Introduction
The relationship between oral health and sleep
quality is a complex and bidirectional phenomenon that has garnered
significant attention in recent years. This interplay affects not
only the quality of life but also has profound implications for
overall health and well-being (1).
Sleep disturbances can significantly impact oral health (2). Individuals with poor sleep quality
often neglect proper oral hygiene practices, increasing the risk of
dental issues such as periodontitis and gingivitis (3). A study, using the National Health and
Nutrition Examination Survey data, found that individuals with
sleep disorders were more likely to report dental pain, periodontal
issues and negative emotions regarding their oral health compared
with those without sleep disorders (4). Poor sleep quality can also lead to
xerostomia, reducing saliva flow and increasing the risk of oral
health problems such as tooth decay and gingivitis (4).
Conversely, poor oral health can significantly
impact sleep quality. Dental issues such as periodontitis,
gingivitis and dental pain can disrupt sleep patterns and lead to
restless nights. Thus, periodontal diseases have been identified as
risk factors for developing sleep disorders, with inflammation
caused by untreated gum disease potentially leading to systemic
health issues that can affect sleep quality (5,6).
Besides, the Oral Health Impact Profile (OHIP)-14 score, which
reflects the subjective interpretation of oral health-related
quality of life, was found to be higher in patients with poor sleep
quality. This suggests that oral health can significantly affect
sleep quality, highlighting the importance of addressing oral
health issues to improve sleep (7).
Several mechanisms underlie the relationship between
oral health and sleep quality. One key mechanism is the
inflammatory pathway (8).
Periodontal disease, for instance, leads to chronic inflammation,
which can affect systemic health and sleep quality. The release of
pro-inflammatory cytokines such as interleukin-6 and tumor necrosis
factor-α can disrupt sleep patterns by altering the immune response
and affecting the hypothalamic-pituitary-adrenal axis (2,4,9). High
cortisol levels can disrupt sleep patterns, and conversely, poor
sleep quality can increase cortisol levels (2). This bidirectional relationship is
crucial in understanding how sleep disturbances affect the stress
response of the body. Studies have shown that elevated cortisol
levels are associated with insomnia, waking up during the night,
and less sleep time overall (2,4).
However, the aforementioned findings are not
universal and certain studies have not found an association between
sleep quality and oral hygiene/health (10,11).
Given the uncertainty regarding the bidirectional nature of this
relationship, a comprehensive meta-analysis is essential to fully
understand the magnitude and mechanisms of the interplay between
oral health and sleep quality. Such an analysis would help to
synthesize data from various studies, providing a clearer picture
of how sleep disorders affect oral health and vice versa. Moreover,
a meta-analysis could help in addressing the limitations of current
studies (12). Therefore, the
present systematic review and meta-analysis was performed to assess
the relationship between sleep quality/disturbances and oral
health/hygiene.
Materials and methods
The present systematic review and meta-analysis were
conducted in accordance with the Preferred Reporting Items for
Systematic Reviews and Meta-Analyses guidelines (13).
Inclusion and exclusion criteria
Studies were included if they met the following
criteria: i) Population: Individuals of any age group, irrespective
of sex, in studies assessing oral health status and sleep quality;
ii) intervention/exposure: Studies examining the relationship
between oral health (such as periodontal disease, dental caries and
oral hygiene) and sleep quality; iii) outcomes: Studies reporting
on sleep quality using validated tools [such as Pittsburgh Sleep
Quality Index (PSQI) and Insomnia Severity Index (ISI)] or on oral
health using validated tools [such as Decayed, Missing, and Filled
Teeth Index (DMFT) or OHIP]; and iv) study design: Cross-sectional,
cohort and case-control studies were included. Review articles,
case reports, commentaries and editorials were excluded. Only
studies published in English were considered. No time limit was
imposed on the publication date. The search was conducted until the
end of September 2024.
Search terms and strategy
A comprehensive search of the literature was
conducted in September 2024 using the following electronic
databases: PubMed (https://pubmed.ncbi.nlm.nih.gov), Embase (https://www.embase.com), Scopus (https://www.scopus.com) and the Cochrane Library
(https://www.cochranelibrary.com).
Additionally, the references of included studies and relevant
review articles were searched to identify any additional studies
not captured in the initial database searches. The search strategy
was developed using a combination of medical subject headings
(MeSH) and free-text terms related to oral health and sleep
quality. The search terms included: i) Oral health-related terms:
‘Oral health’, ‘periodontal disease’, ‘dental caries’, ‘oral
hygiene’, ‘gingivitis’, ‘tooth loss’ and ‘edentulism’; and ii)
sleep quality-related terms: ‘Sleep quality’, ‘sleep disturbance’,
‘insomnia’, ‘sleep disorders’, ‘sleep duration’, ‘Pittsburgh Sleep
Quality Index’, ‘PSQI’, ‘Insomnia Severity Index’ and ‘ISI’.
The specific search strategy for PubMed was as
follows: (‘oral health’[MeSH Terms] OR ‘periodontal disease’[MeSH
Terms] OR ‘dental caries’[MeSH Terms] OR ‘oral hygiene’[MeSH Terms]
OR ‘gingivitis’[MeSH Terms] OR ‘tooth loss’[MeSH Terms] OR
‘edentulism’[MeSH Terms] OR ‘oral health’[Title/Abstract] OR
‘periodontal disease’[Title/Abstract] OR ‘dental
caries’[Title/Abstract] OR ‘oral hygiene’[Title/Abstract] OR
‘gingivitis’[Title/Abstract] OR ‘tooth loss’[Title/Abstract] OR
‘edentulism’[Title/Abstract]) AND (‘sleep quality’[MeSH Terms] OR
‘sleep disturbance’[MeSH Terms] OR ‘insomnia’[MeSH Terms] OR ‘sleep
disorders’[MeSH Terms] OR ‘sleep duration’[MeSH Terms] OR
‘Pittsburgh Sleep Quality Index’[Title/Abstract] OR
‘PSQI’[Title/Abstract] OR ‘Insomnia Severity Index’[Title/Abstract]
OR ‘ISI’[Title/Abstract] OR ‘sleep quality’[Title/Abstract] OR
‘sleep disturbance’[Title/Abstract] OR ‘insomnia’[Title/Abstract]
OR ‘sleep disorders’[Title/Abstract] OR ‘sleep
duration’[Title/Abstract]).
Study selection and data
extraction
The studies identified through the database search
were imported into EndNote X9 (Clarivate) and duplicates were
removed. In total, two independent reviewers screened the titles
and abstracts of the remaining studies. Full texts of potentially
eligible studies were retrieved and assessed according to the
inclusion criteria. Any disagreements between the reviewers were
resolved through discussion or consultation with a third reviewer.
Data were extracted independently by two reviewers using a
standardized data extraction form. Extracted data included: i)
Study characteristics: Author, year of publication, country and
study design; ii) population characteristics: Sample size, age, sex
and oral health status; iii) sleep quality assessment: Tools used
(such as PSQI and ISI) and definitions; and iv) results: Mean ±
standard deviation (SD).
Risk of bias assessment
In total, two independent reviewers assessed the
risk of bias of each study using the Newcastle-Ottawa Scale (NOS)
for observational studies. The NOS evaluates studies based on
selection, comparability and exposure or outcome assessment
(14). Each study was rated as low
(score, 7-9), moderate (score, 4-6) or high (score, 0-3) risk of
bias (14). Discrepancies in the
assessments were resolved through discussion or by consulting a
third reviewer.
