Introduction
Idiopathic pulmonary fibrosis (IPF), a progressive
fibrotic lung ailment (1), exhibits
notably unfavorable prognosis, with a median survival rate of ~3-4
years subsequent to diagnosis (2).
This condition frequently culminates in fatality due to respiratory
failure, primarily caused by the extensive restructuring of the
pulmonary tissue (3). Antifibrotic
treatment has shown effectiveness in reducing the advancement of
the disease and lessening the deterioration of lung function in
individuals with IPF. Furthermore, other studies demonstrated that
the survival rates of patients with IPF treated with antifibrotic
medications were enhanced compared with untreated ones (4,5).
However, several patients stop treatment due to the onset of
adverse effects (6). Genetic
studies suggested that the susceptibility to IPF could be
significantly affected by genetics. Therefore, the investigation of
sporadic and familial cases promoted the successful identification
of multiple IPF-related variants (7). The establishment and verification of
evidence-supported recommendations for gene-oriented screening for
IPF could enable the redefinition and reclassification of this
particular illness based on its molecular characteristics, thus
promoting the adoption of precision medicine strategies (8). Therefore, the timely detection of
possible genetic risk factors could prevent the onset of IPF.
Currently, there are limited methods for the early detection of
IPF. However, the early detection and intervention are crucial for
slowing down the progression of the disease. Due to their powerful
monitoring capabilities, wide applicability and accessible
analysis, serum metabolites have become promising biomarkers for
IPF.
The understanding of disease progression has been
improved with the introduction of transcriptomics, proteomics and
metabolomics, thus providing novel insights into the cellular
mechanisms involved. It has been reported that regulating metabolic
balance could affect IPF (9).
Pulmonary fibrosis can occur due to irregular collagen production
and disrupted airway remodeling caused by metabolic abnormalities
in alveolar epithelial cells, myofibroblasts and macrophages.
Several metabolic pathways are involved in lipid, protein and
carbohydrate metabolism, and more particularly in IPF (10). Metabolomics, via identifying altered
metabolites or metabolic pathways, offer a fresh perspective for
investigating the underlying mechanisms of different diseases, thus
providing valuable insights into the biological processes
associated with these diseases (11). Metabolic changes are not just a
characteristic of fibrosis development, but they can also
significantly affect the development of fibrosis, particularly in
IPF (12). It has been also
suggested that dysfunctions in the metabolic processes of glycine,
glutamine and arginine, along with irregularities in glycolysis,
can activate a pro-fibrotic feature via a TGF-β-dependent mechanism
(10,13,14).
Increased content of extended and moderate chain fatty acids has
been documented in the lungs of patients with IPF. In addition,
previous studies reported an enhanced reprogramming of macrophages,
resulting in IPF improvement via fatty acid β-oxidation (15,16).
Another study demonstrated that metabolites derived from
arachidonic acid could hinder pulmonary fibrosis via altering the
TGF-β1-induced pro-fibrotic signaling pathway (17). The aforementioned finding indicated
that targeting these metabolites could be a promising approach for
treating IPF (17). However, the
effect and relevance of serum metabolomic profiles on IPF remains
understudied.
Investigating metabolites associated with the
pathogenesis and progression of IPF is not just significant for
early detection and prevention, but also serve a key role in
comprehending the biological mechanisms of IPF therapy and
uncovering possible therapeutic objectives. Several metabolites
associated with IPF have been identified, such as Krebs von den
Lungen 6, matrix metalloproteinases and periostin (18). Nevertheless, the uncertain causal
association between metabolites and IPF persists, since there is a
dearth of prospective studies investigating metabolites and IPF.
Randomized controlled trials are widely considered as the benchmark
for establishing causal effects. However, the complexities and
obstacles in this particular scenario pose challenges in reaching
conclusive findings regarding the causal association between
metabolites and IPF. Mendelian randomization (MR) is an extensively
employed and novel epidemiological approach. In the absence of
randomized controlled trials, MR stands out as a highly effective
method for investigating causal associations between exposures of
interest and outcomes. This can be achieved via utilizing genetic
variants as instrumental variables (IVs) to estimate the causal
associations between exposures and outcomes. Furthermore, MR can
help in reducing confounding factors and the bias of reverse
causality, which is inherent in observational studies. Due to the
limited comprehension of the cause-and-effect association between
blood metabolites and IPF, more studies are urgently needed.
