1. Introduction
Obesity, which often results from an imbalance
between energy intake and expenditure, is characterized by
excessive or abnormal fat accumulation and serves as an independent
risk factor for various chronic non-communicable metabolic
diseases, including diabetes, hypertension, osteoarthritis, and
coronary heart disease. This condition exerts substantial adverse
effects on the quality of life of patients and the broader social
economy (1,2). Adipose tissue functions as an energy
storage depot and endocrine organ, secreting bioactive molecules
known as adipokines that broadly affect the metabolic processes of
the body. Adipokines are pivotal in regulating insulin sensitivity,
lipid and glucose metabolism, and energy balance. The PI3K/AKT
signaling pathway, a central mediator of glucose, lipid, and
protein metabolism, is vital for the regulation of cell growth,
survival, and apoptosis. Apoptosis, a controlled form of programmed
cell death, is a ‘double-edged sword’ that occurs under various
physiological and pathological conditions. Central to apoptosis is
the mitochondrial pathway, also known as the ‘executor’ of
apoptosis, which plays an essential role in maintaining tissue
homeostasis. Obesity impairs mitochondrial function and
subsequently affects the process of cell apoptosis. Thus,
investigating how the PI3K/AKT signaling pathway modulates
mitochondrial function to mediate obesity-induced apoptosis is of
substantial theoretical and clinical relevance. This research not
only deepens our understanding of the pathological mechanisms of
obesity but may also identify novel targets and strategies for
treating obesity and its related diseases. By advancing mechanistic
insights, the present review seeks to foster more effective
interventions to improve the metabolic health and quality of life
of individuals with obesity.
2. Epidemiology
From 1975 to 2014, the global average body mass
index (BMI) for men increased from 21.7 to 24.2 kg/m² (3), and for women it increased from 22.1 to
24.4 kg/m². A 2023 study by the Chinese People's Liberation Army
General Hospital (Beijing, China), which included 1,577,094 adults
across China, found that 34.8% of the participants were overweight
and 14.1% were classified as obese, with BMI showing a positive
correlation with obesity-related complications (4).
3. Overview
PI3K/AKT signaling pathway
The PI3K/AKT signaling pathway is integral to
numerous physiological and pathological processes, including cell
growth and proliferation, survival and apoptosis, metabolic
regulation, angiogenesis, immune response, and cell migration and
invasion (5). This pathway plays an
essential role in adipogenesis, adipocyte differentiation, and
energy storage within adipose tissue (6). In the pancreas, it supports the
function and growth of islet β cells. In the muscle tissue, it
regulates glucose uptake, protein synthesis, and vasodilation
(7).
Activation of this pathway consists of multiple
stages. Initially, extracellular signals such as growth factors and
hormones bind to receptors on the cell membrane, initiating signal
transduction. Receptor activation subsequently activates PI3K,
which triggers downstream signaling cascades. PI3K is a heterodimer
composed of catalytic subunits (p110α, p110β, p110δ, p110γ) and
regulatory subunits (p85α, p55α, p50α, p85β, p55γ, p101, p84, p87).
P110α is the main insulin-responsive kinase in adipocytes and
muscle ducts, and the localization of p110δ and p110γ isoenzymes is
mainly limited to immune cells (8,9).
Activated PI3K phosphorylates the precursor protein
phosphatidylinositol 4,5-bisphosphate (PIP2) to
phosphatidylinositol (3,4,5)-triphosphate (PIP3), which
acts as a second messenger, and the conversion of PIP2
to PIP3 promotes the recruitment of 3-phosphoinositide
dependent protein kinase 1 (PDK1) (10). Finally, PDK1 and mammalian rapamycin
target protein complex 2 (mTORC2) activate AKT on the membrane.
PDK1 completely activates AKT by phosphorylation of the AKT
threonine residue at Thr308 and mTORC2 by phosphorylation of AKT's
serine residue of AKT at Ser473(11). AKT1 is generally expressed, AKT2
isoenzymes are characteristic in insulin-sensitive tissues (such as
muscle, fat and liver tissues), and AKT3 is mostly expressed in the
nervous system, pancreas, heart, and kidney (12).
Inhibition of this pathway is orchestrated through
multiple mechanisms, including the dephosphorylation of
PIP3 to PIP2 by phosphatases and inhibitors
such as phosphatase and tensin homolog (PTEN), leading to a
reduction in PIP3 levels, a pivotal modulator of AKT
activation at the membrane (13).
Additionally, negative regulators, such as pro-inflammatory
cytokines (including TNF-α and IL-6), transcription factors, and
microRNAs, play integral roles in the negative regulation of the
pathway (14).
Apoptosis
Under physiological conditions, apoptosis is a
genetically regulated programmed cell death process that
facilitates the timely removal of damaged or dysfunctional cells.
It plays a pivotal role in critical biological processes, including
development, immune surveillance, and tissue homeostasis, and is
indispensable for maintaining cellular equilibrium. Apoptosis
serves as a key defense mechanism in the immune system, enabling
the elimination of infected cells and irreparable DNA damage
(15). The three major apoptotic
pathways are the extrinsic death receptor pathway, the intrinsic
mitochondrial pathway, and the endoplasmic reticulum pathway, which
intersect and interact with one another (16). The hallmarks of apoptosis include
cell shrinkage, membrane blebbing, chromatin condensation,
formation of apoptotic bodies, and subsequent clearance by
phagocytic cells or the immune system (17).
