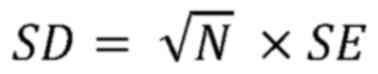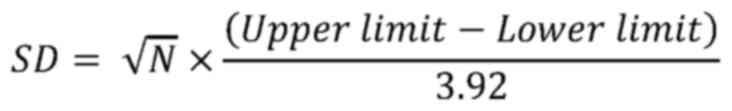Introduction
Type 2 diabetes mellitus (T2DM) is a serious
metabolic disorder affecting millions of individuals worldwide. It
is caused by insulin resistance or dysfunction, leading to
hyperglycemia and an increased risk of cardiovascular
complications, neuropath, and retinopathy, among other serious
risks (1). Management of T2DM
typically involves lifestyle modification, pharmacologic
intervention and insulin therapy to maintain stable blood glucose
levels and minimize diabetes-related complications (2).
One promising class of medications for T2DM
management is glucagon-like peptide-1 receptor agonists (GLP-1
RAs). The GLP-1 receptors found in the pancreas, intestines and
central nervous system are the main targets of these medications.
By doing so, they prevent hepatic gluconeogenesis, boost insulin
secretion, reduce appetite, slow stomach emptying and cause early
satiety (3). Studies have
demonstrated that these medications decrease glycated hemoglobin
(HbA1c) levels, body weight, blood pressure and lipids. GLP-1 RAs
are associated with a minimal risk of hypoglycemia, and the most
frequent adverse outcomes are related to the digestive system
(4). Semaglutide and dulaglutide
are two relatively new GLP-1 RAs that have garnered significant
interest for their favorable efficacy and safety profiles compared
with other medications used for T2DM management (5). Several clinical trials have
demonstrated the efficacy of semaglutide and dulaglutide in
reducing HbA1c levels, with a favorable safety profile.
There is a lack of comparative evidence on the
efficacy and safety of these two drugs in T2DM management. Evidence
emerging from several studies indicates that individuals who
received a weekly dose of semaglutide experienced a significantly
larger reduction in HbA1c compared with those receiving a weekly
dose of dulaglutide. On the other hand, another study showed no
statistically significant difference in glycemic control
achievement between these two medications in patients with T2DM
(6).
The present systematic review and meta-analysis
aimed to comprehensively evaluate studies comparing the
effectiveness of semaglutide and dulaglutide for T2DM management.
The review aimed to critically evaluate the studies, analyze
intervention durations and doses, and compare the clinical outcomes
of the drugs, including glycemic control, weight management and
adverse effects. This information can help clinicians and patients
make informed decisions regarding drug selection and the
personalization of T2DM management.
Materials and methods
The available data were thoroughly examined and
analyzed, and the findings were presented in compliance with the
guidelines outlined in the Preferred Reporting Items for Systematic
Reviews and Meta-Analyses statement (7).
Search strategy
To identify all relevant studies on the
effectiveness and safety of semaglutide and dulaglutide for
glycemic control and weight loss in adult patients with T2DM, a
thorough literature search was conducted using the Embase
(https://www.embase.com), Google Scholar
(https://scholar.google.com/), MEDLINE
(https://www.nlm.nih.gov/medline) and
SCOPUS (https://www.scopus.com/) databases from
their inception until July 2024. Specific search terms were used,
including semaglutide, dulaglutide and T2DM. The search included
studies in all languages but was limited to human subjects.
Inclusion and exclusion criteria
Studies that met the following criteria were only
considered: i) Comparing the effectiveness and safety of various
doses of semaglutide with dulaglutide in achieving glycemic control
and weight loss in patients with T2DM; ii) were conducted in
patients who were at least 18 years old; iii) diagnosed T2DM
according to specific criteria from either the World Health
Organization (8) or the American
Diabetes Association (9); and iv)
were published in a peer-reviewed journal. Meta-analyses, reviews,
case reports, editorials, letters, commentaries, expert opinions
and experimental studies were excluded.
Data extraction
Two authors reviewed the titles and abstracts of the
included publications to determine whether they met specific
requirements for inclusion or exclusion. If a study met the
criteria, the full text was examined. In cases of disagreement
between the two researchers, a third researcher was consulted to
make a conclusive decision. For all included studies, the following
information was extracted: Authorship, year of publication, study
design, study duration, randomization process, intervention method,
sample size, study location, population studied, therapy duration
and follow-up period, patients' body weight or body mass index
(BMI) and adverse events.
Outcome measures for efficacy
The primary outcome of effectiveness was the
difference in HbA1c levels from the initial measurement between the
groups. The secondary outcome was the effect of the medications on
body weight loss (change in body weight from baseline) during the
treatment period.
Quality and risk of bias
assessment
The quality of citations included in the study was
evaluated using the Cochrane Collaboration's Risk of Bias Tool
(10) and Review Manager. The
evaluation considered several factors: Allocation concealment,
blinding of participants and personnel, random sequence generation,
blinding of outcome assessors, selective outcome reporting,
incomplete outcome data and other biases. The studies were
categorized into three levels of risk: high, unclear and low. Two
authors conducted the quality appraisal and consulted a third
reviewer in case of any discrepancies.
Statistical analysis
Comprehensive Meta-Analysis (CMA) 2 was utilized for
all the analyses. Data were expressed as the mean ± standard
deviation (SD). The effect size statistic for continuous
measurement data was the standardized mean difference (SMD). In
cases where SD was not provided, the following formula was used to
convert standard error to SD, where N represents the sample size
(11):
In studies where confidence intervals (CIs) were
provided, the following formula was used to convert CIs to SDs. As
before, N represents the sample size (11):
In the present meta-analysis, a random-effects model
was employed to consider the differences in effect size estimates.
This enables us to make inferences at the population level and is
more rigorous than a fixed-effects model (12). The authors did not rely on the
I2 statistic to indicate the level of heterogeneity in
effect size because it is not designed for that purpose. Unless the
I2 value is zero, it cannot provide information on the
extent of heterogeneity. While the I² statistic measures the
proportion of variability due to heterogeneity, it does not provide
insight into the absolute variation or its practical significance.
By contrast, prediction intervals indicate the range within which
future study results are likely to fall, making them directly
interpretable in real-world contexts. This allows for an improved
understanding of how markedly study results might vary and whether
that variation is meaningful for clinical or practical decisions.
Additionally, prediction intervals are not as sensitive to the
number and size of included studies, addressing some of the
limitations of the I² statistic. Therefore, prediction
intervals were used to report heterogeneity in the data. Prediction
intervals were calculated using a random-effects model, which
considers both within-study and between-study variability. The
interval was derived from the overall effect size estimate,
accounting for the standard error and variability between studies
(13).
A funnel plot was used to determine if there was any
bias in the published material. A balanced funnel plot indicated no
bias, while an unbalanced one suggested that there was bias.
Egger's and Begg's tests were also used to confirm the presence of
bias in the publications because the interpretation of the funnel
plot can be subjective.
Moderator analysis, performed through
meta-regression, explores how study-level characteristics influence
the effect sizes across different studies. In this approach,
potential moderators, such as treatment duration, were identified
and data were collected for each included study. The
meta-regression model was then set up using CMA, with the effect
size as the dependent variable and the moderator as the independent
variable. The analysis estimated a regression line to describe the
relationship, and a significant P-value (typically <0.05)
indicated that the moderator significantly impacts the effect size
(14). If the P-value of either
test was less than 0.05, it indicated bias. P<0.05 was deemed to
indicate a statistically significant difference.