Therefore, the current study employed MR analysis to thoroughly
investigate the potential causality of 486 blood metabolites in the
progression of IPF via using pooled data from genome-wide
association studies (GWAS). Exploring these particular metabolites
and their association with IPF could promote the improved
understanding of the pathogenesis of IPF and lay a solid foundation
for exploring the potential mechanisms and therapeutic targets of
IPF in the future.
Materials and methods
Data of 486 blood metabolites and IPF
from GWAS
Data for 486 metabolites were obtained from the
Metabolomics GWAS summary database (https://metabolomics.helmholtz-muenchen.de/gwas/),
which includes a total of 2,163,597 single nucleotide polymorphisms
(SNPs) associated with them (19).
The names of the 486 metabolites are listed in detail in Table SI. X indicates an unidentified
chemical composition. The FinnGen biospecimen repository
(https://www.finngen.fi/en) was utilized
as an outcome variable for conducting GWAS analysis on IPF. In the
current study the analysis included genotypic data from 1,028
patients with IPF and 196,986 controls (20).
Selection of IVs
To evaluate the possible cause-and-effect
association between circulating metabolites and IPF, a two-sample
MR analysis was conducted (21).
The present study assessed blood metabolites and IPF as factors of
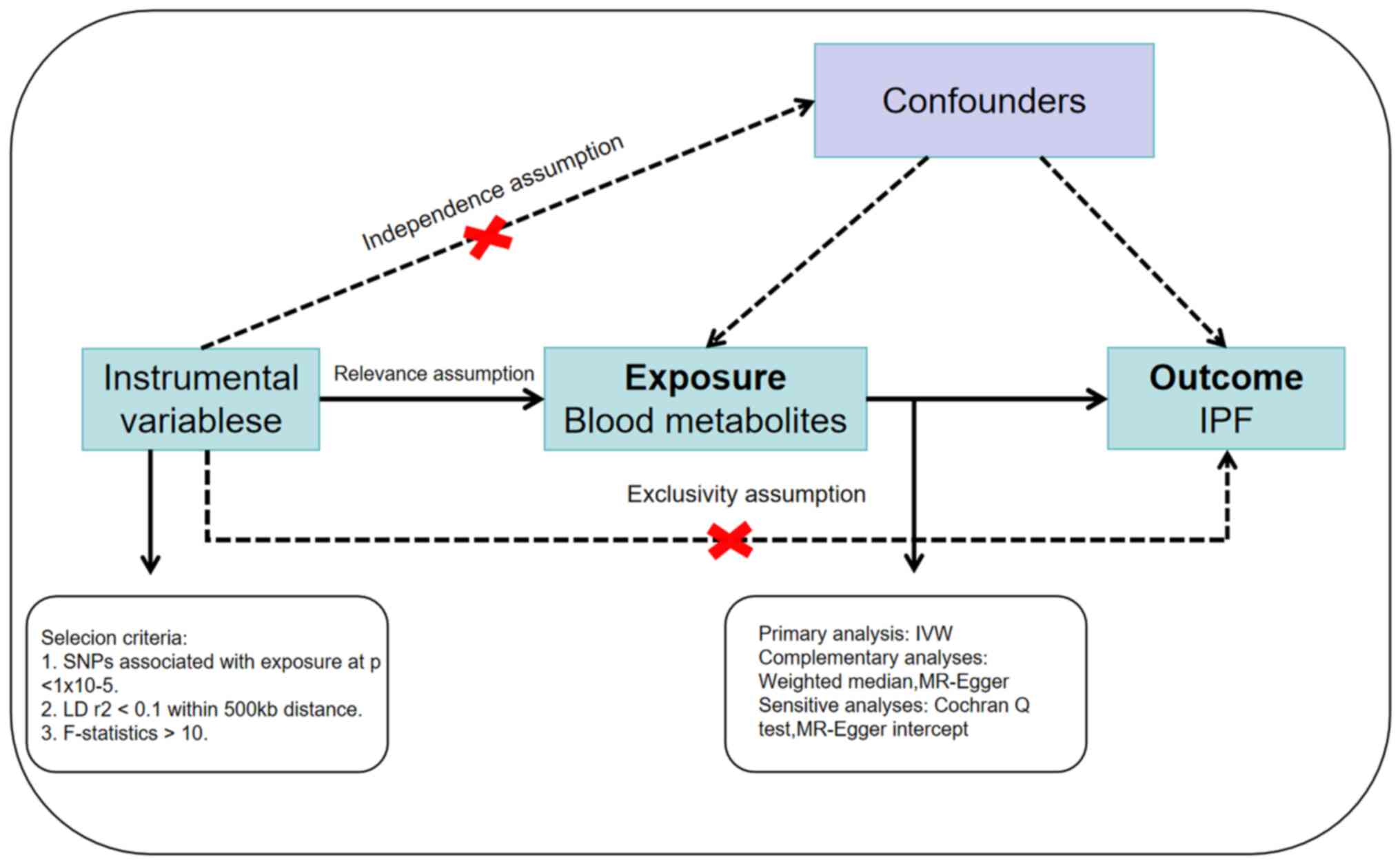
exposure and outcome. The three essential assumptions (22) that should be met for IVs to be valid
are depicted in Fig. 1. Therefore,
it is crucial that the genetic variants employed as IVs exert a
robust association with blood metabolites. Furthermore, genetic
mutations should not be associated with any potential confounding
variables. Thirdly, the genetic variations should exclusively
affect IPF via blood metabolites rather than any alternative
pathways. Therefore, to meet the aforementioned three assumptions,
a significance threshold of P<1x10-5 was set for
autonomy (23). A lower P-value
threshold, such as that of P<1x10-5, could
effectively control the false positive rate. However, it could also
increase the risk of false negative results. Choosing this value
could balance the two to a particular extent, ensuring that the
