Introduction
Lung cancer refers to tumors originating from the
lung parenchyma (1). It is the most
common cancer globally, both in terms of incidence and mortality.
In total, ~2 million individuals are diagnosed with lung cancer
annually, leading to ~1.8 million deaths (2). The mortality rate from lung cancer
exceeds the combined deaths caused by prostate, breast, brain and
colorectal cancers (3). Small-cell
lung cancer (SCLC) accounts for 15% of all cases, while non-small
cell lung cancer (NSCLC) comprises 85%, marking the two primary
subtypes of the disease (4). NSCLC,
in particular, is the most extensively studied form of cancer, with
the highest number of publications recorded in 2022(5). Epidemiological data indicate that the
general population experiences a lung cancer incidence rate of 69
per 100,000 individuals, with this rate increasing substantially to
751 per 100,000 men over the age of 75. This increase is primarily
attributed to both advanced age and the higher prevalence of lung
cancer among males (6).
Effective management planning for lung cancer
requires determining the tumor, lymph node and metastasis (TNM)
staging, as treatment strategies are based on the cancer's stage
and the extent of metastasis (7-9).
Surgical intervention is an option for patients with resectable
NSCLC in stages I, II and IIIA (10). In addition to surgery, the treatment
of NSCLC includes radiotherapy, chemotherapy, immunotherapy and
targeted molecular therapy. Targeted therapy, a relatively new
treatment approach, continues to evolve as researchers identify new
biological targets (7). Among the
targeted therapies, dasatinib, an FDA-approved multi-kinase
inhibitor, is primarily utilized for the treatment of chronic
myeloid leukemia. Additionally, it demonstrates significant
antiproliferative effects on various solid tumors, particularly
when used in combination with other therapeutic agents, including
those for prostate, breast, lung and pancreatic cancers (11).
The present meta-analysis evaluates the efficacy of
dasatinib, both as monotherapy and in combination with other
therapies, in the management of lung cancer. All references have
been checked to exclude any non-peer-reviewed data (12).
Materials and methods
Study design
The present study examines clinical trials
evaluating the efficacy of dasatinib in treatment of lung cancer.
The analysis includes investigations of dasatinib both as a
monotherapy and in combination with other therapies. The
combination groups are classified as follows: Group A (dasatinib
with erlotinib), group B (dasatinib with Osimertinib), group C
(dasatinib with afatinib) and group D (dasatinib with
chemoradiation). The study strictly adhered to the Preferred
Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)
2020 guidelines.
Data sources and search strategy
A comprehensive search was conducted across several
reputable databases, including Google Scholar (https://scholar.google.com/), PubMed/MEDLINE
(https://pubmed.ncbi.nlm.nih.gov/) and
EMBASE (https://www.elsevier.com/en-gb/products/embase). The
search strategy employed a well-defined set of keywords to identify
relevant studies, specifically: (lung OR pulmonary OR bronchi OR
bronchus OR chest OR pleural OR alveolus OR alveoli) AND (Dasatinib
OR SPRYCEL OR Dasanix OR Dasanat). This approach ensured that all
pertinent clinical trials related to dasatinib and lung cancer were
identified for consideration.
Eligibility criteria
For the current meta-analysis, studies were required
to meet specific inclusion and exclusion criteria. Eligible studies
included randomized clinical trials (RCTs) that assessed the
efficacy of dasatinib in the treatment of lung cancer, either as a
monotherapy or in combination with other therapies. Studies were
excluded if they were not RCTs, did not focus on dasatinib as a
treatment for lung cancer, or lacked sufficient data on treatment
efficacy or patient outcomes. These criteria were strictly followed
to ensure that only relevant studies were included in the
analysis.
Study selection process
The study selection process was conducted by two
independent researchers who meticulously screened the titles and
abstracts of identified studies. Each study was evaluated against
the pre-established inclusion and exclusion criteria. In cases
where there was a disagreement regarding the eligibility of a
study, a third researcher was consulted to reach consensus.
Data items
Data extracted from the eligible studies included
comprehensive details, including the first author's name, year of
publication, median age of patients, sex distribution, smoking
status, type of therapy, adverse events, histological
characteristics of lung cancer, and various outcome measures.
Data analysis and synthesis
The extracted data were systematically organized
using a Microsoft Excel (2019) workbook. Qualitative descriptive
statistical analysis was performed utilizing the Statistical
Package for Social Sciences version 26.0 (IBM Corp.). Results were
presented in terms of frequencies, percentages, medians and ranges,
providing a clear depiction of the findings across the included
studies. For categorical variables, statistical comparisons were
made using the Chi-square test and Fisher's exact test, as
appropriate. For continuous variables, such as overall survival and
progression-free survival (PFS), the Mann-Whitney U test was
employed, a non-parametric method that accounts for differences in
data distributions, ensuring that the heterogeneity within the data
was appropriately addressed.