Results
Search results and study
characteristics
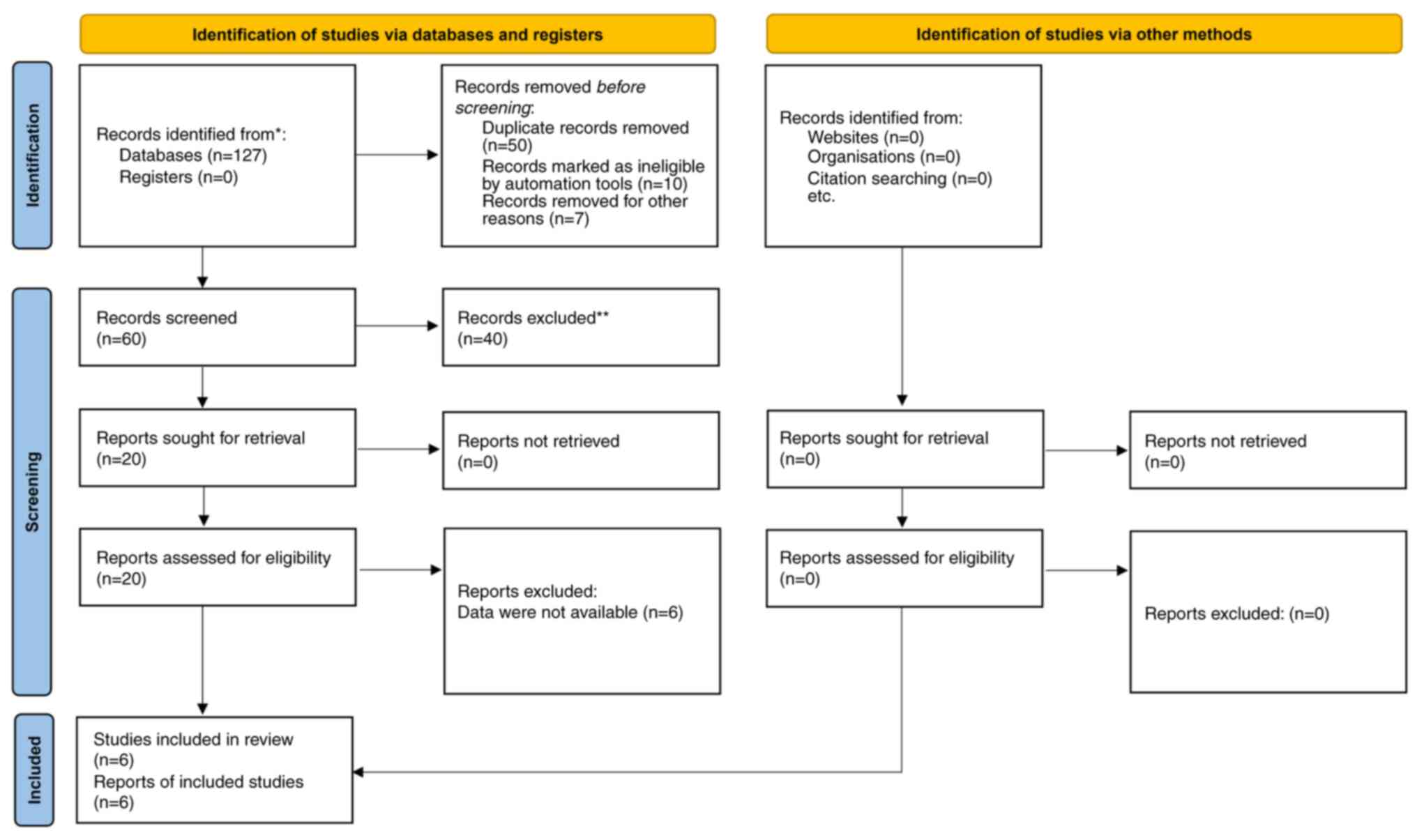
The search for relevant literature yielded 127
publications. After eliminating duplicates and examining the titles
and abstracts of the sources, 20 were selected for a thorough
review. Out of these 20, only six met the criteria for inclusion
(6,15-19).
A total of 3 of these studies had several subgroups and
comparisons, resulting in 10 comparisons in the present
meta-analysis. A total of 2 studies were non-randomized (6,18),
while 4 were randomized clinical trials. A flowchart in Fig. 1 shows the steps involved in
including and excluding the studies. The duration of the studies
ranged between 12-52 weeks. There were 1,038 patients in the
semaglutide group and 847 patients in the dulaglutide group. In the
included trials, semaglutide and dulaglutide doses varied between
0.5-14 mg and between 0.75-1.5 mg, respectively. The study
characteristics are included in Table
I.
 | Table ICharacteristics of the included
studies. Clinical studies comparing semaglutide and dulaglutide in
patients with type 2 diabetes are summarized, focusing on study
design, sample size, location, patient characteristics and
outcomes. Key findings on glycemic control, weight changes and
adverse events are highlighted, showing that semaglutide often
provided improved glycemic control and weight loss compared with
dulaglutide, with similar safety profiles, primarily involving
gastrointestinal issues. |
Table I
Characteristics of the included
studies. Clinical studies comparing semaglutide and dulaglutide in
patients with type 2 diabetes are summarized, focusing on study
design, sample size, location, patient characteristics and
outcomes. Key findings on glycemic control, weight changes and
adverse events are highlighted, showing that semaglutide often
provided improved glycemic control and weight loss compared with
dulaglutide, with similar safety profiles, primarily involving
gastrointestinal issues.
| First author,
year | Study design | Duration | Randomization | Intervention | Sample size | Location | Population | Duration of
therapy/follow up | BMI/Weight | Adverse events | (Refs.) |
|---|
| Takahashi et
al, 2023 | Multicentre,
prospective, randomized, open-label | 24 weeks | Third-party, 1:1
randomization | Semaglutide vs.
