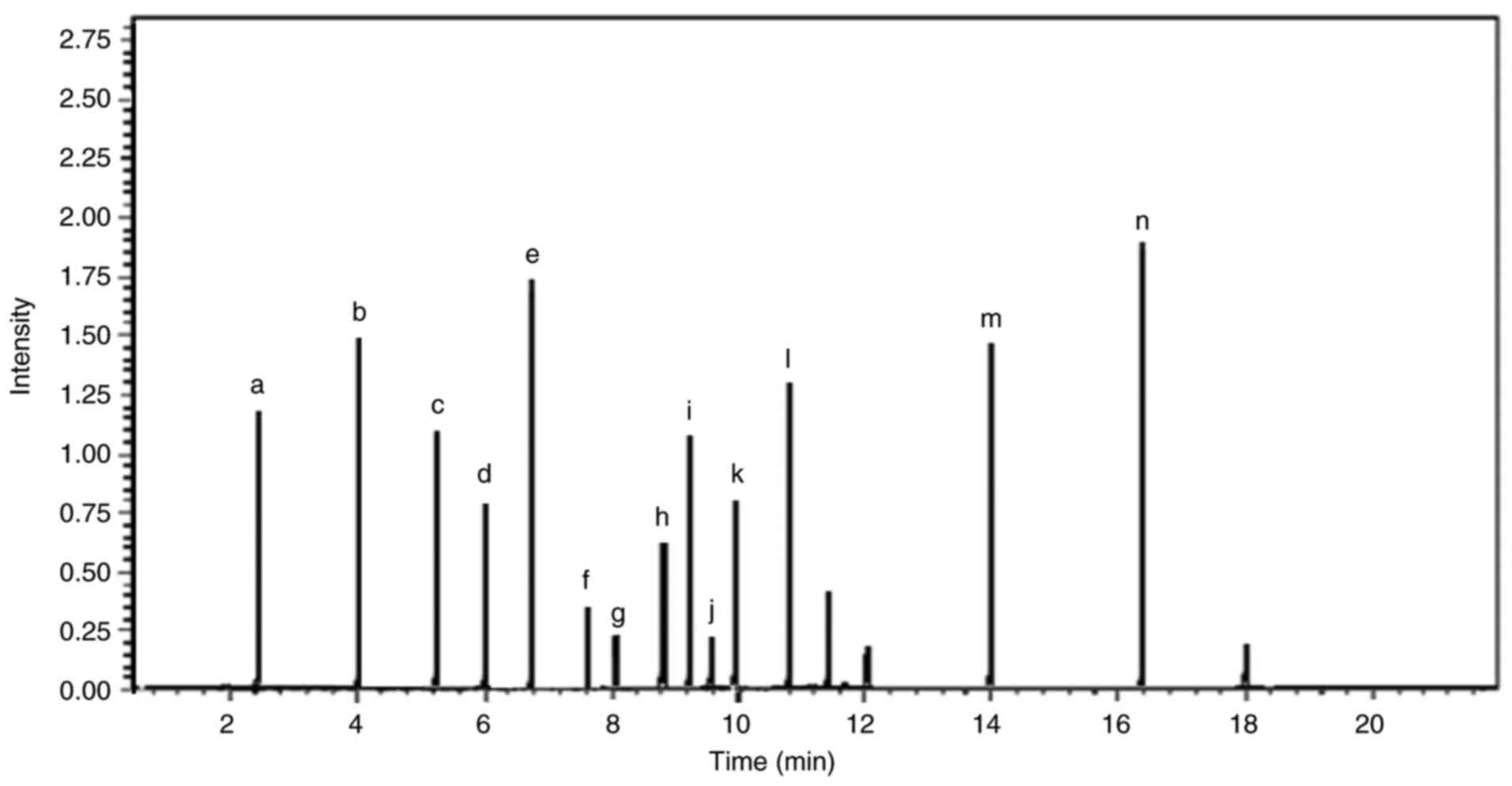 | Figure 9Liquid chromatography-mass
spectrometry chromatogram of the components in the Amphimedon
chloros extract. Chromatogram of the Amphimedon chloros
extract with peaks that correspond to individual compounds, which
were identified based on their mass-to-charge ratio. Each peak
provided detail on the chemical composition of the extract and the
relative abundance of its components. The chromatogram revealed the
molecular profile of Amphimedon chloros and highlighted key
compounds such as (a) purine, (b) methoxyhexadecanoic acid, (c)
hachijodine, (d) tricosenal, (e) kermaphidine B, (f) tricosenoic
acid, (g) pentacosenal, (h) zamamidine, (i) karamamine, (j)
pentacosenic acid, (k) ircinol, (l) pyrinodemin, (m) nakinadine and
(n) hydroxytricosanoic acid. The chromatogram corresponds to ions
with an m/z of 193.00, which have a relative intensity of 81.82%
compared with the total ion intensity across all detected m/z
values. The maximum intensity range or full-scale limit of the
detector was 1,000,000,000. m/z, mass-to-charge ratio. |
 | Table IAnalysis of the Stylissa
carteri extract components using liquid chromatography-mass
spectrometry. |
Table I
Analysis of the Stylissa
carteri extract components using liquid chromatography-mass
spectrometry.
| Compound name | Molecular
formula | M/Z | Composition
(%) |
|---|
| Calthramide |
C2H15BrN4O3 | 343.10 | 11.0 |
| Agelongine |
C13H11BrN2O4 | 339.10 | 9.6 |
| Manzacidine A |
C12H14BrN3O4 | 344.16 | 13.0 |
|
Debromohymenialdisine |
C11H11N5O2 | 245.20 | 12.5 |
| Spongiacidine |
C11H11Br2N5O2 | 405.05 | 10.5 |
| Hymenialdisine |
C11H10BrN5O2 | 324.13 | 17.8 |
|
3-Bromohymenialdisine |
C11H9Br2N5O2 | 403.03 | 9.1 |
| Ageliferin |
C22H24Br2N10O2 | 620.30 | 9.5 |
 | Table IIAnalysis of the Amphimedon
chloros extract components using liquid chromatography-mass
spectrometry. |
Table II
Analysis of the Amphimedon
chloros extract components using liquid chromatography-mass
spectrometry.
| Compound name | Molecular
formula | M/Z | Composition
(%) |
|---|
| Tricosenal |
C23H44O | 336.6 | 6.4 |
| Tricosenoic
acid |
C23H44O2 | 352.6 | 4.1 |
| Pentacosenal |
C25H48O | 364.6 | 3.5 |
| Pentacosenic
acid |
C25H48O3 | 396.6 | 3.2 |
| Methoxyhexadecanoic
acid |
C18H36O3 | 300.5 | 9.1 |
| Hydroxytricosanoic
acid |
C23H46O3 | 370.6 | 11.4 |
| Zamamidine |
C49H60N6O | 749.0 | 5.1 |
| Keramamine |
C23H33N3 | 380.0 | 8.7 |
| Ircinol |
C26H40N2O2 | 412.6 | 6.4 |
| Keramaphidin B |
C26H40N2 | 351.5 | 10.2 |
| Purine |
C5H4N4 | 120.1 | 7.1 |
| Pyrinodemin |
C37H57N3O | 559.9 | 8.9 |
| Nakinadine |
C27H40N2O2 | 424.6 | 8.1 |
| Hachijodine |
C19H34N2O | 306.5 | 3.4 |
Molecular docking reveals strong
binding affinities of marine compounds with EGFR and TrkA kinases,
highlighting the inhibitory potential of keramaphidin B
The present study investigated the docking
interactions of six marine compounds (debromohymenialdisine,
hydroxytricosanoic acid, hymenialdisine, keramaphidin B,
manzacidine A and methoxyhexadecanoic acid) with the kinase domains
of EGFR and TrkA, which have been previously revealed to be
overexpressed in different cancer cells such as A549, HT-29, MCF7
and PANC-1 (63-68).