Statistical analysis
Meta-analyses were performed using Comprehensive
Meta-Analysis version 3 software (https://meta-analysis.com). The relationship between
oral health and sleep quality was synthesized using Standardized
Mean Differences (SMDs) to compare outcomes across studies with
different measurement scales. The choice between a fixed-effect and
a random-effects (in this case) meta-analysis should never be made
on the basis of a statistical test for heterogeneity. In addition,
since heterogeneity is expected for the intervention effects among
multiple studies from different groups and geographical locations,
a random effects model was used to calculate the SMDs.
Heterogeneity among the studies was assessed using the precision
interval approach, which examines the variability in effect sizes
across studies. Unlike the I² statistic, the precision interval
provides a range of plausible true effects, giving a more direct
interpretation of heterogeneity. This method was chosen due to its
robustness in handling the diverse study designs and populations
included in the analysis (15).
Publication bias was assessed through Egger's, and Begg and
Mazumdar rank correlation tests and visually using funnel plots.
Begg's test evaluates the asymmetry of the funnel plot, with
P<0.05 indicating potential publication bias. In cases where
publication bias was suggested, the trim-and-fill method was
applied to estimate the impact of missing studies on the overall
effect size.
Results
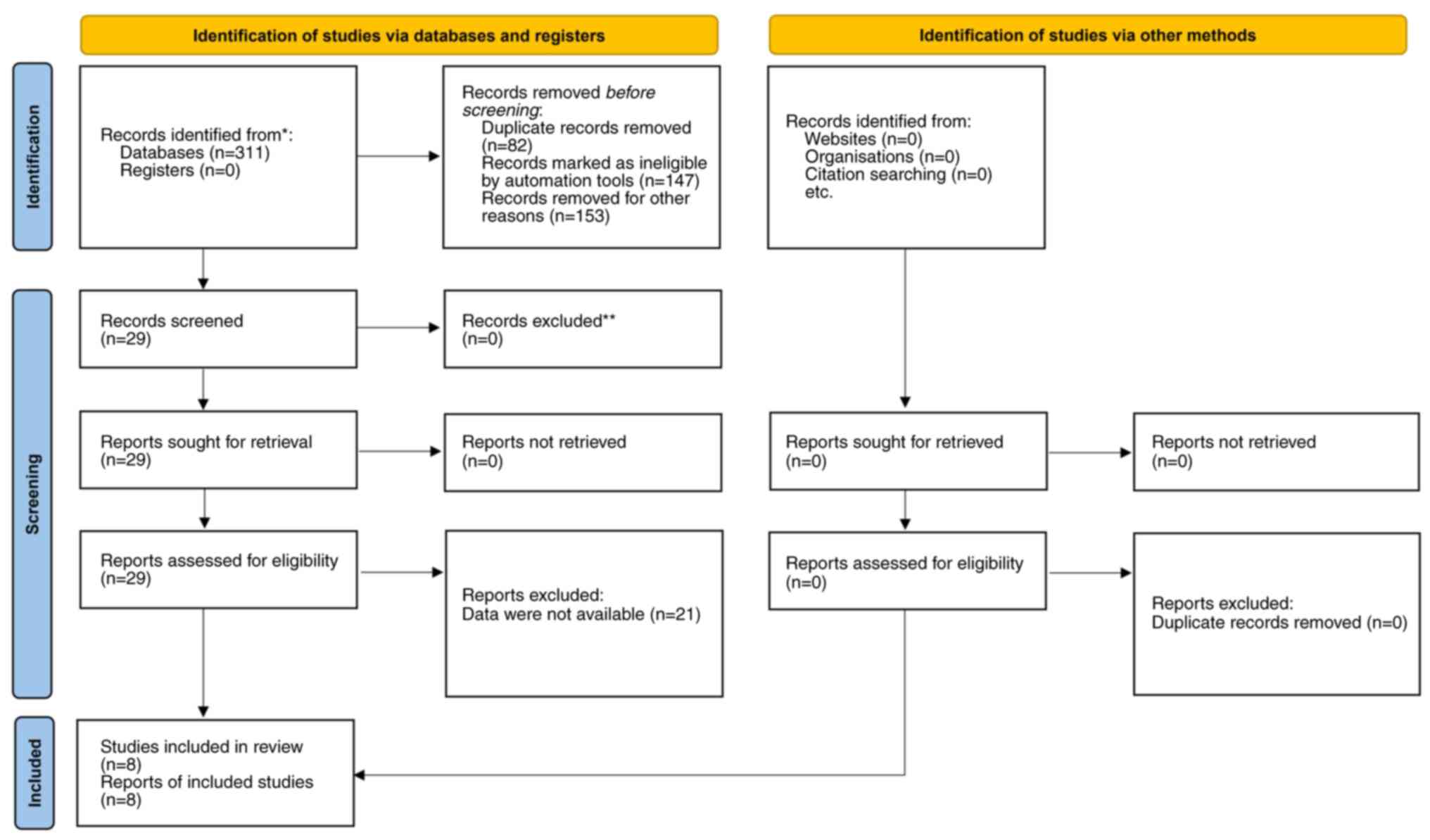
Using the predefined search strategy, a total of 311
articles were identified. After removing duplicates, irrelevant
studies, reviews and editorials, 29 articles were deemed eligible
for full-text assessment. Of these, 8 articles, encompassing 18
different comparisons, met the criteria for inclusion in the
meta-analysis (Fig. 1) (5,6,16-21).
Some studies included multiple comparisons with different
populations and have therefore been split into multiple studies in
the present analysis. The characteristics of the included studies
are presented in Table I. The table
includes 8 cross-sectional studies conducted between 2015 and 2023
in countries such as Greece, India, Spain, Italy, France, South
Korea and the United States. Sample sizes ranged from 60 to 29,870
participants, with age groups spanning from young children (2-5
years) to adults. The total number of patients included across all
the studies was 36,559. Oral health assessments used various
indices, including the DMFT, Community Periodontal Index and
gingival indices. Additionally, sleep quality was measured using
tools such as the PSQI, Pediatric Sleep Questionnaire and Epworth
Sleepiness Scale. The results showed that poorer oral health, such
as higher DMFT scores and gingival inflammation, was often
associated with poorer sleep quality, including increased sleep
disturbances and reduced sleep duration.
 | Table ICharacteristics of the included
studies, population characteristics, sleep quality assessment and
the results. |
Table I
Characteristics of the included
studies, population characteristics, sleep quality assessment and
the results.