liraglutide/dulaglutide | 110 (100
completed) | Japan | Patients with T2DM on
GLP-1 therapy | 24 weeks | BMI ≥22 kg/m² | Hypoglycemia,
gastrointestinal issues | (19) |
| Iacobellis and
Villasante Fricke, 2019 | Controlled,
parallel study | 12 weeks | Based on
preference/insurance coverage | Semaglutide 1 mg
vs. Dulaglutide 1.5 mg vs. Metformin | 80 | USA | Overweight/obese
type 2 diabetics | 12 weeks | BMI ≥27 kg/m²,
weight loss noted | No significant
difference between groups in HbA1c improvement, gastrointestinal
issues | (18) |
| Iijima et
al, 2023 | Open-label,
randomized, parallel-group | 26 weeks | 1:1
randomization | Semaglutide 0.5 mg
vs. Dulaglutide 0.75 mg | 32 (30
completed) | Japan | Patients with T2DM
on liraglutide | 26 weeks | Weight loss in
semaglutide group (-2.6 kg) | Higher adverse
events in semaglutide group, severe vomiting | (17) |
| Pratley et
al, 2018 | Randomized,
open-label, phase 3b | 40 weeks | Interactive
web-response system | Semaglutide (0.5
mg, 1.0 mg) vs. Dulaglutide (0.75 mg, 1.5 mg) | 1201 | 16 countries
(Global) | Patients with T2DM
on metformin | 40 weeks | Body weight
reduction: semaglutide > dulaglutide | Gastrointestinal
disorders, similar safety profiles | (15) |
| Seijas-Amigo et
al, 2023 | Non-randomized,
multicenter study | 3 months | Not randomized | GLP-1 RAs:
Semaglutide (oral/subcutaneous), Dulaglutide | 94 | Spain | Patients with T2DM
with BMI >30 kg/m² | 3 months | Mean body weight:
99.3 kg; BMI, 36.7 kg/m² | Gastrointestinal
disorders, more frequent in dulaglutide group | (6) |
| Yabe et al,
2020 | Randomized,
open-label, phase 3a | 52 weeks | 2:2:2:1
randomization | Oral Semaglutide
vs. Dulaglutide 0.75 mg | 458 | Japan | Patients with T2DM
with uncontrolled glucose | 52 weeks | Body weight change:
varied by dose | Gastrointestinal
events, mostly constipation | (16) |
Efficacy of semaglutide vs.
dulaglutide in the achievement of glycemic control and weight
loss
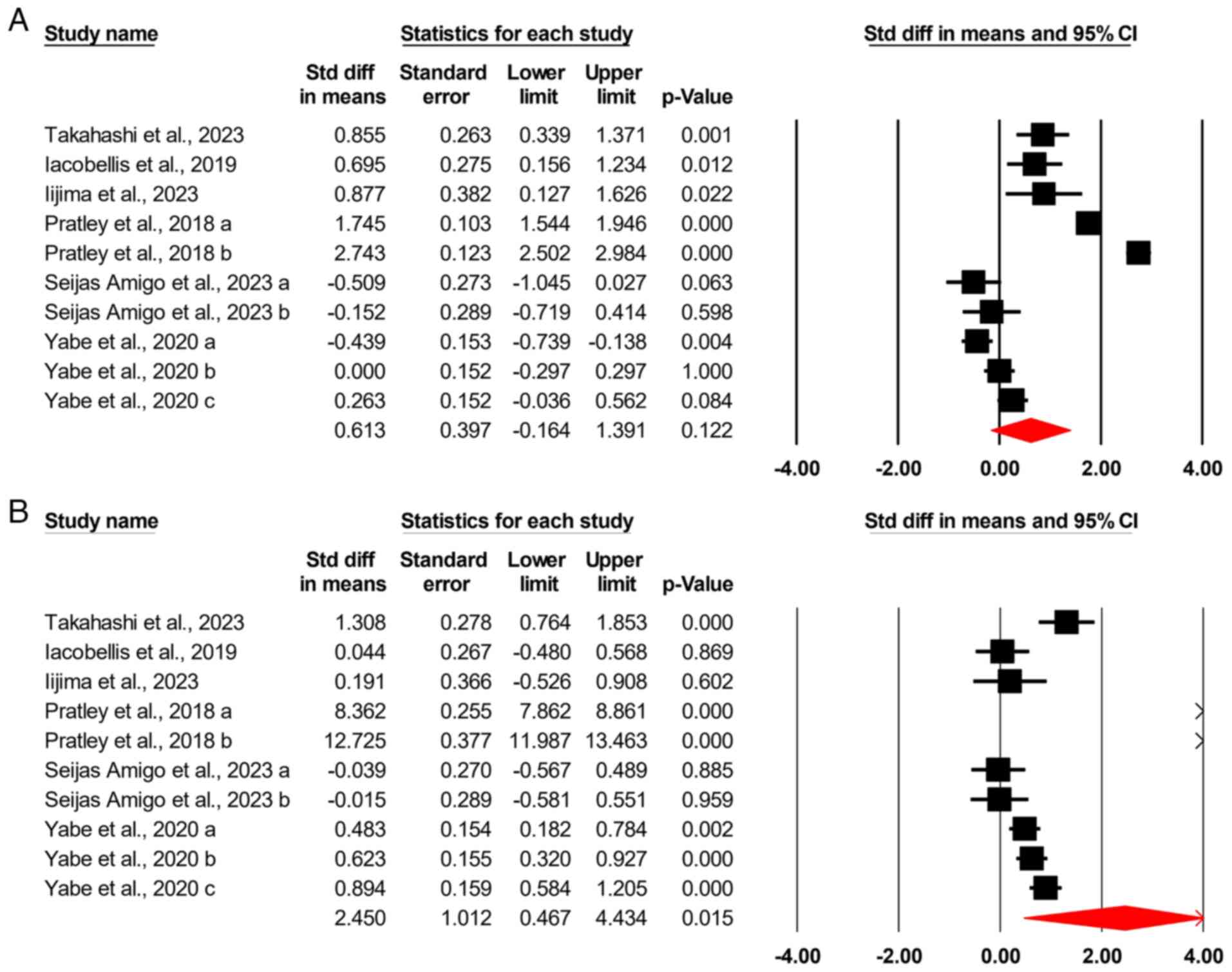
The analysis included 10 different comparisons. The
initial global examination of all glycemic control measures using a
CMA required the inclusion of each study only once in a nested
analysis. The results demonstrated no difference between
semaglutide and dulaglutide in achieving glycemic control in
patients with T2DM, with an SMD of 0.613 (95% CI, -0.164-1.391;
P=0.122) (Fig. 2A).
Similar to the previous analysis, this examination
included 10 different comparisons. It was found that semaglutide
was more effective than dulaglutide in inducing weight loss in
patients with T2DM, with an SMD of 2.45 (95% CI, 0.467-4.434;
P=0.015) (Fig. 2B).