results would have a certain level of reliability when discovering
potential related SNPs. In large sample studies, lower P-value
thresholds, such as P<5x10-8, are commonly used in
GWAS, since they can effectively reduce false positive results
caused by multiple comparisons. However, if the sample size is
small, using overly strict thresholds can miss out several truly
relevant SNPs. Therefore, choosing a P<1x10-5 could
be more suitable for the current research scenario. Additionally,
to guarantee the autonomy of SNPs and eliminate linkage
disequilibrium (LD) (24), a LD
threshold of r2<0.001 and a distance of 10,000 kb
were implemented. r² is a statistical measure of LD between two
loci. Therefore, the closer the value is to 1, the stronger the LD
between these two loci. r²<0.001 indicates that there is almost
no linkage imbalance between these two loci and they can be
therefore considered independent from each other. Setting
r2<0.001 could help identify markers that are
independent of the phenotype and avoid LD-caused bias. This strict
selection could improve the accuracy of the results, ensuring that
the discovered effects are independent and not caused by the
effects of other loci. Furthermore, the PhenoScanner (25) (http://www.phenoscanner.medschl.cam.ac.uk/) website
was utilized to evaluate if these SNPs were associated with
confounding variables in IPF. Currently, several risk factors for
IPF have been identified, such as smoking, exposure to dust and
reflux esophagitis. Therefore, in the present study, when any
associations were identified, individuals with the aforementioned
variations were excluded.
Statistical analysis
Various strong statistical methods were employed to
guarantee the dependability and authenticity of the results.
However, owing to its exceptional resilience, the inverse variance
weighted (IVW) method was used as the main analytical approach
(26). The IVW method is commonly
applied in fixed- and random-effect models, which help mitigate the
heterogeneity-related bias (27).
Furthermore, the robustness of the findings was assessed via
employing the MR-Egger regression and weighted median techniques.
Several studieshave argued that the MR-Egger regression technique
lacks statistical validity for estimating causality (27,28).