The excessive inhibition or aberrant activation of
apoptosis can have deleterious effects. For instance, cancer cells
evade normal cell death mechanisms by inhibiting apoptosis, thereby
facilitating unchecked cell proliferation (18). In patients with systemic lupus
erythematosus, dysregulation of apoptosis prevents the effective
clearance of autoantigenic cells, triggering an autoimmune response
that attacks self-tissues (19).
Research has shown that the adipose tissue in obese mice and
humans, induced by high-fat diets, exhibits a pro-apoptotic
phenotype. Caspase activation and adipocyte apoptosis are
significantly increased, which is considered a key initiating event
leading to macrophage infiltration and insulin resistance in obese
adipose tissue (20). Furthermore,
increased hepatocyte apoptosis has been observed in high-fat
diet-induced obese mice, and is recognized as a critical factor in
the progression of obesity (21).
Mitochondrial pathway
Mitochondria, as cellular powerhouses, are central
to ATP production and utilize most of the oxygen during oxidative
phosphorylation. A small amount of residual oxygen forms superoxide
anions, initiating the generation of reactive oxygen species (ROS).
Excessive ROS accumulation can trigger mitochondrial swelling,
alter membrane permeability, and activate apoptotic pathways by
releasing cytochrome c (Cyt c) and other apoptotic factors
(22).
Changes in the mitochondrial membrane potential
(MMP) is a critical event that initiates apoptosis. Typically, the
MMP is maintained at a stable level to support efficient ATP
production. However, when cells are subjected to various stressors,
the MMP becomes depolarized, which increases membrane permeability.
The loss of MMP triggers the release of key pro-apoptotic factors,
such as Cyt c, through a process known as mitochondrial outer
membrane permeabilization, a key ‘point of no return’ in apoptosis.
The B-cell lymphoma 2 (Bcl-2) protein family is integral to MMP
regulation. For example, the anti-apoptotic protein, Bcl-2, blocks
MMP depolarization, thereby shielding cells from apoptosis
(23).
The specific mechanism of mitochondrial pathway
activation under obesity is as follows. First, this classical
intrinsic apoptotic pathway is triggered by endogenous signals in
response to cellular stress or damage (24). Pro-apoptotic members of the Bcl-2
family translocate to the inner mitochondrial membrane, inducing
alterations in membrane permeability. These changes facilitate the
release of endogenous apoptotic factors such as Cyt c into the
cytoplasm. Cyt c binds to the apoptotic protease-activating factor
1 (Apaf-1) to form an apoptosome complex. Formation of the
apoptosome activates caspase-9, which in turn activates the
downstream effector caspase-3(25).
Caspase-3 cleaves multiple cellular substrates and is a key enzyme
in apoptosis (Fig. 1).
Subsequently, effector caspases degrade critical cellular proteins,
leading to membrane blebbing, cell fragmentation into apoptotic
bodies, and eventual phagocyte clearance without inducing
inflammation. The release of Cyt c and activation of caspases
generate a feedback loop in which caspase-3 cleaves anti-apoptotic
Bcl-2 proteins, further amplifying Cyt c release and reinforcing
the apoptotic cascade (25).
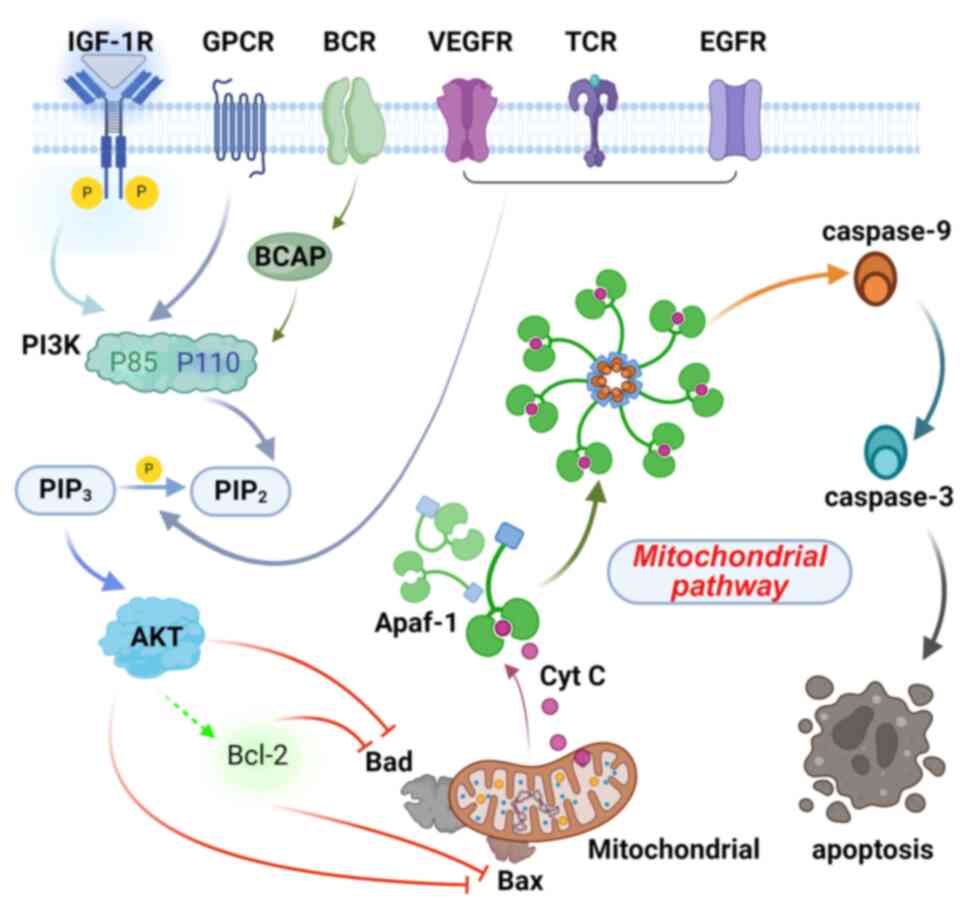 | Figure 1Mechanism of apoptosis regulation in
the mitochondrial pathway by PI3K/AKT. IGF-1R, insulin-like growth
factor 1 receptor; GPCR, G-protein coupled receptor; BCR, B-cell
receptor; VEGFR, vascular endothelial growth factor receptor; TCR,
T-cell receptor; EGFR, epidermal growth factor receptor; IRS-1,
insulin receptor substrate 1; PI3K, phosphoinositide 3-kinase;
PIP2, phosphatidylinositol 4,5-bisphosphate;
PIP3, phosphatidylinositol (3,4,5)-triphosphate;
AKT, protein kinase B; Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2-associated X protein; Bad, Bcl-2-associated death promoter;
Cyt c, cytochrome c, Apaf-1, apoptotic protease activating
factor 1; caspase-9, cysteinyl aspartate specific protease 9;
caspase-3, cysteinyl aspartate specific protease 3. (Created in
BioRender. Li, J. (2024) https://BioRender.com/y00e363). |
4. Summary of mechanistic analysis
Mechanism of apoptosis induced by
obesity
Obesity is strongly associated with the development
of various diseases, particularly through its effect on apoptosis,
