Introduction
Atopic dermatitis (AD) is a common chronic
inflammatory skin disease characterized by dry, itchy and inflamed
skin (1). Although AD commonly
develops during childhood, it can develop at any age. The precise
cause of AD is not fully understood; however, it is considered to
result from a complex interplay between genetic, environmental and
dysregulated immune systems (1).
Various systemic and topical medications, including
immunosuppressants, Janus kinase (JAK) inhibitors, and therapeutic
monoclonal antibodies (Abs), have been developed for the treatment
of AD (2-4).
However, potentially unwanted side effects limit their long-term
applicability (5,6). Given the multifactorial aspects of the
pathogenesis of AD, the identification of additional molecular
targets with minimal side effects is required for optimal AD
treatment.
Toll-like receptors (TLRs) are pattern recognition
receptors primarily responsible for recognizing microbial
pathogen-associated molecular patterns (PAMPs), thus initiating an
innate immune response to defend the host against invading
pathogens. TLRs also recognize internal danger signals known as
damage-associated molecular patterns (DAMPs), which are released by
cells that are activated or damaged during the inflammatory process
(7). The activation of TLR pathways
plays a role in the development of AD (8). Among the TLR members, TLR2 is involved
in sensing a broad range of PAMPs (9).
Tomaralimab is a novel humanized monoclonal Ab
targeting TLR2(10). The authors'
previous findings showed that intradermal injection of Tomaralimab
effectively improved AD-like skin inflammation in BALB/c mice
exposed to house dust mites (11).
However, it remains unclear whether allergenic hapten-induced AD
can be effectively treated with a systemically administered
humanized monoclonal Ab targeting TLR2. The primary objective of
the present study was to evaluate the therapeutic potential of
systemically administered Tomaralimab for treating AD-like skin
inflammation triggered by the contact allergen
2,4-dinitrochlorobenzene (DNCB) in BALB/c mice.
Materials and methods
Materials
Tomaralimab (OPN-305; cat. no. NM-103), a humanized
anti-TLR2 Ab, was provided by Neuramedy Co., Ltd. DNCB (cat. no.
237329), toluidine blue (TB; cat. no. 89640) and hematoxylin (cat.
no. H3136) and eosin B (cat. no. 212954) were purchased from
MilliporeSigma. Anti-thymic stromal lymphopoietin (TSLP) Ab (cat.
no. NBP1-76754) was obtained from Novus Biologicals, LLC and
anti-HMGB1 Ab (cat. no. ab18256) was obtained from Abcam. Anti-IL1β
Ab (cat. no. 2022) and anti-F4/80 Ab (cat. no. 70076) were obtained
from Cell Signaling Technology, Inc., and anti-IL33 Ab (cat. no.
12372-1-AP) was purchased from Proteintech Group, Inc. Anti-MPO Ab
(code no. A0398) was obtained from Dako; Agilent Technologies, Inc.
Anti-GAPDH (cat. no. sc-32233) and anti-IL17 (cat. no. sc-374218)
Abs were purchased from Santa Cruz Biotechnology, Inc. Anti-IL31 Ab
(cat. no. 701082) and secdondary Abs conjugated with horseradish
peroxidase (anti-rabbit IgG, cat. no. 31460; anti-mouse IgG, cat.
no. 31430) and rhodamine Red-X (cat. no. R6394) were obtained from
Thermo Fisher Scientific, Inc.
Induction of AD-like skin lesions in a
mouse model
A total of 18 male BALB/c mice (body weight range,
18-23 g), aged ~7 weeks, were purchased from Orient Bio, Inc. The
mice were housed in a controlled environment at 22±2˚C with
constant humidity (40-60%), maintained under a 12-h light-dark
cycle. They were provided with unrestricted access to sterile water
and food in a specific pathogen-free grade laboratory. In the
induction of AD with DNCB in BALB/c mice, 4% SDS and 1% DNCB are
typically used initially to create skin barrier disruption and
sensitization, respectively (12,13).
The commonly used dose of neutralizing Abs for intravenous (IV)
administration in mice usually ranges from 1 to 25 mg/kg (14,15).
In preliminary experiments, two doses of Tomaralimab were tested,
25 and 50 mg/kg. It was observed that administrating 25 mg/kg
effectively reduced DNCB-induced skin inflammation, comparable to a
higher dose of 50 mg/kg. Consequently, the 25 mg/kg dosage of
Tomaralimab was selected for investigation. The mice were divided
into three groups in a random manner: Group I, Control; Group II,
DNCB + PBS; and Group III, DNCB + Tomaralimab (n=6 in each group).
The method of inducing skin inflammation similar to AD through the
topical application of DNCB was carried out as previously described
(16). Group I mice were treated
with a vehicle (acetone:olive oil, 3:1 v/v), and Group II and III
mice were sensitized with 4% SDS on the dorsal skin to disrupt the
skin barrier. After 4 h, SDS-sensitized areas were subjected to
daily topical application of 1% DNCB in vehicle for 3 days.
Following a 4-day break, 0.5% DNCB was applied topically to the
same area seven times at 2-day intervals, and the mice were
sacrificed the next day (on day 20). Group III mice received an IV
dose of Tomaralimab (25 mg/kg) following each DNCB application. On
day 20, all mice were euthanized in a chamber with CO2
at a fill rate of 50% of the chamber volume per min. After visual
confirmation of death, the mice were further exposed to
CO2 for an additional minute to ensure the absence of a
heartbeat for confirmation of complete euthanasia and final death.
The animal studies followed the guidelines approved (IACUC;
approval number KU26036) by the Konkuk University Institutional
Animal Care and Use Committee (Seoul, Korea).
Tissue preparation and histopathologic
examination
Skin lesions from the dorsal surface were surgically
excised and fixed overnight at 25˚C with 100% acetone, then
embedded in paraffin. Paraffin blocks were sliced at a thickness of
5 µm with a microtome (Leica Microsystems GmbH). As previously
described, paraffin-embedded tissues were deparaffinized with
xylene and rehydrated using a graded series of ethanol (17). Rehydrated sections were immersed in
hematoxylin solution for 1 min at 25˚C, rinsed under running tap
water, and subsequently immersed in eosin solution for 30 sec at
25˚C. Dehydration was performed by passing the sections through a
graded ethanol series (80, 90, 95 and 100%) for 30 sec each at
25˚C. The slides were then rinsed in xylene three times for 5 min
each at 25˚C and mounted with coverslips.
The mast cells infiltrated were stained with 0.1% TB
for 3 min at 25˚C. Stained images were observed using a light
microscope (EVOS FL Auto; Thermo Fisher Scientific, Inc.).
Epidermal thickness was measured using ImageJ 1.52a software
(National Institutes of Health).
Immunohistochemical staining
After blocking the intracellular peroxidase activity
for 1 h at 25˚C with 3% hydrogen peroxide, tissue sections were
incubated overnight at 4˚C with primary antibodies against F4/80
(1:200) and MPO (1:500), followed by avidin/biotin complex-mediated
DAB staining using VECTASTAIN Elite ABC-HRP Kit (cat. no. PK-6101;
Vector Laboratories, Inc.), and counterstained with hematoxylin, as
previously described (11).