Results
Study selection process and
eligibility criteria
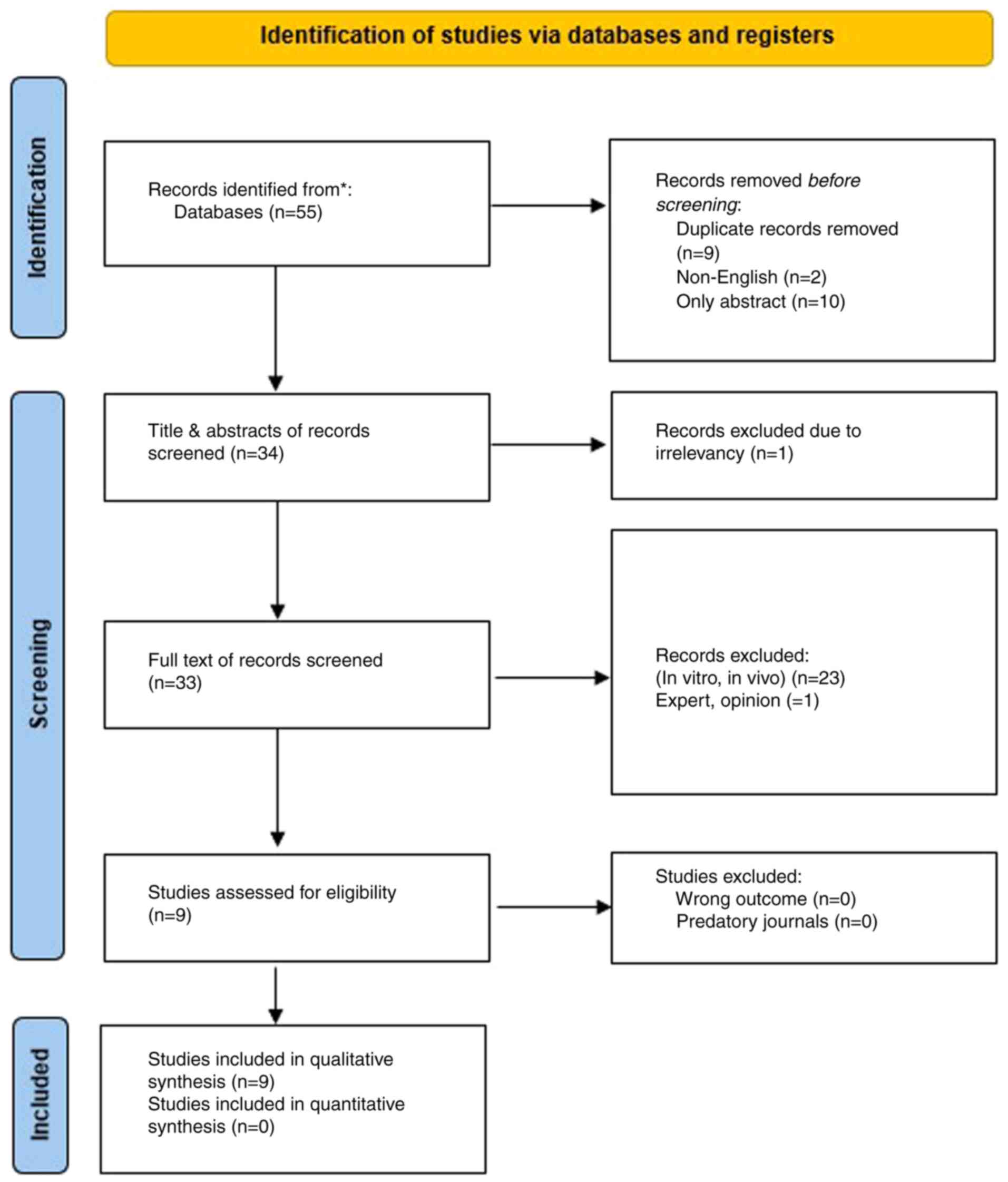
The systematic search initially identified 55
studies related to dasatinib and lung cancer. After removing nine
duplicates, two non-English publications, and 10 abstract-only
studies, 34 studies remained for title and abstract screening.
Following the initial assessment, one article was excluded due to
irrelevance. Among the remaining 33 studies, 24 were excluded for
not meeting the predefined inclusion criteria, resulting in a final
total of nine eligible studies for analysis (13-21)
(Fig. 1).
Characteristics of included RCTs
All the studies included were RCTs, consisting of
four phase II trials, three phase I trials, and two studies that
encompass both phase I and II designs. The raw data, along with key
characteristics of each study, are summarized in Table I, Table
II and Table III. A
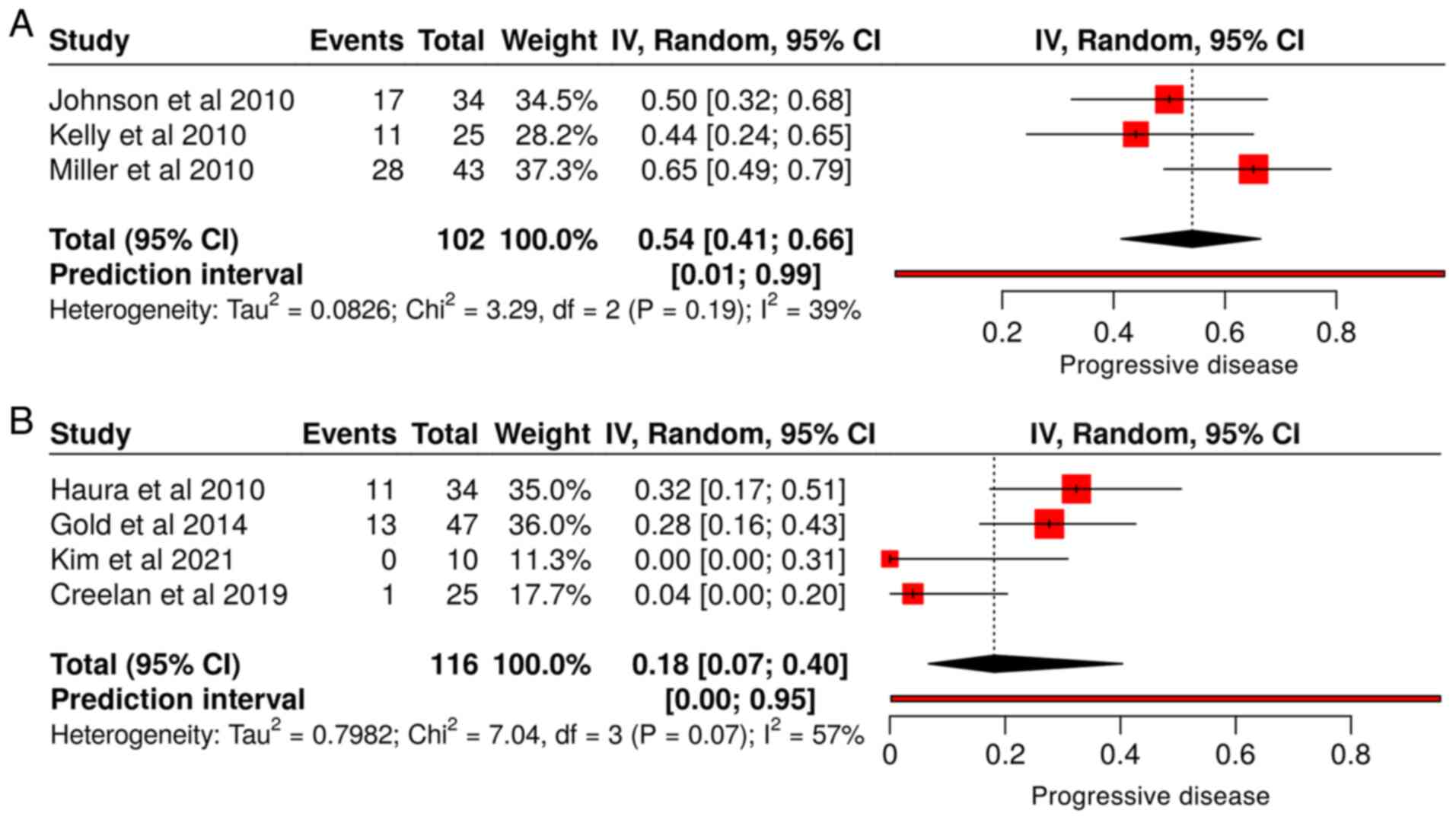
meta-analysis of studies that utilized dasatinib alone for
progressive disease, showing a pooled estimate of 0.54 [95%
confidence interval (CI), 0.41-0.66] with moderate heterogeneity
(I²=39%, Tau²=0.0826) (Fig. 2A). By
contrast, studies that employed combination therapy, resulting in a
lower pooled estimate of 0.18 [95% CI, 0.07-0.40] but with greater
heterogeneity (I²=57%, Tau²=0.7982) (Fig. 2B). The increased heterogeneity in
these studies may be attributed to the variability in treatment
protocols across the included studies, a limitation that may impact
the consistency of the findings.