Therefore, it was suggested that this method should only serve as a
sensitivity analysis to assess the violation of core assumptions of
IVs, rather than being used as a substitute for the IVW method
(27). It is generally recognized
that SNPs with F-statistics <10 are weakly instrumented
variables, while those of >10 indicate no significant weakly
instrumented variable bias. In the present study, SNPs with
F-statistics of <10 in the MR analysis were excluded. To
calculate the F value, the following formula was used:
F=R2 x (n-k-1)/(1-R2) x k. The sample size,
denoted by n, indicates the genetic variance denoted by
R2, while k indicates the number of SNPs present in the
sample (29).
Sensitivity analysis
The heterogeneity of IVW and MR Egger regression
methods was evaluated using Cochran's Q test and funnel plots. The
results of Cochran's Q test (30)
with P>0.05 indicated the absence of significant heterogeneity.
The intercept in the MR-Egger regression represents the magnitude
of horizontal pleiotropy. Therefore, the smaller the horizontal
pleiotropy, the more the intercept tends to 0. In addition, to
evaluate whether the findings were significantly affected by a
solitary SNP, a leave-one-out examination was performed (31). This involved excluding each SNP at a
time and subsequently conducting an MR analysis.
Results
Analysis of the associations between
486 blood metabolites and risk of IPF
After rigorous quality evaluation of the IVs, a
total of 486 blood metabolites were obtained for MR analysis. LD
analysis and removal of palindromic sequences to enhance accuracy
and reliability of the selected IVs were also performed. Among the
486 blood metabolites identified, five appeared in two forms, thus
resulting in a total of 491 metabolites. All SNPs demonstrated
adequate validity as indicated by the F-statistics, which exceeded
the empirical threshold of 10. The results of the analysis of the
486 metabolites are displayed in Table
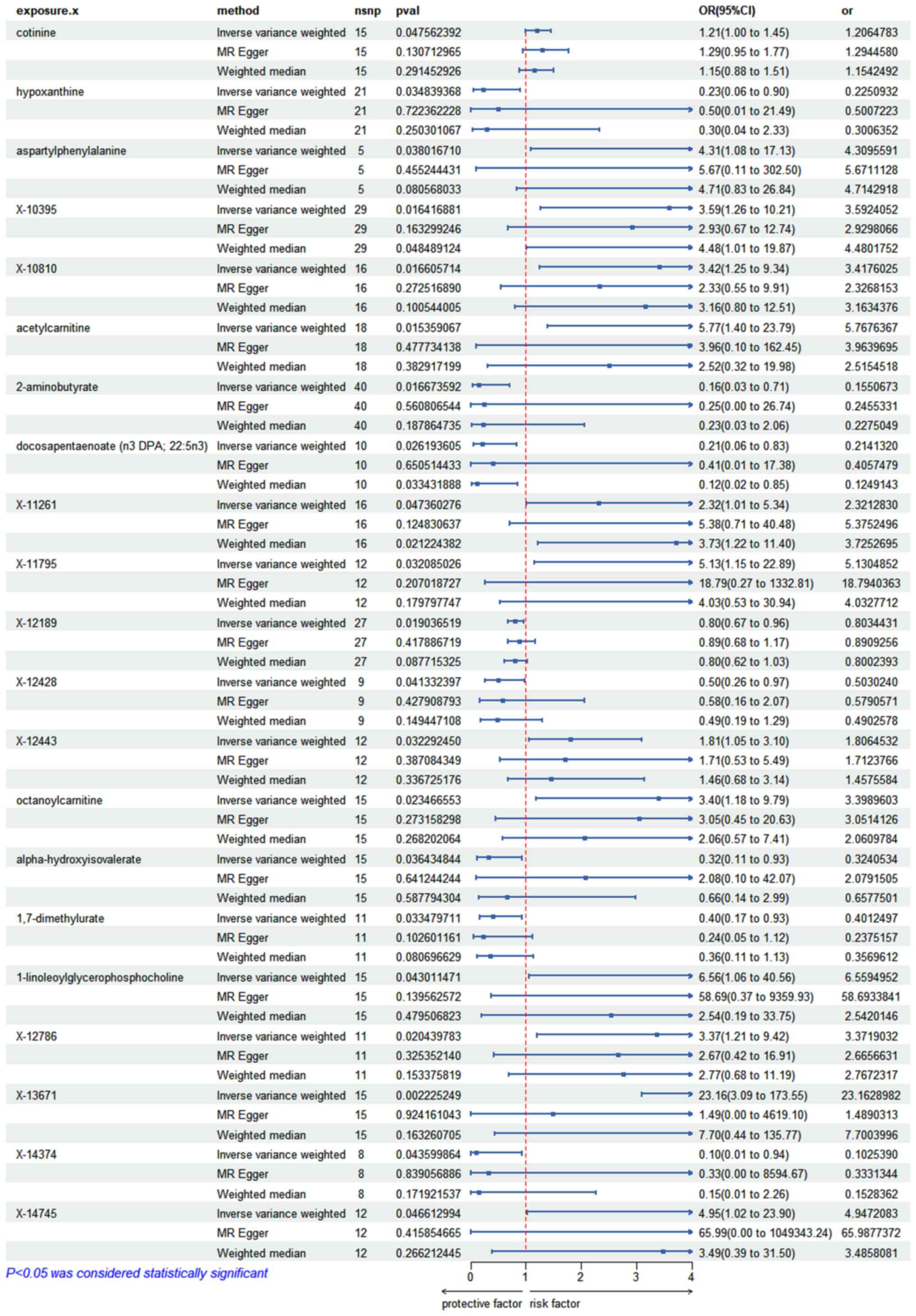
SII. Additionally, using the IVW methodology, a total of 21