| First author/s,
year | Study
characteristics | Population
characteristics | Sleep quality
assessment | Results, Mean ±
SD | (Refs.) |
|---|
| Apessos et al,
2020 | Country: Greece;
study design: Cross-sectional observational clinical study. | Sample size: 177;
age: 18-30 years; sex: Male; oral health status: Good general
health, no medical problems. | Tools used: ESS,
PSQI, BQ, SB questionnaire and XI. | Age: 23.10±2.86
years; BMI: 25.43±3.69; MST: 24.97±10.61; XI: 19.41±5.88; ESS:
6.97±3.50; PSQI: 5.91±3.17 | (16) |
| Arroyo Buenestado and
Ribas-Pérez, 2023 | Country: Spain; Study
design: Analytical cross-sectional study. | Sample Size: 80; age:
2-5 years; sex: Male (50%) and female (50%); oral health status:
Evaluated using DMFT index, with a mean of 1.73±2.34 | Tools Used: PSQ and
AAPD guideline-based questionnaire. | Occasional snorers:
DMFT, 2.63±2.50; non-snorers: DMFT, 1.02±1.96; Significant
relationships with noisy breathing, sleepwalking and
nightmares. | (17) |
| Grillo et al,
2019 | Country: Italy; study
design: . Cross-sectional study | Sample size: 122;
age: 8-17 years; sex: Male (54.1%) and female (45.9%); oral health
status: Evaluated using DMFS, PPD and BOP indices | Tools used: (PSQ) and
COHIP. | DMFS: SDB+ (13.6±4.7)
and SDB- (3.5±2.2); COHIP (parent): SDB+ (24.5±5.6) and SDB-
(16.7±4.3); COHIP (child): SDB+ (23.2±4.6) and SDB- (15.9±3.8) | (5) |
| Carra et al,
2017 | Country: France;
study design: Cross-sectional epidemiological study. | Sample size: 29,870;
mean age: 45.3 years; sex: Male (47.9%) and female (52.1%); oral
health status: Evaluated using plaque index, calculus index,
gingival inflammation and masticatory function. | Tools used:
Self-reported questionnaire on sleep disorders and sleep
duration. | Age: 45.3±15.19
years; BMI: 25.73±4.93; sleep duration: 6.02±1.48 h; gingival
inflammation: Higher in individuals with sleep disorders [AOR 1.22
(1.13-1.32)] | (6) |
| Chacko et al,
2021 | Country: India; study
design: Cross-sectional study. | Sample size: 85; age:
15-60 years; sex: Male (65.9%) and female (34.1%); oral health
status: Categorized into clinically healthy, gingivitis and
periodontitis groups. | Tools used:
PSQI. | PSQI: Healthy
(3.03±1.14), gingivitis (4.44±1.28) and periodontitis (9.03±2.17).
GI: Healthy (0.67±0.44), gingivitis (1.31±0.19) and periodontitis
(2.05±0.51); PD: Healthy (2.16±0.33), gingivitis (2.73±0.38) and
periodontitis (5.19±1.31). | (18) |
| Grover et al,
2015 | Country: India; study
design: Cross-sectional study. | Sample size: 60; age:
25-50 years; sex: Male (43.3%) and female (56.7%); oral health
status: Categorized into clinically healthy, gingivitis and
periodontitis groups. | Tools used:
PSQI. | PSQI: Healthy
(1.20±0.83), gingivitis (1.88±0.18) and periodontitis (7.39±1.33);
GI: Healthy (0), gingivitis (1.39±0.32) and periodontitis
(1.88±0.18). PD: Healthy (2.08±0.06), gingivitis (2.34±0.17) and
periodontitis (3.58±0.63). | (19) |
| Romandini et
al, 2017 | Country: South Korea;
study design: Cross-sectional study. | Sample size: 5,812;
Age: Adults (≥19 years); sex: Not specified; oral health status:
Evaluated using Community Periodontal Index. | Tools used:
Self-reported average daily sleep duration. | Sleep duration:
6.89±0.004 h; Periodontitis prevalence: Higher in those with longer
sleep duration. | (20) |
| Tamasa et al,
2018 | Country: United
States; study design: Cross-sectional study. | Sample size: 123;
age: 8-17 years; sex: Male (52%) and female (48%); Oral health
status: Evaluated using COHIP, DMFS and periodontal indices. | Tools Used: PSQ. | COHIP: SDB+ (24.5±12)
and SDB- (11.6±9.2); DMFS: SDB+ (15.7±15.7) and SDB- (3.7±6.2);
Periodontal PD: SDB+ (2.0±1.0) and SDB- (0.0±0.7); Bleeding on
probing: SDB+ (90%) and SDB- (20%). | (21) |
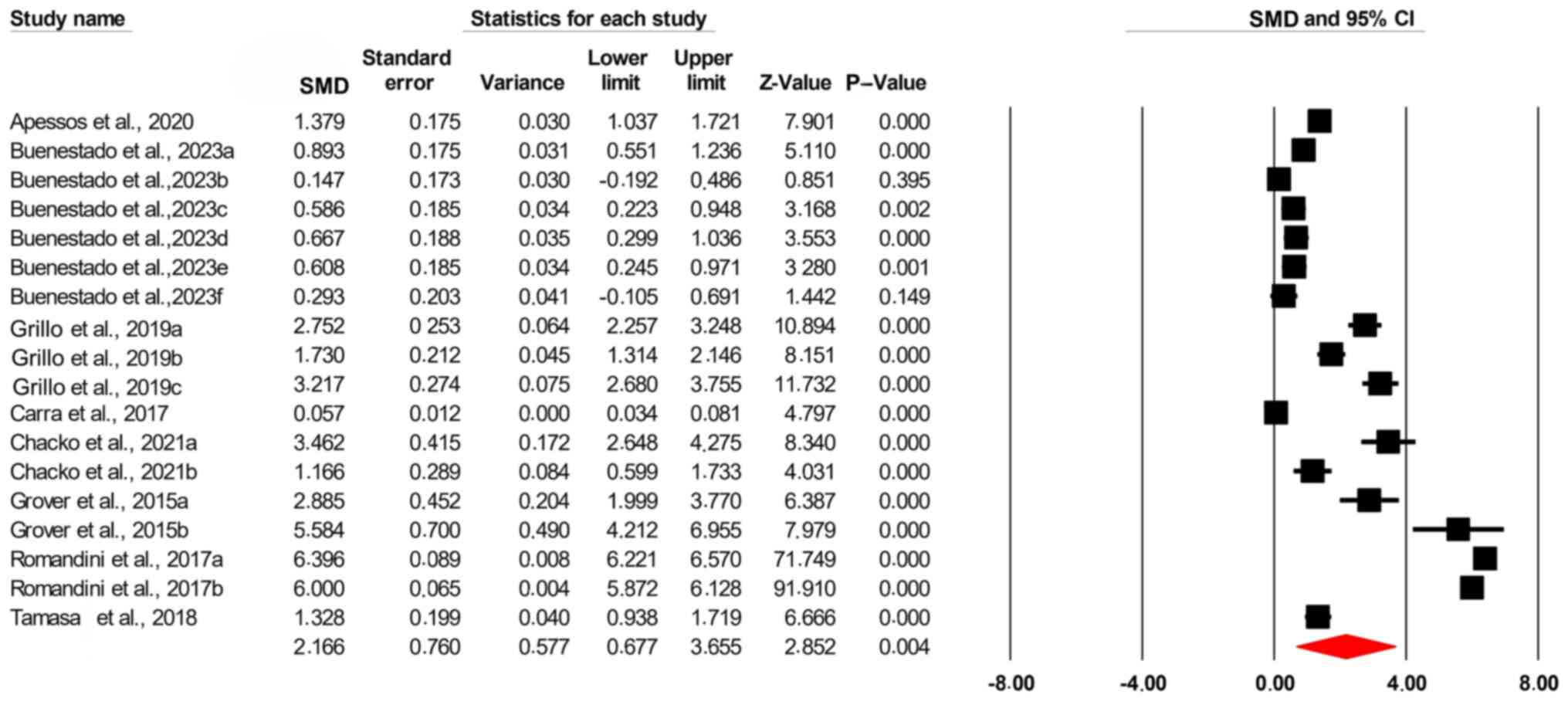
The results of the present study showed a
significant association between oral health and sleep quality [SMD,
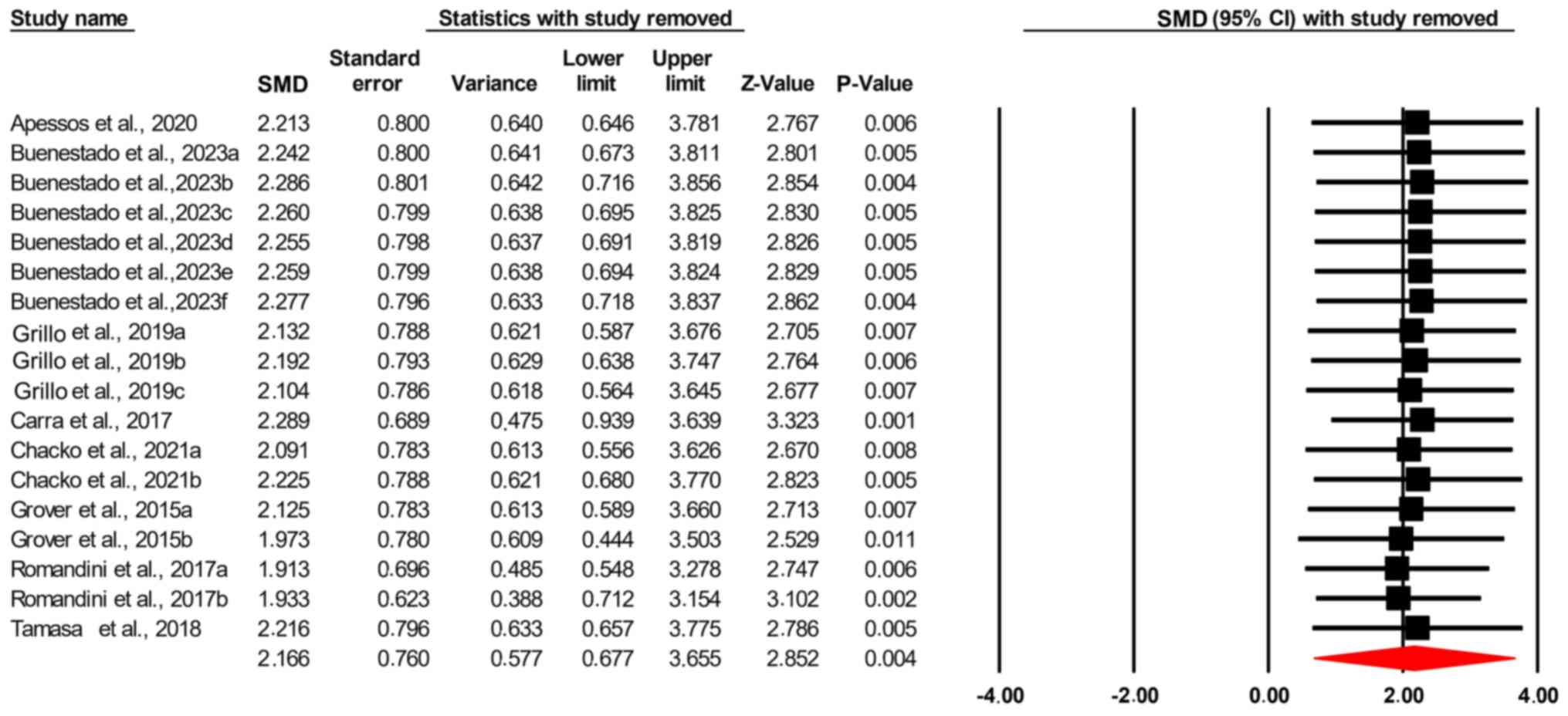
2.166; confidence interval (95% CI), 0.677-3.655; P=0.004; Fig. 2]. The Leave-One-Out sensitivity
analysis showed that the exclusion of no single study significantly
affected the total effect size of the studies and its significance
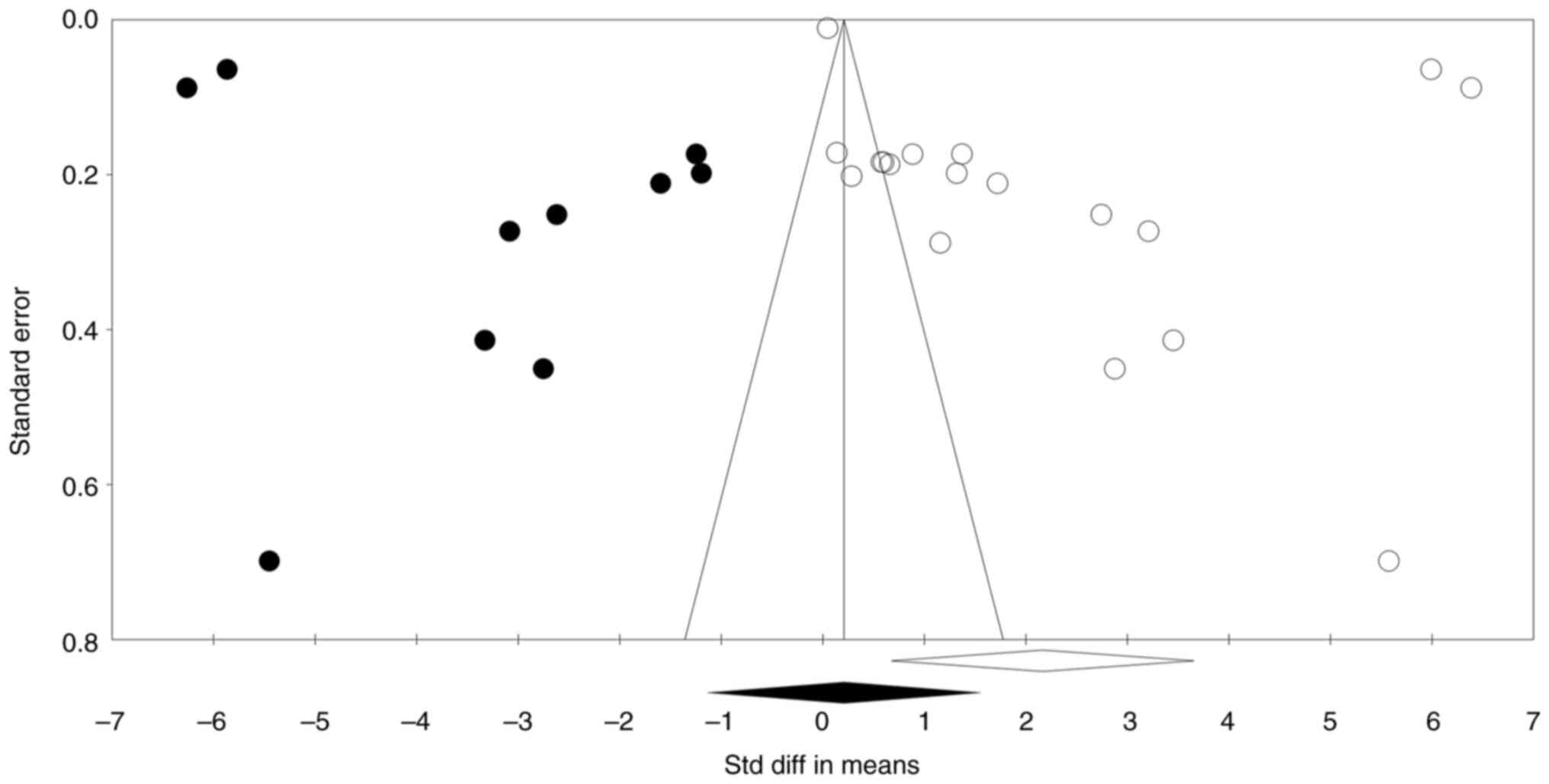
(Fig. 3). Additionally, the funnel
plot was reasonably symmetric and showed no evidence of publication
bias (Fig. 4). The observed and
adjusted point estimates were also the same (point estimate:
0.387). In the present analysis, the trim-and-fill method was
applied to assess potential publication bias by evaluating the
symmetry of the funnel plot. This method involves ‘trimming’
asymmetric studies and ‘filling’ in estimated missing studies to
achieve symmetry. The results indicated that no studies needed to
be trimmed or imputed on either side of the mean, suggesting an
absence of publication bias. Consequently, both the observed and
adjusted point estimates remained consistent, with a fixed-effect
estimate of 0.387 and a random-effect estimate of 2.166. This
consistency implies that the data distribution is symmetric, and