 | Table IBaseline characteristics of patients
enrolled in clinical trials evaluating dasatinib and combination
therapies for lung cancer. |
Table I
Baseline characteristics of patients
enrolled in clinical trials evaluating dasatinib and combination
therapies for lung cancer.
| | Sex | | Smoking status | |
|---|
| First author/s,
year | Type of therapy | Phase of clinical
trial | No. of patients | Male | Female | Median age | Former | Active | Never (<100 in
lifetime) | N/A | (Refs.) |
|---|
| Johnson et
al, 2010 | Dasatinib | 2 | 34 | 24 | 10 | 69 | 1 | N/A | N/A | 33 | (13) |
| Haura et al,
2010 | Dasatinib +
Erlotinib | 1 and 2 | 34 | 18 | 16 | 61 | 30 | 3 | 1 | 0 | (14) |
| Gold et al,
2014 | Dasatinib +
Erlotinib | 1 and 2 | 47 | 26 | 21 | 62 | N/A | N/A | N/A | 47 | (15) |
| Kelly et al,
2016 | Dasatinib | 2 | 25 | 14 | 11 | 62 | N/A | N/A | N/A | 25 | (16) |
| Kim et al,
2021 | Dasatinib +
Osimertinib | 1 | 10 | 1 | 9 | 70.5 | 3 | 0 | 7 | 0 | (17) |
| Creelan et
al, 2019 | Dasatinib +
Afatinib | 1 | 25 | 10 | 15 | 66 | N/A | N/A | N/A | 25 | (18) |
| Miller et
al, 2010 | Dasatinib | 2 | 43 | 17 | 26 | 64 | N/A | 0 | 0 | 43 | (19) |
| Khurshid et
al, 2012 | Dasatinib +
chemoradiation | 1 | 11 | 8 | 3 | 63 | N/A | N/A | N/A | 11 | (20) |
| Brunner et
al, 2013 | Dasatinib | 2 | 5 | 3 | 2 | 59 | 5 | N/A | N/A | 0 | (21) |
 | Table IIDistribution of ECOG performance
status and histological classification of lung cancers among study
participants. |
Table II
Distribution of ECOG performance
status and histological classification of lung cancers among study
participants.
| | Histology of lung
cancers | |
|---|
| ECOG Status | | NSCLC | |
|---|
| 0 | 1 | 2 | 3 | N/A | SCLC | Adenocarcinoma | Squamous cell
carcinoma | Large cell
carcinoma | NSCLC
NOSa | Adenosquamous | Others |
|---|
| 10 | 24 | 0 | 0 | 0 | 0 | 25 | 6 | 0 | 3 | 0 | 0 |
| 24 | 10 | 0 | 0 | 0 | 0 | 17 | 7 | 0 | 10 | 0 | 0 |
| 10 | 37 | 0 | 0 | 0 | 1 | 25 | 11 | 0 | 5 | 0 | 5 |
| 3 | 17 | 5 | 0 | 0 | 0 | 15 | 5 | 0 | 4 | 1 | 0 |
| 5 | 5 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 1 | 1 | 0 |
| N/A | N/A | N/A | N/A | 25 | 0 | 23 | 2 | 0 | 0 | 0 | 0 |
| 12 | 31 | 0 | 0 | 0 | 43 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 9 | 0 | 0 | 0 | 0 | 5 | 5 | 0 | 1 | 0 | 0 |
| 2 | 3 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 |
 | Table IIITreatment outcomes, prior therapies,
and survival data for patients with lung cancer treated with
dasatinib and combination therapies. |
Table III
Treatment outcomes, prior therapies,
and survival data for patients with lung cancer treated with
dasatinib and combination therapies.
| | Previous
chemoradiation/surgery for lung cancer | | Outcome | Median survivalin
months | |
|---|
| First author/s,
year | No. of patients
(evaluable for response) | Yes | No. | Type of
therapy | Partial
response | Stable disease | Progressive
disease | Not mentioned | Overall
survival | Progression-free
survival | (Refs.) |
|---|
| Johnson et
al, 2010 | 34(30) | 13 | 21 | Dasatinib
alone | 1 | 12 | 17 | 0 | 11.4 | 1.36 | (13) |
| Haura et al,
2010 | 34(29) | 34 (Chemo) | 0 | Dasatinib +
Erlotinib | 2 | 16 | 11 | 0 | 5.6 | 2.7 | (14) |
| Gold et al,
2014 | 47(33) | N/A | N/A | Dasatinib +
Erlotinib | 5 | 15 | 13 | 0 | 13 | 3.3 | (15) |
| Kelly et al,
2017 | 25(16) | 25 (chemo) | 0 | Dasatinib
alone | 0 | 5 | 11 | 9 | 3.7 | N/A | (16) |
| Kim et al,
2021 | 10(10) | N/A | N/A | Dasatinib +
Osimertinib | 9a | 1 | 0 | 0 | 36.1 | 19.4 | (17) |
| Creelan et
al, 2018 | 25(25) | 25 | 0 | Dasatinib +
Afatinib | 0 | 17 | 1 | 7 | 14.7 | 3.7 | (18) |
| Miller et
al, 2010 | 43(35) | 43 (Chemo) 34
(Radiation) | 0 | Dasatinib
alone | 0 | 7 | 28 | 0 | 3.9 | 1.4 | (19) |
| Khurshid et
al, 2012 | 11(10) | 0 | 11 | Dasatinib +
chemoradiation | 2 | N/A | N/A | 8 | 18 | N/A | (20) |
| Brunner et
al, 2013 | 5 (0) | 5 | 0 | Dasatinib
alone | N/A | N/A | N/A | 5 | N/A | N/A | (21) |
Baseline characteristics and treatment
group distribution in the included studies
A total of 234 patients were included across the
eligible studies. These patients were categorized into two groups:
107 patients (45.7%) received dasatinib as a monotherapy, while 127
patients (54.3%) were treated with dasatinib in combination with
other therapies. The median age of the patients in the dasatinib
alone group and combination groups was 63 years, with an
interquartile range of 6 for the dasatinib alone group and 6.75 for
the combination group.
Patient demographics, histological
subtypes and treatment response
Among the 234 patients, 121 (51.7%) patients were
male. Smoking status was reported for only 50 patients (21.4%); of
these, 39 (16.7%) were former smokers, three (1.3%) were active
smokers, and 8 (3.4%) were never-smokers (<100 cigarettes in a
lifetime). The smoking status of the remaining 184 (78.6%) patients
was undocumented. Most patients had an Eastern Cooperative Oncology
Group (ECOG) status of one [136 patients, (58.1%)], followed by
scores of zero [68 patients, (29.1%)] and two [5 patients, (2.1%)].
