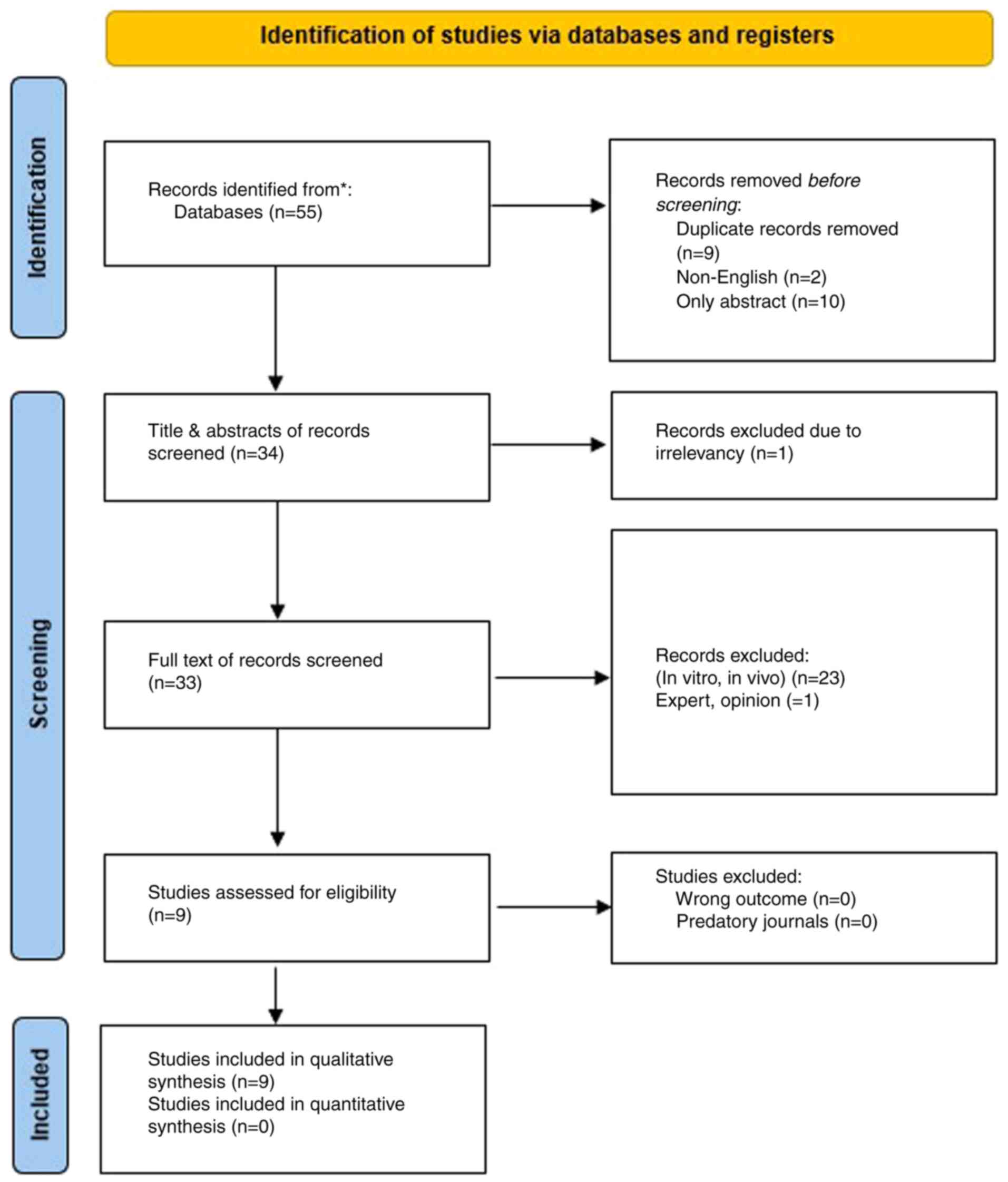
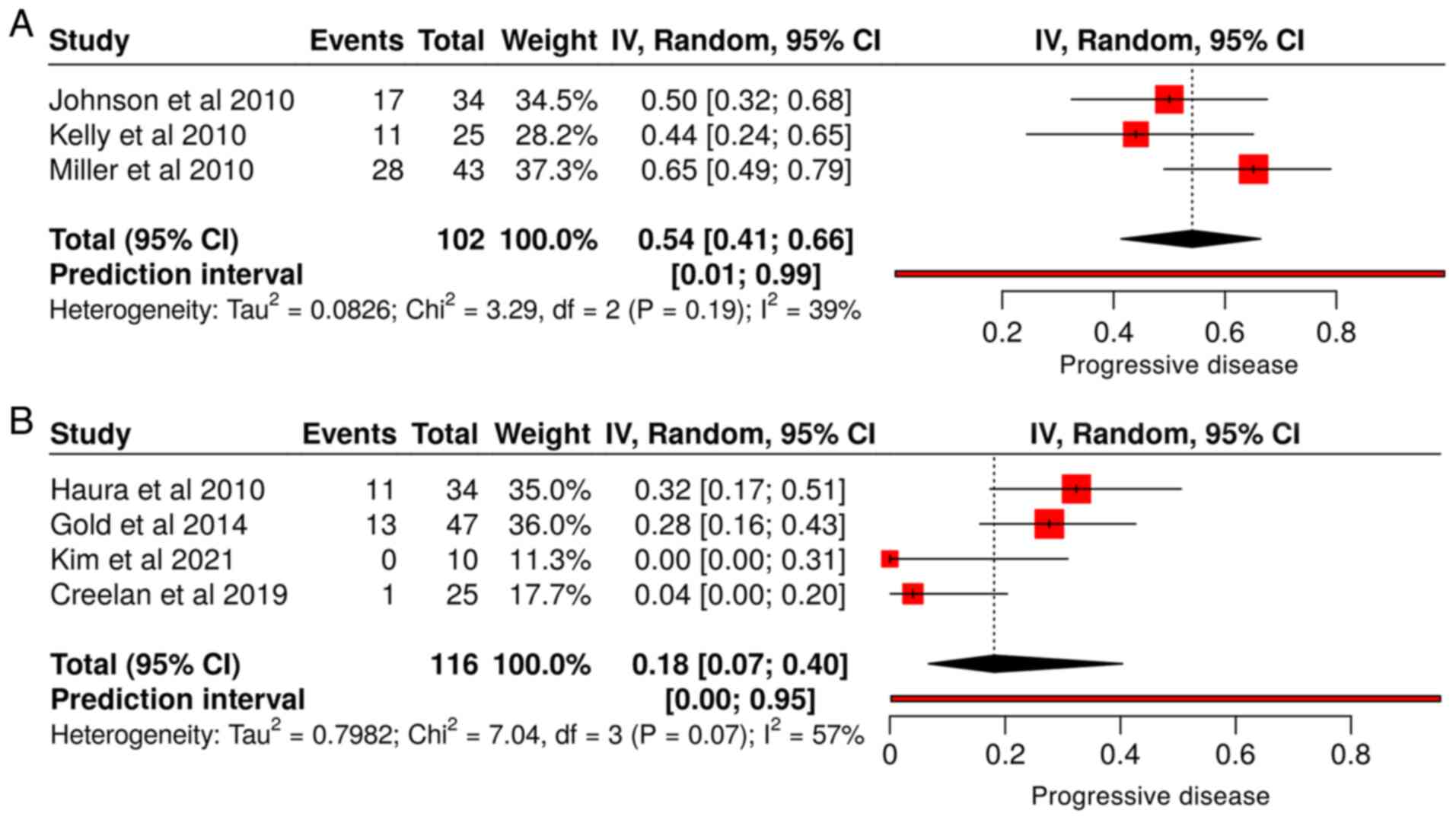
|
1
|
Siddiqui F, Vaqar S and Siddiqui AH: Lung
Cancer. StatPearls Publishing, Treasure Island, FL, 2024.
|
|
2
|
Thandra KC and Barsouk A, Saginala K,
Aluru JS and Barsouk A: Epidemiology of lung cancer. Contemp Oncol
(Pozn). 25:45–52. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Siegel RL, Miller KD, Goding Sauer A,
Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA and Jemal
A: Colorectal cancer statistics, 2020. CA Cancer J Clin.