which has been extensively studied. In individuals with obesity,
the prevalence of apoptotic cells in the cardiac tissue is higher
than that in those with normal weight, which correlates with
obesity-induced cardiac remodeling and dysfunction (26). Obesity also contributes to
pancreatic β-cell failure, primarily due to increased apoptosis. In
addition, the obesity-induced apoptosis of ovarian granulosa cells
may affect fertility. Thus, obesity-induced apoptosis is a result
of metabolic dysregulation and is a key mechanism in disease
pathogenesis (27). The
multifactorial mechanisms driving obesity-related apoptosis involve
oxidative stress, inflammation, endoplasmic reticulum (ER) stress,
mitochondrial dysfunction, lipotoxicity, cytokine signaling, and
death receptor activation (Table
I).
 | Table ISummary of mechanisms through which
obesity induces cell apoptosis. |
Table I
Summary of mechanisms through which
obesity induces cell apoptosis.
| Mechanism | In obesity | Inducing apoptosis
mechanism | (Refs.) |
|---|
| Insulin
resistance | Insufficient
activation of the PI3K/AKT signaling pathway prevents glucose from
entering cells effectively, leading to impaired glucose metabolism
and excessive ROS production. | Elevated ROS levels
cause cellular damage and activate pro-apoptotic pathways: ①
Dephosphorylation of Bad enhances its binding with Bcl-2 family
members; ② FOXO activation promotes the expression of pro-
apoptotic genes such as Bim and FasL; ③ dephosphorylation of GSK-3β
inhibits the anti- apoptotic function of Bcl-2. | (24) |
| Oxidative
stress | Excessive fat
accumulation, increased fatty acid oxidation, and enhanced
adipocyte metabolism contribute to a greater production of
ROS. | Excessive ROS
results in: ① DNA damage (including DNA strand breaks and base
oxidation), triggering DNA repair mechanisms or apoptosis; ②
protein damage (loss of protein function or conformational
changes), leading to cellular dysfunction; ③ lipid peroxidation
(generation of lipid peroxides), disrupting membrane integrity and
intracellular signaling. | (49-51) |
| Inflammatory
response | ① Macrophage
infiltration into adipose tissue is a hallmark of chronic low-grade
inflammation; ② increased secretion of pro-inflammatory cytokines,
chemokines, and adipokines. | ① Pro-inflammatory
cytokines activate the NF-κB signaling pathway (upregulating
pro-apoptotic genes such as FasL and Bax); ② activation of the JNK
pathway promotes the expression of Bim and Bid; ③ activation of the
p38 MAPK pathway upregulates the expression of p53 and Bax,
enhancing apoptosis. | (52,53) |
| Endoplasmic
reticulum stress | Adipocyte expansion
induces ER stress: ① Increased protein folding and processing load;
② activation of the UPR. | ① The UPR
transcription factor CHOP is upregulated, promoting apoptosis; ②
JNK activation enhances Bax and Bim expression, leading to
apoptosis. | (54-56) |
| Lipotoxicity | Accumulation of
free fatty acids and triglycerides exacerbates lipotoxicity,
damaging cell membranes and organelles. | ① Increased cell
membrane permeability causes cell rupture; ② Excessive lipid
droplet accumulation disrupts cellular signaling, amplifying
oxidative stress and inflammation; ③ Cross-talk with mitochondrial
dysfunction mechanisms contributes to apoptosis. | (57,58) |
| Death receptor
pathway | Binding of death
receptors with ligands plays a critical role in chronic
inflammation and directly initiates apoptosis. | ① Recruitment of
TRADD, FADD, and caspase-8; ② activation of caspase-8 initiates the
apoptotic process; ③ formation of the DISC, leading to cytochrome
c release from mitochondria through tBid (truncated form of
Bid protein). | (59) |
Impact of obesity on the PI3K/AKT
pathway
Obesity disrupts PI3K/AKT signaling through insulin
resistance, chronic inflammation, and lipotoxicity. It increases
the serine phosphorylation of insulin receptor substrates (IRS),
inhibits tyrosine phosphorylation, blocks PI3K p85 binding, and
impairs PI3K activation. Serine 307 phosphorylation of IRS-1 is a
known marker (28) of insulin
resistance. Obesity is often accompanied by chronic low-grade
inflammation, characterized by elevated pro-inflammatory cytokines,
such as TNF-α and IL-6, intensifying local inflammation and
impairing systemic metabolic homeostasis. These cytokines activate
signaling pathways, including the mitogen-activated protein kinase