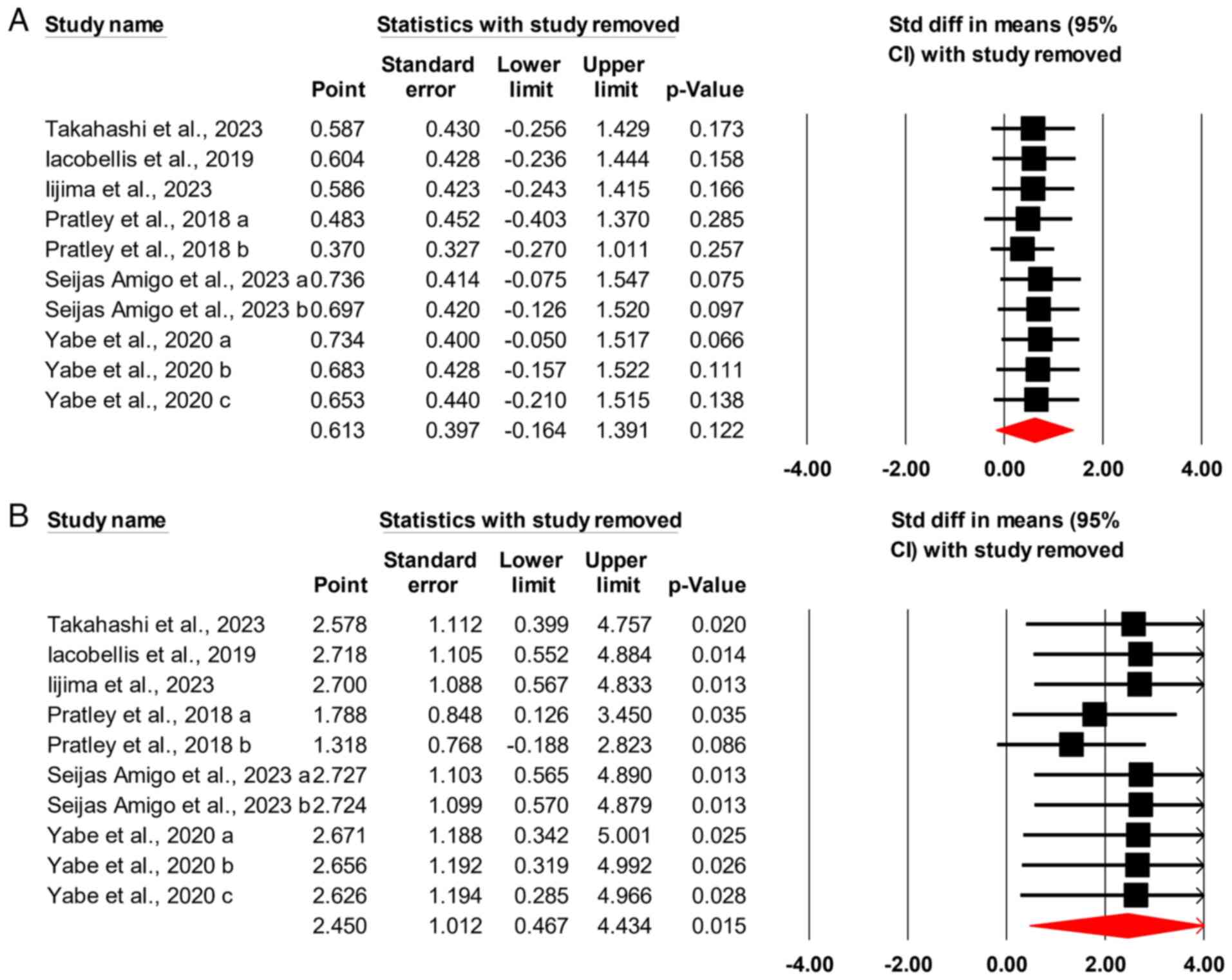
Sensitivity analysis
The analysis was conducted 10 times, with each
iteration excluding one study to demonstrate the impact of that
particular study on the outcome. It was found that the exclusion of
no single study affects the outcome of glycemic control efficacy
data (Fig. 3A). However, the
exclusion of one of the comparisons conducted by Pratley et
al (15) caused a change in the
initial assumption that semaglutide had higher efficacy than
dulaglutide in inducing weight loss in patients with T2DM. This
means that these results should be interpreted with caution
(Fig. 3B).
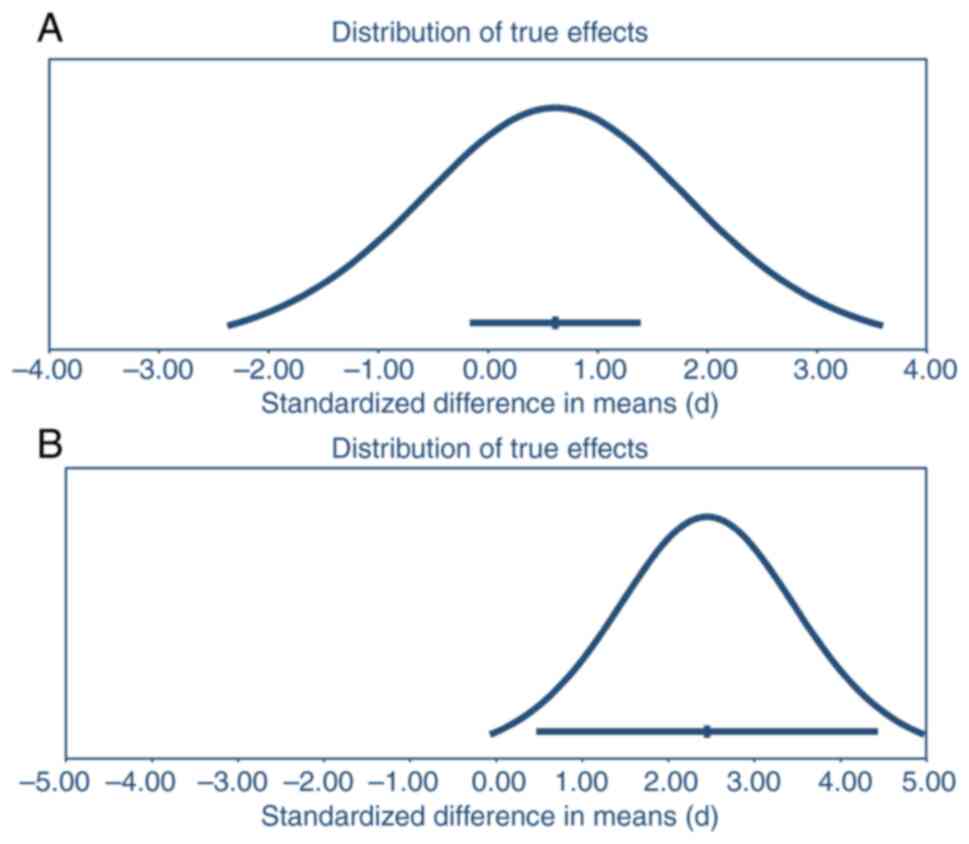
Data heterogeneity
The prediction interval analysis for glycemic
control data found that the mean effect size was 0.61, with a 95%
CI of -0.164-1.39. In 95% of similar populations, the true effect
size falls between -2.37-3.60 (Fig.
4A). This indicates a large amount of heterogeneity among the
studies analyzed, as the prediction interval is wider than the CI
and suggests a broader range of potential treatment effects. The
presence of a statistically significant effect is not confirmed
here, as all values in the CI and prediction interval do not fall
on one side of the null. The mean effect size for the weight loss
data was found to be 2.45, with a 95% CI of 0.47-4.43. The true
effect size in 95% of all comparable populations fell from
-0.07-4.97. Again, the data heterogeneity was considered high
because the prediction interval was wider than the CI. All CI
values on one side of the null confirm the presence of a
statistically significant effect. At the same time, the prediction
interval suggests the possibility of values on both sides of the
null (Fig. 4B).