The binding energies, specific amino acid interactions, presence of
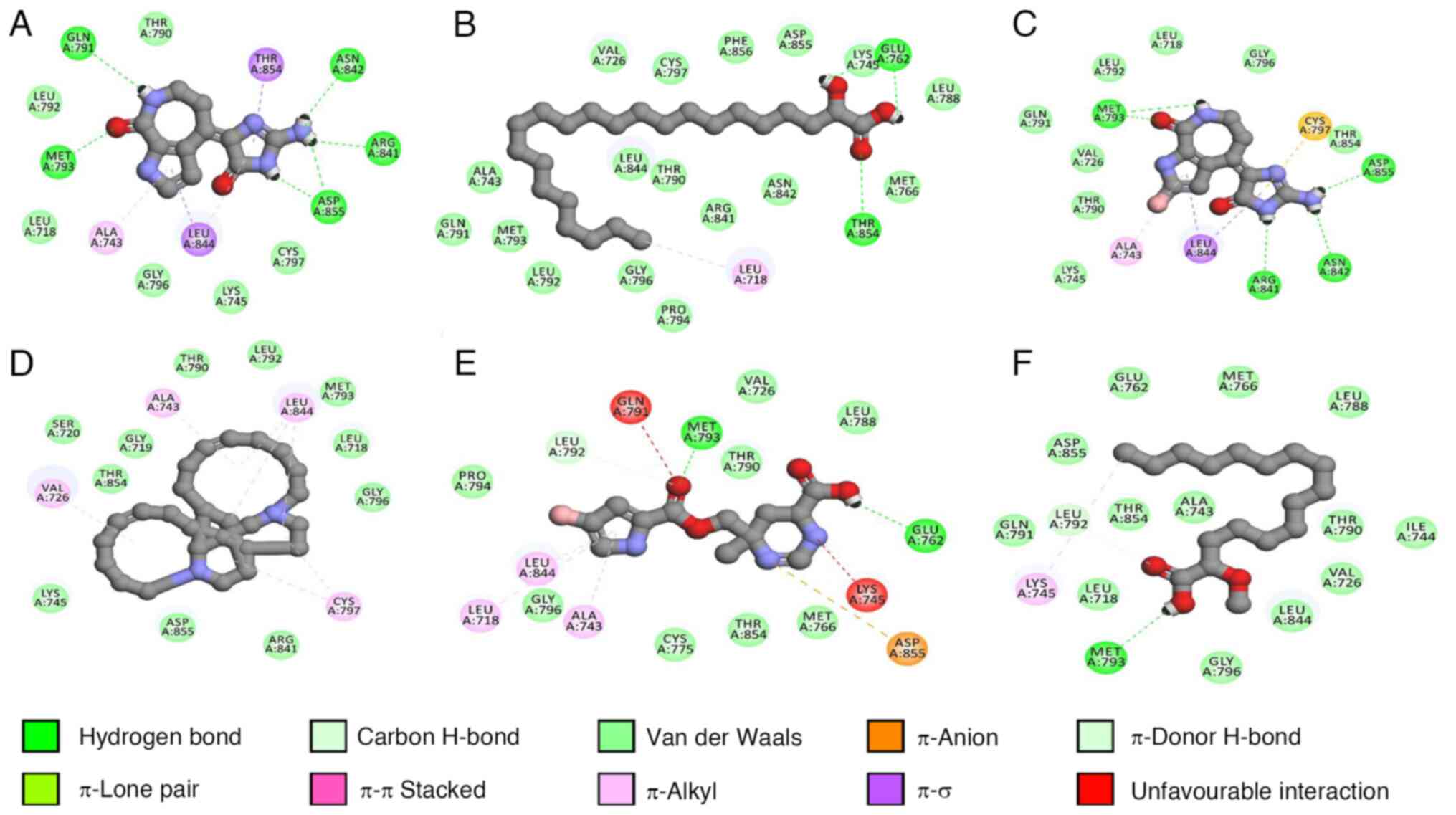
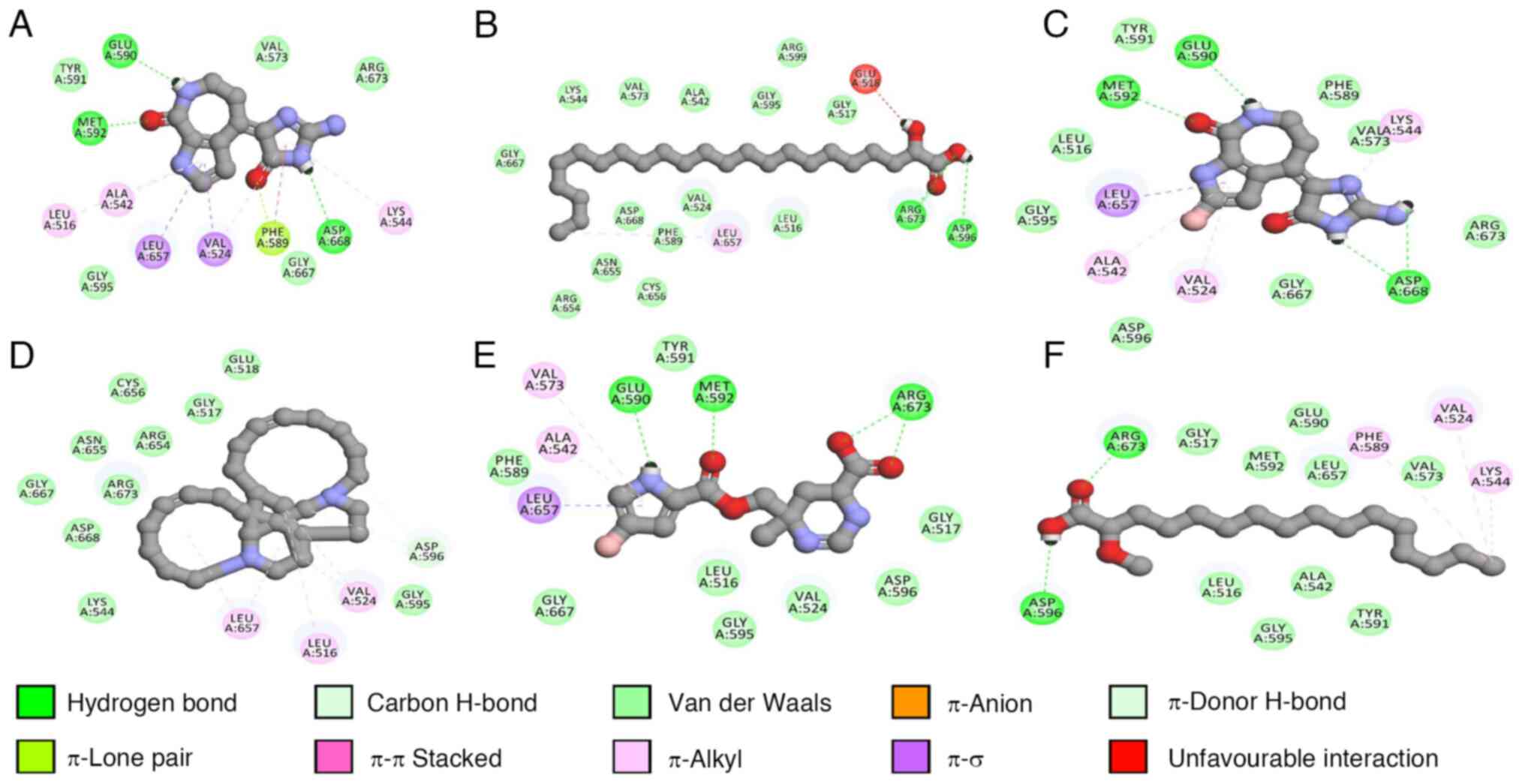
ionic bonds, hydrogen bonds and halogens were analyzed (Table III; Figs. 11 and 12).
 | Table IIIBinding interactions and affinity
analysis of the selected marine components against the EGFR kinase
domain and TrkA after molecular docking. |
Table III
Binding interactions and affinity
analysis of the selected marine components against the EGFR kinase
domain and TrkA after molecular docking.