the influence of publication bias on the present findings is
minimal. Regarding the funnel plot visualization, it is important
to note that the plot displays the original studies without any
imputed data points, as no imputations were necessary. The
appearance of points outside the funnel could be attributed to
factors such as heterogeneity among studies or variations in study
precision. While the trim-and-fill method did not identify missing
studies, the presence of points outside the funnel suggests that
other factors, such as study heterogeneity, may be influencing the
plot's appearance. Therefore, while the trim-and-fill results
suggest minimal publication bias, the funnel plot should be
interpreted with caution, considering these potential influences
(Fig. 4). Begg and Mazumdar rank
correlation confirmed these findings by showing no evidence of
publication bias (two-tailed P=0.225). Similarly, Egger's
regression intercept revealed no evidence of publication bias
(two-tailed P=0.065) (Table
II).
 | Table IIBegg and Mazumdar's rank correlation
and Egger's regression intercept. |
Table II
Begg and Mazumdar's rank correlation
and Egger's regression intercept.
| Measure | Value |
|---|
| Kendall's S statistic
(P-Q) | 33.000 |
| Kendall's tau without
continuity correction | |
|
Tau | 0.215 |
|
Z-value for
tau | 1.249 |
|
P-value
(1-tailed) | 0.105 |
|
P-value
(2-tailed) | 0.211 |
| Kendall's tau with
continuity correction | |
|
Tau | 0.209 |
|
Z-value for
tau | 1.212 |
|
P-value
(1-tailed) | 0.112 |
|
P-value
(2-tailed) | 0.225 |
| Egger's regression
intercept | |
|
Intercept | 13.578 |
|
Standard
error | 6.871 |
|
95% Lower
limit (2-tailed) | -0.987 |
|
95% Upper
limit (2-tailed) | 28.144 |
|
t-value | 1.976 |
|
Degrees of
freedom | 16.000 |
|
P-value
(1-tailed) | 0.032 |
|
P-value
(2-tailed) | 0.065 |
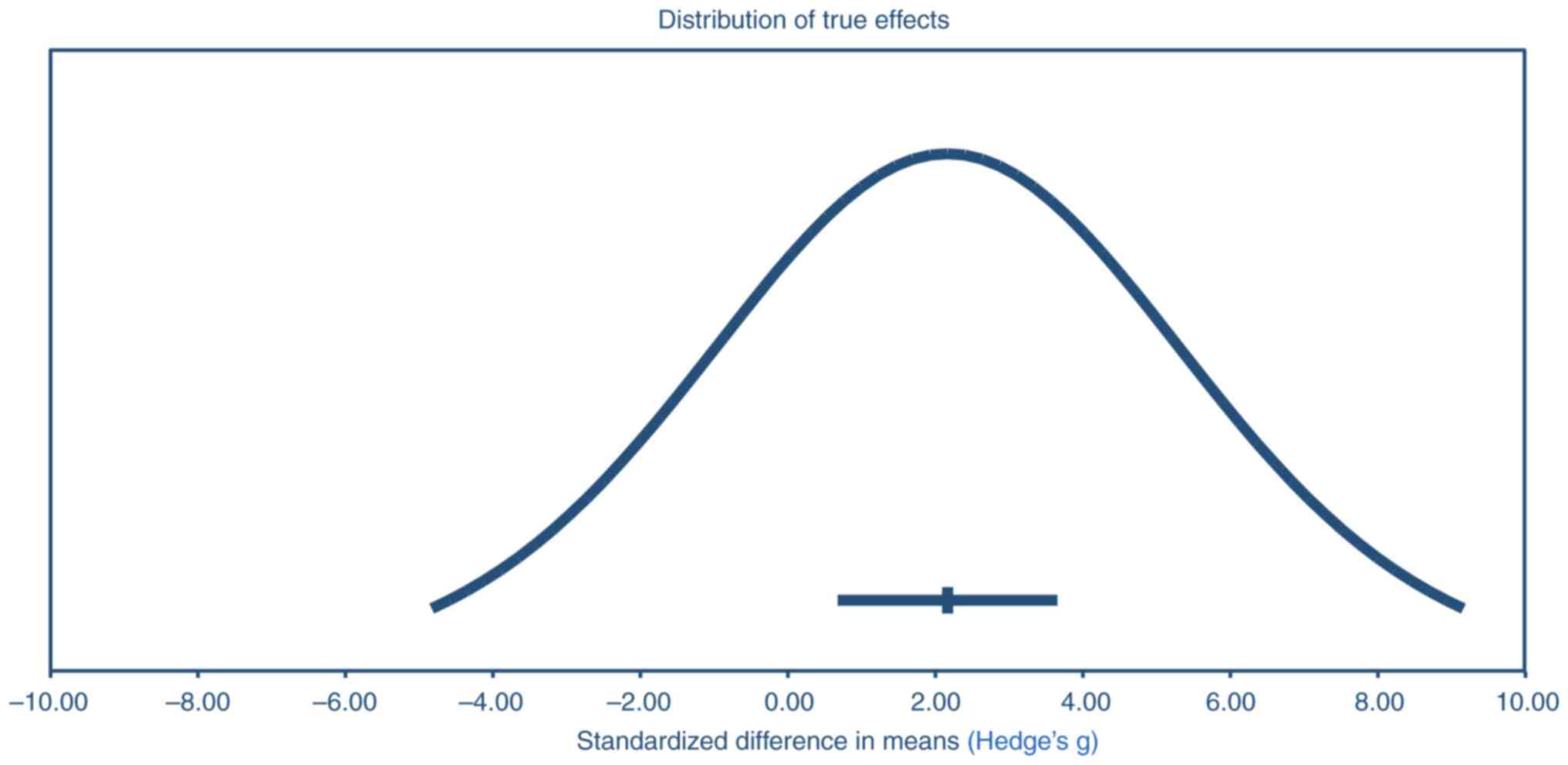
The precision interval analysis yielded a mean
effect size of 2.17 with a 95% confidence interval (CI) ranging
from 0.68 to 3.66. This suggests that, based on the present sample
data, it is with 95% confidence that the true effect size lies
between 0.68 and 3.66. Additionally, the analysis provided a
prediction interval from -4.83 to 9.16, indicating that in 95% of
comparable populations, the true effect size is expected to fall
within this range. The broader prediction interval reflects the
variability observed across different populations, highlighting the
potential for diverse outcomes in similar studies (Fig. 5).