Fluorescent immunohistochemical staining was carried
out as previously described (11).
A secondary Ab labeled with rhodamine Red-X (1:500) was incubated
for 1 h at 25˚C for the fluorescence staining. Hoechst 33258 (10
µg/ml) was employed to counterstain the nuclear DNA. Subsequently,
the slides were treated with a fluorescence mounting medium
(ProLong Gold Antifade Reagent; Invitrogen; Thermo Fisher
Scientific, Inc.) after washing with PBS. Fluorescence images were
taken with an EVOS FL Auto system, and the fluorescence levels were
quantified using ImageJ software (National Institutes of
Health).
Stimulation of HaCaT cells
Human keratinocyte HaCaT cells were obtained from
the CLS Cell Line Service GmbH. The cells were cultured in
Dulbecco's modified Eagle's medium supplemented with 10% fetal
bovine serum (HyClone; Cytiva) and penicillin-streptomycin
(Sigma-Aldrich; Merck KGaA) in a humidified atmosphere with 5%
CO2 at 37˚C. DNCB were treated with varying
concentrations (0-50 nM) for 18 h (for detecting mRNA levels) and
24 h (for detecting protein levels), or 10 nM DNCB for various
periods (0-36 h). Tomaralimab at concentrations of 0.5, 1, 5 and 10
µg/ml was pretreated 30 min before DNCB stimulation.
Immunoblotting
HaCaT cells were lysed in lysis buffer (50 mM
Tris-HCl pH 7.4, 400 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate,
1 mM EDTA, 1 mM NaF, 1 mM NaVO4) as previously described
(18). The proteins (20 µg/lane)
were electrophoresed on 10% SDS-PAGE and transferred to
nitrocellulose membranes. After incubation with the specific
primary Abs overnight at 4˚C, HRP-conjugated secondary Abs (goat
anti-rabbit IgG, 1:8,000; goat anti-mouse IgG, 1:8,000) were
incubated for 1 h at 25˚C. The blots were visualized utilizing an
enhanced chemiluminescence (ECL) detection system (cat. no. 34580;
Thermo Fisher Scientific, Inc.). Densitometric analysis was
performed using ImageJ 1.52a software (National Institutes of
Health).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA isolation, cDNA synthesis, and PCR
reaction were carried out as previously described (19). The PCR primers used in the present
study were as follows: TSLP forward, 5'-TAGCAATCGGCCACATTGCCT-3'
and reverse, 5'-GAAGCGACGCCACAATCCTTG-3'; IL1β forward,
5'-AAACAGATGAAGTGCTCCTTCCAGG-3' and reverse,
5'-TGGAGAACACCACTTGTTGCTCCA-3'; IL17 forward, 5'-CCATAGTGAAGGCAGGAA
TC-3' and reverse, 5'-GAGGTGGATCGGTTGTAGTA-3'; IL31 forward,
5'-TCGAGGAATTACAGTCCCTCT-3' and reverse,
5'-TGTCGAGGTGCTCTATGATCTC-3'; IL33 forward,
5'-CAAAGAAGTTTGCCCCATGT-3' and reverse, 5'-AAGGCAAAGCACTCCACAGT-3';
HMGB1 forward, 5'-ATATGGCAAAAGCGGACAAG-3' and reverse,
5'-AGGCCAGGATGTTCTCCTTT-3'; and GAPDH forward,
5'-ACCCACTCCTCCACCTTTG-3' and reverse, 5'-CTCTTGTGCTCTTGCTGGG-3'.
The amplified PCR products were visualized under UV
transillumination.
Cell counting kit-8 (CCK-8) assay
Cell viability was measured using a EZ-Cytox assay
kit (cat. no. EZ-3000; DoGenBio). HaCaT cells cultured in 96-well
plates (5,000 cells/100 µl) were treated with varying
concentrations of DNCB (0, 5, 10, 25 and 50 nM) for 24 h. The assay
solution (10 µl per 100 µl culture medium) was added to each plate
and incubate for 1 h in a 37˚C CO2 incubator, after
which the absorbance was measured at 450 nm using an Emax Endpoint
ELISA Microplate Reader (Molecular Devices, LLC).
Statistical analysis
The data are presented as the average value ±
standard deviation (SD). To compare multiple groups, a one-way
ANOVA followed by Dunnett's multiple comparisons test or Tukey's
multiple comparisons test was performed using GraphPad Prism
V10.1.2 software (GraphPad Software Inc.; Dotmatics). P<0.05 was
considered to indicate a statistically significant difference.
Results
IV administration of Tomaralimab
attenuates DNCB-induced AD-like skin lesions in BALB/c mice
To assess the therapeutic effectiveness of systemic
administration of Tomaralimab for allergenic hapten-induced AD, a
mouse model was used, in which the allergenic hapten DNCB was
applied topically to the back skin of BALB/c mice (11). Topical application of DNCB leads to
typical AD-like skin inflammation with superficial redness and
edema (11,20). Under these experimental conditions,
Tomaralimab was injected IV through the lateral tail vein every
other day until day 19 (Fig. S1).