Performance status was not reported for 25 patients (10.7%).
Histologically, 185 cases (79.1%) were diagnosed as
NSCLC, while 44 cases (18.8%) were classified as SCLC. Within the
NSCLC cohort, adenocarcinoma was the most prevalent subtype,
identified in 118 patients (63.8%), followed by squamous cell
carcinoma in 41 patients (22.1%).
The majority of the cohort presented with advanced
disease, with 167 patients (71.4%) at stage IV, eight patients
(3.4%) at stage IIIB, and six patients (2.6%) at stage IIIA.
Disease stage was not documented for the remaining 53 patients
(22.6%).
Regarding treatment, 127 patients (54.3%) received
combination therapies, while 107 patients (45.7%) were treated with
dasatinib alone. In the dasatinib alone group, 56 patients (52.4%)
experienced progressive disease, 24 patients (22.4%) had stable
disease, one patient (0.9%) demonstrated a partial response, and 26
patients (24.3%) had an undocumented response status. In group A,
comprising patients treated with dasatinib and erlotinib, 31
patients (38.3%) achieved stable disease, 24 patients (29.6%)
experienced progressive disease, seven patients (8.6%) had a
partial response, and 19 patients (23.5%) had an undocumented
response status. In group B, involving dasatinib and osimertinib,
nine patients (90%) demonstrated a partial response, one patient
(10%) achieved stable disease, and no patients (0%) expressed
progressive disease (Table IV).
The lack of data on certain factors (for example, smoking status
and disease stage) limits a comprehensive understanding of outcomes
across different subgroups.
 | Table IVPatient demographics, lung cancer
characteristics and treatment responses for various therapies. |
Table IV
Patient demographics, lung cancer
characteristics and treatment responses for various therapies.
| Variables | Number of patients
(234) |
|---|
| Sex | |
|
Male | 121 (51.7%) |
|
Female | 113 (48.3%) |
| Smoking status | |
|
Former | 39 (16.7%) |
|
Active | 3 (1.3%) |
|
Never
(<100 in a lifetime) | 8 (3.4%) |
|
Not
mentioned | 184 (78.6%) |
| ECOG status | Number of patients
(234) |
|
ECOG status
(0) | 68 (29.1%) |
|
ECOG status
(1) | 136 (58.1%) |
|
ECOG status
(2) | 5 (2.1%) |
|
Not
mentioned | 25 (10.7%) |
| Histology of lung
cancers | |
|
Small cell
lung cancer | 44 (18.8%) |
|
NSCLC | 185 (79.1%) |
|
Others | 5 (2.1%) |
| Histology of
NSCLC | Number of patients
(185) |
|
Squamous
cell carcinoma | 41 (22.1%) |
|
Adenocarcinoma | 118 (63.8%) |
|
Adenosquamous | 2 (1.1%) |
|
NSCLC
NOS | 24 (13.0%) |
| Lung cancer
stage | Number of patients
(234) |
|
Stage
IIIA | 6 (2.6%) |
|
Stage
IIIB | 8 (3.4%) |
|
Stage
IV | 167 (71.4%) |
|
not
mentioned | 53 (22.6%) |
| Treatment
group | Number of patients
(234) |
|
Dasatinib
alone | 107 (45.7%) |
|
Combination
group | 127 (54.3%) |
| Response to
dasatinib alone | Number of patients
(107) |
|
Partial
response | 1 (0.9%) |
|
Stable
disease | 24 (22.4%) |
|
Progressive
disease | 56 (52.4%) |
|
Not
mentioned | 26 (24.3%) |
| Response to
dasatinib combined with Erlotinib | Number of patients
(81) |
|
Partial
response | 7 (8.6%) |
|
Stable
disease | 31 (38.3%) |
|
Progressive
disease | 24 (29.6%) |
|
Not
mentioned | 19 (23.5%) |
| Response to
dasatinib combined with Osimertinb | Number of patients
(10) |
|
Partial
response | 9 (90%) |
|
Stable
disease | 1 (10%) |
|
Progressive
disease | 0 (0.0%) |
|
Not
mentioned | 0 (0.0%) |
| Response to
dasatinib combined with Afatinib | Number of patients
(25) |
|
Partial
response | 0 (0.0%) |
|
Stable
disease | 17 (68%) |
|
Progressive
disease | 1 (4%) |
|
Not
mentioned | 7 (28%) |
| Response to
dasatinib combined with chemoradiation | Number of patients
(11) |
|
Partial
response | 2 (18.2%) |
|
Stable
disease | N/A |
|
Progressive
disease | N/A |
|
Not
mentioned | 9 (81.8%) |
Adverse events in different treatment
groups
In terms of adverse events, anemia was the most
frequent hematological adverse event, reported in 57 patients
(42.2%). Specifically, the dasatinib monotherapy group reported in
4 cases (3.7%), while combination therapy groups reported 41 cases
(50.6%) in group A, four cases (40.0%) in group B, six cases
(24.0%) in group C, and two cases (18.3%) in group D.
Among gastrointestinal adverse events, diarrhea was
the most common, affecting 86 patients (35.4%). This included three
cases (2.8%) in the dasatinib monotherapy group, 59 cases (72.8%)
in group A, eight cases (80.0%) in group C, and 15 cases (60.0%) in
group C.
In dermatological adverse events, skin rash was
frequently reported, affecting 80 patients (85.1%). Of these, three
cases (2.8%) were in the dasatinib monotherapy group, 57 cases
(70.4%) in group A, seven cases (70.0%) in group B, and 13 cases
(52.0%) in group C. Additionally, fatigue was documented in 82
cases (28.8%), with 50 cases (61.7%) observed in group A, and 21
cases (19.6%) in the dasatinib monotherapy group (Table V).
 | Table VAdverse events in patients with lung
cancer treated with dasatinib and combination therapies across
treatment groups. |
Table V
Adverse events in patients with lung
cancer treated with dasatinib and combination therapies across
treatment groups.