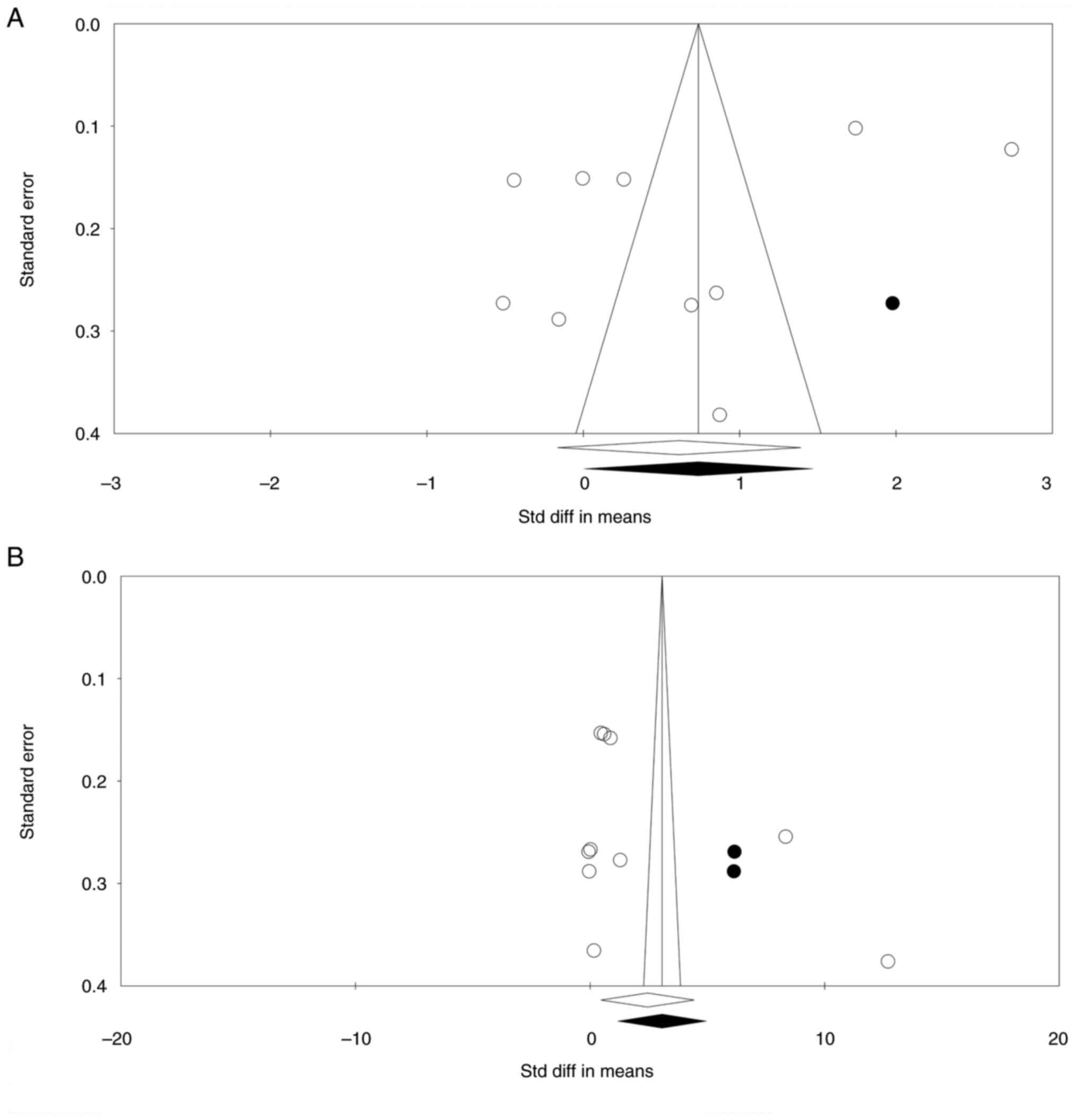
Risk of bias
For the first set of comparisons, the funnel plot
was reasonably symmetric. However, one study was found to be
missing to the right of the mean, while no study was missing to the
left of the mean (Fig. 5A). The
Begg's and Mazumdar rank correlation showed no publication bias
(2-tailed P=0.05 in Kendall's tau with continuity correction).
Similarly, Egger's regression revealed no sign of publication bias
(two-tailed P=0.22).
For the second set of comparisons, the shape of the
funnel plot was also symmetric. Nevertheless, two studies were
missing to the right of the mean in the trim-and-fill analysis. No
study was found missing to the left of the mean (Fig. 5B). The Begg's and Mazumdar rank
correlation showed no publication bias (2-tailed P=0.05 in
Kendall's tau with continuity correction). Similarly, Egger's
regression revealed no sign of publication bias (two-tailed
P=0.22).
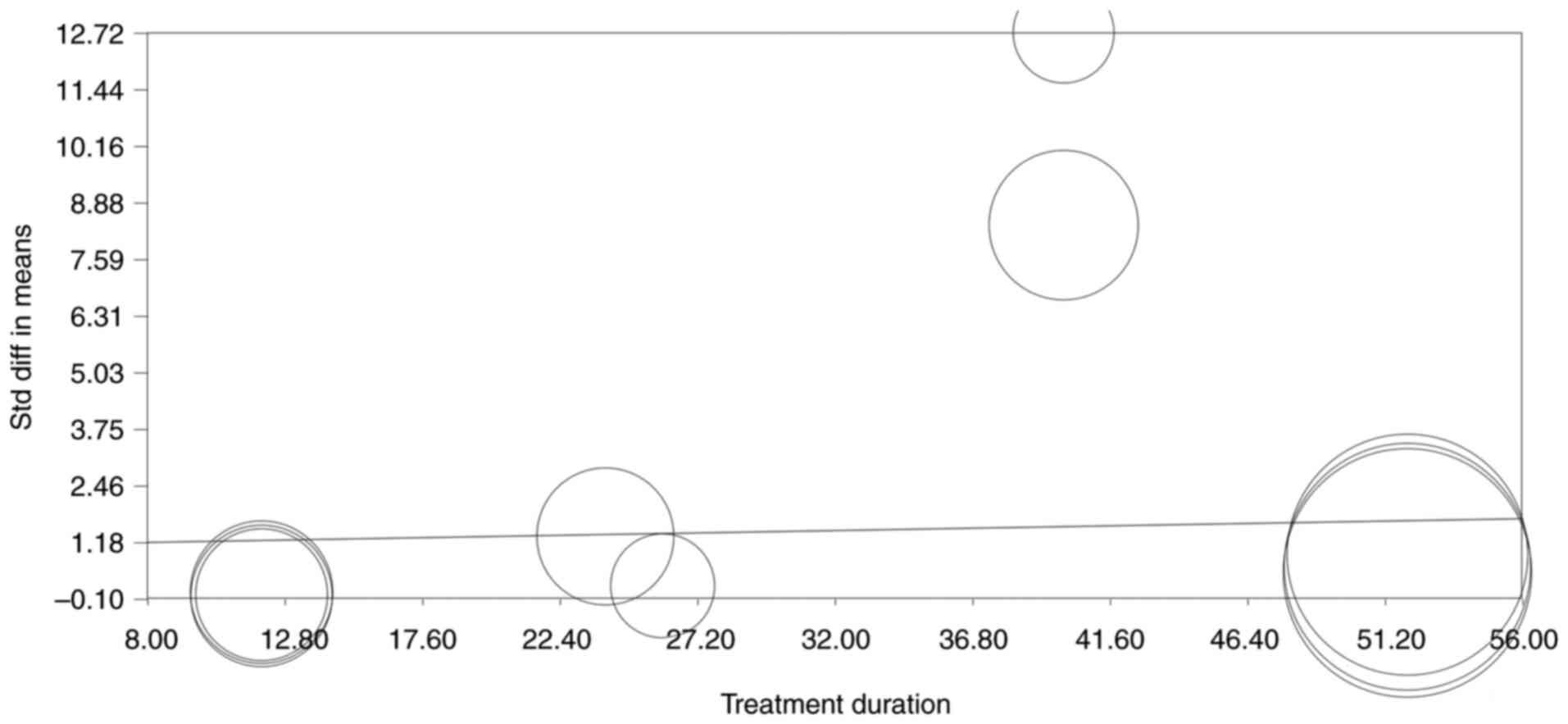
Meta-regression or moderator
analysis
No correlation was found between treatment duration
or semaglutide/dulaglutide doses and glycemic control (slope
P>0.05 for all comparisons; data not shown). However, a
correlation was found between treatment duration and weight loss,
indicating that a longer treatment duration was associated with
higher weight loss (slope P=0.02; Fig.