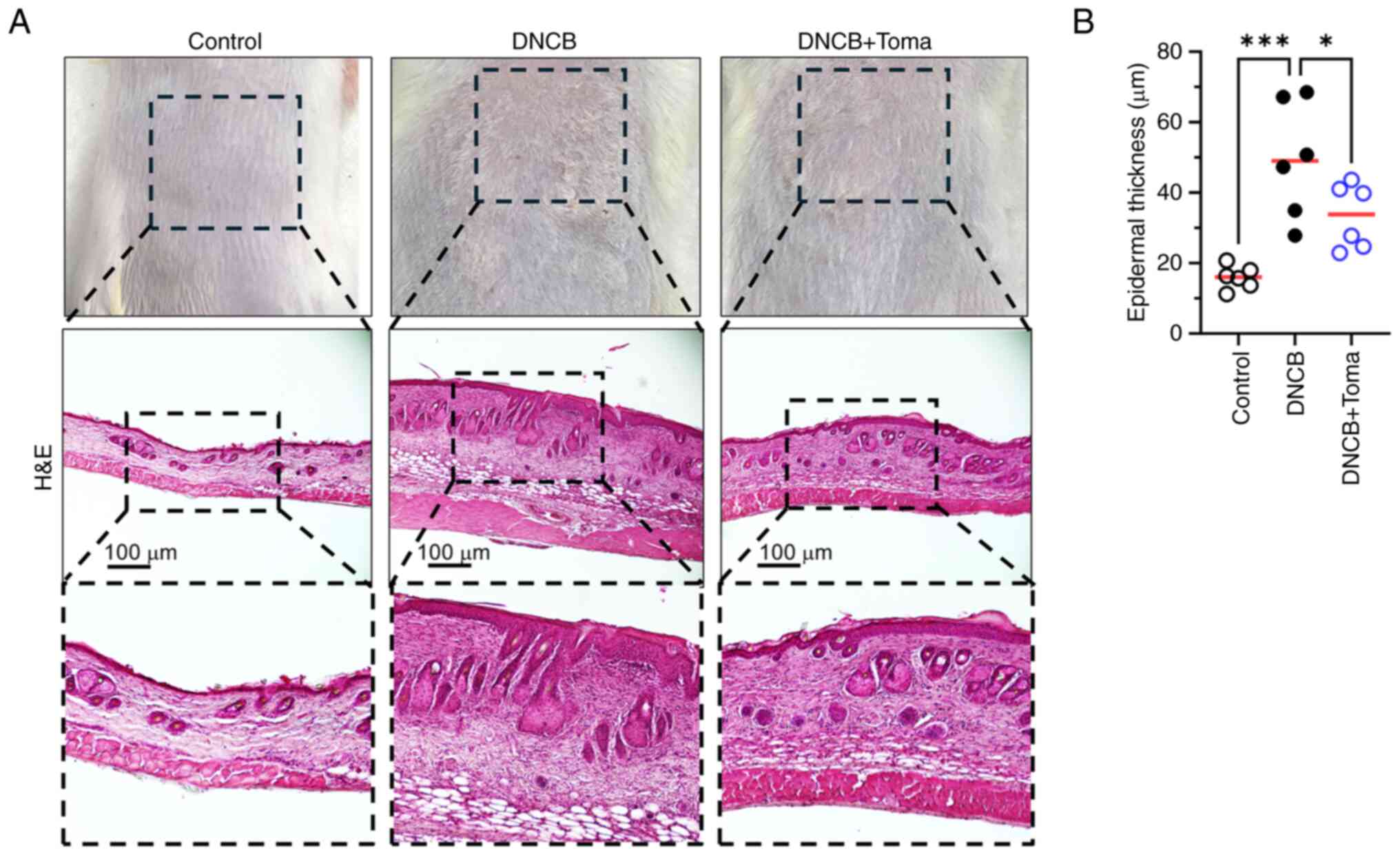
H&E staining revealed that DNCB significantly increased
epidermal hyperplasia, which was reduced by systemic administration
of Tomaralimab (Fig. 1A).
Measurement of epidermal thickness using ImageJ demonstrated that
Tomaralimab administration resulted in a significant reduction
(P=0.043 by Dunnett's multiple comparisons test, n=6) in
DNCB-induced epidermal thickness (Fig.
1B).
IV administration of Tomaralimab
restores filaggrin (FLG) and TSLP expression in DNCB-induced
AD-like skin lesions in BALB/c mice
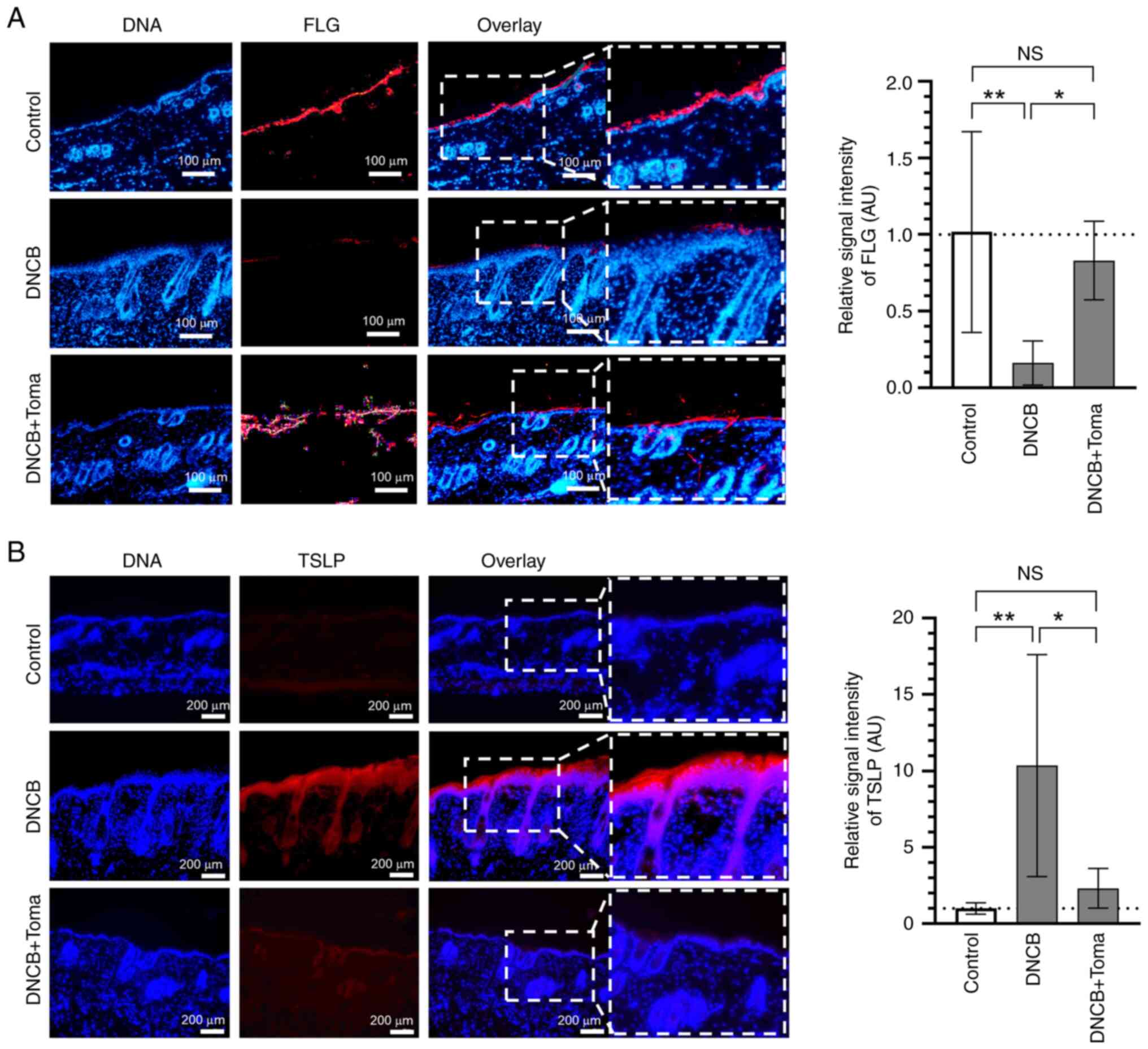
FLG is an epidermal protein responsible for
maintaining skin barrier function (21), and its deficiency contributes
critically to AD (22). TSLP is an
epithelial-derived pro-inflammatory cytokine that triggers
dendritic cells, T-lymphocytes and mast cells to stimulate the
release of various inflammatory cytokines and is considered a
crucial marker in the early pathogenesis of AD (23,24).