70:145–164. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sher T, Dy GK and Adjei AA: Small cell
lung cancer. Mayo Clin Proc. 83:355–367. 2008.
|
|
5
|
Mingomataj E, Krasniqi M, Dedushi K,
Sergeevich KA, Kust D, Qadir AA, Abdullah AS, Ahmed MK and Fatah
GM: Cancer publications in one year (2023): A cross-sectional
study. Barw Med J. 2:3–11. 2024.
|
|
6
|
Ali RM, Omar SS, Ahmed HK, Omar DA,
Mahmood YM, Mustafa MQ, Abdullah AS, Hassan MN, Hussein DA, Kakamad
SH, et al: Effect of sunitinib in the management of lung cancer: A
systematic review of clinical trials. Barw Med J. 2:57–64.
2024.
|
|
7
|
Alduais Y, Zhang H, Fan F, Chen J and Chen
B: Non-small cell lung cancer (NSCLC): A review of risk factors,
diagnosis, and treatment. Medicine (Baltimore).
102(e32899)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Valk PE, Pounds TR, Hopkins DM, Haseman
MK, Hofer GA, Greiss HB, Myers RW and Lutrin CL: Staging non-small
cell lung cancer by whole-body positron emission tomographic
imaging. Ann Thorac Surg. 60:1573–1581; discussion 1581-1582.
1995.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zappa C and Mousa SA: Non-small cell lung
cancer: Current treatment and future advances. Transl Lung Cancer
Res. 5:288–300. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ye Q, Gui C, Jin D, Zhang J, Zhang J, Ma N
and Xu L: Synergistic effect of cannabidiol with dasatinib on lung
cancer by SRC/PI3K/AKT signal pathway. Biomed Pharmacother.
173(116445)2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Abdullah HO, Abdalla BA, Kakamad FH, Ahmed
JO, Baba HO, Hassan MN, Bapir R, Rahim HM, Omar DA, Kakamad SH, et
al: Predatory publishing lists: A review on the ongoing battle
against fraudulent actions. Barw Med J. 2:26–30. 2024.
|
|
13
|
Johnson FM, Bekele BN, Feng L, Wistuba I,
Tang XM, Tran HT, Erasmus JJ, Hwang LL, Takebe N, Blumenschein GR,
et al: Phase II study of dasatinib in patients with advanced
non-small-cell lung cancer. J Clin Oncol. 28:4609–4615.
2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Haura EB, Tanvetyanon T, Chiappori A,
Williams C, Simon G, Antonia S, Gray J, Litschauer S, Tetteh L,
Neuger A, et al: Phase I/II study of the Src inhibitor dasatinib in
combination with erlotinib in advanced non-small-cell lung cancer.
J Clin Oncol. 28:1387–1394. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gold KA, Lee JJ, Harun N, Tang X, Price J,
Kawedia JD, Tran HT, Erasmus JJ, Blumenschein GR, William WN, et
al: A phase I/II study combining erlotinib and dasatinib for
non-small cell lung cancer. Oncologist. 19:1040–1041.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kelley MJ, Jha G, Shoemaker D, Herndon JE
II, Gu L, Barry WT, Crawford J and Ready N: Phase II study of
dasatinib in previously treated patients with advanced non-small
cell lung cancer. Cancer Invest. 35:32–35. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kim C, Liu SV, Crawford J, Torres T, Chen
V, Thompson J, Tan M, Esposito G, Subramaniam DS and Giaccone G: A
phase I trial of dasatinib and osimertinib in TKI naïve patients
with advanced EGFR-mutant non-small-cell lung cancer. Front Oncol.
11(728155)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Creelan BC, Gray JE, Tanvetyanon T,
Chiappori AA, Yoshida T, Schell MJ, Antonia SJ and Haura EB: Phase
1 trial of dasatinib combined with afatinib for epidermal growth
factor receptor-(EGFR-) mutated lung cancer with acquired tyrosine
kinase inhibitor (TKI) resistance. Br J Cancer. 120:791–796.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Miller AA, Pang H, Hodgson L, Ramnath N,
Otterson GA, Kelley MJ, Kratzke RA and Vokes EE: Cancer and
Leukemia Group B (CALGB). A phase II study of dasatinib in patients
with chemosensitive relapsed small cell lung cancer (cancer and
leukemia group B 30602). J Thorac Oncol. 5:380–384. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Khurshid H, Dipetrillo T, Ng T,
Mantripragada K, Birnbaum A, Berz D, Radie-Keane K, Perez K,
Constantinou M, Luppe D, et al: A phase I study of dasatinib with
concurrent chemoradiation for stage III non-small cell lung cancer.