| Adverse events | Total | Dasatinib
(107) | Group A (81) | Group B (10) | Group C (25) | Group D (11) |
|---|
| Hematological | | | | | | |
|
Anemia | 57 (42.2%) | 4 (3.7%) | 41(50.6%) | 4 (40.0%) | 6 (24.0%) | 2 (18.3%) |
|
Leukopenia | 8 (5.9%) | 1 (0.9%) | 4 (4.9%) | 3 (30.0%) | N/A | N/A |
|
Neutropenia | 14 (10.4%) | 1 (0.9%) | 4 (4.9%) | 7 (70.0%) | N/A | 2 (18.2%) |
|
Lymphopenia | 29 (21.5%) | 7 (6.5%) | 22 (27.2%) | N/A | N/A | N/A |
|
Thrombocytopenia | 27 (20.0%) | 0 (0.0%) | 18 (22.0%) | 7 (70.0%) | 2 (8.0%) | 0 (0.0%) |
|
Gastrointestinal | | | | | | |
|
Nausea | 71 (29.2%) | 7 (6.5%) | 50 (61.7%) | 4 (40.0%) | 8 (32.0%) | 2 (18.2%) |
|
Vomiting | 35 (14.4%) | 4 (3.7%) | 23 (28.4%) | N/A | 7 (28.0%) | 1 (9.1%) |
|
Diarrhea | 86 (35.4%) | 3 (2.8%) | 59 (72.8%) | 8 (80.0%) | 15 (60.0%) | 1 (9.1%) |
|
Anorexia | 51 (20.9%) | 5 (4.7%) | 35 (43.2%) | 4 (40.0%) | 6 (24.0%) | 1 (9.1%) |
| Dermatological | | | | | | |
|
Skin
rash | 80 (85.1%) | 3 (2.8%) | 57 (70.4%) | 7 (70.0%) | 13 (52.0%) | N/A |
|
Alopecia | 14 (14.9%) | N/A | 12 (14.8%) | 2 (20.0%) | N/A | N/A |
| Others | | | | | | |
|
Fatigue | 82 (28.8%) | 21 (19.6%) | 50 (61.7%) | 4 (40.0%) | 5 (20.0%) | 2 (18.2%) |
|
Cough | 9 (3.2%) | 1 (0.9%) | 3 (3.7%) | 2 (20.0%) | 3 (12.0%) | N/A |
|
Dyspnea | 54 (18.9%) | 23 (21.5%) | 24 (29.6%) | 3 (30.0%) | 3 (12.0%) | 1 (9.1%) |
|
Pain | 39 (13.7%) | 3 (2.8%) | 28 (34.6%) | 4 (40.0%) | 3 (12.0%) | 1 (9.1%) |
|
Edema | 13 (4.5%) | 1 (0.9%) | 5 (6.2%) | 5 (50.0%) | 2 (8.0%) | N/A |
|
Pleural
effusion | 68 (23.9%) | 22 (20.6%) | 26 (32.1%) | 10 (100%) | 7 (28.0%) | 3 (27.3%) |
|
Pericardial
effusion | 6 (2.1%) | 4 (3.7%) | 1 (1.2%) | N/A | N/A | 1 (9.1%) |
|
Mucositis/stomatitis | 14 (4.9%) | N/A | 4 (17.3%) | 3 (30.0%) | 7 (28.0%) | N/A |
Clinical outcomes revealed a significantly higher
partial response in the combination therapy group (94.7%, 18/19)
compared with the dasatinib alone group (5.3%, 1/19) with
P<0.001. Stable disease was observed in 49 (67.1%) patients
receiving combination therapy vs. 24 (32.9%) patients treated with
dasatinib monotherapy. Conversely, progressive disease was more
prevalent in the dasatinib monotherapy group (69.1%, 56/81)
compared with the combination therapy group (30.9%, 25/81).
Survival outcomes favored combination therapy, with
overall survival significantly longer (13.60±7.71 months) than
dasatinib monotherapy (mean 6.35±3.59 months, P<0.001).
Similarly, PFS was greater in the combination therapy group
(4.60±4.58 months vs. 1.38±0.02 months, P<0.001).
Anemia was significantly more frequent in the
combination therapy group 93.0% (53/57) than in the dasatinib-alone
group 7.0% (4/57, P=0.033). Other gastrointestinal and
dermatological adverse events, such as nausea, and vomiting,
diarrhea, skin rash, alopecia showed no significant differences
between groups. Fatigue was more common in combination therapy
patients (74.4%, 61/82) compared with the dasatinib group (25.6%,
21/82). Additionally, pleural effusion was present in 67.6% (46/68)
of the combination therapy group vs. 32.4% (22/68) in the dasatinib
group (P<0.001) (Table VI).
 | Table VIComparison of clinical outcomes and
adverse events between dasatinib monotherapy and combination
therapy. |
Table VI
Comparison of clinical outcomes and
adverse events between dasatinib monotherapy and combination
therapy.
| | Type of
therapy | |
|---|
| Variables | | Dasatinib
alone | Combination
therapy | P-value |
|---|
| Outcome N (%) | Partial
response | 1 (5.3%) | 18 (94.7%) | <0.001 |
| | Stable disease | 24 (32.9%) | 49 (67.1%) | |
| | Progressive
disease | 56 (69.1%) | 25 (30.9%) | |
| | Not mentioned | 14 (48.3%) | 15 (51.7%) | |
| Overall survival
(Mean ± SD) | | 6.35±3.59 | 13.60±7.71 | <0.001 |
| Progression-free
survival (Mean ± SD) | | 1.38±0.02 | 4.60±4.58 | <0.001 |
| Hematological
adverse events, N (%) | Anemia | 4 (7.0%) | 53 (93.0%) | 0.027 |
| | Leukopenia | 1 (12.5%) | 7 (87.5%) | |
| | Neutropenia | 1 (7.1%) | 13 (92.9%) | |
| | Lymphopenia | 7 (24.1%) | 22 (75.9%) | |
| |
Thrombocytopenia | 0 (0.0%) | 27 (100.0%) | |
| Gastrointestinal
adverse events, N (%) | Nausea | 7 (9.9%) | 64 (90.1%) | 0.247 |
| | Vomiting | 4 (11.4%) | 31 (88.6%) | |
| | Diarrhea | 3 (3.5%) | 83 (96.5%) | |
| | Anorexia | 5 (9.8) | 46 (90.2%) | |
| Dermatological
adverse events, N (%) | Skin rash | 3 (3.7%) | 77 (96.3%) | 0.613 |
| | Alopecia | 0 (0.0%) | 14 (100.0%) | |
| Others, N (%) | Fatigue | 21 (25.6%) | 61 (74.4%) | <0.001 |
| | Cough | 1 (11.1%) | 8 (88.9%) | |
| | Dyspnea | 23 (42.6%) | 31 (57.4%) | |
| | Pain | 3 (7.7%) | 36 (92.3%) | |
| | Edema | 1 (7.7%) | 12 (92.3%) | |
| | Pleural
effusion | 22 (32.4%) | 46 (67.6%) | |
| | Pericardial
effusion | 4 (66.7%) | 2 (33.3%) | |
| |
Mucositis/stomatitis | 0 (0.0%) | 14 (100.0%) | |
Discussion
Cancer continues to pose a significant societal,
public health, and economic challenge in the 21st century,
accounting for nearly one in six deaths globally (16.8%) and one in
four deaths (22.8%) attributed to non-communicable diseases (NCDs)
(22). Traditionally viewed
primarily as a genetic disease, this perspective fails to address
complex clinical phenomena such as recurrence and drug resistance.