Immunofluorescence staining revealed that administration of
Tomaralimab led to the recovery of DNCB-induced suppression of FLG
levels (Fig. 2A) and decreased
DNCB-induced TSLP expression (Fig.
2B). These data suggest that systemic administration of
Tomaralimab has a potential impact on the recovery of damaged skin
barrier function and inhibiting the onset of allergic skin
inflammation.
IV administration of Tomaralimab
decreases the infiltration of inflammatory cells in DNCB-induced
AD-like skin lesions in BALB/c mice
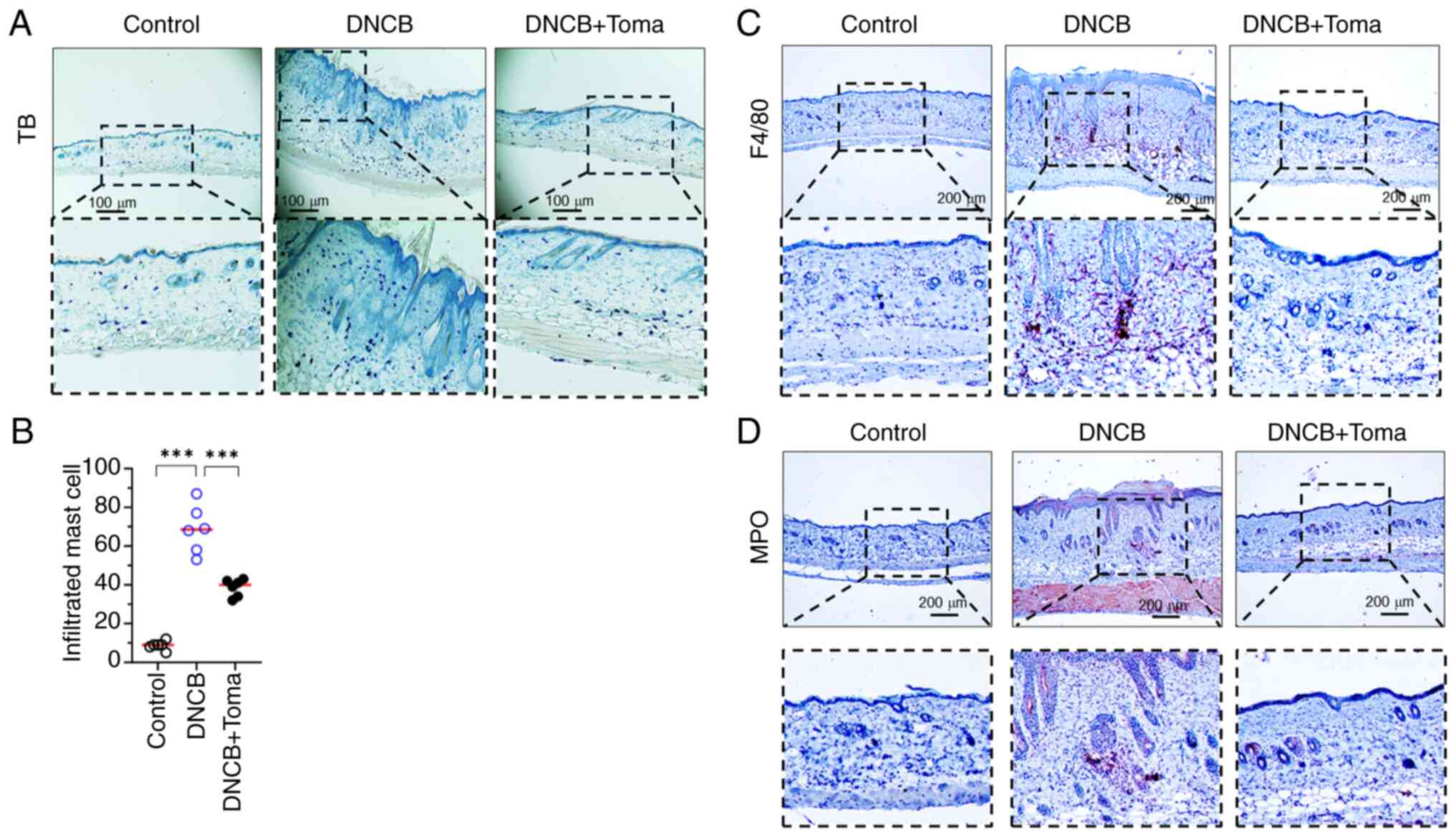
The increased infiltration of immune cells,
including T lymphocytes and mast cells, at inflammatory sites is
closely linked to the pathogenesis of AD (25). It was also observed that the
administration of Tomaralimab significantly (P<0.001 by
Dunnett's multiple comparisons test, n=6) reduced the population of
infiltrated mast cells stained by TB (Fig. 3A and B). Furthermore, Tomaralimab led to a
notable decrease in the presence of F4/80-positive macrophages
(Fig. 3C) and myeloperoxidase
(MPO)-positive immune cells, including basophils and neutrophils
(Fig. 3D).