(MAPK) and Janus kinase/signal transducers and activators of
transcription (JAK/STAT) pathways, which can indirectly modulate
the PI3K/AKT cascade. Furthermore, they upregulate suppressors of
cytokine signaling (SOCS), which bind to IRS, promote IRS
degradation, weaken insulin signaling, and ultimately drive
metabolic dysfunction. Additionally, high free fatty acids (FFAs)
and ceramides induce lipotoxicity: FFAs activate Toll-like
receptors (TLRs), aggravating inflammation and insulin resistance,
while ceramides inhibit AKT. Moreover, in obesity, adipose tissue
secretes various adipokines, such as adiponectin and leptin, which
modulate PI3K/AKT pathway activity and influence cellular insulin
sensitivity (29). Finally, obesity
upregulates microRNAs, such as miR-221, which suppress PI3K and
increase insulin resistance (30).
These aforementioned pathways modulate the PI3K/AKT
signaling cascade through interactions with cell surface receptors,
principally engaging receptor tyrosine kinases (RTKs), TLRs, and
B-cell antigen receptors (BCRs). RTKs (31), including epidermal growth factor
receptors (EGFRs), vascular endothelial growth factor receptors
(VEGFRs), and fibroblast growth factor receptors (FGFRs), are
activated by ligands such as growth factors, cytokines, and
hormones. The BCR is essential for B cell development, activation,
and differentiation, and activates downstream pathways through
B-cell receptor-associated protein (BCAP). Additionally,
G-protein-coupled receptors (GPCRs), the largest family of cell
surface receptors, activate specific signaling cascades by
recognizing and responding to a wide variety of ligands (32).
Flavonoids, including quercetin (33), kaempferol (34), and puerarin (35); terpenoids, such as astragaloside IV
(36) and ginsenoside Rb2(37); and alkaloids, such as capsaicin
(38) and berberine (39), regulate glucose and lipid
metabolism, facilitate weight loss, and exert anti-inflammatory
effects by modulating the PI3K/AKT signaling pathway (Table II). Glinides, a class of drugs that
are frequently used in endocrinology, enhance insulin secretion and
ameliorate insulin resistance by activating the PI3K/AKT pathway
(40). Buparlisib, which is used to
treat cancer, functions as a pan-PI3K inhibitor and improves
obesity-related metabolic disorders (41).
PI3K/AKT pathway mediates the
mitochondrial pathway
The PI3K/AKT pathway is a critical signaling cascade
that regulates mitochondrial apoptosis by modulating the expression
of various proteins, including members of the Bcl-2, Bax, Bad, and
caspase families (Fig. 1).
AKT regulates mitochondrial membrane permeability by
influencing the Bcl-2 family proteins and Bad. It enhances the
anti-apoptotic functions of the Bcl-2 family members, such as Bcl-2
and Bcl-xL, through phosphorylation (42). At the same time, AKT inhibits the
pro-apoptotic activity of proteins such as Bax and Bak, preventing
their ability to form membrane pores. This reduces the release of
mitochondrial apoptotic signals such as Cyt c and diminishes
apoptotic body formation. Additionally, AKT phosphorylates Bad,
facilitating its binding to 14-3-3 proteins, which reduces
competition with Bcl-2/Bcl-xL, further stabilizing the
mitochondrial membrane and reinforcing its anti-apoptotic function.
Furthermore, AKT activates mTORC1, thereby improving the cellular
energy supply. Mitochondrial function is preserved by upregulating
antioxidant enzymes such as SOD2, thereby reducing oxidative
stress-induced mitochondrial damage (43).
Research has demonstrated that the activation of the
PI3K/AKT pathway effectively inhibits apoptosis and promotes cell
growth and proliferation. In numerous types of cancer, the aberrant
activation of this pathway enables tumor cells to bypass normal
apoptotic mechanisms, facilitating sustained tumor growth and
metastasis. Research has confirmed that astragaloside IV (44), tanshinone IIA (15), and curcumin (45) modulate mitochondrial apoptosis by
regulating the PI3K/AKT pathway. Benazepril, a commonly prescribed
antihypertensive agent used for cardiovascular diseases, indirectly
alleviates PI3K/AKT pathway dysregulation and mitochondrial
dysfunction by lowering blood pressure and improving cardiac
function (46). Gefitinib, an EGFR
inhibitor, promotes apoptosis in cancer cells by downregulating the
PI3K/AKT signaling cascade (47).