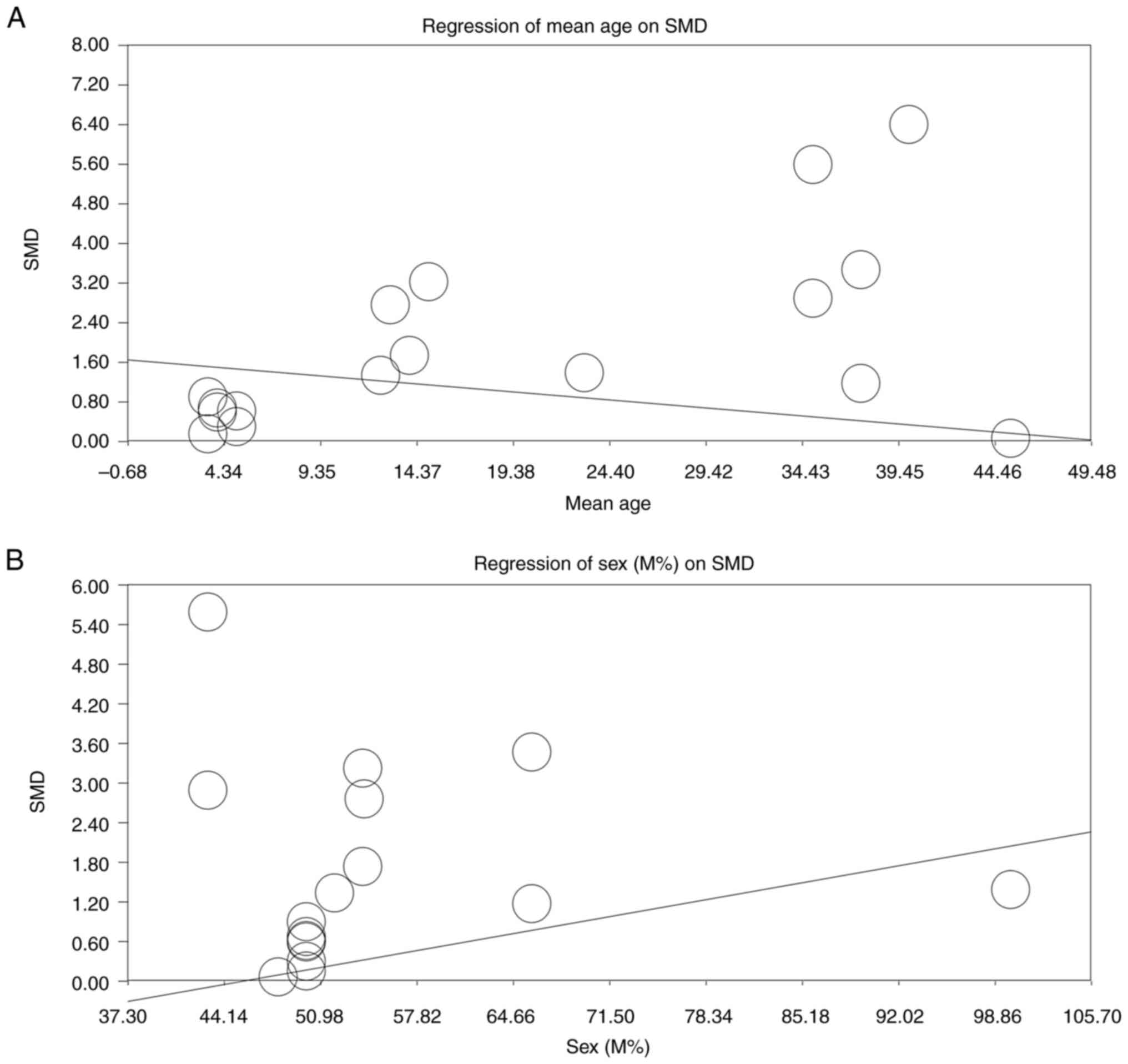
Due to the high heterogeneity of the included
studies, a moderator analysis was conducted to examine the effects
of age (mean ± SD) and sex (percentage of male participants) on the
overall effect size. The analysis revealed that the mean age of
participants significantly influenced the study outcomes, with
higher ages associated with lower SMDs (slope, P<0.00001;
Fig. 6A). Conversely, an increase
in the proportion of male participants was associated with a higher
SMDs (slope, P<0.00001; Fig.
6B). To address this issue further, subgroup analyses based on
the continent of origin (Europe, Asia and North America) and year
of publication of the studies were performed. However, the
heterogeneity of the subgroups remained high even at subgroup level
(data not shown).
Based on the NOS study quality appraisal, 2 studies
were deemed to have low quality while others were found to be of
high quality (Table III). The
decision to include the 2 studies of low quality was made to
provide a comprehensive overview of the existing literature on the
topic. Excluding these studies could have led to an incomplete
synthesis of available evidence, potentially omitting valuable
insights.
 | Table IIIMethodological quality assessment of
cross-sectional studies using the Newcastle-Ottawa
Scalea. |
Table III
Methodological quality assessment of
cross-sectional studies using the Newcastle-Ottawa
Scalea.
| | Criteria | |
|---|
| | Selection | Comparability | Outcome | |
|---|
| First author/s,
year | Representativeness
of the sample |
Non-respondents | Sample size | Ascertainment of
the exposure (risk factor) | The study controls
for the most important factor | The study control
for any additional factor | Assessment of the
outcome | Statistical
test | Total score | (Refs.) |
|---|
| Apessos, et
al, 2020 | The study sample
consisted of 177 Greek male soldiers without medical problems,
ensuring a homogenous population. | Four participants
did not agree to undergo the specific test and were excluded. | 177 participants,
divided into a study group (n=63) and a control group (n=110). | Morning
hyposalivation was assessed using the MST. | Age and BMI were
controlled, showing no significant difference between the study and
control groups. | Smoking status was
considered, with smokers prevailing in the study group. | Sleep quality,
daytime sleepiness, risk of OSA, and sleep bruxism were assessed
using validated questionnaires (PSQI, ESS and BQ, SB
questionnaire). | The correlation
between variables was tested using the Pearson χ2 test,
Mann-Whitney U test and Kruskal-Wallis test. The level of
significance was set at P<0.05, with Bonferroni correction
applied for multiple tests. | 8 | (16) |
| Buenestado and
Ribas-Pérez, 2023 | Included healthy
children aged 2-5 years from Pozoblanco, Córdoba. | 40 children did not
meet inclusion criteria. | 80 | Sleep disorders.
assessed using the PSQ | Age and BMI were
controlled. | Exclusion of
children with regular medication or orthopedic treatment. | Caries risk factors
and DMFT index evaluated using clinical examination and
questionnaires. | Mann-Whitney U
test, Kruskal-Wallis test, Spearman's correlation and two-way
ANOVA | 8 | (17) |
| Grillo et
al, 2019 | Included children
aged 8-17 years from a university-based dental clinic. | Not explicitly
mentioned. | 122 | Sleep-disordered
breathing assessed using the PSQ. | Sex,
caregiver's. | None mentioned.