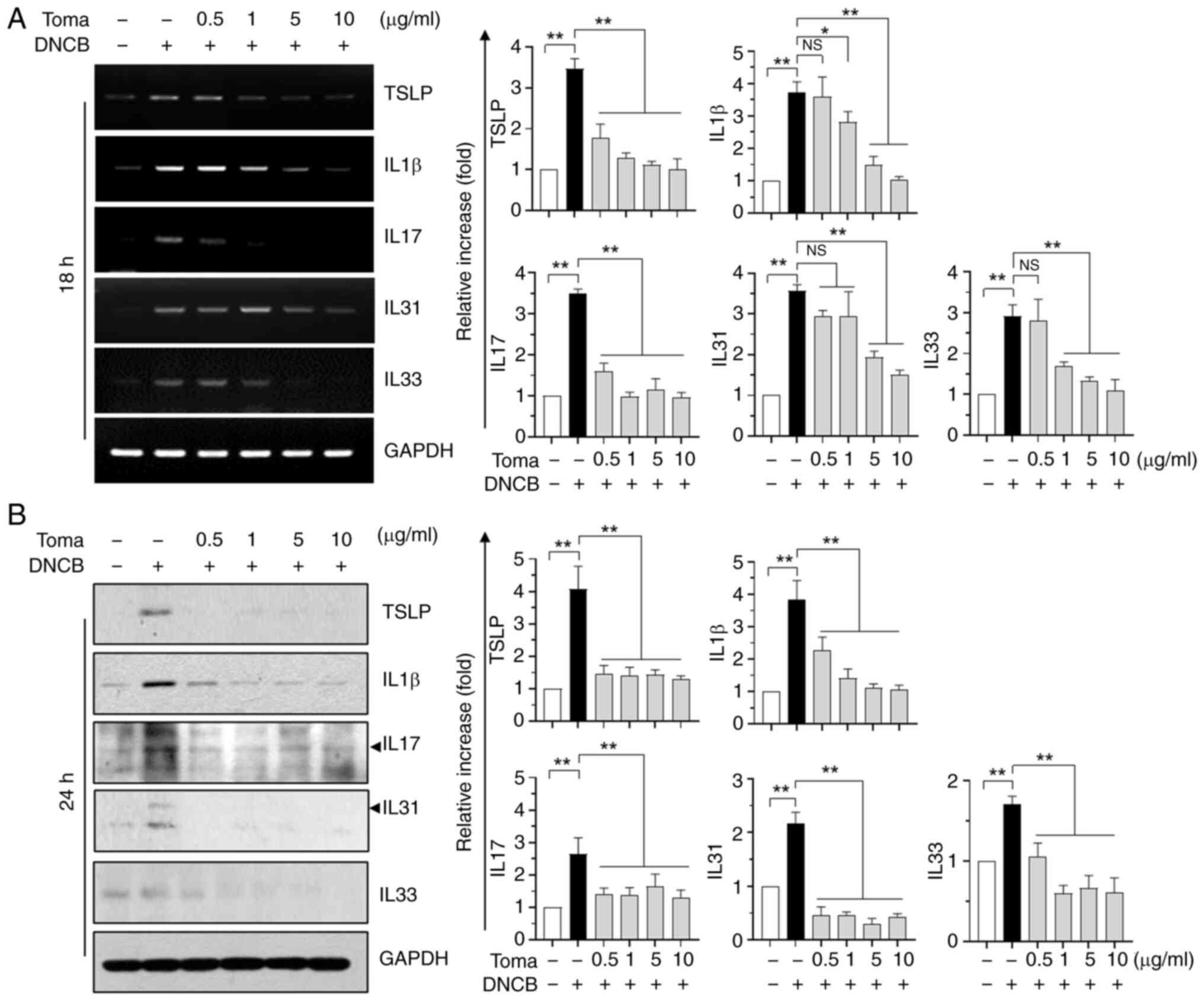
Tomaralimab decreases the expression
of DNCB-induced pro-inflammatory cytokines in HaCaT
keratinocytes
Keratinocytes play crucial roles in the pathogenesis
of AD (26). To investigate how
Tomaralimab impacts DNCB-induced inflammation on a cellular
level,inflammatory responses in HaCaT keratinocytes were triggered
using DNCB. Previous studies have demonstrated that keratinocytes
release multiple pro-inflammatory cytokines involved in the
pathogenesis of AD, including TSLP, IL-1β, IL-17 and IL-33
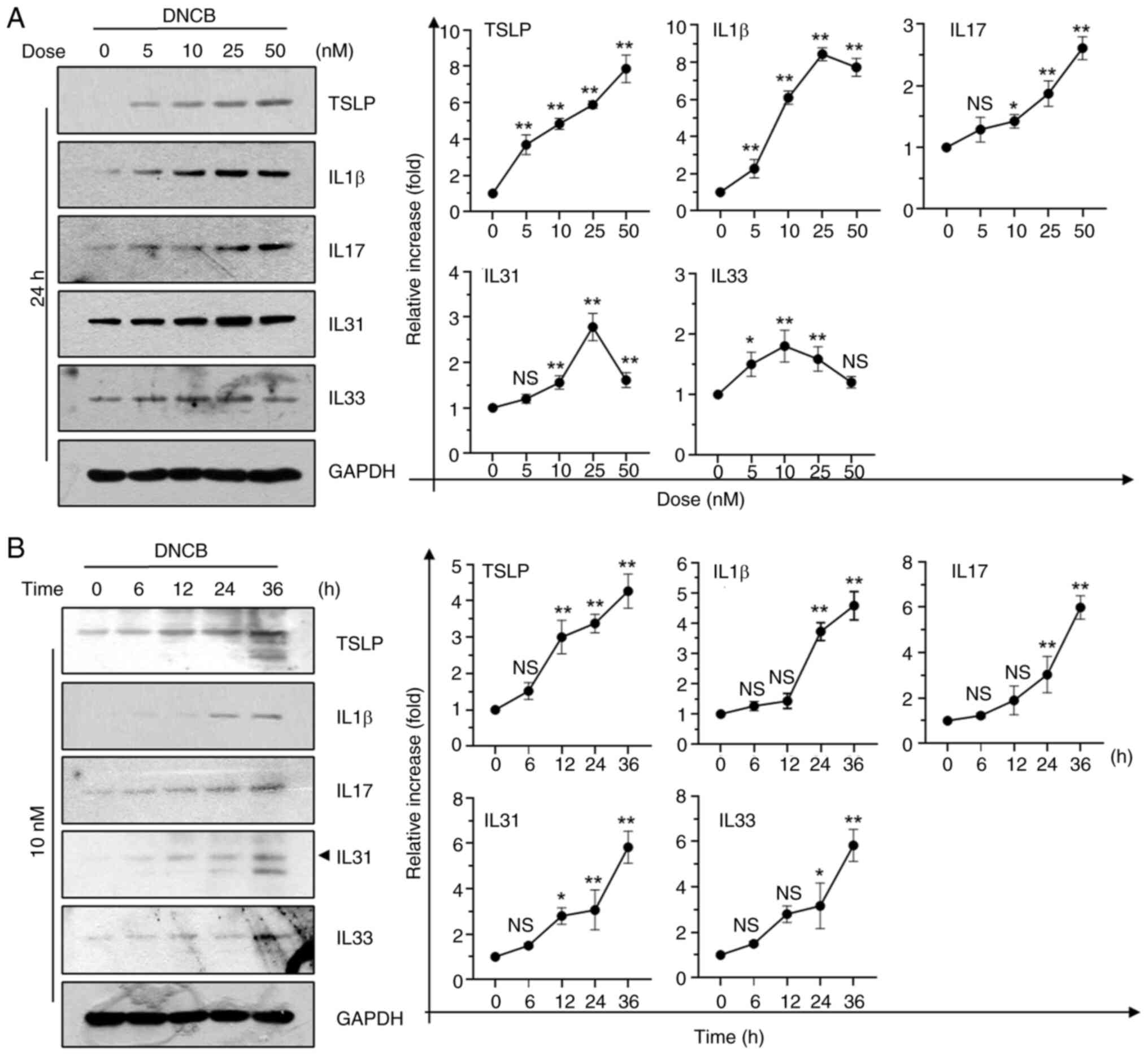
(16,27). Immunoblot analysis demonstrated that
DNCB increased levels of TSLP, IL-1β, IL-17, IL-31 and IL-33 in a
dose- (Fig. 4A) and time-dependent
manner (Fig. 4B). When HaCaT cells
were exposed to varying concentrations (0-50 nM) of DNCB, no
cytotoxicity was observed; instead, DNCB exposure exhibited slight
increases in survival rate (Fig.