Additionally, MK-2206, a selective AKT inhibitor, directly induces
apoptosis in cells (48).
5. Conclusion and outlook
The present review summarized various mechanisms
underlying obesity-induced apoptosis, focusing on the role of the
PI3K/Akt-mediated mitochondrial pathway. Analysis of drugs and
natural compounds that may act on this pathway revealed its
bidirectional regulatory function in maintaining cellular survival
and metabolic homeostasis; it promotes cell growth and survival
under normal conditions but becomes dysregulated under oxidative
stress and inflammatory conditions, leading to the initiation of
apoptosis. This highlights key directions for future research:
First, to investigate the interactions between the PI3K/Akt pathway
and other cell death pathways, and second, to develop
small-molecule drugs or biologics targeting this pathway to improve
clinical efficacy. Additionally, the influence of genetic,
environmental, and lifestyle factors on the PI3K/Akt signaling
pathway could provide deeper insights into the prevention and
reversal of obesity and its metabolic complications.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the 2020
Heilongjiang Provincial Natural Science Foundation Joint Guidance
Project (grant no. LH2020H085) and the Heilongjiang University of
Chinese Medicine ‘Outstanding Innovative Talent Support Project’
(grant no. 2018RCD13).
Availability of data and materials
Not applicable.
Authors' contributions
The study was conceived and designed by JL. The
original draft of the manuscript was written by JL, MS, MT and XS.
The literature review was conducted by KZ and CL. The design of the
tables was performed by TM. The manuscript was reviewed and edited
by JL and LD. Data authentication is not applicable. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gustafson B, Nerstedt A and Smith U:
Reduced subcutaneous adipogenesis in human hypertrophic obesity is
linked to senescent precursor cells. Nat Commun.
10(2757)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kivimäki M, Strandberg T, Pentti J, Nyberg
ST, Frank P, Jokela M, Ervasti J, Suominen SB, Vahtera J, Sipilä
PN, et al: Body-mass index and risk of obesity-related complex
multimorbidity: An observational multicohort study. Lancet Diabetes
Endocrinol. 10:253–263. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
NCD Risk Factor Collaboration (NCD-RisC).
Trends in adult body-mass index in 200 countries from 1975 to 2014:
A pooled analysis of 1698 population-based measurement studies with
19·2 million participants. Lancet. 387:1377–1396. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chen K, Shen Z, Gu W, Lyu Z, Qi X, Mu Y
and Ning Y: Meinian Investigator Group. Prevalence of obesity and
associated complications in China: A cross-sectional, real-world
study in 15.8 million adults. Diabetes Obes Metab. 25:3390–3399.
2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Glaviano A, Foo ASC, Lam HY, Yap KCH,
Jacot W, Jones RH, Eng H, Nair MG, Makvandi P, Geoerger B, et al:
PI3K/AKT/mTOR signaling transduction pathway and targeted therapies
in cancer. Mol Cancer. 22(138)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kim J, Han D, Byun SH, Kwon M, Cho SJ, Koh
YH and Yoon K: Neprilysin facilitates adipogenesis through
potentiation of the phosphatidylinositol 3-kinase (PI3K) signaling
pathway. Mol Cell Biochem. 430:1–9. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu J, Quan L, Wang J, Zhang G, Cai L, Pan
Z, Liu S, Zhu C, Wu R, Wang L, et al: Knockdown of VEGF-B improves
HFD-induced insulin resistance by enhancing glucose uptake in
vascular endothelial cells via the PI3K/Akt pathway. Int J Biol
Macromol. 285(138279)2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Araiz C, Yan A, Bettedi L, Samuelson I,
Virtue S, McGavigan AK, Dani C, Vidal-Puig A and Foukas LC:
Enhanced β-adrenergic signalling underlies an age-dependent
beneficial metabolic effect of PI3K p110alpha inactivation in
adipose tissue. Nat Commun. 10(1546)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lanahan SM, Wymann MP and Lucas CL: The
role of PI3Kγ in the immune system: New insights and translational
implications. Nat Rev Immunol. 22:687–700. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Arasi MB, De Luca G, Chronopoulou L,
Pedini F, Petrucci E, Flego M, Stringaro A, Colone M, Pasquini L,
Spada M, et al: MiR126-targeted-nanoparticles combined with
PI3K/AKT inhibitor as a new strategy to overcome melanoma
resistance. Mol Ther. 32:152–167. 2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hermida MA, Dinesh Kumar J and Leslie NR:
GSK3 and its interactions with the PI3K/AKT/mTOR signalling
network. Adv Biol Regul. 65:5–15. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Manning BD and Toker A: AKT/PKB Signaling:
Navigating the network. Cell. 169:381–405. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Carnero A, Blanco-Aparicio C, Renner O,
Link W and Leal JF: The PTEN/PI3K/AKT signalling pathway in cancer,
therapeutic implications. Curr Cancer Drug Targets. 8:187–198.
2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Al-Hawary SIS, Ruzibakieva M, Gupta R,
Malviya J, Toama MA, Hjazi A, Alkhayyat MRR, Alsaab HO, Hadi A and
Alwaily ER: Detailed role of microRNA-mediated regulation of
PI3K/AKT axis in human tumors. Cell Biochem Funct.