education, family social class, obesity, Mallampati classification,
Brodsky score and Angle's malocclusion classification were
controlled | Oral health status
and oral health-related quality of life evaluated using dental
exams and the COHIP questionnaire. | χ2 test,
Mann-Whitney test, Student t-test and regression analysis. | 6 | (5) |
| Carra et al,
2017 | Included a French
cohort of individuals who underwent medical and oral examinations
between 2012 and 2013. | Not explicitly
mentioned. | 29,870 | Sleep disorders
assessed based on self-reported data. | Age, sex and BMI
were controlled. | Smoking status,
diabetes, EPICES score, depression and stress were also
considered. | Oral health
variables such as plaque, calculus, gingival inflammation and
masticatory function were evaluated. | Multivariate
logistic regression and general linear models. | 7 | (6) |
| Chacko et
al, 2021 | Included subjects.
from SMBT Dental College & Hospital, Sangamner, Maharashtra,
India | Not explicitly
mentioned. | 85 | Sleep deprivation
assessed using the PSQI. | Age, sex and.
socioeconomic status were controlled | Exclusion of
subjects with systemic diseases, recent periodontal therapy or
medication use. | Periodontal status
assessed using GI and pocket probing depth. | ANOVA F test,
Tukey's post hoc test, χ2 test, Pearson correlation
coefficient. | 7 | (18) |
| Romandini et
al, 2017 | Representative
sample of the South Korean population. | Not explicitly
mentioned. | 5,812 | Sleep duration
assessed through self-reported average daily sleep duration. | Age, sex,
education, smoking status, alcoholism and consumption frequency of
coffee, tea, chocolate and red wine were controlled. | None
mentioned. | Periodontal status
assessed using the CPI. | Multivariate
logistic regressions. | 6 | (20) |
| Tamasa et
al, 2018 | Included children
aged 8-17 years from a university-based pediatric dental
clinic. | Not explicitly
mentioned. | 123 | Sleep-disordered
breathing assessed using the PSQ. | Age, sex and
parental education were considered. | Exclusion of
subjects with craniofacial diagnoses, systemic conditions,
neurologic disease or cognitive impairment. | Oral health status
assessed using dental examinations and the COHIP
questionnaire. | χ2
tests, t-tests and linear and logistic regression analyses. | 7 | (21) |
Discussion
The meta-analysis conducted in the present study on
the association between oral health and sleep quality yielded
significant findings, highlighting a robust link between these two
critical aspects of overall health. The results indicated that
individuals with poorer oral health, as measured by indices such as
the DMFT and gingival indices, were more likely to experience sleep
disturbances and reduced sleep duration. The Leave-One-Out
sensitivity analysis showed that the exclusion of any single study
did not significantly affect the total effect size, suggesting that
the findings were robust and not heavily influenced by any one
study. This consistency was further supported by the symmetry of
the funnel plot and the absence of evidence for publication bias,
as confirmed by the Begg and Mazumdar rank correlation and Egger's
regression intercept. The absence of adjusted values indicated that
the observed distribution of studies was balanced around the pooled
effect size. Therefore, the results of the trim and fill analysis
supported the robustness of the study findings, reinforcing that
the significant association between oral health and sleep quality
(SMD, 2.166; 95% CI, 0.677-3.655; P=0.004) was not notably impacted
by potential publication bias.
A wide precision interval, as observed in the
present study, suggests variability in the strength of the
association across different populations, indicating that the true
effect size may vary depending on the specific characteristics of
each study. This reflects a high degree of heterogeneity, which the
moderator analysis performed in the present study aimed to address
(15). For instance, the analysis
revealed that age played a significant role, with studies involving
older populations tending to show lower SMDs. This finding suggests
that age-related factors may influence the strength of the
association between oral health and sleep quality. Additionally,
the moderator analysis highlighted that the proportion of male
participants also affected the results, with a higher percentage of
males associated with larger SMDs. These findings indicate that
demographic factors such as age and sex contribute to the observed
variability, leading to the wide precision interval. This
variability could arise due to different age groups and sex
distributions responding differently to the factors influencing
both sleep quality and oral health.
The association between sleep disorders and oral
health is well-documented in the literature. For instance, a study
using self-reported questionnaires found that individuals with
sleep disorders reported worse self-perceived oral health,
including a higher prevalence of tooth and temporomandibular joint
pain (22). Another study
highlighted that both short and long sleep durations are
significantly associated with poor oral health status.
Specifically, sleep durations of ≤5 h and ≥9 h per night were
linked to higher odds of poor oral health compared with a normal
sleep duration of 6-8 h (23). This
supports the results of the present meta-analysis, suggesting that
sleep quality and duration are critical factors influencing oral
health. Additionally, a study examining the relationship between
sleep duration and dental caries found a statistically significant
negative relationship, indicating that individuals who sleep ≥7 h
per night are less likely to experience dental caries compared with
those who sleep <7 h (24). This
finding is consistent with the broader theme that adequate sleep is
essential for maintaining good oral health.
Poor oral health can directly affect sleep quality
through various mechanisms. For instance, dental issues such as
tooth decay, gum disease and oral infections can cause discomfort,
leading to difficulty falling asleep or frequent awakenings
(25). Additionally, conditions
such as sleep apnea, which can be linked to oral health issues such
as misalignment of the jaw or the tongue falling back into the
throat, further disrupt sleep patterns (25). Poor sleep quality can exacerbate
stress and inflammation, which in turn can worsen oral health.
Chronic sleep deprivation is known to increase levels of
inflammatory markers, which can contribute to conditions such as
periodontitis and other oral health issues (22). The relationship between oral health
and sleep quality may also be influenced by behavioral factors. For
instance, individuals with poor sleep quality might have reduced
motivation or ability to maintain good oral hygiene practices,
leading to a vicious cycle of deteriorating oral health and sleep
quality (25).
The present meta-analysis included a diverse range
of studies conducted across different countries and populations,
providing a comprehensive overview of the association between oral
health and sleep quality. The use of SMD and sensitivity analyses
ensured that the findings were robust and not unduly influenced by
any single study. The absence of publication bias further
strengthens the reliability of the results. The majority of the
included studies were deemed to be of high quality according to the
NOS study quality appraisal, which enhances the credibility of the
findings. However, the present study had several shortcomings. The
wide precision interval (-4.83 to 9.16) indicated significant
variability in the strength of the association across different
populations. This variability could be due to differences in study
designs, population demographics and the measurement tools used. A
key limitation is the reliance on self-reported sleep measures in a
number of the included studies. Self-reported data can be prone to
recall and social desirability biases, leading to possible
misclassification of sleep quality or disorders, which may
underestimate the true impact of sleep disturbances on oral health.
Moreover, self-reports often lack the precision of objective
measures (including polysomnography or actigraphy), which are more
accurate in diagnosing conditions such as obstructive sleep apnea.
This could result in the underreporting of certain sleep disorders,
further influencing the observed association between sleep quality
and oral health. The included studies were predominantly
cross-sectional, which limits the ability to establish causality
between oral health and sleep quality. Longitudinal studies are
necessary to determine the temporal relationship between these
variables. The use of various indices for oral health and sleep
quality might also introduce some heterogeneity in the results.