circulating metabolites were identified, that could exert a causal
role in IPF risk. Among the aforementioned circulating metabolites,
10 were known compounds and 11 were unknown (Fig. 2). Namely, the circulating
metabolites identified were the following: Cotinine (OR=1.206; 95%
CI, 1.002-1.452; P=0.047), hypoxanthine (OR=0.225; 95% CI,
0.056-0.899; P=0.034), aspartyl phenylalanine (OR=4.309; 95% CI,
1.084-17.131; P=0.038), acetyl-carnitine (OR=5.767; 95% CI,
1.398-23.789; P=0.015), 2-aminobutyrate (OR=0.155; 95% CI,
0.033-0.713; P=0.016), docosapentaenoate (OR=0.214; 95% CI,
0.055-0.833; P=0.026), octanoyl-carnitine (OR=3.398; 95% CI,
1.179-9.794; P=0.023), alpha-hydroxy-isovalerate (OR=0.324; 95% CI,
0.112-0.931; P=0.036), 1,7-dimethylurate (OR=0.401; 95% CI,
0.172-0.931; P=0.033), 1-linoleoylglycerophosphocholine (OR=6.559;
95% CI, 1.060-40.557; P=0.043), X-10395 (OR=3.592; 95% CI,
1.263-10.210; P=0.016), X-10810 (OR=3.42; 95% CI, 1.263-10.210;
P=0.016), X-11261 (OR=2.321; 95% CI, 1.009-5.335; P=0.047), X-11795
(OR=5.13; 95% CI, 1.150-22.885; P=0.032), X-12189 (OR=0.803; 95%
CI; 0.669-0.964; P=0.019), X-12428 (OR=0.503; 95% CI, 0.259-0.973;
P=0.041), X-12443 (OR=1.806; 95% CI, 1.051-3.104; P=0.032), X-12786
(OR=3.371; 95% CI, 1.206-9.423, P=0.020), X-13671 (OR=23.162; 95%
CI; 3.091-173.553; P=0.002), X-14374 (OR=0.102; 95% CI;
0.011-0.936; P=0.043) and X-14745 (OR=4.947; 95% CI; 1.024-23.895;
P=0.046). The analysis also revealed that the high levels of
cotinine, aspartyl-phenylalanine, acetyl-carnitine,
octanoyl-carnitine and 1-lipoyllglycophorophosphacholine were
potential risk metabolites for IPF. By contrast, the high levels of
hypoxanthine, 2-aminobutyrate, docosapentaenoate alpha
hydroxy-isovalerate and 1,7-dimethylurate could reduce the risk of
IPF. These findings could be helpful for detecting the incidence
and predicting the prognosis of IPF in clinical practice. The
results of the MR analysis are visualized in Table SIII, while those of sensitivity
analysis are listed in Table I.