42(e3904)2024.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Tai H, Cui XZ, He J, Lan ZM, Li SM, Li LB,
Yao SC, Jiang XL, Meng XS and Kuang JS: Renoprotective effect of
tanshinone IIA against kidney injury induced by
ischemia-reperfusion in obese rats. Aging (Albany NY).
14:8302–8320. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tait SW and Green DR: Mitochondria and
cell death: Outer membrane permeabilization and beyond. Nat Rev Mol
Cell Biol. 11:621–632. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Obeng E: Apoptosis (programmed cell death)
and its signals-A review. Braz J Biol. 81:1133–1143.
2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dakkak BE, Taneera J, El-Huneidi W,
Abu-Gharbieh E, Hamoudi R, Semreen MH, Soares NC, Abu-Rish EY,
Alkawareek MY, Alkilany AM, et al: Unlocking the therapeutic
potential of BCL-2 associated protein family: Exploring BCL-2
inhibitors in cancer therapy. Biomol Ther (Seoul). 32:267–280.
2024.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang W, Wu H, Liao Y, Zhu C and Zou Z:
Caspase family in autoimmune diseases. Autoimmun Rev.
24(103714)2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Alkhouri N, Gornicka A, Berk MP, Thapaliya
S, Dixon LJ, Kashyap S, Schauer PR and Feldstein AE: Adipocyte
apoptosis, a link between obesity, insulin resistance, and hepatic
steatosis. J Biol Chem. 285:3428–3348. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Reis-Barbosa PH, Marinho TS, Matsuura C,
Aguila MB, de Carvalho JJ and Mandarim-de-Lacerda CA: The obesity
and nonalcoholic fatty liver disease mouse model revisited: Liver
oxidative stress, hepatocyte apoptosis, and proliferation. Acta
Histochem. 124(151937)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang B, Wang Y, Zhang J, Hu C, Jiang J, Li
Y and Peng Z: ROS-induced lipid peroxidation modulates cell death
outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis.
Arch Toxicol. 97:1439–1451. 2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Qian S, Wei Z, Yang W, Huang J, Yang Y and
Wang J: The role of BCL-2 family proteins in regulating apoptosis
and cancer therapy. Front Oncol. 12(985363)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cai Y, Liu P, Xu Y, Xia Y, Peng X, Zhao H
and Chen Q: Biomarkers of obesity-mediated insulin resistance:
Focus on microRNAs. Diabetol Metab Syndr. 15(167)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kaur H, Singh A, Kaur K, Kumar A, Attri S,
Rashid F, Singh S, Bedi N, Tuli HS, Haque S, et al:
4-methylthiobutyl isothiocyanate synergize the antiproliferative
and pro-apoptotic effects of paclitaxel in human breast cancer
cells. Biotechnol Genet Eng Rev. 40:3780–3804. 2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li FJ, Abudureyimu M, Zhang ZH, Tao J,
Ceylan AF, Lin J, Yu W, Reiter RJ, Ashrafizadeh M, Guo J and Ren J:
Inhibition of ER stress using tauroursodeoxycholic acid rescues
obesity-evoked cardiac remodeling and contractile anomalies through
regulation of ferroptosis. Chem Biol Interact.
398(111104)2024.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Good AL and Stoffers DA: Stress-induced
translational regulation mediated by RNA binding proteins: Key
links to β-cell failure in diabetes. Diabetes. 69:499–507.
2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hirosumi J, Tuncman G, Chang L, Görgün CZ,
Uysal KT, Maeda K, Karin M and Hotamisligil GS: A central role for
JNK in obesity and insulin resistance. Nature. 420:333–336.
2002.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Barberio MD, Nadler EP, Sevilla S, Lu R,
Harmon B and Hubal MJ: Comparison of visceral adipose tissue DNA
methylation and gene expression profiles in female adolescents with
obesity. Diabetol Metab Syndr. 11(98)2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Huang F, Zhu P, Wang J, Chen J and Lin W:
Postnatal overfeeding induces hepatic microRNA-221 expression and
impairs the PI3K/AKT pathway in adult male rats. Pediatr Res.
89:143–149. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lemieux SM and Hadden MK: Targeting the
fibroblast growth factor receptors for the treatment of cancer.
Anticancer Agents Med Chem. 13:748–761. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu F, Zhou H, Li X, Zhou L, Yu C, Zhang
H, Bu D and Liang X: GPCR-BSD: A database of binding sites of human
G-protein coupled receptors under diverse states. BMC
Bioinformatics. 25(343)2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu H, Guan H, Tan X, Jiang Y, Li F,
Sun-Waterhouse D and Li D: Enhanced alleviation of insulin
resistance via the IRS-1/Akt/FOXO1 pathway by combining quercetin
and EGCG and involving miR-27a-3p and miR-96-5p. Free Radic Biol
Med. 181:105–117. 2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Rebollo-Hernanz M, Zhang Q, Aguilera Y,
Martín-Cabrejas MA and Gonzalez de Mejia E: Phenolic compounds from
coffee by-products modulate adipogenesis-related inflammation,
mitochondrial dysfunction, and insulin resistance in adipocytes,
via insulin/PI3K/AKT signaling pathways. Food Chem Toxicol.