6).
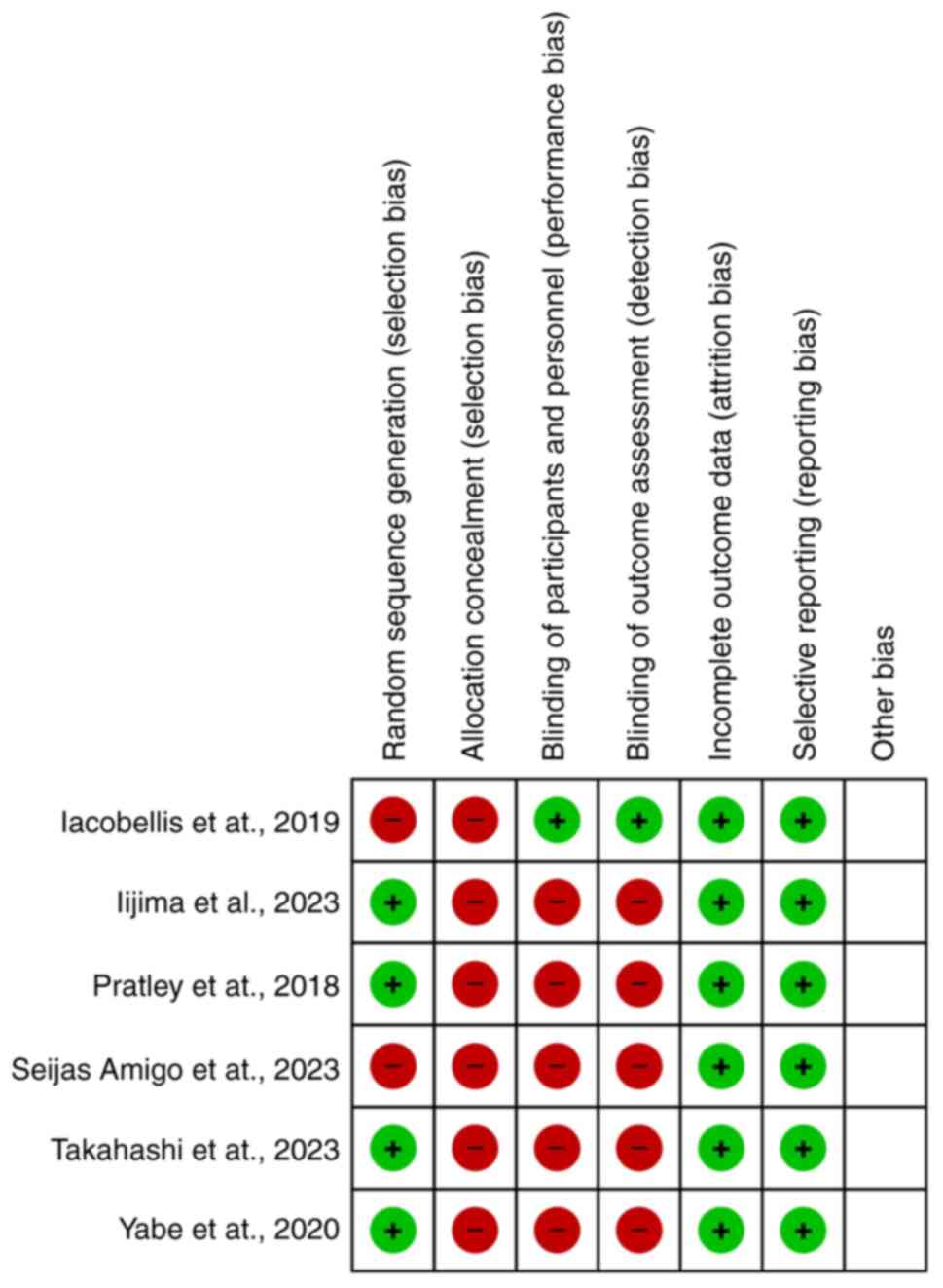
Quality of the studies
Based on the Cochrane risk of bias assessment tool,
the general quality of the included studies was low. Only one of
the studies was blinded (18), and
randomization was performed in four of the studies. None of the
studies implemented allocation concealment (Fig. 7).
Safety and adverse events
No serious adverse events were reported in the study
of Takahashi et al (19).
During the study period, three patients experienced hypoglycemia
(one with dulaglutide and two with semaglutide). However, none of
these incidents required the patients to stop their treatment
(19). In line with that Iacobellis
and Villasante Fricke (18)
demonstrated no adverse events or need for hospitalization. Iijima
et al (17) reported
treatment discontinuation in one patient due to intractable
vomiting and weight loss. In the course of the study by Pratley
et al (15), six
participants lost their lives: One and two individuals succumbed
while using semaglutide and dulaglutide, respectively. The
incidence of serious adverse effects was similar across all
treatment groups. Gastrointestinal problems were the most common
adverse effects (6,16), with similar rates in all groups
except for those receiving dulaglutide 0.75 mg, where fewer
patients experienced gastrointestinal issues (15). Seijas-Amigo et al (6) reported one cardiovascular-related
death during dulaglutide treatment.
Discussion
One of the most important T2DM therapy options is
using GLP-1 RAs. The present meta-analysis aimed to compare the
efficacy of semaglutide and dulaglutide in achieving glycemic
control and weight loss in patients with T2DM. This analysis
revealed no significant difference in glycemic control between
semaglutide and dulaglutide, while semaglutide was more effective
in inducing weight loss than dulaglutide.
Glycemic control efficacy
Previous studies have shown mixed results regarding
the efficacy of these medications in achieving glycemic control in
patients with T2DM, with some indicating that there is no
significant difference between the two medications (6), while others show that semaglutide has
improved efficacy in reducing HbA1c levels compared with
dulaglutide (15,16). When semaglutide was compared with
other GLP-1 RAs in a meta-analysis, it was identified that it
lowers HbA1c and fasting plasma glucose levels more effectively and
increases the likelihood of achieving both targeted and intense
HbA1c level reductions by >2 times. Although the likelihood of
significant HbA1c reductions with semaglutide was comparable to
that of liraglutide, the absolute reduction in HbA1c and fasting
plasma glucose levels obtained with semaglutide was not larger than
that attained with dulaglutide (20). In another meta-analysis, despite
substantial variation in the length of the trials and the therapy
regimens employed, semaglutide was shown to be more efficient than
dulaglutide in terms of glycemic control, with a significant
decrease in HbA1c by 0.47% (21).