| A, EGFR kinase
domain |
|---|
| | Interacting amino
acid per interaction type |
|---|
| Compound | LEB (kcal/mol) | Ionic | H-bond | Halogen | Other binding
residues |
|---|
|
Debromohymenialdisine | -6.68 | None | Gln791, Met793,
Arg841, Asn842 and Asp855 | None | Leu 718, Ala 743,
Lys 745, Thr 790, Leu792, Gly 796, Cys 797 and Thr 854 |
| Hydroxytricosanoic
acid | -3.77 | None | Glu762 and
Thr854 | None | Leu718, Val726,
Ala743, Lys 745, Met 766, Leu 788, Thr 790, Gln 791, Leu 792, Met
793,Pro 794, Gly 796, Cys 797,Arg 841,Asn 842, Leu 844, Asp 855 and
Phe 856 |
| Hymenialdisine | -6.65 | None | Met 793, Arg 841,
Asn 842 and Asp 855 | None | Leu 718, Val 726,
Ala 743, Lys 745, Thr 790, Gln791, Leu 792, Gly 796, Cys797, Leu
844 and Thr 854 |
| Keramaphidin B | -7.15 | None | None | None | Leu 718, Gly719,
Ser 720, Val 726, Ala 743, Lys 745, Thr 790, Leu 792, Met 793, Gly
796, Cys 797, Asp 800, Arg 841, Leu 844, Thr 854 and Asp 855 |
| Manzacidine A | -6.16 | Asp 855 | Glu 762 and Met
793 | None | Leu 718, Val 726,
Ala 743, Thr 754, Met 766, Cys 775, Leu 788, Thr 790, Leu 792, Pro
794, Gly 796 and Leu 844 |
| Methoxyhexadecanoic
acid | -4.62 | None | Met 793 | None | Leu 718, Val 726,
Ala 743, Ile 744, Lys 745, Glu762, Met 766, Leu788, Thr790, Gln
791, Leu792, Gly796, Leu 844, Thr 854 and Asp 855 |
| Dacomitinib
(control) | -7.48 | None | Thr 790 and Met
793 | Glu 762 | Leu718, Val726,
Ala743, Lys745, Met766, Leu788, Gln791, Leu792, Pro794, Gly 796,Cys
797, Leu 844), Thr 854 and Asp 855 |
| Repotrectinib
(control) | - | - | - | - | - |
| B, TrkA |
| | Interacting amino
acid per interaction type |
| Compound | LEB (kcal/mol) | Ionic | H-bond | Halogen | Other binding
residues |
|
Debromohymenialdisine | -6.71 | None | Glu 590, Met 592
and Asp 668 | None | Leu 516, Val 524,
Ala542, Lys 544, Val 573, Phe 589, Tyr 591, Gly 595, Leu 657, Gly
667 and Arg 673 |
| Hydroxytricosanoic
acid | -4.60 | None | Asp 596 and Arg
673 | None | Leu 516, Gly 517,
Val 524, Ala 542, Lys 544, Val 573, Phe 589, Gly 595, Arg 599, Arg
654, Asn 655, Cys 656, Leu 657, Gly 667 and Asp 668 |
| Hymenialdisine | -6.95 | None | Glu 590, Met 592
and Asp 668 | None | Leu 516, Val 524,
Ala 542, Lys 544, Val 573, Phe 589, Tyr 591, Gly 595, Asp 596, Leu
657, Gly 667 and Arg 673 |
| Keramaphidin B | -7.08 | None | None | None | Leu 516, Gly 517,
Glu 518, Val 524, Lys 544, Asp 558, Gly 595, Asp 596, Arg 654, Asn
655, Cys 656, Leu 657, Gly 667 and Arg 673 |
| Manzacidine A | -6.32 | None | Glu 590, Met 592
and Arg 673 | None | Leu 516, Gly 517,
Val 524, Ala 542, Val 573, Phe 589, Tyr 591, Gly 595, Asp 596, Leu
657 and Gly 667 |
| Methoxyhexadecanoic
acid | -4.37 | None | Asp 596 and Arg
673 | None | Leu 516, Gly 517,
Val 524, Ala 542, Lys 544, Val 573, Phe 589, Glu 590, Tyr 591, Met
592, Gly 595 and Leu 657 |
| Dacomitinib
(control) | - | - | - | - | - |
| Repotrectinib
(control) | -9.23 | None | Met 592 and Gly
667 | Asn 655 and Cys
656 | Leu 516, Gly 517,
Val 524, Ala 542, Lys 544, Val 573, Phe 589, Glu 590, Tyr 591, Gly
595, Asp 596, Arg654, Leu 657, Asp 668 and Arg 673 |
All compounds except keramaphidin B formed hydrogen
bonds with key amino acid residues such as Leu718, Lys745 or Met793
within EGFR. The results indicated that keramaphidin B had the most
favorable binding energy (-7.15 kcal/mol), followed by
debromohymenialdisine (-6.68 kcal/mol) and hymenialdisine (-6.65
kcal/mol). Dacomitinib, the control compound, indicated a binding
energy of -7.48 kcal/mol and formed hydrogen bonds with Thr790 and
Met793. However, it lacked several interactions observed in the
marine compounds, such as interactions with Leu718 and Lys745.
Furthermore, keramaphidin B also demonstrated the
strongest binding affinity (-7.08 kcal/mol), followed by
hymenialdisine (-6.95 kcal/mol) and debromohymenialdisine (-6.71
kcal/mol), against TrkA. Similar to EGFR, all compounds except
keramaphidin B formed hydrogen bonds with residues such as Leu516
and Val524.
The findings suggested that several marine
compounds, particularly hymenialdisine and debromohymenialdisine,
may potentially interact with and inhibit both EGFR and TrkA. These
results indicated that these compounds interact with specific
residues (Leu718 and Lys745 in EGFR; Leu516 and Val524 in TrkA),
which was not observed with the control compounds. Therefore, this
may indicate a unique binding mode.
Co-administering sponge extracts with
AgNPs enhances the antibacterial activity
Sponge ethyl acetate extracts combined with AgNPs
were tested against six bacterial species using the disc diffusion
method (Table IV). In addition, 20
µg/disc AgNPs or sponge ethyl acetate extract alone was
administered. The AgNPs and sponge ethyl acetate extract were
combined in proportions of 5 or 10 µg/disc of AgNPs with 10 µg/disc
of ethyl acetate extract for synergistic tests. Using 20 µg/disc
AgNPs alone resulted in ~10 mm-diameter inhibition zones against
the majority of bacteria, except for Enterobacter aerogenes,
which had an increased susceptibility and an inhibition zone of
28.33 mm. All bacterial species, with inhibition zone diameters
ranging from 21.43-13.58 mm, were suppressed by the 20 µg/disc
Stylissa carteri ethyl acetate extract. However, the 20
µg/disc Amphimedon chloros extract had no effect on
Enterobacter aerogenes or Klebsiella oxytoca, and the
remaining bacterial species had inhibition zones that ranged in
diameter from 7.34-15.15 mm. When using the extracts alone, the
Stylissa carteri extract had an increased inhibitory effect
against the six investigated bacterial species compared with the
Amphimedon chloros extract. Additionally, when combining 10
µg/disc sponge extract with 5 or 10 µg/disc AgNPs, the majority of
the bacterial species investigated had increased inhibition zones
and increased fold area (IFA) values when using the Stylissa
carteri extract compared with the Amphimedon chloros
extract, except for E. coli in which the opposite results
were demonstrated. The results indicated that the bacterial species
exhibited different responses, which were caused either by the
combined treatment of AgNPs with the sponge extract or by the
effect of each treatment alone. These results suggest the
possibility of using AgNPs in combination with a sponge ethyl
acetate extract to treat bacterial infections in hydatid cysts.
 | Table IVAntibacterial and synergistic effects
of Stylissa carteri or Amphimedon chloros ethyl
acetate extracts and AgNPs against bacteria isolated from
echinococcal hydatid cyst fluid. |
Table IV
Antibacterial and synergistic effects
of Stylissa carteri or Amphimedon chloros ethyl
acetate extracts and AgNPs against bacteria isolated from
echinococcal hydatid cyst fluid.