132(110672)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Liu Y, Qiu Y, Chen Q, Han X, Cai M and Hao
L: Puerarin suppresses the hepatic gluconeogenesis via activation
of PI3K/Akt signaling pathway in diabetic rats and HepG(2) cells.
Biomed Pharmacother. 137(111325)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gong P, Xiao X, Wang S, Shi F, Liu N, Chen
X, Yang W, Wang L and Chen F: Hypoglycemic effect of astragaloside
IV via modulating gut microbiota and regulating AMPK/SIRT1 and
PI3K/AKT pathway. J Ethnopharmacol. 281(114558)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Dai S, Hong Y, Xu J, Lin Y, Si Q and Gu X:
Ginsenoside Rb2 promotes glucose metabolism and attenuates fat
accumulation via AKT-dependent mechanisms. Biomed Pharmacother.
100:93–100. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bort A, Sánchez BG, Mateos-Gómez PA,
Díaz-Laviada I and Rodríguez-Henche N: Capsaicin targets
lipogenesis in HepG2 cells through AMPK activation, AKT inhibition
and PPARs regulation. Int J Mol Sci. 20(1660)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Dou Y, Huang R, Li Q, Liu Y, Li Y, Chen H,
Ai G, Xie J, Zeng H, Chen J, et al: Oxyberberine, an absorbed
metabolite of berberine, possess superior hypoglycemic effect via
regulating the PI3K/Akt and Nrf2 signaling pathways. Biomed
Pharmacother. 137(111312)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lv W, Wang X, Xu Q and Lu W: Mechanisms
and characteristics of sulfonylureas and glinides. Curr Top Med
Chem. 20:37–56. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hanker AB, Kaklamani V and Arteaga CL:
Challenges for the clinical development of PI3K inhibitors:
Strategies to improve their impact in solid tumors. Cancer Discov.
9:482–491. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Cao Y, Wen H, Leng C and Feng S: MiR-29a
mediates the apoptotic effects of TNF-α on endothelial cells
through inhibiting PI3K/AKT/BCL-2 axis. J Biochem Mol Toxicol.
38(e23598)2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Jaiswal N, Gavin M, Loro E, Sostre-Colón
J, Roberson PA, Uehara K, Rivera-Fuentes N, Neinast M, Arany Z,
Kimball SR, et al: AKT controls protein synthesis and oxidative
metabolism via combined mTORC1 and FOXO1 signalling to govern
muscle physiology. J Cachexia Sarcopenia Muscle. 13:495–514.
2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xu F, Cui WQ, Wei Y, Cui J, Qiu J, Hu LL,
Gong WY, Dong JC and Liu BJ: Astragaloside IV inhibits lung cancer
progression and metastasis by modulating macrophage polarization
through AMPK signaling. J Exp Clin Cancer Res.
37(207)2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Feng Y, Ren Y, Zhang X, Yang S, Jiao Q, Li
Q and Jiang W: Metabolites of traditional Chinese medicine
targeting PI3K/AKT signaling pathway for hypoglycemic effect in
type 2 diabetes. Front Pharmacol. 15(1373711)2024.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zhan L, Wang X, Zhang Y, Zhu G, Ding Y,
Chen X, Jiang W and Wu S: Benazepril hydrochloride protects against
doxorubicin cardiotoxicity by regulating the PI3K/Akt pathway. Exp
Ther Med. 22(1082)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhao ZQ, Yu ZY, Li J and Ouyang XN:
Gefitinib induces lung cancer cell autophagy and apoptosis via
blockade of the PI3K/AKT/mTOR pathway. Oncol Lett. 12:63–68.
2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wang Z, Luo G and Qiu Z: Akt inhibitor
MK-2206 reduces pancreatic cancer cell viability and increases the
efficacy of gemcitabine. Oncol Lett. 19:1999–2004. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chiaramonte A, Testi S, Pelosini C,
Micheli C, Falaschi A, Ceccarini G, Santini F and Scarpato R:
Oxidative and DNA damage in obese patients undergoing bariatric
surgery: A one-year follow-up study. Mutat Res.
827(111827)2023.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Alcalá M, Calderon-Dominguez M, Bustos E,
Ramos P, Casals N, Serra D, Viana M and Herrero L: Increased
inflammation, oxidative stress and mitochondrial respiration in
brown adipose tissue from obese mice. Sci Rep.
7(16082)2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yuzefovych LV, Musiyenko SI, Wilson GL and
Rachek LI: Mitochondrial DNA damage and dysfunction, and oxidative
stress are associated with endoplasmic reticulum stress, protein
degradation and apoptosis in high fat diet-induced insulin
resistance mice. PLoS One. 8(e54059)2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Bhatia K, Tiwari S, Gupta VK, Sapariya NM
and Upadhyay SK: An in vitro model of adipose tissue-associated
macrophages. J Biosci. 49(79)2024.PubMed/NCBI
|
|
53
|
Kirichenko TV, Markina YV, Bogatyreva AI,
Tolstik TV, Varaeva YR and Starodubova AV: The role of adipokines
in inflammatory mechanisms of obesity. Int J Mol Sci.
23(14982)2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Fernandes-da-Silva A, Miranda CS,
Santana-Oliveira DA, Oliveira-Cordeiro B, Rangel-Azevedo C,
Silva-Veiga FM, Martins FF and Souza-Mello V: Endoplasmic reticulum
stress as the basis of obesity and metabolic diseases: Focus on
adipose tissue, liver, and pancreas. Eur J Nutr. 60:2949–2960.