Another meta-analysis revealed that semaglutide 1 mg significantly
outperformed other GLP-1 RAs, such as dulaglutide, in decreasing
HbA1c, with a similar drop of 0.38% (22). In a similar vein, a network
meta-analysis showed that semaglutide 1 mg was more substantially
associated with a decrease in HbA1c among individuals with T2DM
than dulaglutide (23).
Several factors could cause the variations in the
results provided. These could include differences in study design,
sample sizes and patient populations. Additionally, variations
could be caused by differences in dosage, administration route
(oral vs. subcutaneous) and formulation. Other factors, such as
patient adherence, treatment duration and lifestyle modifications,
could also contribute to the differences observed in the results.
It is thus important to carefully consider these factors when
interpreting and comparing the results of different studies.
Weight loss efficacy
Moderate weight reduction can improve blood glucose
control, decrease the need for hypoglycemic medications, and is
linked to a substantial decrease in the risk of various
obesity-connected complications in overweight or obese patients
with T2DM. Therefore, the impact on weight should be considered
when glucose-lowering medications are prescribed for overweight or
obese patients with T2DM. GLP-1 RAs exhibit both the effectiveness
of weight reduction and a favorable hypoglycemic impact (24). The results concerning the efficacy
of semaglutide vs. dulaglutide in inducing weight loss are more
consistent. According to the findings of Patoulias et al
(20), semaglutide was found to be
more effective than dulaglutide when looking at absolute weight
loss, probabilities for attaining weight loss between 5-10%, and
BMI decrease. Similarly, in two other aforementioned meta-analyses,
semaglutide was substantially more effective than dulaglutide in
reducing body weight. In direct connection with these findings,
Andreadis et al (25) found
that compared with dulaglutide, semaglutide 1 mg was more effective
at lowering body weight.
Safety
Due to a lack of data, a safety analysis could not
be performed in the present study. It was found that the most
common side effect of treatment in these therapies was
gastrointestinal complications such as nausea and vomiting.
However, a direct comparison between medications was not possible.
Mishriky et al (22) showed
that, compared with other GLP-1 RAs, semaglutide was considerably
more likely to cause nausea and vomiting. On the other hand, Shi
et al's (21) meta-analysis
of semaglutide and other GLP-1 RAs (for example, dulaglutide)
failed to detect a higher incidence of gastrointestinal side
effects in the former as opposed to the latter (21). In addition, a meta-analysis by
Mishriky et al (22) found
no statistically significant difference between semaglutide and
other GLP-1 RAs in the incidence of any serious adverse events.
Data heterogeneity and sensitivity
analysis
Heterogeneity is a common issue in meta-analyses,
arising from differences in study design, patient characteristics,
interventions and outcome measures among included studies. In the
present study, high heterogeneity was found among the studies
included in the meta-analysis, as evidenced by prediction interval
analysis, which suggested significant between-study variability.
This heterogeneity is important to acknowledge, as it can affect
the precision, validity and generalizability of the meta-analysis
results.
The I2 statistic is commonly used in
meta-analyses to quantify the percentage of total variation across
studies due to heterogeneity rather than chance. It measures the
inconsistency or diversity among studies and can range from 0-100%,
with higher values indicating greater heterogeneity (26). However, it has been recently argued
that the I2 statistic does not adequately reveal the
degree of variation in effect size in a meta-analysis (27). In that light, prediction interval
analysis was used instead. Prediction interval analysis is a
statistical method used to calculate CIs for the true effect size
based on the distribution of the data and the sampling error. Given
the observed data and sampling variability, it provides a range of
values within which the true effect size is likely to fall.
Prediction interval analysis is particularly useful when estimating
small or large effect sizes, as CIs are narrower for larger samples
and effect sizes and wider for smaller samples and effect
sizes.
To assess the potential impact of individual studies
on the meta-analysis results, a sensitivity analysis was conducted
by sequentially removing one study at a time and recalculating the
effect size and pooled estimate. The sensitivity analysis results
showed that removing one study (15) had a substantial effect on the
overall effect size of the weight loss data and the statistical
significance of the meta-analysis. This underscores the importance
of cautious interpretation of meta-analysis results and the
potential impact of individual studies on the outcome.
Limitations
The present study has several limitations that must
be acknowledged. Despite the comprehensive search strategy used, it
is still possible that some eligible studies may have been missed,
which may influence the precision of the meta-analysis results.
Besides, the number of studies available for this meta-analysis was
limited which might be attributed to several factors. Firstly,
semaglutide and dulaglutide are relatively new therapeutic options
for the management of T2DM, and head-to-head comparisons between
these two drugs have only recently become the focus of clinical
research. This has resulted in a smaller pool of studies that
directly compare their efficacy and safety. Additionally, our
strict inclusion criteria, which required randomized controlled
trials with specific outcome measures such as glycemic control and
weight loss, further narrowed the range of eligible studies. As a
result, some potentially relevant studies may have been excluded
due to differences in study design, populations, or intervention
protocols that did not align with the objectives of the current
analysis.
Additionally, the quality of the included studies
was variable, with some studies being of lower quality. This
variability could have contributed to the observed heterogeneity
and the sensitivity analysis results. The heterogeneity of the
included data was also high. Although random-effects model was used
to account for heterogeneity, it is important to acknowledge that
some residual heterogeneity may remain, affecting the precision of
the estimates. The high degree of heterogeneity observed in the
present meta-analysis is likely influenced by differences in study
design, patient populations, treatment regimens and dosage levels
among the included studies. Specifically, variations in baseline
HbA1c levels, treatment duration and dosing strategies (for
example, semaglutide doses ranging from 0.5-14 mg vs. dulaglutide
doses between 0.75-1.5 mg) may have contributed to the observed
variability in outcomes. While subgroup analyses could provide
further insights into the impact of these factors, the limited
number of studies and variability in study characteristics made
such analyses impractical. Instead, using moderator analysis
allowed us to identify a significant relationship between longer
treatment duration and greater weight loss, suggesting that
duration may be an important factor to consider when choosing
between these treatments. The broad prediction intervals indicate
that, while semaglutide generally shows an advantage in weight
loss, results may vary across different clinical settings and
patient populations, highlighting the need for individualized
treatment decisions. Future studies should aim for more consistent
study designs and larger sample sizes to better delineate the
comparative effectiveness of these therapies. The current
sensitivity analysis identified that the exclusion of one study
could significantly impact the overall results of weight loss data.
This suggests that the present conclusions depend on including all
studies and that one or more studies may have an undue influence on
the overall estimate. Finally, meta-analyses are inherently limited
by the quality and availability of the primary studies and the
limitations of the meta-analytic methods used. While it was aimed
to minimize potential biases and increase transparency in our
review process, the present results may be subject to publication
bias, incomplete reporting of results and other methodological
limitations.