S2). Certain cytokines, such as IL-1β and IL-31, reached peak
levels when the DNCB concentration exceeded 25 nM, and all tested
cytokines approached sub-peak levels after 24 h of treatment. Cells
were treated at a concentration of 10 nM for 24 h to maximize the
inhibitory action of Tomaralimab. Under these experimental
conditions, Tomaralimab treatment resulted in a dose-dependent
decrease in mRNA levels of DNCB-induced inflammatory cytokines
(Fig. 5A). In line with the RT-qPCR
findings, the protein levels of inflammatory cytokines were reduced
in a dose-dependent manner (Fig.
5B).
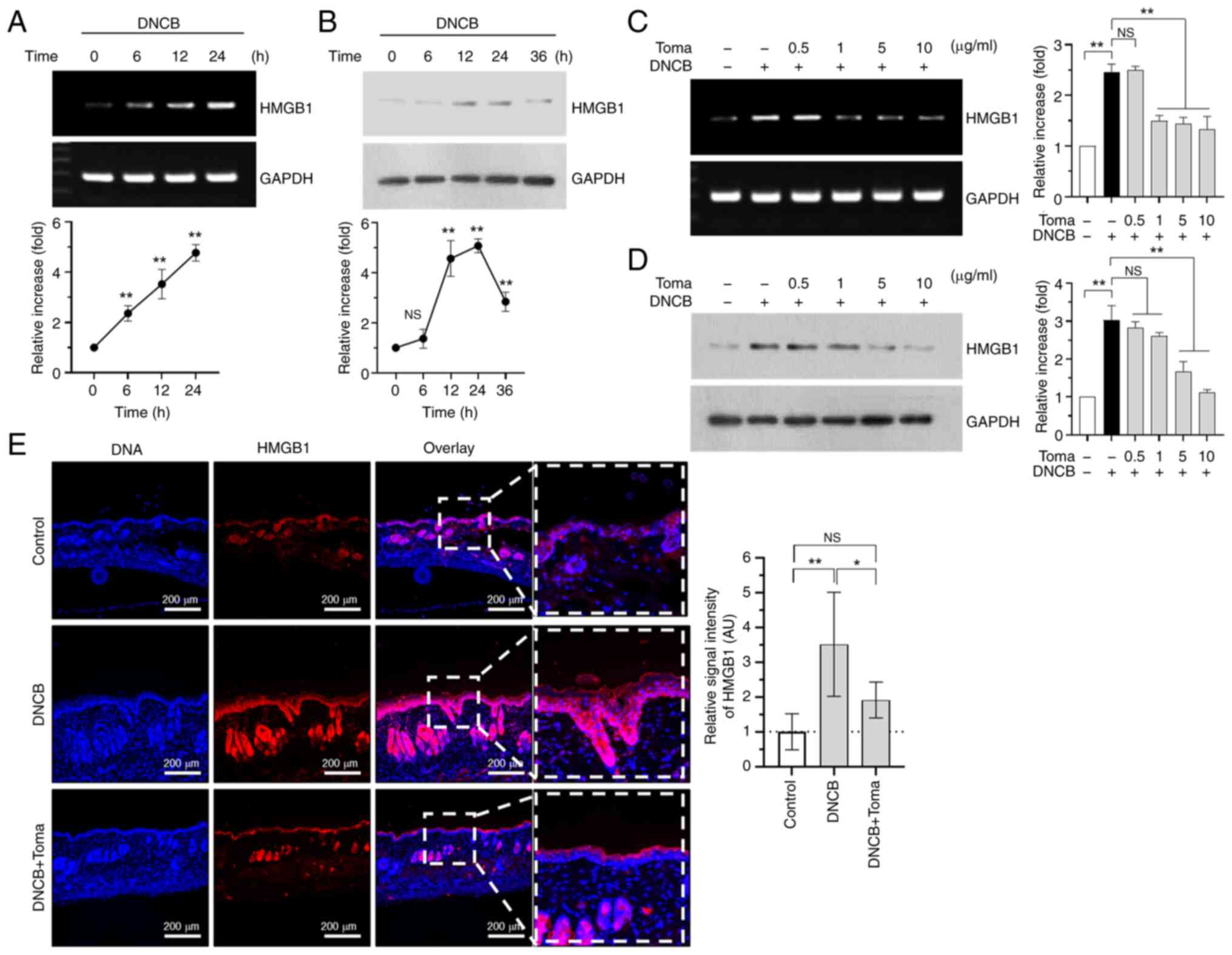
Tomaralimab inhibits DNCB-induced high
mobility group box 1 (HMGB1) expression in HaCaT keratinocytes
HMGB1 is a chromatin-binding nuclear protein
involved in DNA bending and assembly of proteins at specific DNA
sites (28). HMGB1 can be produced
from necrotic cells and implicated as a mediator of inflammation by
induction of various pro-inflammatory cytokines (29). AS HMGB1 is an endogenous TLR2 ligand
involved in TLR2-induced inflammation (30), it was examined whether DNCB induces
HMGB1 expression. It was observed that DNCB increased mRNA
(Fig. 6A) and protein (Fig. 6B) levels in a time-dependent manner.
Under this experimental condition, treatment with Tomaralimab
dose-dependently reduced DNCB-induced HMGB1 expression at mRNA
(Fig. 6C) and protein (Fig. 6D) levels. To evaluate the impact of
Tomaralimab on HMGB1 expression in vivo, immunofluorescence
analysis was conducted on skin tissues from BALB/c mice challenged
with DNCB. Topical application of DNCB markedly increased the
staining intensity of HMGB1 in the epidermis (Fig. 6E, left panel). By contrast, the
administration of Tomaralimab led to a significant decrease in
DNCB-induced HMGB1 intensity (P<0.05, n=6) (Fig. 6E, right graph). These data suggest
that the decrease in HMGB1 levels may be associated with the
inhibition of DNCB-induced skin lesions by the humanized anti-TLR2
Ab, Tomaralimab.
Discussion
At least 10 TLRs (TLR1-10) have been identified
(31). A strong connection has been
observed between the quantity of TLR2 ligands in the skin and the
extent of severity of AD (32).
Also, innate TLR2 ligands promote Th2 cell-mediated chronic AD
(33). These findings emphasize the
pivotal role of TLR2 in the development of AD and show promise as a
possible target for AD treatment. Previously, it was demonstrated
that intradermal injection of Tomaralimab effectively relieved
AD-like skin inflammation in BALB/c mice exposed to house dust mite
extracts (11), offering promise
for AD treatment through systemic Tomaralimab therapy. However, it
remains unclear whether allergenic hapten-induced AD can be
effectively treated with a systemically administered humanized
monoclonal Ab targeting TLR2. In the present study, it was aimed to
elucidate the effects of systemic Tomaralimab administration on
DNCB-induced AD in a BALB/c mouse model.
The skin lesions in AD are characterized by marked
infiltration of inflammatory cells, including mast cells and
T-lymphocytes, and the concurrent production of large amounts of
various inflammatory cytokines (34). In the induction of AD with DNCB in
BALB/c mice, 4% SDS and 1% DNCB were used to create skin barrier
disruption and sensitization (12,13).