Front Oncol. 2(56)2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Brunner AM, Costa DB, Heist RS, Garcia E,
Lindeman NI, Sholl LM, Oxnard GR, Johnson BE and Hammerman PS:
Treatment-related toxicities in a phase II trial of dasatinib in
patients with squamous cell carcinoma of the lung. J Thorac Oncol.
8:1434–1437. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Luo W: Nasopharyngeal carcinoma ecology
theory: Cancer as multidimensional spatiotemporal ‘unity of ecology
and evolution’ pathological ecosystem. Theranostics. 13:1607–1631.
2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
World Health Organization: Lung cancer.
https://www.who.int/news-room/fact-sheets/detail/lung-cancer.
Accessed November 4, 2024.
|
|
25
|
Chen BT, Chen Z, Ye N, Mambetsariev I,
Fricke J, Daniel E, Wang G, Wong CW, Rockne RC, Colen RR, et al:
Differentiating peripherally-located small cell lung cancer from
non-small cell lung cancer using a CT radiomic approach. Front
Oncol. 10(593)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ruano-Ravina A, Provencio M, Calvo de Juan
V, Carcereny E, Estival A, Rodríguez-Abreu D, Benítez G,
López-Castro R, Belver M, Guirado-Risueño M, et al: Are there
differences by sex in lung cancer characteristics at diagnosis? -a
nationwide study. Transl Lung Cancer Res. 10:3902–3911.
2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sehgal K, Gill RR, Widick P, Bindal P,
McDonald DC, Shea M, Rangachari D and Costa DB: Association of
performance status with survival in patients with advanced
non-small cell lung cancer treated with pembrolizumab monotherapy.
JAMA Netw Open. 4(e2037120)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bitenc M, Cufer T, Kern I, Miklavcic M,
Petrovic S, Groznik V and Sadikov A: Real-life long-term outcomes
of upfront surgery in patients with resectable stage I-IIIA
non-small cell lung cancer. Radiol Oncol. 56:346–354.
2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lee SH: Chemotherapy for lung cancer in
the era of personalized medicine. Tuberc Respir Dis (Seoul).
82:179–189. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Petrella F, Rizzo S, Attili I, Passaro A,
Zilli T, Martucci F, Bonomo L, Del Grande F, Casiraghi M, De
Marinis F and Spaggiari L: Stage III non-small-cell lung cancer: An
overview of treatment options. Curr Oncol. 30:3160–3175.
2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Guo Q, Liu L, Chen Z, Fan Y, Zhou Y, Yuan
Z and Zhang W: Current treatments for non-small cell lung cancer.
Front Oncol. 12(945102)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Omar SS, Ali RH, Abdullah SH, Hussein DM,
Radha BMM, Latif AB, Ali SM, Hiwa DS, Ahmed HK, Hamasaeed AG, et
al: Durvalumab (Anti-PD-1) in the management of mesothelioma: A
systematic review of the current literature. Barw Med J. 1:32–39.
2023.
|
|
33
|
Ali RM, Kakamad FH, Abdullah HO, Abdulla
SH, Ahmed SF, Amin BJH, Hassan MN, Hasan SJ, Hamasalih HM, Abdalla
BA, et al: Pembrolizumab (Anti-PD-1) immunotherapy in malignant
pleural mesothelioma: A systematic review of the current
literature. Barw Med J. 1:6–13. 2023.
|
|
34
|
Park NS, Park YK, Yadav AK, Shin YM,
Bishop-Bailey D, Choi JS, Park JW and Jang BC: Anti-growth and
pro-apoptotic effects of dasatinib on human oral cancer cells
through multi-targeted mechanisms. J Cell Mol Med. 25:8300–8311.
2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Conchon M, Freitas CM, Rego MA and Braga
Junior JW: Dasatinib-clinical trials and management of adverse
events in imatinib resistant/intolerant chronic myeloid leukemia.
Rev Bras Hematol Hemoter. 33:131–139. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Shyam Sunder S, Sharma UC and Pokharel S:
Adverse effects of tyrosine kinase inhibitors in cancer therapy:
Pathophysiology, mechanisms and clinical management. Signal
Transduct Target Ther. 8(262)2023.PubMed/NCBI View Article : Google Scholar
|