Recent advancements suggest a more integrative framework,
characterizing cancer as a ‘multidimensional spatiotemporal unity
of ecology and evolution’, a dynamic pathological ecosystem which
cancer cells interact with their microenvironment and undergo
evolutionary changes over time. This paradigm offers critical
insights into cancer progression, providing novel strategies to
tackle clinical challenges such as therapeutic resistance and
disease relapse (23). The disease
accounts for 30.3% of premature deaths due to NCDs among
individuals aged 30-69 years, ranking as one of the top three
causes of death within this age group across 177 of 183 countries
(22). For lung cancer
specifically, exposure to cigarette smoke, radiation, asbestos, and
metals such as nickel, chromium and arsenic significantly elevates
risk (2). Smoking remains the
primary cause, accounting for ~85% of all lung cancer cases
(24). In the present study, 39
(16.7%) out of the 234 patients reported a history of smoking.
Lung cancer is broadly categorized into two primary
types: SCLC and NSCLC (25). Within
NSCLC, adenocarcinoma, squamous cell carcinoma and large cell
carcinoma represent the major histological subtypes, with
adenocarcinoma constituting 40%, squamous cell carcinoma 25-30% and
large cell carcinoma 5-10% (5). The
findings of the present study revealed that adenocarcinoma was
present in 118 patients (63.8%), while squamous cell carcinoma was
identified in 41 patients (22.1%). Sex distribution patterns also
vary across studies. A study conducted by Ruano-Ravina et al
indicated that among 13,950 participants, 25.6% were female, with
female participants tending to be younger, while smoking prevalence
was higher among males (26). By
contrast, the current study found no significant sex disparity,
with males comprising 51.7% and female 48.3% of the cohort.
Sehgal et al (27) conducted a study on patients with
advanced-stage NSCLC undergoing treatment with pembrolizumab,
demonstrating that those with an ECOG performance status of 2 or
higher had significantly lower disease control rates, shorter
median overall survival and reduced PFS. These findings underscore
the critical importance of baseline ECOG status as a determinant of
treatment effectiveness and patient outcomes. In the present study,
the majority of patients had an ECOG status of one (58.1%), with
fewer patients having a status of zero (29.1%) or two (2.1%).
Treatment strategies for lung cancer are according
to the tumors' characteristics and stage of progression. Options
include immunotherapy, chemotherapy, radiotherapy, molecular
targeted therapy and surgical intervention. Surgical resection with
curative intent is typically reserved for patients with early TNM
stages (I or II) who are in sufficient overall health to endure the
procedure (28,29). Despite transformative advancements
in molecular targeted therapy and immuno-oncology, chemotherapy
continues to serve as a cornerstone of lung cancer management.
Studies consistently demonstrate its positive effect on both
survival and quality of life, spanning early and advanced disease
stages, whether administered as a monotherapy or in combination
with other treatments. Moreover, emerging combination regimens and
innovative drug delivery systems hold significant promise for
enhancing treatment efficacy (30).
Radiotherapy plays a critical role in managing stage
III NSCLC, though its effectiveness depends on the extent of the
disease. For Pancoast tumors, a treatment regimen combining
induction chemo-radiotherapy followed by surgery has demonstrated
improved survival rates. However, in resectable N2-NSCLC,
neoadjuvant chemoradiotherapy does not provide a survival benefit
over chemotherapy alone. Postoperative radiotherapy does not
generally improve outcomes in completely resected N2 disease,
except in cases with positive margins. Concurrent
chemoradiotherapy, often supplemented by durvalumab, remains the
standard approach for unresectable N2 or N3 NSCLC. Advanced
techniques such as intensity-modulated radiation therapy can
mitigate the risk of severe pneumonitis; though their overall
effectiveness varies. For patients at higher risk, radiotherapy
alone may provide a modest survival benefit (31). Targeted therapy has emerged as a
crucial strategy for treating NSCLC, based on the understanding
that multiple oncogenic mutations drive lung cancer development.
Identifying these specific genetic alterations enables the
development of targeted therapies to directly combat these
mutations (32). Beyond NSCLC,
targeted therapies have also shown efficacy in treating other
cancers, with pembrolizumab and durvalumab demonstrating
effectiveness in mesothelioma (33,34).
Dasatinib inhibits cell proliferation and survival
while inducing apoptosis in hematologic malignancies and solid
tumors. At a concentration of 10 µM, it reduces viability and
induces apoptosis in HSC-3 cells. Additionally, it significantly
inhibits the proliferation and survival of YD-8, YD-10B, HSC-3 and
YD-38 cells, with YD-38 cells being the most sensitive,
underscoring its broad effectiveness across various cell lines
(35). Johnson et al
(13) conducted a phase II trial
evaluating dasatinib in advanced NSCLC. Patients received dasatinib
as first-line therapy, with response assessments performed via CT
and PET imaging. Pre-treatment tissue samples were analyzed for
EGFR and KRAS mutations as well as expression of phosphorylated Src
family kinases (SFK). Among 34 patients, the disease control rate
was 43%, with one partial response and a metabolic response rate of
32%. Notably, EGFR and KRAS mutations did not predict treatment
response (13). In the
aforementioned study, 56 patients (52.4%) in the dasatinib-alone
group exhibited progressive disease, 24 patients (22.4%) had stable
disease, one patient (0.9%) had a partial response, and the
response status of 26 patients (24.3%) was unknown. Several factors
could account for these outcomes. One possibility is the inherent
aggressiveness of the lung cancer in these patients, particularly
in those with advanced stages of the disease. Dasatinib, though
effective in some malignancies, may have limited efficacy as a
monotherapy in specific lung cancer subtypes without actionable
mutations (for example, EGFR or KRAS), which could partially
explain the limited disease control observed. Another potential
explanation is treatment-induced hyper-progression, where certain
therapies, including tyrosine kinase inhibitors and immune
checkpoint inhibitors, accelerate tumour growth in a subset of
patients. While dasatinib has demonstrated efficacy in hematologic
malignancies and certain solid tumors, it may induce
hyper-progression in a minority of patients with lung cancer,
leading to higher rates of progression (36). Further researches are needed to
clarify the role of hyper-progression in dasatinib-treated patients
with lung cancer, particularly concerning specific genetic profiles
or pre-existing conditions.
Dasatinib has also been evaluated with other
therapies such as osimertinib, afatinib and other targeted
therapies. Haura et al (14)
conducted a phase I trial involving group A combination therapy,
where patients with advanced NSCLC received erlotinib for one week
before dasatinib was introduced. Pharmacokinetics were assessed at
weeks 1 and 2, across four dasatinib dosage cohorts. Adverse events
included gastrointestinal issues, skin rash, cytopenia, pleural
effusions and fatigue. The study reported two partial responses and
one bone response, with a disease control rate of 63%.