| A, Stylissa
carteri |
|---|
| | Inhibition zones
(mm) |
|---|
| Bacteria | AgNPs (20
µg/disc) | Sponge (20
µg/disc) | AgNPs (5 µg/disc)
plus sponge (10 µg/disc) | AgNPs (10 µg/disc)
plus sponge (10 µg/disc) | IFAa |
|---|
| Staphylococcus
xylosus | 10.40±0.15 | 21.43±0.17 | 16.00±0.12 | 20.00±0.18 | 2.70±0.13 |
| Klebsiella
oxytoca | 10.65±0.14 | 15.92±0.16 | 12.77±0.13 | 16.54±0.19 | 1.40±0.08 |
| Enterobacter
aerogenes | 28.33±0.18 | 13.58±0.15 | 17.14±0.14 | 30.79±0.16 | 0.18±0.02 |
| Micrococcus
spp. | 9.75±0.13 | 21.00±0.18 | 13.60±0.12 | 20.52±0.17 | 3.43±0.14 |
| Pseudomonas
aeruginosa | 9.40±0.16 | 16.09±0.15 | 13.38±0.11 | 14.96±0.13 | 1.53±0.10 |
| Escherichia
coli | 10.17±0.14 | 13.93±0.17 | 12.44±0.16 | 16.71±0.18 | 1.70±0.09 |
| B, Amphimedon
chloros |
| | Inhibition zones
(mm) |
| Bacteria | AgNPs (20
µg/disc) | Sponge (20
µg/disc) | AgNPs (5 µg/disc)
plus sponge (10 µg/disc) | AgNPs (10 µg/disc)
plus sponge (10 µg/disc) | IFAa |
| Staphylococcus
xylosus | 10.40±0.13 | 9.00±0.12 | 11.19±0.15 | 13.23±0.14 | 0.62±0.05 |
| Klebsiella
oxytoca | 10.65±0.16 | 0.00±0.00 | 9.48±0.14 | 12.28±0.15 | 0.33±0.03 |
| Enterobacter
aerogenes | 28.33±0.17 | 0.00±0.00 | 8.88±0.12 | 15.93±0.16 | 0.00±0.00 |
| Micrococcus
spp. | 9.75±0.12 | 8.77±0.13 | 8.90±0.14 | 11.99±0.15 | 0.51±0.05 |
| Pseudomonas
aeruginosa | 9.40±0.14 | 15.00±0.16 | 9.66±0.11 | 11.39±0.13 | 0.47±0.06 |
| Escherichia
coli | 10.17±0.15 | 7.34±0.12 | 18.00±0.19 | 19.71±0.18 | 2.76±0.13 |
Discussion
To the best of our knowledge, the present study was
the first to investigate the combined effects of AgNPs with the
extracts of two sponge species, Stylissa carteri and
Amphimedon chloros, in order to evaluate their potential as
antibacterial and anticancer agents. In the present study, four
cancer cell lines (namely A549, PANC-1, HT-29 and MCF7 from lung,
pancreatic, colorectal and breast cancer, respectively). These
cancer types were selected due to their notable incidence rates,
and across all types of cancer diagnoses globally, have incidence
rates of 18.4% for lung cancer, 13.1% for breast cancer, 9.7% for
pancreatic cancer and 11.2% for colorectal (colon) cancer. In terms
of the global cancer-related mortality rates, lung cancer accounts
for 32.8% of mortalities, followed by pancreatic and colorectal
cancers at 7.4% each, and breast cancer at 4.0% (2,69).
Furthermore, these tumors frequently have non-specific symptoms
such as, fatigue, weight loss, pain and gastrointestinal disorders,
which can interfere with the correct diagnosis as these symptoms
are observed in a number of different types of cancer (69,70).
In addition, they have genetic and epigenetic changes, such as
mutations in the Myc and Ras oncogenes, which are considered
notable targets for developing treatments (71-73).
Treatment of these types of cancer cells is made more difficult by
their metastatic propensity, which requires an alternative
treatment approach (74).
Therefore, investigating these cell lines is necessary in order to
develop treatment approaches and reduce their impact on public
health (75,76).
To investigate the anticancer activities of the
Amphimedon chloros and Stylissa carteri marine sponge
extracts, the cytotoxic effects were investigated using the
extracts separately and in combination with AgNPs. Various cancer
cell lines were used including A549, HT-29, MCF7 and PANC-1, as
well as the HUVEC normal cell line. To investigate the
antibacterial activity of the Amphimedon chloros and
Stylissa carteri extracts alone or with AgNPs, pathogenic
bacteria were used, which were isolated from hydatid cyst fluid
from damaged anatomical sites including the lung and liver
(55). Several previous studies
investigate the anticancer and antimicrobial properties of
naturally occurring AgNPs derived from fungal origins (77-86).
The present study used LC-MS analysis, which
revealed the main active chemical components in both sponge
species, and molecular docking, which indicated the potential
processes that may underlie their bioactivity. Cytotoxicity was
observed when Amphimedon chloros or Stylissa carteri
were combined with AgNPs and applied to different cancer cell
lines. However, the impact of AgNPs in combination with Stylissa
carteri was only significant in the A549 cell line. This
synergistic effect may be explained by the presence of the
bioactive chemicals present in the sponge extract, which may have
increased the efficiency of the AgNPs by facilitating their contact
with or absorption by the cancer cells. The LC-MS experiment
indicated that Amphimedon chloros and Stylissa
carteri had different chemical components, which may be the
reason for the differences in their synergistic effects. Substances
such as debromohymenialdisine, hymenialdisine and keramaphidin B
may act in combination with AgNPs to inhibit the growth of cancer
cells (87-92).
Recently, certain sponge components, such as stylissamide,
6-bromotrisindoline, N-(2-hydroxyphenyl)-acetamide, petrocidin A,
2,3-dihydroxybenzamid, 6-bromotrisindoline and geodiataurine, have
been revealed to have anti-proliferative capabilities against
cancer cells (93,94). Additionally, keramaphidin B, which
is known to be an important constituent of Amphimedon
chloros, has a cytotoxic impact on a number of cancer cell
lines, including P388 murine leukemia cells and KB human epidermoid
carcinoma cells (89). However,
neither the mechanism nor the possible synergistic effects of
keramaphidin B in combination with AgNPs have been investigated.
Furthermore, hymenialdisine, which is identified as the main
component of Stylissa carteri, inhibited the growth of
ovarian cancer cell lines, and demonstrated an antiangiogenic
activity by blocking NF-κB activity and angiogenic factors such as
vascular endothelial growth factor and IL-8 (91,92).
Furthermore, debromohymenialdisine, which is also indicated to be a
component of Stylissa carteri, increases cell cycle arrest
by inhibiting the G2 phase in the MCF7 cell line (87).
Due to their function in cancer cell signaling
pathways, their overexpression or mutation in a variety of types of
cancer (including lung, pancreatic, colorectal and breast cancers)
and their potential as therapeutic targets for inhibiting tumor
growth and progression, EGFR and TrkA were chosen for docking
experiments (95). We hypothesize
that the activity of the sponge components (such as
debromohymenialdisine, hydroxytricosanoic acid, hymenialdisine,
keramaphidin B, manzacidine A and methoxyhexadecanoic acid)
originates from the possible interaction of these components with
TrkA and EGFR, which in turn may inhibit various pathways that
promote the growth of cancer, including the
phoshoinositide-3-kinase/Akt and mitogen-activated protein
kinase/extracellular signal-regulated kinase signaling pathways.
This may accelerate the process of apoptosis, cause cell cycle
arrest, oppose the metastatic stage and prevent cancer cells from
proliferating. Furthermore, the present study also demonstrated the
cytotoxicity of combining the sponge extracts with AgNPs. The
combination of 6 µg/ml Stylissa carteri extract with 0.75
µg/ml AgNPs revealed a relatively low cytotoxicity in HUVEC cells,
with a 25.97% cell cytotoxicity, suggesting minimal toxicity at
this dose. However, this cytotoxic effect was not significantly
different compared with the Stylissa carteri extract alone
at the same concentration. By contrast, a significant reduction in
the cytotoxicity of the HUVEC cells was revealed when the
Amphimedon chloros extract was used across concentrations
ranging from 6-200 µg/ml in combination with 0.75 µg/ml AgNPs
compared with the Amphimedon chloros extract alone. This
selectivity suggested that the combination of sponge extracts with
AgNPs has a possible therapeutic potential as a low cytotoxicity
against HUVEC normal cells is an important factor for the
development of safer anticancer agents (96). The present study highlighted the
potential of analyzing marine products, indicating that they may be
used to find new drugs among the vast resources that are present in
the water of the Aqaba Gulf and other seas located within the
sub-tropical arid climate.
The combination of the Stylissa carteri
extract with AgNPs demonstrated the strongest synergistic action
against Micrococcus spp., with an IFA of 3.43. The catalytic
reactivity of nanoparticles is primarily dependent on their surface
area and increases as the surface energy increases (97). However, AgNPs can have harmful
effects (such as DNA damage, inflammation and apoptosis) due to
their ability to induce the production of reactive oxygen species
(such as hydrogen peroxide and superoxide anions) (57,98).
The antibacterial activities of the AgNPs, the sponge ethyl acetate
extracts and the AgNPs combined with the sponge ethyl acetate
extracts demonstrated inconsistencies between the different
treatment groups, regardless of Gram status of the bacteria.
Therefore, it was hypothesized that the structural variations of
the membranes could not be the reason behind this variation. A
number of compounds, such as debromohymenialdisine,
hydroxytricosanoic acid, hymenialdisine, keramaphidin B,
manzacidine A and methoxyhexadecanoic acid, extracted using ethyl
acetate, which is a semi-polar solvent, have a high level of
antibacterial activity (99-104).