Conclusion
The present meta-analysis compared the efficacy of
two important GLP-1 RAs, semaglutide and dulaglutide, in achieving
glycemic control and weight loss in patients with T2DM. The results
revealed that while there was no significant difference in glycemic
control between the two drugs, semaglutide was more effective in
inducing weight loss than dulaglutide. It is important to note that
although random-effects model was used to account for
heterogeneity, some residual heterogeneity may have remained, which
could affect the precision of the estimates. Despite this, the
present findings suggested that semaglutide may be a more favorable
treatment option for patients with T2DM requiring weight management
and glycemic control. Further research is needed to investigate the
long-term anti-diabetic and cardiovascular benefits of GLP-1 RAs,
the optimal dosages and regimens, and individual factors that may
influence treatment response and outcomes. Nevertheless, the
present meta-analysis provides valuable insights for clinicians and
patients when making treatment decisions for T2DM.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LX, LS and HA drafted and wrote the manuscript,
collected and analyzed data. LS revised the manuscript for
intellectual content, provided general supervision and gave final
approval for publication. SH performed data analysis, language
editing and obtained materials. LX and HA developed the study
protocol and confirm the authenticity of all the raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tönnies T, Rathmann W, Hoyer A, Brinks R
and Kuss O: Quantifying the underestimation of projected global
diabetes prevalence by the International Diabetes Federation (IDF)
Diabetes Atlas. BMJ Open Diabetes Res Care.
9(e002122)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
American-diabetes-association. Improving
care and promoting health in populations: Standards of medical
care in diabetes-2021. Diabetes Care. 44:S7–S14.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Campbell JE and Drucker DJ: Pharmacology,
physiology, and mechanisms of incretin hormone action. Cell Metab.
17:819–837. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nauck MA and Meier JJ: The incretin effect
in healthy individuals and those with type 2 diabetes: Physiology,
pathophysiology, and response to therapeutic interventions. Lancet
Diabetes Endocrinol. 4:525–536. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hinnen D: Glucagon-like peptide 1 receptor
agonists for type 2 diabetes. Diabetes Spectr. 30:202–210.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Seijas-Amigo J, Salgado-Barreira Á,
Castelo-Dominguez R, Pérez-Álvarez MT, Ponce-Piñón B,
Fernández-Silva M, Rodríguez-Barreiro M, Pereira-Pía M,
Iglesias-Moreno JM, Gago-García M, et al: Differences in weight
loss and safety between the glucagon-like peptide-1 receptor
agonists: A non-randomized multicenter study from the titration
phase. Prim Care Diabetes. 17:366–372. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: an updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Kononenko IV, Smirnova OM, Mayorov AY and
Shestakova MV: Classification of diabetes. World Health
Organization 2019. What's new? Diabetes Mellitus. 23:329–339.
2020.
|
|
9
|
American-diabetes-association. 2.
Classification and diagnosis of diabetes: Standards of medical care
in diabetes-2022. Diabetes Care. 45:S17–S38. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC, et
al: The Cochrane Collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Higgins JP and Green S (eds): Cochrane
handbook for systematic reviews of interventions. The Cochrane
Collaboration, 2008.
|
|
12
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Graham PL and Moran JL: Robust
meta-analytic conclusions mandate the provision of prediction
intervals in meta-analysis summaries. J Clin Epidemiol. 65:503–510.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bloch MH: Meta-analysis and moderator
analysis: Can the field develop further? J Am Acad Child Adolesc
Psychiatry. 53:135–137. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pratley RE, Aroda VR, Lingvay I, Lüdemann
J, Andreassen C, Navarria A and Viljoen A: SUSTAIN 7 investigators.
Semaglutide versus dulaglutide once weekly in patients with type 2
diabetes (SUSTAIN 7): A randomised, open-label, phase 3b trial.
Lancet Diabetes Endocrinol. 6:275–286. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yabe D, Nakamura J, Kaneto H, Deenadayalan
S, Navarria A, Gislum M and Inagaki N: PIONEER 10 Investigators.
Safety and efficacy of oral semaglutide versus dulaglutide in
Japanese patients with type 2 diabetes (PIONEER 10): An open-label,
randomised, active-controlled, phase 3a trial. Lancet Diabetes
Endocrinol. 8:392–406. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Iijima T, Shibuya M, Ito Y and Terauchi Y:
Effects of switching from liraglutide to semaglutide or dulaglutide
in patients with type 2 diabetes: A randomized controlled trial. J
Diabetes Investig. 14:774–781. 2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Iacobellis G and Villasante Fricke AC:
Effects of semaglutide versus dulaglutide on epicardial fat
thickness in subjects with type 2 diabetes and obesity. J Endocr
Soc. 4(bvz042)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Takahashi Y, Nomoto H, Yokoyama H, Takano
Y, Nagai S, Tsuzuki A, Cho KY, Miya A, Kameda H, Takeuchi J, et al:
Improvement of glycaemic control and treatment satisfaction by
switching from liraglutide or dulaglutide to subcutaneous
semaglutide in patients with type 2 diabetes: A multicentre,
prospective, randomized, open-label, parallel-group comparison
study (SWITCH-SEMA 1 study). Diabetes Obes Metab. 25:1503–1511.
2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Patoulias D, Popovic DS, Stoian AP, Janez
A, Sahebkar A and Rizzo M: Effect of semaglutide versus other
glucagon-like peptide-1 receptor agonists on cardio-metabolic risk
factors in patients with type 2 diabetes: A systematic review and
meta-analysis of head-to-head, phase 3, randomized controlled
trials. J Diabetes Complications. 37(108529)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shi FH, Li H, Cui M, Zhang ZL, Gu ZC and
Liu XY: Efficacy and safety of once-weekly semaglutide for the
treatment of type 2 diabetes: A systematic review and meta-analysis
of randomized controlled trials. Front Pharmacol.
9(576)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mishriky BM, Cummings DM, Powell JR,
Sewell KA and Tanenberg RJ: Comparing once-weekly semaglutide to
incretin-based therapies in patients with type 2 diabetes: A
systematic review and meta-analysis. Diabetes Metab. 45:102–109.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Witkowski M, Wilkinson L, Webb N, Weids A,
Glah D and Vrazic H: A systematic literature review and network
meta-analysis comparing once-weekly semaglutide with other glp-1
receptor agonists in patients with type 2 diabetes previously
receiving 1-2 oral anti-diabetic drugs. Diabetes Ther. 9:1149–1167.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tang Y, Zhang L, Zeng Y, Wang X and Zhang
M: Efficacy and safety of tirzepatide in patients with type 2
diabetes: A systematic review and meta-analysis. Front Pharmacol.
13(1016639)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Andreadis P, Karagiannis T, Malandris K,
Avgerinos I, Liakos A, Manolopoulos A, Bekiari E, Matthews DR and
Tsapas A: Semaglutide for type 2 diabetes mellitus: A systematic
review and meta-analysis. Diabetes Obes Metab. 20:2255–2263.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
West SL, Gartlehner G, Mansfield AJ, Poole
C, Tant E, Lenfestey N, Lux LJ, Amoozegar J, Morton SC, Carey TC,
et al: AHRQ methods for effective health care. In: Comparative
effectiveness review methods: Clinical heterogeneity. Agency for
Healthcare Research and Quality (US), Rockville (MD), 2010.
|
|
27
|
Borenstein M: In a meta-analysis, the
I-squared statistic does not tell us how much the effect size
varies. J Clin Epidemiol. 152:281–284. 2022.PubMed/NCBI View Article : Google Scholar
|