SDS is a surfactant that disrupts the skin barrier, facilitating
the penetration of DNCB into the skin. This disruption helps ensure
that the DNCB adequately triggers the immunological response. The
higher concentration of DNCB (>1%) in the initial applications
sensitizes the immune system effectively. This first exposure is
critical as it primes the immune system by creating an allergic
response, stimulating T-cells, and setting the stage for an
inflammatory reaction typical of AD (12). After sensitization, a lower
concentration of DNCB (0.5%) is used in repeated applications to
sustain the inflammatory response. This lower concentration can
trigger an immune response in already-sensitized skin without
causing excessive irritation or toxicity, allowing for a controlled
and sustained AD-like condition in the mice. Therefore, the
combination of 4% SDS and higher concentrations of DNCB initially
followed by a lower concentration helps establish a robust model of
AD that mimics both the sensitization and chronic phases of the
disease (12). The findings of the
present study demonstrated that systemic administration of
Tomaralimab in BALB/c mice effectively alleviated DNCB-induced
AD-like skin lesions by reducing the infiltration of inflammatory
cells, including TB-positive mast cells, F4/80-positive macrophages
and MPO-positive neutrophils and basophils, into skin lesions and
suppressing the production of multiple inflammatory cytokines
closely associated with the pathogenesis of AD. Furthermore,
administration of Tomaralimab reduces DNCB-induced TSLP expression,
crucial for mast cell development, immune responses mediated by
mast cells, initiating Th2 response, and upregulating itch-related
factors such as IL-31 and IL-33, responsible for activating sensory
neurons to trigger itchiness (35-38).
These results demonstrate that the administration of Tomaralimab
effectively reduces the pathogenesis of hapten-induced AD in a
BALB/c mouse model.
The mechanism by which TLR2-targeting Abs suppress
hapten-induced skin inflammation remains largely unknown. Continual
contact with chemical allergens on the skin may lead to the
secretion of TLR ligands, such as DAMP molecules, from injured
cells (39). HMGB1, a molecule
known as a DAMP molecule, binds to various TLRs, including
TLR2(40), and functions as a
pro-inflammatory mediator to initiate inflammation by promoting the
production of multiple inflammatory cytokines (41). In this context, it was demonstrated
that the mRNA expression level of HMGB1 was enhanced by DNCB
and dose-dependently reduced by Tomaralimab, suggesting that
TLR2-targeting Abs could block the inflammatory response elicited
by allergenic hapten-induced DAMPs. The current results support the
notion that TLR2 plays a crucial role in allergenic hapten-induced
AD-like skin inflammation and that targeting TLR2 with humanized
monoclonal Abs is a feasible therapeutic approach. Further studies
are necessary to explore the effects of targeting TLR2 on skin
inflammation induced by multiple allergenic haptens in the
pathogenesis of AD.
In conclusion, the present findings further support
the feasibility of Ab therapy using humanized anti-TLR2 monoclonal
Ab, Tomaralimab, as a promising candidate for systemic therapy in
treating allergenic hapten-induced AD-like skin disorders.
Supplementary Material
Illustration of the experimental
schedule for the induction of atopic dermatitis-like skin lesions
and intravenous administration of Tomaralimab.
HaCaT cells were treated with varying
concentrations of DNCB (0, 5, 10, 25 and 50 nM) for 24 h and cell
viability was determined. The cell viability for the control group
received with the vehicle (DMSO) was set to 100%. Results are
expressed as the mean ± SD (n=3). DNCB,
2,4-dinitrochlorobenzene.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National
Research Foundation of Korea (NRF) grant funded by the Korean
Government (MSIT) (grant no. 2023R1A2C1003601). The article was
also supported by the KU Research Professor Program at Konkuk
University (Seoul, Republic of Korea).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HY carried out biochemical analysis, animal
experiments, histological examination, ethodology, formal analysis
and data curation. EJ conducted investigation, histological
examination and data curation. TYK performed formal analysis and
visualization SYS conceptualized and supervised the study, acquired
funding, wrote, reviewed and edited the manuscript. All authors
read and approved the final version of the manuscript. All athors
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
All animal studies were conducted following the
guidelines for animal experiments and procedures approved (IACUC;
approval no. KU26036) by the Konkuk University Institutional Animal
Care and Use Committee (Seoul, Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Weidinger S and Novak N: Atopic
dermatitis. Lancet. 387:1109–1122. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Newsom M, Bashyam AM, Balogh EA, Feldman
SR and Strowd LC: New and emerging systemic treatments for atopic
dermatitis. Drugs. 80:1041–1052. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bieber T: Atopic dermatitis: An expanding
therapeutic pipeline for a complex disease. Nat Rev Drug Discov.
21:21–40. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhou G, Huang Y and Chu M: Clinical trials
of antibody drugs in the treatments of atopic dermatitis. Front Med
(Lausanne). 10(1229539)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hong J, Buddenkotte J, Berger TG and
Steinhoff M: Management of itch in atopic dermatitis. Semin Cutan
Med Surg. 30:71–86. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kychygina A, Cassagne M, Tauber M, Galiacy
S, Paul C, Fournié P and Simon M: Dupilumab-associated adverse
events during treatment of allergic diseases. Clin Rev Allergy
Immunol. 62:519–533. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gong T, Liu L, Jiang W and Zhou R:
DAMP-sensing receptors in sterile inflammation and inflammatory
diseases. Nat Rev Immunol. 20:95–112. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tamagawa-Mineoka R: Toll-like receptors:
Their roles in pathomechanisms of atopic dermatitis. Front Immunol.
14(1239244)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801.
2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Garcia-Manero G, Jabbour EJ, Konopleva MY,
Daver NG, Borthakur G, DiNardo CD, Bose P, Patel P, Komrokji RS,
Shastri A, et al: A clinical study of tomaralimab (OPN-305), a
toll-like receptor 2 (TLR-2) antibody, in heavily pre-treated
transfusion dependent patients with lower risk myelodysplastic
syndromes (MDS) that have received and failed on prior
hypomethylating agent (HMA) therapy. Blood. 132(798)2018.
|
|
11
|
Yeo H, Ahn SS, Ou S, Yun SJ, Lim Y, Koh D,
Lee YH and Shin SY: The EGR1-Artemin axis in keratinocytes enhances
the innervation of epidermal sensory neurons during skin
inflammation induced by house dust mite extract from
Dermatophagoidesfarinae. J Invest Dermatol. 144:1817–1828.e17.
2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Riedl R, Kühn A, Rietz D, Hebecker B,
Glowalla KG, Peltner LK, Jordan PM, Werz O, Lorkowski S, Wiegand C
and Wallert M: Establishment and characterization of mild atopic
dermatitis in the DNCB-induced mouse model. Int J Mol Sci.