2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Li Y, Guo Y, Tang J, Jiang J and Chen Z:
New insights into the roles of CHOP-induced apoptosis in ER stress.
Acta Biochim Biophys Sin (Shanghai). 46:629–640. 2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Yilmaz E: Endoplasmic reticulum stress and
obesity. Adv Exp Med Biol. 960:261–276. 2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Ma XM, Geng K, Law BY, Wang P, Pu YL, Chen
Q, Xu HW, Tan XZ, Jiang ZZ and Xu Y: Lipotoxicity-induced mtDNA
release promotes diabetic cardiomyopathy by activating the
cGAS-STING pathway in obesity-related diabetes. Cell Biol Toxicol.
39:277–299. 2023.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Kusminski CM, Shetty S, Orci L, Unger RH
and Scherer PE: Diabetes and apoptosis: Lipotoxicity. Apoptosis.
14:1484–1495. 2009.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Green DR: The death receptor pathway of
apoptosis. Cold Spring Harb Perspect Biol.
14(a041053)2022.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Huang TL, Jiang WJ, Zhou Z, Shi TF, Yu M,
Yu M, Si JQ, Wang YP and Li L: Quercetin attenuates
cisplatin-induced mitochondrial apoptosis via PI3K/Akt mediated
inhibition of oxidative stress in pericytes and improves the blood
labyrinth barrier permeability. Chem Biol Interact.
393(110939)2024.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Tang P, Tang Y, Liu Y, He B, Shen X, Zhang
ZJ, Qin DL and Tian J:
Quercetin-3-O-α-L-arabinopyranosyl-(1→2)-β-D-glucopyranoside
isolated from eucommia ulmoides leaf relieves insulin resistance in
HepG2 cells via the IRS-1/PI3K/Akt/GSK-3β pathway. Biol Pharm Bull.
46:219–229. 2023.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zhu M, Zhou X and Zhao J: Quercetin
prevents alcohol-induced liver injury through targeting of
PI3K/Akt/nuclear factor-κB and STAT3 signaling pathway. Exp Ther
Med. 14:6169–6175. 2017.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Imran M, Rauf A, Shah ZA, Saeed F, Imran
A, Arshad MU, Ahmad B, Bawazeer S, Atif M, Peters DG and Mubarak
MS: Chemo-preventive and therapeutic effect of the dietary
flavonoid kaempferol: A comprehensive review. Phytother Res.
33:263–275. 2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Yao YX, Yu YJ, Dai S, Zhang CY, Xue XY,
Zhou ML, Yao CH and Li YX: Kaempferol efficacy in metabolic
diseases: Molecular mechanisms of action in diabetes mellitus,
obesity, non-alcoholic fatty liver disease, steatohepatitis, and
atherosclerosis. Biomed Pharmacother. 175(116694)2024.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Mao J, Li M, Wang X, Wang B, Luo P, Wang G
and Guo X: Exploring the mechanism of Pueraria lobata (Willd.) Ohwi
in the regulation of obesity. J Ethnopharmacol.
335(118703)2024.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Guo X, Yin T, Chen D, Xu S, Ye R and Zhang
Y: Astragaloside IV regulates insulin resistance and inflammatory
response of adipocytes via modulating MIR-21/PTEN/PI3K/AKT
signaling. Endocr Metab Immune Disord Drug Targets. 23:1538–1547.
2023.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Wu D, Jia H, Zhang Z and Li S: Capsaicin
suppresses breast cancer cell viability by regulating the
CDK8/PI3K/Akt/Wnt/β-catenin signaling pathway. Mol Med Rep.
22:4868–4876. 2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Zhang N, Liu X, Zhuang L, Liu X, Zhao H,
Shan Y, Liu Z, Li F, Wang Y and Fang J: Berberine decreases insulin
resistance in a PCOS rats by improving GLUT4: Dual regulation of
the PI3K/AKT and MAPK pathways. Regul Toxicol Pharmacol.
110(104544)2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Zhang XY, Yu L, Wang K, Wang M, Li P,
Zheng ZG and Yang H: The combination of berberine and
isoliquiritigenin synergistically improved adipose inflammation and
obesity-induced insulin resistance. Phytother Res. 38:3839–3855.
2024.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Qin C, Liu S, Zhou S, Xia X, Hu J, Yu Y
and Ma D: Tanshinone IIA promotes vascular normalization and boosts
Sorafenib's anti-hepatoma activity via modulating the PI3K-AKT
pathway. Front Pharmacol. 14(1189532)2023.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Kim Y, Rouse M, González-Mariscal I, Egan
JM and O'Connell JF: Dietary curcumin enhances insulin clearance in
diet-induced obese mice via regulation of hepatic PI3K-AKT axis and
IDE, and preservation of islet integrity. Nutr Metab (Lond).
16(48)2019.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Zoi V, Kyritsis AP, Galani V, Lazari D,
Sioka C, Voulgaris S and Alexiou GA: The role of curcumin in
cancer: A Focus on the PI3K/Akt Pathway. Cancers (Basel).
16(1554)2024.PubMed/NCBI View Article : Google Scholar
|
