Using the Stylissa carteri extract alone produced inhibitory
zones in the six bacterial species investigated, and had an
increased antibacterial efficacy compared with the extract of
Amphimedon chloros. Using marine natural components reveals
synergistic effects with antimicrobial medicines (99,105).
For example, several marine sources have produced natural compounds
such as equisetin, D-mannose, cis-vaccenic acid,
trans-13-octadecenoic acid, stigmasterol and retinoyl-β-glucuronide
with antibiotic-resistant microorganism-fighting capabilities
(94,106,107). A number of them have stronger
antimicrobial activity compared with therapeutic
antibacterial/antifungal drugs. For example, an epoxy sponge
sterol, 9α,11α-epoxycholest-7-ene-3β,5α,6α,19-tetrol-6 acetate
(ECTA) was the first marine natural substance to reverse the
multidrug efflux pump-mediated fluconazole resistance of Candida
albicans (108,109). Combining fluconazole with ECTA
(3.8 µM) increases its antifungal efficacy by 35 times (105). Marine sponges are the animal
kingdoms that create the largest quantities of bioactive chemicals
(110). Currently, it is unclear
if the combination of AgNPs and sponge ethyl acetate extracts will
be additive or synergistic, as their ability to penetrate the
bacterial envelope is still poorly understood.
Although the present study offered insights
regarding the potential synergistic effects of AgNPs combined with
the ethyl acetate extracts of marine sponges Amphimedon
chloros or Stylissa carteri, a number of limitations
should be addressed. Firstly, there was a lack of results on the
combined effect of Stylissa carteri and Amphimedon
chloros extracts with and without AgNPs, as well as a lack of
an evaluation of these combinations on a control ‘normal’ human
cell line. Additionally, in vivo validation is required to
investigate the therapeutic potential and safety. Furthermore, the
mechanisms proposed in the present study regarding TrkA and EGFR
requires further comprehensive mechanistic investigation. Although
important chemical components were identified using LC-MS analysis,
the entire spectrum of bioactive chemicals was not fully
investigated. In addition, the number of cancer cell lines and
bacterial strains investigated in the presence of these compounds
was limited in the present study. Therefore, the present study was
not sufficient in demonstrating the therapeutic prospects of these
components for cancer and bacterial infections. However, it
provided a framework for future investigations and highlighted the
possibility of combining AgNPs with marine sponge extracts as
potential options for antibacterial and cancer therapy.
In conclusion, the observed cytotoxic effects of
Amphimedon chloros and Stylissa carteri extracts
combined with AgNPs against a variety of cancer cell lines and
bacterial strains suggests that further investigation on the
anticancer and antibacterial potential is warranted.
Future studies should focus on elucidating the
precise mechanisms of the interaction between the sponge-derived
compounds and AgNPs. Furthermore, the combination ratios between
the sponge extracts (such as Stylissa carteri and
Amphimedon chloros) and between the sponges extracts and
AgNPs should be optimized for the maximum efficacy. Additionally,
the therapeutic potential should be evaluated in vivo. The
results of the present study indicated the importance of marine
biodiversity as a source of novel therapeutic agents and
highlighted the potential of combining natural products with
nanotechnology in order to increase their anticancer and
antibacterial properties.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
found in Figshare under accession number 26255297 or at the
following URL: https://doi.org/10.6084/m9.figshare.26255297.
Authors' contributions
MA, KK, AAl-S, DA, YQ and AAls participated in the
conception and design of the study. Material preparation and
analysis were carried out by MA, KK, AAl-S, DA and AF. The docking
experiment was carried out by BA and FS. The manuscript was written
by MA, YQ and AAls. All authors read and approved the final version
of the manuscript. MA and KK confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gerstberger S, Jiang Q and Ganesh KJC:
Metastasis. Cell. 186:1564–1579. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, CA Cancer J. Clin. 73:17–48. 2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bazeed AY, Day CM and Garg SJC: Pancreatic
cancer: Challenges and opportunities in locoregional therapies.
Cancers (Basel). 14(4257)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Vasan N, Baselga J and Hyman DM: A view on
drug resistance in cancer. Nature. 575:299–309. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hossain CM, Gera M and Ali KA: Current
status and challenges of herbal drug development and regulatory
aspect: A global perspective. Asian J Pharmaceutical Clin Res.
15:31–41. 2022.
|
|
6
|
Abdussalam-Mohammed W: Review of
therapeutic applications of nanotechnology in medicine field and
its side effects. J Chem Rev. 1:243–251. 2019.
|
|
7
|
Nirmala MJ, Kizhuveetil U, Johnson A,
Balaji G, Nagarajan R and Muthuvijayan V: Cancer nanomedicine: A
review of nano-therapeutics and challenges ahead. RSC Adv.
13:8606–8629. 2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bhattacharjee S: Craft of co-encapsulation
in nanomedicine: A struggle to achieve synergy through reciprocity.
ACS Pharmacol Transl Sci. 5:278–298. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fan H, Sun Q, Dukenbayev K, Benassi E,
Manarbek L, Nurkesh AA, Khamijan M, Mu C, Li C, Razbekova M, et al:
Carbon nanoparticles induce DNA repair and PARP inhibitor
resistance associated with nanozyme activity in cancer cells. Res
Square. 13(39)2022.
|
|
10
|
Elmehrath S, Nguyen HL, Karam SM, Amin A
and Greish YE: BioMOF-based anti-cancer drug delivery systems.
Nanomaterials. 13(953)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
El-kharrag R, Abdel Halim SS, Amin A,
Greish YE and Biomaterials P: Synthesis and characterization of
chitosan-coated magnetite nanoparticles using a modified wet method
for drug delivery applications. Int J Polymeric Materials Polymeric
Biomaterials. 68:73–82. 2019.
|
|
12
|
Ibrahim S, Baig B, Hisaindee S, Darwish H,
Abdel-Ghany A, El-Maghraby H, Amin A and Greish Y: Development and
evaluation of crocetin-functionalized pegylated magnetite
nanoparticles for hepatocellular carcinoma. Molecules.
28(2882)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shaimoldina A, Sergazina A, Myrzagali S,
Nazarbek G, Omarova Z, Mirza O, Fan H, Amin A, Zhou W and Xie Y:
Carbon nanoparticles neutralize carbon dioxide (CO2) in
cytotoxicity: Potent carbon emission induced resistance to
anticancer nanomedicine and antibiotics. Ecotoxicol Environ Saf.
273(116024)2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
El-Kharrag R, Amin A and Greish YEJCI: Low
temperature synthesis of monolithic mesoporous magnetite
nanoparticles. Ceramics Int. 38:627–634. 2012.
|
|
15
|
Benassi E, Fan H, Sun Q, Dukenbayev K,
Wang Q, Shaimoldina A, Tassanbiyeva A, Nurtay L, Nurkesh A,
Kutzhanova A and Mu C: Generation of particle assemblies mimicking
enzymatic activity by processing of herbal food: The case of
rhizoma polygonati and other natural ingredients in traditional
Chinese medicine. Nanoscale Adv. 3:2222–2235. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nazarbek G, Kutzhanova A, Nurtay L, Mu C,
Kazybay B, Li X, Ma C, Amin A and Xie Y: Nano-evolution and
protein-based enzymatic evolution predicts novel types of natural
product nanozymes of traditional Chinese medicine: Cases of
herbzymes of Taishan-Huangjing (Rhizoma polygonati) and Goji
(Lycium chinense). Nanoscale Adv. 3:6728–6738.
2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xie Y, Shaimoldina A, Fan H, Myrzagali S,
Nazarbek G, Myrzagalieva A, Orassay A, Amin A and Benassi E:
Characterisation of a phosphatase-like nanozyme developed by baking
cysteine and its application in reviving mung bean sprouts damaged
by ash. Environ Sci.: Nano. 11:266–277. 2024.
|
|
18
|
Paiva L, Fidalgo T, Da Costa L, Maia LC,
Balan L, Anselme K, Ploux L and Thiré RMSM: Antibacterial
properties and compressive strength of new one-step preparation
silver nanoparticles in glass ionomer cements (NanoAg-GIC). J Dent.
69:102–109. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Huy TQ, Thanh NTH, Thuy NT, Chung PV, Hung
PN, Le AT and Hong Hanh NT: Cytotoxicity and antiviral activity of
electrochemical-synthesized silver nanoparticles against
poliovirus. J Virol Methods. 241:52–57. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pawar A, Korde SK, Rakshe DS, William P,
Jawale M and Deshpande N: Analysis of Silver Nanoparticles as
Carriers of Drug Delivery System. J Nano-Electron Phys.