24(12325)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Toyama S, Moniaga CS, Nakae S, Kurosawa M,
Ogawa H, Tominaga M and Takamori K: Regulatory T cells exhibit
interleukin-33-dependent migratory behavior during skin barrier
disruption. Int J Mol Sci. 22(7443)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Paci A, Desnoyer A, Delahousse J, Blondel
L, Maritaz C, Chaput N, Mir O and Broutin S:
Pharmacokinetic/pharmacodynamic relationship of therapeutic
monoclonal antibodies used in oncology: Part 1, monoclonal
antibodies, antibody-drug conjugates and bispecific T-cell
engagers. Eur J Cancer. 128:107–118. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tao Z, Liu W, Chen Q, Zhang L, She K, Zhao
G, Liang L, Chen X, Yang Y, Song Q and Lu F: Blocking Th2 signaling
pathway alleviates the clinical symptoms and inflammation in
allergic conjunctivitis. Invest Ophthalmol Vis Sci.
64(30)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yeo H, Ahn SS, Lee JY, Jung E, Jeong M,
Kang GS, Ahn S, Lee Y, Koh D, Lee YH, et al: Disrupting the DNA
binding of EGR-1 with a small-molecule inhibitor ameliorates 2,
4-dinitrochlorobenzene-induced skin inflammation. J Invest
Dermatol. 141:1851–1855. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yeo H, Lee YH, Koh D, Lim Y and Shin SY:
Chrysin inhibits NF-κB-dependent CCL5 transcription by targeting
IκB kinase in the atopic dermatitis-like inflammatory
microenvironment. Int J Mol Sci. 21(7348)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yeo H, Lee YH, Ahn SS, Jung E, Lim Y and
Shin SY: Chrysin inhibits TNFα-induced TSLP expression through
downregulation of EGR1 expression in keratinocytes. Int J Mol Sci.
22(4350)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ahn SS, Lee YH, Yeo H, Jung E, Lim Y and
Shin SY: Saikosaponin A and saikosaponin C reduce TNF-α-induced
TSLP expression through inhibition of MAPK-mediated EGR1 expression
in HaCaT keratinocytes. Int J Mol Sci. 23(4857)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ahn SS, Yeo H, Jung E, Lim Y, Lee YH and
Shin SY: FRA1:c-JUN:HDAC1 complex down-regulates filaggrin
expression upon TNFα and IFNγ stimulation in keratinocytes. Proc
Natl Acad Sci USA. 119(e2123451119)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sandilands A, Sutherland C, Irvine AD and
McLean WHI: Filaggrin in the frontline: Role in skin barrier
function and disease. J Cell Sci. 122:1285–1294. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cabanillas B and Novak N: Atopic
dermatitis and filaggrin. Curr Opin Immunol. 42:1–8.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu YJ: Thymic stromal lymphopoietin:
Master switch for allergic inflammation. J Exp Med. 203:269–273.
2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Brandt EB and Sivaprasad U: Th2 cytokines
and atopic dermatitis. J Clin Cell Immunol. 2(110)2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ando T, Matsumoto K, Namiranian S,
Yamashita H, Glatthorn H, Kimura M, Dolan BR, Lee JJ, Galli SJ,
Kawakami Y, et al: Mast cells are required for full expression of
allergen/SEB-induced skin inflammation. J Invest Dermatol.
133:2695–2705. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Girolomoni G and Pastore S: The role of
keratinocytes in the pathogenesis of atopic dermatitis. J Am Acad
Dermatol. 45 (1 Suppl):S25–S28. 2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Imai Y: Interleukin-33 in atopic
dermatitis. J Dermatol Sci. 96:2–7. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bianchi ME and Beltrame M: Upwardly mobile
proteins. Workshop: the role of HMG proteins in chromatin
structure, gene expression and neoplasia. EMBO Rep. 1:109–114.
2000.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Scaffidi P, Misteli T and Bianchi ME:
Release of chromatin protein HMGB1 by necrotic cells triggers
inflammation. Nature. 418:191–195. 2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yu M, Wang H, Ding A, Golenbock DT, Latz
E, Czura CJ, Fenton MJ, Tracey KJ and Yang H: HMGB1 signals through
toll-like receptor (TLR) 4 and TLR2. Shock. 26:174–179.
2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kawai T and Akira S: The role of
pattern-recognition receptors in innate immunity: Update on
Toll-like receptors. Nat Immunol. 11:373–384. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Travers JB, Kozman A, Mousdicas N, Saha C,
Landis M, Al-Hassani M, Yao W, Yao Y, Hyatt AM, Sheehan MP, et al:
Infected atopic dermatitis lesions contain pharmacologic amounts of
lipoteichoic acid. J Allergy Clin Immunol. 125:146–152.e1-e2.
2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kaesler S, Volz T, Skabytska Y, Köberle M,
Hein U, Chen KM, Guenova E, Wölbing F, Röcken M and Biedermann T:
Toll-like receptor 2 ligands promote chronic atopic dermatitis
through IL-4-mediated suppression of IL-10. J Allergy Clin Immunol.
134:92–99. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Werfel T, Allam JP, Biedermann T, Eyerich
K, Gilles S, Guttman-Yassky E, Hoetzenecker W, Knol E, Simon HU,
Wollenberg A, et al: Cellular and molecular immunologic mechanisms
in patients with atopic dermatitis. J Allergy Clin Immunol.
138:336–349. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ebina-Shibuya R and Leonard WJ: Role of
thymic stromal lymphopoietin in allergy and beyond. Nat Rev
Immunol. 23:24–37. 2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wilson SR, Thé L, Batia LM, Beattie K,
Katibah GE, McClain SP, Pellegrino M, Estandian DM and Bautista DM:
The epithelial cell-derived atopic dermatitis cytokine TSLP
activates neurons to induce itch. Cell. 155:285–295.
2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gibbs BF, Patsinakidis N and Raap U: Role
of the pruritic cytokine IL-31 in autoimmune skin diseases. Front
Immunol. 10(1383)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Meng J, Moriyama M, Feld M, Buddenkotte J,
Buhl T, Szöllösi A, Zhang J, Miller P, Ghetti A, Fischer M, et al:
New mechanism underlying IL-31-induced atopic dermatitis. J Allergy
Clin Immunol. 141:1677–1689.e8. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
McFadden J, Dearman R, White J, Basketter
D and Kimber I: The hapten-atopy hypothesis II: The ‘cutaneous
hapten paradox’. Clin Exp Allergy. 41:327–337. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Park JS, Gamboni-Robertson F, He Q,
Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama
I, Banerjee A, et al: High mobility group box 1 protein interacts
with multiple Toll-like receptors. Am J Physiol Cell Physiol.
290:C917–C924. 2006.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Venereau E, Casalgrandi M, Schiraldi M,
Antoine DJ, Cattaneo A, De Marchis F, Liu J, Antonelli A, Preti A,
Raeli L, et al: Mutually exclusive redox forms of HMGB1 promote
cell recruitment or proinflammatory cytokine release. J Exp Med.
209:1519–1528. 2012.PubMed/NCBI View Article : Google Scholar
|