Additionally, plasma angiogenic markers decreased during treatment,
correlating with disease control. This combination therapy was
tolerable and demonstrated potential efficacy in NSCLC, warranting
further exploration of personalized SFK-targeting strategies
(14). In the present study, 127
patients (54.3%) underwent combination therapies, while 107
patients (45.7%) received dasatinib alone. In group A, 31 patients
(38.3%) achieved stable disease, 24 patients (29.6%) experienced
progression, and seven patients (8.6%) exhibited a partial
response. The response status of the remaining 19 patients (23.5%)
was unspecified.
The utilization of dasatinib in lung cancer
treatment remains a topic of ongoing debate within the oncology
community. While dasatinib is primarily approved for chronic
myeloid leukemia and acute lymphoblastic leukemia with BCR-ABL
positivity, its off-label use in various solid tumors, including
lung cancer, raises important questions regarding efficacy and
safety. Studies have indicated that dasatinib exhibits significant
antiproliferative effects against a range of malignancies,
particularly when used in conjunction with other therapeutic
agents. These effects are largely attributed to its inhibition of
SFKs, which induces cytotoxicity and apoptosis in tumor cells
(11). Despite these promising
findings, the lack of large-scale clinical trials specifically
assessing effectiveness of dasatinib in lung cancer necessitates
caution. Challenges such as the potential for drug resistance and
the need for personalized treatment strategies highlight the
complexities of its off-label use. As ongoing research continues to
explore the therapeutic potential of dasatinib in various
malignancies, a nuanced understanding of its role in lung cancer is
essential to guide clinical decision-making and optimize patient
outcomes.
Dasatinib is generally well tolerated, with adverse
events that are typically manageable and often occur early in the
course of treatment. These events are often mild to moderate,
resolving either spontaneously, with supportive care, or through
temporary treatment interruption or dose adjustments. Effective
management of adverse events is crucial for ensuring treatment
adherence and optimizing therapeutic outcomes. Adverse events are
categorized using common terminology criteria, with the most
frequent being cytopenia, fluid retention, pleural effusion,
dyspnea, gastrointestinal problems, skin rash, headache and fatigue
(34). In the current study, anemia
was the most common hematological adverse event, affecting 57
patients (42.2%). The incidence of anemia was particularly higher
in the combination therapy group, with 41 cases (50.6%) reported in
group A, compared with only 4 cases (3.7%) in the dasatinib
monotherapy group. Diarrhea was also a significant adverse event
within the gastrointestinal group, affecting 86 patients (35.4%)
overall. It was especially prevalent in the group A combination
therapy cohort, where 59 patients (72.8%) experienced diarrhea,
compared with only 3 cases (2.8%) in the dasatinib monotherapy
group, 8 cases (80.0%) in group B, and 15 cases (60.0%) in the
group C. Dermatological adverse events were also common, with skin
rash reported in 80 cases (85.1%). Group A demonstrated the highest
incidence, with 57 cases (70.4%), compared with 3 cases (2.8%) in
the monotherapy group, 7 cases (70.0%) in group B, and 13 cases
(52.0%) in group C.
In addition to the limitation of including only one
published article on the role of dasatinib in lung cancer over the
last five years, other limitations of the study include the small
sample size, with only nine eligible studies included in the
meta-analysis, which may reduce the generalizability of the
findings. Additionally, significant heterogeneity across studies,
particularly in the combination therapy group, complicates the
interpretation of results and suggests variability in treatment
protocols.
In conclusion, dasatinib treatment may improve
overall survival and have fewer adverse events compared with
combination therapies in patients with lung cancer. The present
study holds significant practical implications by advancing the
understanding and potential applications of targeted therapies such
as dasatinib in lung cancer management. By systematically
evaluating its efficacy and safety as a standalone treatment,
current findings can inform clinical decision-making and optimize
treatment strategies to improve patient outcomes. Furthermore, the
present research highlights the need for large-scale trials to
validate these findings, which could pave the way for more
personalized and effective therapeutic approaches in oncology. If
confirmed, the standalone use of dasatinib could offer a
streamlined treatment option, reducing the complexity and adverse
effects associated with combination therapies, thereby improving
patient quality of life. The present study represents a critical
step in expanding therapeutic options and guiding future research
in this vital area of medicine.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
DJHR, SOK, YMM and AGH were responsible for data
collection and analysis, and final approval of the manuscript. FHK
and FHF were major contributors to the conception of the study, as
well as to the literature search for related studies. ReMA, HKA,
RaMA, AMA and HAY were involved in the literature review, the
design of the study, and the critical revision of the manuscript.
FHK, SJH and BAA were involved in the literature review, the
writing of the manuscript, and design of the study and data
interpretation. BAA and FHK confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siddiqui F, Vaqar S and Siddiqui AH: Lung
Cancer. StatPearls Publishing, Treasure Island, FL, 2024.
|
|
2
|
Thandra KC and Barsouk A, Saginala K,
Aluru JS and Barsouk A: Epidemiology of lung cancer. Contemp Oncol
(Pozn). 25:45–52. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Siegel RL, Miller KD, Goding Sauer A,
Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA and Jemal
A: Colorectal cancer statistics, 2020. CA Cancer J Clin.
70:145–164. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sher T, Dy GK and Adjei AA: Small cell
lung cancer. Mayo Clin Proc. 83:355–367. 2008.
|
|
5
|
Mingomataj E, Krasniqi M, Dedushi K,
Sergeevich KA, Kust D, Qadir AA, Abdullah AS, Ahmed MK and Fatah
GM: Cancer publications in one year (2023): A cross-sectional
study. Barw Med J. 2:3–11. 2024.
|
|
6
|
Ali RM, Omar SS, Ahmed HK, Omar DA,
Mahmood YM, Mustafa MQ, Abdullah AS, Hassan MN, Hussein DA, Kakamad
SH, et al: Effect of sunitinib in the management of lung cancer: A
systematic review of clinical trials. Barw Med J. 2:57–64.
2024.
|
|
7
|
Alduais Y, Zhang H, Fan F, Chen J and Chen
B: Non-small cell lung cancer (NSCLC): A review of risk factors,
diagnosis, and treatment. Medicine (Baltimore).
102(e32899)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Valk PE, Pounds TR, Hopkins DM, Haseman
MK, Hofer GA, Greiss HB, Myers RW and Lutrin CL: Staging non-small
cell lung cancer by whole-body positron emission tomographic
imaging. Ann Thorac Surg. 60:1573–1581; discussion 1581-1582.
1995.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zappa C and Mousa SA: Non-small cell lung
cancer: Current treatment and future advances. Transl Lung Cancer
Res. 5:288–300. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ye Q, Gui C, Jin D, Zhang J, Zhang J, Ma N
and Xu L: Synergistic effect of cannabidiol with dasatinib on lung
cancer by SRC/PI3K/AKT signal pathway. Biomed Pharmacother.