15(04015)2023.
|
|
21
|
Naseer F, Ahmed M, Majid A, Kamal W and
Phull AR: Green nanoparticles as multifunctional nanomedicines:
Insights into anti-inflammatory effects, growth signaling and
apoptosis mechanism in cancer. Semin Cancer Biol. 86:310–324.
2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sharma P, Hasan MR, Khanuja M, Rawal R,
Shivani Pilloton R and Narang J: Aptamer-based silver nanoparticle
decorated paper platform for electrochemical detection ovarian
cancer biomarker PDGF. Materials Chemistry Physics.
306(128114)2023.
|
|
23
|
Zhang Y, Han X, Liu Y, Wang S, Han X and
Cheng C: Research progress on nano-sensitizers for enhancing the
effects of radiotherapy. Materials Adv. 3:3709–3725. 2022.
|
|
24
|
Kitic D, Miladinovic B, Randjelovic M,
Szopa A, Seidel V, Prasher P, Sharma M, Fatima R, Arslan Ateşşahin
D, Calina D and Sharifi-Rad J: Anticancer and chemopreventive
potential of Morinda citrifolia L. bioactive compounds: A
comprehensive update. Phytother Res. 38:1932–1950. 2024.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Minhas LA, Kaleem M, Farooqi HMU, Kausar
F, Waqar R, Bhatti T, Aziz S, Jung DW and Mumtaz AS: Algae-derived
bioactive compounds as potential nutraceuticals for cancer therapy:
A comprehensive review. Algal Res. 78(103396)2024.
|
|
26
|
Al-Hrout A, Baig B, Hilal-Alnaqbi A and
Amin A: Cancer and biotechnology: A matchup that should never
slowdown. In: Biotechnology and Production of Anti-Cancer
Compounds, Springer International, (pp.73-97), 2017.
|
|
27
|
Sahoo A, Mandal AK, Kumar M, Dwivedi K and
Singh D: Prospective challenges for patenting and clinical trials
of anticancer compounds from natural products: Coherent review.
Recent Pat Anticancer Drug Discov. 18:470–494. 2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Amin A and Buratovich M: The anti-cancer
charm of flavonoids: A cup-of-tea will do! Recent Pat Anticancer
Drug Discov. 2:109–117. 2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xie Y, Mu C, Kazybay B, Sun Q, Kutzhanova
A, Nazarbek G, Xu N, Nurtay L, Wang Q, Amin A and Li X: Network
pharmacology and experimental investigation of Rhizoma
polygonati extract targeted kinase with herbzyme activity for
potent drug delivery. Drug Deliv. 28:2187–2197. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Badran MM, Alouny NN, Aldosari BN,
Alhusaini AM and Abou El Ela AES: Transdermal glipizide delivery
system based on chitosan-coated deformable liposomes: development,
ex vivo, and in vivo studies. Pharmaceutics. 14(826)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Murali C, Mudgil P, Gan CY, Tarazi H,
El-Awady R, Abdalla Y, Amin A and Maqsood S: Camel whey protein
hydrolysates induced G2/M cellcycle arrest in human colorectal
carcinoma. Sci Rep. 11(7062)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mathew BT, Torky Y, Amin A, Mourad AI,
Ayyash MM, El-Keblawy A, Hilal-Alnaqbi A, AbuQamar SF and
El-Tarabily KA: Halotolerant marine rhizosphere-competent
actinobacteria promote Salicornia bigelovii growth and seed
production using seawater irrigation. Front Microbiol.
11(552)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ortigosa-Palomo A, Quiñonero F, Ortiz R,
Sarabia F, Prados J and Melguizo C: Natural products derived from
marine sponges with antitumor potential against lung cancer: A
systematic review. Mar Drugs. 22(101)2024.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shady NH, Fouad MA, Salah Kamel M,
Schirmeister T and Abdelmohsen UR: Natural product repertoire of
the genus Amphimedon. Mar Drugs. 17(19)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hardani IN, Damara FA, Nugrahani AD and
Bashari MH: Ethanol extract of Stylissa carteri induces cell
death in parental and paclitaxel-resistant cervical cancer cells.
IJIHS. 6:91–96. 2018.
|
|
36
|
Al-Soub A, Khleifat K, Al-Tarawneh A,
Al-Limoun M, Alfarrayeh I, Sarayreh AA, Qaisi YA, Qaralleh H,
Alqaraleh M and Albashaireh A: Silver nanoparticles biosynthesis
using an airborne fungal isolate, Aspergillus flavus:
Optimization, characterization and antibacterial activity. Iran J
Microbiol. 14:518–528. 2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jaidev L and Narasimha G: Fungal mediated
biosynthesis of silver nanoparticles, characterization and
antimicrobial activity. Colloids Surf B Biointerfaces. 81:430–433.
2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hooper JN and Van Soest RW: Systema
Porifera. A guide to the classification of sponges. In: Systema
Porifera: A guide to the classification of sponges. Springer,
pp1-7, 2002.
|
|
39
|
Helmy T and Van Soest R: Amphimedon
species (Porifera: Niphatidae) from the Gulf of Aqaba, Northern Red
Sea: Filling the gaps in the distribution of a common pantropical
genus. Zootaxa. 859(1)2005.
|
|
40
|
O'Rourke A, Kremb S, Duggan BM, Sioud S,
Kharbatia N, Raji M, Emwas AH, Gerwick WH and Voolstra CR:
Identification of a 3-alkylpyridinium compound from the red sea
sponge Amphimedon chloros with in vitro inhibitory activity
against the West Nile Virus NS3 protease. Molecules.
23(1472)2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bashari MH, Huda F, Tartila TS, Shabrina
S, Putri T, Qomarilla N, Atmaja H, Subhan B, Sudji IR and Meiyanto
E: Bioactive compounds in the ethanol extract of marine sponge
Stylissa carteri demonstrates potential anti-cancer activity
in breast cancer cells. Asian Pac J Cancer Prev. 20:1199–1206.
2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kandler NM, Wooster MK, Leray M, Knowlton
N, de Voogd NJ, Paulay G and Berumen ML: Hyperdiverse macrofauna
communities associated with a common sponge, Stylissa
carteri, shift across ecological gradients in the Central Red
Sea. Diversity. 11(18)2019.
|
|
43
|
Ebada SS, Edrada RA, Lin W and Proksch P:
Methods for isolation, purification and structural elucidation of
bioactive secondary metabolites from marine invertebrates. Nat
Protoc. 3:1820–1831. 2008.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bayona LM, Videnova M and Choi YH:
Increasing metabolic diversity in marine sponges extracts by
controlling extraction parameters. Mar Drugs.
16(393)2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Al-Tawarah NM, Qaralleh H, Khlaifat AM,
Nebih Nofal M, Khleifat KM, Al-Limoun MO, Alqaraleh M and Ahmed Al
Shhab M: Anticancer and antibacterial properties of verthemia
iphionides essential oil/silver nanoparticles. Biomed Pharmacol J.
13:1175–1185. 2020.
|
|
46
|
Alqaraleh M, Khleifat KM, Abu Hajleh MN,
Farah HS and Ahmed KAA: Fungal-mediated silver nanoparticle and
biochar synergy against colorectal cancer cells and pathogenic
bacteria. Antibiotics (Basel). 12(597)2023.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Nikalje APG and Gadikar R: A simple liquid
chromatographic method for simultaneous determination of
aceclofenac, methyl salicylate, and benzyl alcohol in
pharmaceuticals. J Pharmacy Res. 12(283)2018.
|
|
48
|
Murray BW, Rogers E, Zhai D, Deng W, Chen
X, Sprengeler PA, Zhang X, Graber A, Reich SH, Stopatschinskaja S,
et al: Molecular characteristics of repotrectinib that enable
potent inhibition of TRK fusion proteins and resistant mutations.
Mol Cancer Ther. 20:2446–2456. 2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Gajiwala KS, Feng J, Ferre R, Ryan K,
Brodsky O, Weinrich S, Kath JC and Stewart A: Insights into the
aberrant activity of mutant EGFR kinase domain and drug
recognition. Structure. 21:209–219. 2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Morris GM, Huey R, Lindstrom W, Sanner MF,
Belew RK, Goodsell DS and Olson AJ: AutoDock4 and AutoDockTools4:
Automated docking with selective receptor flexibility. J Comput
Chem. 30:2785–2791. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Bouabdallah S, Brinza I, Boiangiu RS,
Ibrahim MH, Honceriu I, Al-Maktoum A, Cioanca O, Hancianu M, Amin
A, Ben-Attia M and Hritcu L: The effect of a Tribulus-based
formulation in alleviating cholinergic system impairment and
scopolamine-induced memory loss in zebrafish (Danio rerio):
Insights from molecular docking and in vitro/in vivo approaches.