Therefore, standardization of measurement tools across studies
could help in reducing this variability. While the present study
adjusted for several covariates, there could be other unmeasured
confounding variables that influence the relationship between oral
health and sleep quality. Future studies should aim to control for
a broader range of potential confounders.
Several gaps in knowledge remain in the field of
oral health and sleep quality. One key area is the mechanistic
pathways through which sleep quality impacts oral health, such as
the role of inflammatory processes and immune responses.
Additionally, more research is needed to understand the impact of
specific sleep disorders, such as obstructive sleep apnea, on
various oral health conditions beyond periodontitis
The findings of the present meta-analysis highlight
important clinical implications. Clinicians should consider
incorporating basic sleep assessments into routine dental
check-ups, as poor sleep quality is linked to worsened oral health
outcomes. Collaboration with sleep specialists for patients with
severe oral health issues and suspected sleep disorders can help
optimize patient care. Additionally, educating patients about the
relationship between sleep and oral health can encourage improved
sleeping habits, which may improve oral health maintenance and
recovery. For older patients and those with chronic oral
conditions, screening for sleep issues may be particularly
beneficial in managing their overall health.
In conclusion, the present meta-analysis provided
strong evidence for a significant association between poorer oral
health and poorer sleep quality. This relationship is supported by
various studies in the literature, which highlight the direct and
indirect mechanisms through which oral health can impact sleep and
vice versa. The robust statistical analysis and the high quality of
the included studies are notable strengths of the present study.
However, the variability in study populations and the
cross-sectional nature of the included studies are limitations that
need to be addressed in future research. Understanding this
association can inform public health strategies aimed at improving
both oral health and sleep quality, ultimately contributing to
improved overall health outcomes. Given the cross-sectional nature
of most included studies, future research should focus on
longitudinal studies to establish causality between oral health and
sleep quality. Long-term studies that follow individuals over time
can clarify whether poor sleep quality leads to deteriorating oral
health, or if existing oral health issues contribute to sleep
disturbances.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XH conceived, designed, revised, supervised and
edited the manuscript, as well as analyzed and interpreted the
data. FL acquired the data. FL and XH confirm the authenticity of
the data. Both authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Asawa K, Sen N, Bhat N, Tak M, Sultane P
and Mandal A: Influence of sleep disturbance, fatigue, vitality on
oral health and academic performance in Indian dental students.
Clujul Med. 90:333–343. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Schroeder K and Gurenlian JR: Recognizing
poor sleep quality factors during oral health evaluations. Clin Med
Res. 17:20–28. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Alqaderi H, Tavares M, Hartman M and
Goodson JM: Effect of sleep and salivary glucose on gingivitis in
children. J Dent Res. 95:1387–1393. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Movahed E, Moradi S, Mortezagholi B,
Shafiee A, Moltazemi H, Hajishah H, Siahvoshi S, Monfared AB, Amini
MJ, Safari F and Bakhtiyari M: Investigating oral health among US
adults with sleep disorder: A cross-sectional study. BMC Oral
Health. 23(996)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Grillo C, La Mantia I, Zappala G, Cocuzza
S, Ciprandi G and Andaloro C: Oral health in children with
sleep-disordered breathing: A cross-sectional study. Acta Biomed.
90:52–59. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Carra MC, Schmitt A, Thomas F, Danchin N,
Pannier B and Bouchard P: Sleep disorders and oral health: A
cross-sectional study. Clin Oral Investig. 21:975–983.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Safak ED, Celik F, Mazicioglu MM, Akin S,
Manav TY, Kesim S and Ozturk A: The relationship between oral
health and sleep quality in community-dwelling older adults. Niger
J Clin Pract. 26:1449–1455. 2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Taştan Eroğlu Z, Özkan Şen D, Uçan Yarkac
F and Altiparmak F: The association between sleep quality, fatigue
and periodontal status: A pilot study. Odovtos Int J Dent Sci.
25:99–117. 2023.
|
|
9
|
Hirotsu C, Tufik S and Andersen ML:
Interactions between sleep, stress, and metabolism: From
physiological to pathological conditions. Sleep Sci. 8:143–152.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wiener RC: Relationship of routine
inadequate sleep duration and periodontitis in a nationally
representative sample. Sleep Disord. 2016(9158195)2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Beydoun HA, Hossain S, Beydoun MA, Weiss
J, Zonderman AB and Eid SM: Periodontal disease, sleep duration,
and white blood cell markers in the 2009 to 2014 National Health
and Nutrition Examination Surveys. J Periodontol. 91:582–595.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Corker KS: Strengths and weaknesses of
meta-analyses. In: Research Integrity: Best Practices for the
Social and Behavioral Sciences. Jussim L, Krosnick JA and Stevens
ST (eds). Oxford University Press, Oxford, pp150-174, 2022.
|
|
13
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Peterson J, Welch V, Losos M and Tugwell
P: The Newcastle-Ottawa scale (NOS) for assessing the quality of
nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital
Research Institute. 2:1–12. 2011.
|
|
15
|
IntHout J, Ioannidis JP, Rovers MM and
Goeman JJ: Plea for routinely presenting prediction intervals in
meta-analysis. BMJ Open. 6(e010247)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Apessos I, Andreadis D, Steiropoulos P,
Tortopidis D and Angelis L: Investigation of the relationship
between sleep disorders and xerostomia. Clin Oral Investig.
24:1709–1716. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Arroyo Buenestado A and Ribas-Pérez D:
Early childhood caries and sleep disorders. J Clin Med.
12(1378)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chacko NL, Raje M, Rakhewar P and Bhamare
R: The association between sleep deprivation and periodontitis: A
cross sectional study. IOSR J Dent Med Sci (IOSR-JDMS). 20:1–8.
2021.
|
|
19
|
Grover V, Malhotra R and Kaur H: Exploring
association between sleep deprivation and chronic periodontitis: A
pilot study. J Indian Soc Periodontol. 19:304–307. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Romandini M, Gioco G, Perfetti G, Deli G,
Staderini E and Laforì A: The association between periodontitis and
sleep duration. J Clin Periodontol. 44:490–501. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tamasa B, Godfrey G, Nelson T and Chen M:
Oral health status of children with high risk of sleep-disordered
breathing. J Dent Sleep Med. 5:31–38. 2018.
|
|
22
|
Pereira D, Progiante P, Pattussi M, Grossi
P and Grossi M: Study on the association between sleep disorders
versus oral health related variables. Med Oral Patol Oral Cir
Bucal. 26:e164–e71. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Han S, Jee D, Kang YJ, Park YJ and Cho JH:
Possible association between oral health and sleep duration: A
cross-sectional study based on the Korean National Health and
Nutrition Examination Surveys from 2010 to 2015. Medicine
(Baltimore). 100(e28035)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Alawady A, Alharbi A, Alharbi H, Almesbah
S, Alshammari N, Alkandari A, Alhazmi H and Alqaderi H: Association
between sleep duration and dental caries in a nationally
representative U.S. population. BMC Oral Health.
23(497)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ju X, Hedges J, Sethi S and Jamieson LM:
Poor self-rated sleep quality and quantity associated with poor
oral health-related quality of life among indigenous Australian
adults. Int J Environ Res Public Health. 21(453)2024.PubMed/NCBI View Article : Google Scholar
|