173(116445)2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Abdullah HO, Abdalla BA, Kakamad FH, Ahmed
JO, Baba HO, Hassan MN, Bapir R, Rahim HM, Omar DA, Kakamad SH, et
al: Predatory publishing lists: A review on the ongoing battle
against fraudulent actions. Barw Med J. 2:26–30. 2024.
|
|
13
|
Johnson FM, Bekele BN, Feng L, Wistuba I,
Tang XM, Tran HT, Erasmus JJ, Hwang LL, Takebe N, Blumenschein GR,
et al: Phase II study of dasatinib in patients with advanced
non-small-cell lung cancer. J Clin Oncol. 28:4609–4615.
2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Haura EB, Tanvetyanon T, Chiappori A,
Williams C, Simon G, Antonia S, Gray J, Litschauer S, Tetteh L,
Neuger A, et al: Phase I/II study of the Src inhibitor dasatinib in
combination with erlotinib in advanced non-small-cell lung cancer.
J Clin Oncol. 28:1387–1394. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gold KA, Lee JJ, Harun N, Tang X, Price J,
Kawedia JD, Tran HT, Erasmus JJ, Blumenschein GR, William WN, et
al: A phase I/II study combining erlotinib and dasatinib for
non-small cell lung cancer. Oncologist. 19:1040–1041.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kelley MJ, Jha G, Shoemaker D, Herndon JE
II, Gu L, Barry WT, Crawford J and Ready N: Phase II study of
dasatinib in previously treated patients with advanced non-small
cell lung cancer. Cancer Invest. 35:32–35. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kim C, Liu SV, Crawford J, Torres T, Chen
V, Thompson J, Tan M, Esposito G, Subramaniam DS and Giaccone G: A
phase I trial of dasatinib and osimertinib in TKI naïve patients
with advanced EGFR-mutant non-small-cell lung cancer. Front Oncol.
11(728155)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Creelan BC, Gray JE, Tanvetyanon T,
Chiappori AA, Yoshida T, Schell MJ, Antonia SJ and Haura EB: Phase
1 trial of dasatinib combined with afatinib for epidermal growth
factor receptor-(EGFR-) mutated lung cancer with acquired tyrosine
kinase inhibitor (TKI) resistance. Br J Cancer. 120:791–796.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Miller AA, Pang H, Hodgson L, Ramnath N,
Otterson GA, Kelley MJ, Kratzke RA and Vokes EE: Cancer and
Leukemia Group B (CALGB). A phase II study of dasatinib in patients
with chemosensitive relapsed small cell lung cancer (cancer and
leukemia group B 30602). J Thorac Oncol. 5:380–384. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Khurshid H, Dipetrillo T, Ng T,
Mantripragada K, Birnbaum A, Berz D, Radie-Keane K, Perez K,
Constantinou M, Luppe D, et al: A phase I study of dasatinib with
concurrent chemoradiation for stage III non-small cell lung cancer.
Front Oncol. 2(56)2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Brunner AM, Costa DB, Heist RS, Garcia E,
Lindeman NI, Sholl LM, Oxnard GR, Johnson BE and Hammerman PS:
Treatment-related toxicities in a phase II trial of dasatinib in
patients with squamous cell carcinoma of the lung. J Thorac Oncol.
8:1434–1437. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Luo W: Nasopharyngeal carcinoma ecology
theory: Cancer as multidimensional spatiotemporal ‘unity of ecology
and evolution’ pathological ecosystem. Theranostics. 13:1607–1631.
2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
World Health Organization: Lung cancer.
https://www.who.int/news-room/fact-sheets/detail/lung-cancer.
Accessed November 4, 2024.
|
|
25
|
Chen BT, Chen Z, Ye N, Mambetsariev I,
Fricke J, Daniel E, Wang G, Wong CW, Rockne RC, Colen RR, et al:
Differentiating peripherally-located small cell lung cancer from
non-small cell lung cancer using a CT radiomic approach. Front
Oncol. 10(593)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ruano-Ravina A, Provencio M, Calvo de Juan
V, Carcereny E, Estival A, Rodríguez-Abreu D, Benítez G,
López-Castro R, Belver M, Guirado-Risueño M, et al: Are there
differences by sex in lung cancer characteristics at diagnosis? -a
nationwide study. Transl Lung Cancer Res. 10:3902–3911.
2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sehgal K, Gill RR, Widick P, Bindal P,
McDonald DC, Shea M, Rangachari D and Costa DB: Association of
performance status with survival in patients with advanced
non-small cell lung cancer treated with pembrolizumab monotherapy.
JAMA Netw Open. 4(e2037120)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bitenc M, Cufer T, Kern I, Miklavcic M,
Petrovic S, Groznik V and Sadikov A: Real-life long-term outcomes
of upfront surgery in patients with resectable stage I-IIIA
non-small cell lung cancer. Radiol Oncol. 56:346–354.
2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lee SH: Chemotherapy for lung cancer in
the era of personalized medicine. Tuberc Respir Dis (Seoul).
82:179–189. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Petrella F, Rizzo S, Attili I, Passaro A,
Zilli T, Martucci F, Bonomo L, Del Grande F, Casiraghi M, De
Marinis F and Spaggiari L: Stage III non-small-cell lung cancer: An
overview of treatment options. Curr Oncol. 30:3160–3175.
2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Guo Q, Liu L, Chen Z, Fan Y, Zhou Y, Yuan
Z and Zhang W: Current treatments for non-small cell lung cancer.
Front Oncol. 12(945102)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Omar SS, Ali RH, Abdullah SH, Hussein DM,
Radha BMM, Latif AB, Ali SM, Hiwa DS, Ahmed HK, Hamasaeed AG, et
al: Durvalumab (Anti-PD-1) in the management of mesothelioma: A
systematic review of the current literature. Barw Med J. 1:32–39.
2023.
|
|
33
|
Ali RM, Kakamad FH, Abdullah HO, Abdulla
SH, Ahmed SF, Amin BJH, Hassan MN, Hasan SJ, Hamasalih HM, Abdalla
BA, et al: Pembrolizumab (Anti-PD-1) immunotherapy in malignant
pleural mesothelioma: A systematic review of the current
literature. Barw Med J. 1:6–13. 2023.
|
|
34
|
Park NS, Park YK, Yadav AK, Shin YM,
Bishop-Bailey D, Choi JS, Park JW and Jang BC: Anti-growth and
pro-apoptotic effects of dasatinib on human oral cancer cells
through multi-targeted mechanisms. J Cell Mol Med. 25:8300–8311.
2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Conchon M, Freitas CM, Rego MA and Braga
Junior JW: Dasatinib-clinical trials and management of adverse
events in imatinib resistant/intolerant chronic myeloid leukemia.
Rev Bras Hematol Hemoter. 33:131–139. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Shyam Sunder S, Sharma UC and Pokharel S:
Adverse effects of tyrosine kinase inhibitors in cancer therapy:
Pathophysiology, mechanisms and clinical management. Signal
Transduct Target Ther. 8(262)2023.PubMed/NCBI View Article : Google Scholar
|