Pharmaceuticals (Basel). 17(200)2024.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Saqallah FG, Hamed WM, Talib WH, Dianita R
and Wahab HA: Antimicrobial activity and molecular docking
screening of bioactive components of Antirrhinum majus
(snapdragon) aerial parts. Heliyon. 8(e10391)2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Shtaiwi M, Alemleh M, Abu-Safieh KA,
Salameha BA, Shtaiwi A, Alwahsh M, Hamadneh L and Khanfar MA:
Design, synthesis, crystal structure, biological activity and
molecular modeling of novel schiff bases derived from chalcones and
5-Hydrazino-1,3-Dimethyl-4-Nitropyrazole as anticancer agents.
Polycyclic Aromatic Compounds. 44:4178–4196. 2023.
|
|
54
|
Biovia: Discovery Studio Modeling
Environment. Dassault-Systèmes, San Diego, CA, 2016.
|
|
55
|
Al Qaisi YT, Khleifat KM, Oran SA, Al
Tarawneh AA, Qaralleh H, Al-Qaisi TS and Farah HS: Ruta graveolens,
Peganum harmala, and Citrullus colocynthis methanolic extracts have
in vitro protoscolocidal effects and act against bacteria isolated
from echinococcal hydatid cyst fluid. Arch Microbiol.
204(228)2022.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Clinical and Laboratory Standards
Institute (CLSI): Performance standards for antimicrobial
susceptibility testing. CLSI, Wayne, PA, 2011.
|
|
57
|
Qaralleh H, Khleifat K, Al-Limoun M,
Al-Tarawneh A, Khleifat W, Almajali L, Buqain R, Shadid KA and
Aslowayeh N: Antibacterial activity of airborne fungal mediated
nanoparticles in combination with Foeniculum vulgare essential oil.
J Herbmed Pharmacol. 11:419–427. 2022.
|
|
58
|
Amirjani A, Firouzi F and Haghshenas DF:
Predicting the size of silver nanoparticles from their optical
properties. J Plasmonics. 15:1077–1082. 2020.
|
|
59
|
Abbas R, Luo J, Qi X, Naz A, Khan IA, Liu
H, Yu S and Wei J: Silver nanoparticles: Synthesis, structure,
properties and applications. Nanomaterials (Basel).
14(1425)2024.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Al-Samydai A, Abu Hajleh MN, Al-Sahlawi F,
Nsairat H, Khatib AA, Alqaraleh M and Ibrahim AK: Advancements of
metallic nanoparticles: A promising frontier in cancer treatment.
Sci Prog. 107(368504241274967)2024.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Leary M, Heerboth S, Lapinska K and Sarkar
S: Sensitization of drug resistant cancer cells: A matter of
combination therapy. Cancers. 10(483)2018.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Chenthamara D, Subramaniam S, Ramakrishnan
SG, Krishnaswamy S, Essa MM, Lin FH and Qoronfleh MW: Therapeutic
efficacy of nanoparticles and routes of administration. Biomater
Res. 23(20)2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Brunetto de Farias C, Rosemberg DB, Heinen
TE, Koehler-Santos P, Abujamra AL, Kapczinski F, Brunetto AL,
Ashton-Prolla P, Meurer L, Reis Bogo M, et al: BDNF/TrkB content
and interaction with gastrin-releasing peptide receptor blockade in
colorectal cancer. Oncology. 79:430–439. 2011.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Seo JH, Jung KH, Son MK, Yan HH, Ryu YL,
Kim J, Lee JK, Hong S and Hong SS: Anti-cancer effect of HS-345, a
new tropomyosin-related kinase A inhibitor, on human pancreatic
cancer. Cancer Lett. 338:271–281. 2013.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Chen B, Liang Y, He Z, An Y, Zhao W and Wu
J: Autocrine activity of BDNF induced by the STAT3 signaling
pathway causes prolonged TrkB activation and promotes human
non-small-cell lung cancer proliferation. Sci Rep.
6(30404)2016.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Kyker-Snowman K, Hughes RM, Yankaskas CL,
Cravero K, Karthikeyan S, Button B, Waters I, Rosen DM, Dennison L,
Hunter N, et al: TrkA overexpression in non-tumorigenic human
breast cell lines confers oncogenic and metastatic properties.
Breast Cancer Res. 179:631–642. 2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Griffin N, Marsland M, Roselli S,
Oldmeadow C, Attia J, Walker MM, Hondermarck H and Faulkner S: The
receptor tyrosine kinase TrkA is increased and targetable in
HER2-positive breast cancer. Biomolecules. 10(1329)2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Nwaefulu ON, Sagineedu S, Islam MK and
Stanslas J: Pancreatic cancer treatment with targeted therapies:
Are we there yet? Eur Rev Med Pharmacol Sci. 26:367–381.
2022.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI View Article : Google Scholar
|
|
70
|
de Chiffre JMD, Ormstrup TE, Kusk MW and
Hess S: Patients from general practice with non-specific cancer
symptoms: A retrospective study of symptoms and imaging. BJGP Open.
8(BJGPO.2023.0058)2024.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Park JW and Han JW: Targeting epigenetics
for cancer therapy. Arch Pharm Res. 42:159–170. 2019.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Grześ M, Jaiswar A, Grochowski M, Wojtyś
W, Kaźmierczak W, Olesiński T, Lenarcik M, Nowak-Niezgoda M, Kołos
M, Canarutto G, et al: A common druggable signature of oncogenic
c-Myc, mutant KRAS and mutant p53 reveals functional redundancy and
competition among oncogenes in cancer. Cell Death.
15(638)2024.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Perurena N, Situ L and Cichowski K:
Combinatorial strategies to target RAS-driven cancers. Nat Rev
Cancer. 24:316–337. 2024.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Liu B, Zhou H, Tan L, Siu KTH and Guan XY:
Exploring treatment options in cancer: Tumor treatment strategies.
Signal Transduction Targeted Ther. 9(175)2024.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Mattiuzzi C and Lippi G: Current cancer
epidemiology. J Epidemiol Glob Health. 9:217–222. 2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
De Angelis R, Demuru E, Baili P, Troussard
X, Katalinic A, Chirlaque Lopez MD, Innos K, Santaquilani M, Blum
M, Ventura L, et al: Complete cancer prevalence in Europe in 2020
by disease duration and country (EUROCARE-6): A population-based
study. Lancet Oncol. 25:293–307. 2024.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Naqvi SZH, Kiran U, Ali MI, Jamal A,
Hameed A, Ahmed S and Ali N: Combined efficacy of biologically
synthesized silver nanoparticles and different antibiotics against
multidrug-resistant bacteria. Int J Nanomedicine. 8:3187–3195.
2013.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Govindaraju K, Tamilselvan S, Kiruthiga V
and Singaravelu G: Biogenic silver nanoparticles by Solanum torvum
and their promising antimicrobial activity. J Biopesticides.
3:394–399. 2010.
|
|
79
|
Bhainsa KC and D'souza SF: Extracellular
biosynthesis of silver nanoparticles using the fungus Aspergillus
fumigatus. Colloids Surf B Biointerfaces. 47:160–164.
2006.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Ahmad A, Mukherjee P, Senapati S, Mandal
D, Khan MI, Kumar R and Sastry M: Extracellular biosynthesis of
silver nanoparticles using the fungus Fusarium oxysporum.
Colloids Surfaces B: Biointerfaces. 28:313–318. 2003.
|
|
81
|
Jain N, Bhargava A, Majumdar S, Tarafdar J
and Panwar J: Extracellular biosynthesis and characterization of
silver nanoparticles using Aspergillus flavus NJP08: A mechanism
perspective. Nanoscale. 3:635–641. 2011.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Zhang X, Yan S, Tyagi R and Surampalli
RJC: Synthesis of nanoparticles by microorganisms and their
application in enhancing microbiological reaction rates.
Chemosphere. 82:489–494. 2011.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Al-Limoun M, Qaralleh HN, Khleifat KM,
Al-Anber M, Al-Tarawneh A, Al-sharafa K, Kailani MH, Zaitoun MA,
Matar SA and Al-soub T: Culture media composition and reduction
potential optimization of mycelia-free filtrate for the
biosynthesis of silver nanoparticles using the fungus
Tritirachium oryzae W5H. Curr Nanosci. 16:757–769. 2020.
|
|
84
|
Khleifat K, Alqaraleh M, Al-Limoun M,
Alfarrayeh I, Khatib R, Qaralleh H, Alsarayreh A, Al Qaisi Y and
Abu Hajleh M: The ability of Rhizopus stolonifer MR11 to
biosynthesize silver nanoparticles in response to various culture
media components and optimization of process parameters required at
each stage of biosynthesis. J Ecol Eng. 23:89–100. 2022.
|
|
85
|
Khleifat K, Qaralleh H and Al-Limoun M:
Antibacterial activity of silver nanoparticles synthesized by
Aspergillus flavus and its synergistic effect with
antibiotics. J Pure Appl Microbiol. 16:1722–1735. 2022.
|
|
86
|
Abu Hajleh MN, Al-Limoun M, Al-Tarawneh A,
Hijazin TJ, Alqaraleh M, Khleifat K, Al-Madanat OY, Qaisi YA,
AlSarayreh A, Al-Samydai A, et al: Synergistic effects of AgNPs and
biochar: A potential combination for combating lung cancer and
pathogenic bacteria. Molecules. 28(4757)2023.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Curman D, Cinel B, Williams DE, Rundle N,
Block WD, Goodarzi AA, Hutchins JR, Clarke PR, Zhou BB, Lees-Miller
SP, et al: Inhibition of the G2 DNA damage checkpoint and of
protein kinases Chk1 and Chk2 by the marine sponge alkaloid
debromohymenialdisine. J Biol Chem. 276:17914–17919.
2001.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Leirós M, Alonso E, Rateb ME, Houssen WE,
Ebel R, Jaspars M, Alfonso A and Botana LM: Bromoalkaloids protect
primary cortical neurons from induced oxidative stress. ACS Chem
Neurosci. 6:331–338. 2015.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Jakubec P, Farley AJ and Dixon DJ: Towards
the total synthesis of keramaphidin B. Beilstein J Org Chem.
12:1096–1100. 2016.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Lee SM, Kim NH, Lee S, Kim YN, Heo JD, Rho
JR and Jeong EJ: (10 Z)-Debromohymenialdisine from marine sponge
stylissa sp. regulates intestinal inflammatory responses in
Co-culture model of epithelial Caco-2 cells and THP-1 macrophage
cells. Molecules. 24(3394)2019.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Abdullah N, Al Balushi N, Hasan SI, Al
Bahlani S, Dobretsov S, Tamimi Y and Burney IA: Hymenialdisine is
cytotoxic against cisplatin-sensitive but not against
cisplatin-resistant cell lines. Sultan Qaboos Univ Med J.
21:632–634. 2021.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Ueda G, Matsuo Y, Murase H, Aoyama Y, Kato
T, Omi K, Hayashi Y, Imafuji H, Saito K, Tsuboi K, et al:
10Z-Hymenialdisine inhibits angiogenesis by suppressing NF-κB
activation in pancreatic cancer cell lines. Oncol Rep.
47(48)2022.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Esposito R, Federico S, Glaviano F, Somma
E, Zupo V and Costantini M: Bioactive compounds from marine sponges
and algae: Effects on cancer cell metabolome and chemical
structures. Int J Mol Sci. 23(10680)2022.PubMed/NCBI View Article : Google Scholar
|
|
94
|
El-Naggar HA, Bashar MA, Rady I, El-Wetidy
MS, Suleiman WB, Al-Otibi FO, Al-Rashed SA, Abd El-Maoula LM, Salem
EL-S, Attia EMH, et al: Two red sea sponge extracts (Negombata
magnifica and Callyspongia siphonella) induced
anticancer and antimicrobial activity. Applied Sci.
12(1400)2022.
|
|
95
|
Murugesan A, Mani SK, Koochakkhani S,
Subramanian K, Kandhavelu J, Thiyagarajan R, Gurbanov AV, Mahmudov
KT and Kandhavelu M: Design, synthesis and anticancer evaluation of
novel arylhydrazones of active methylene compounds. Int J Biol
Macromol. 254(127909)2024.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Ramezani-Aliakbari M, Soltanabadi A,
Sadeghi-aliabadi H, Varshosaz J, Yadollahi B, Hassanzadeh F and
Rostami M: Eudesmic acid-polyoxomolybdate Organo-conjugate as novel
anticancer agent. J Mol Structure. 1240(130612)2021.
|
|
97
|
Cuenya BR and Behafarid F: Nanocatalysis:
Size-and shape-dependent chemisorption and catalytic reactivity.
Surface Sci Rep. 70:135–187. 2015.
|
|
98
|
Lakkim V, Reddy MC, Pallavali RR, Reddy
KR, Reddy CV, Inamuddin Bilgrami AL and Lomada D: Green synthesis
of silver nanoparticles and evaluation of their antibacterial
activity against multidrug-resistant bacteria and wound healing
efficacy using a murine model. Antibiotics (Basel).
9(902)2020.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Rahman NIA, Ramzi MM, Rawi NN, Siong JYF,
Bakar K, Bhubalan K, Ariffin F, Saidin J, Azemi AK and Ismail N:
Characterization of antibiofilm compound from marine sponge
Stylissa carteri. Environ Sci Pollut Res Int.
31:37552–37563. 2024.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Hawas UW, Shaher F, Ghandourah M, Abou
El-Kassem LT, Satheesh S and Al-Sofyani AMA: Lipids and free fatty
acids of Red Sea Avrainvillea amadelpha, Holothuria atra, and
Sarcocornia fruticosa inhibit marine bacterial biofilms.
Lett Organic Chemistry. 17:466–471. 2020.
|
|
101
|
Hamed AN, Schmitz R, Bergermann A, Totzke
F, Kubbutat M, Müller WEG, Youssef DTA, Bishr MM, Kamel MS,
Edrada-Ebel R, et al: Bioactive pyrrole alkaloids isolated from the
Red Sea: Marine sponge Stylissa carteri. Z Naturforsch C J
Biosci. 73:199–210. 2018.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Althagbi HI, Alarif WM, Al-Footy KO and
Abdel-Lateff A: Marine-derived macrocyclic alkaloids (MDMAs):
Chemical and biological diversity. Mar Drugs.
18(368)2020.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Júnior ACV, de Castro Nogueira Diniz
Pontes M, Barbosa JP, Höfling JF, Araújo RM, Boniek D, de Resende
Stoianoff MA and Andrade VS: Antibiofilm and Anti-candidal
activities of the extract of the marine sponge agelas dispar.
Mycopathologia. 186:819–832. 2021.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Al-Shamayleh W, Qaralleh H, Al-Madadheh
OA, Al Qaisi Y and AlSarayreh A: Inhibitory effect of the marine
sponge amphimidon chloros extracts against multidrug-resistant
bacteria. Tropical J Natural Product Res. (8)2024.
|
|
105
|
Barbosa F, Pinto E, Kijjoa A, Pinto M and
Sousa E: Targeting antimicrobial drug resistance with marine
natural products. Int J Antimicrob Agents.
56(106005)2020.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Chen S, Liu D, Zhang Q, Guo P, Ding S,
Shen J, Zhu K and Lin W: A marine antibiotic kills
multidrug-resistant bacteria without detectable high-level
resistance. ACS Infect Dis. 7:884–893. 2021.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Schneider YK: Bacterial natural product
drug discovery for new antibiotics: strategies for tackling the
problem of antibiotic resistance by efficient bioprospecting.
Antibiotics (Basel). 10(842)2021.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Jacob MR, Hossain CF, Mohammed KA, Smillie
TJ, Clark AM, Walker LA and Nagle DG: Reversal of fluconazole
resistance in multidrug efflux-resistant fungi by the dysidea a
renaria sponge sterol 9α, 11α-epoxycholest-7-ene-3β, 5α, 6α,
19-tetrol 6-acetate. J Nat Prod. 66:1618–1622. 2003.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Saravanakumar K, Abinaya M, Mehnath S,
Shanmuga Priya V, Jeyaraj M, Al-Rashed S and Muthuraj V: Nano Ag@
bioactive microspheres from marine sponge clathria frondifera:
Fabrication, fortification, characterization, anticancer and
antibacterial potential evaluation. Environ Res.
206(112282)2022.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Bayona LM, de Voogd NJ and Choi YH:
Metabolomics on the study of marine organisms. Metabolomics.
18(17)2022.PubMed/NCBI View Article : Google Scholar
|