Cochran's Q-test yielded insignificant heterogeneity, while
MR-Egger's intercept analysis verified the lack of horizontal
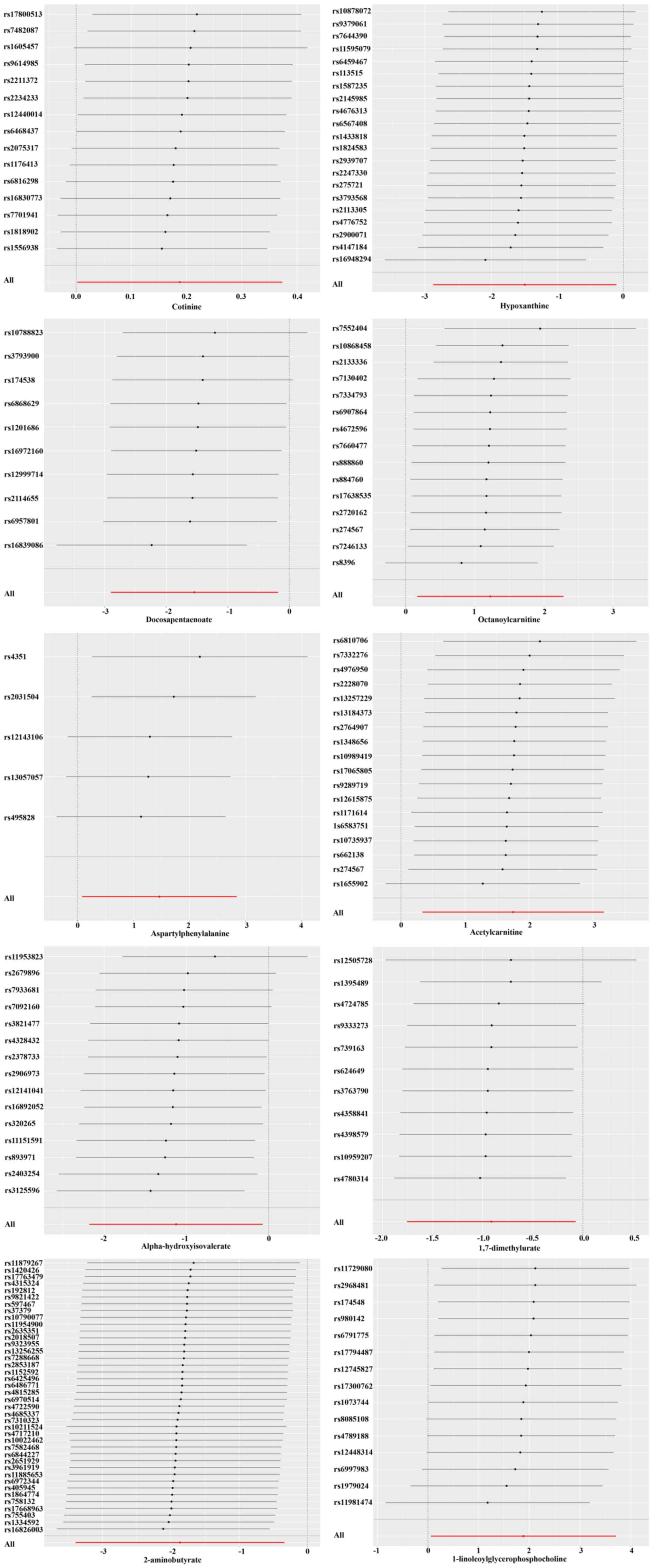
pleiotropy. The reliability of the MR analysis was also verified by
the leave-one-out graphing (Fig.
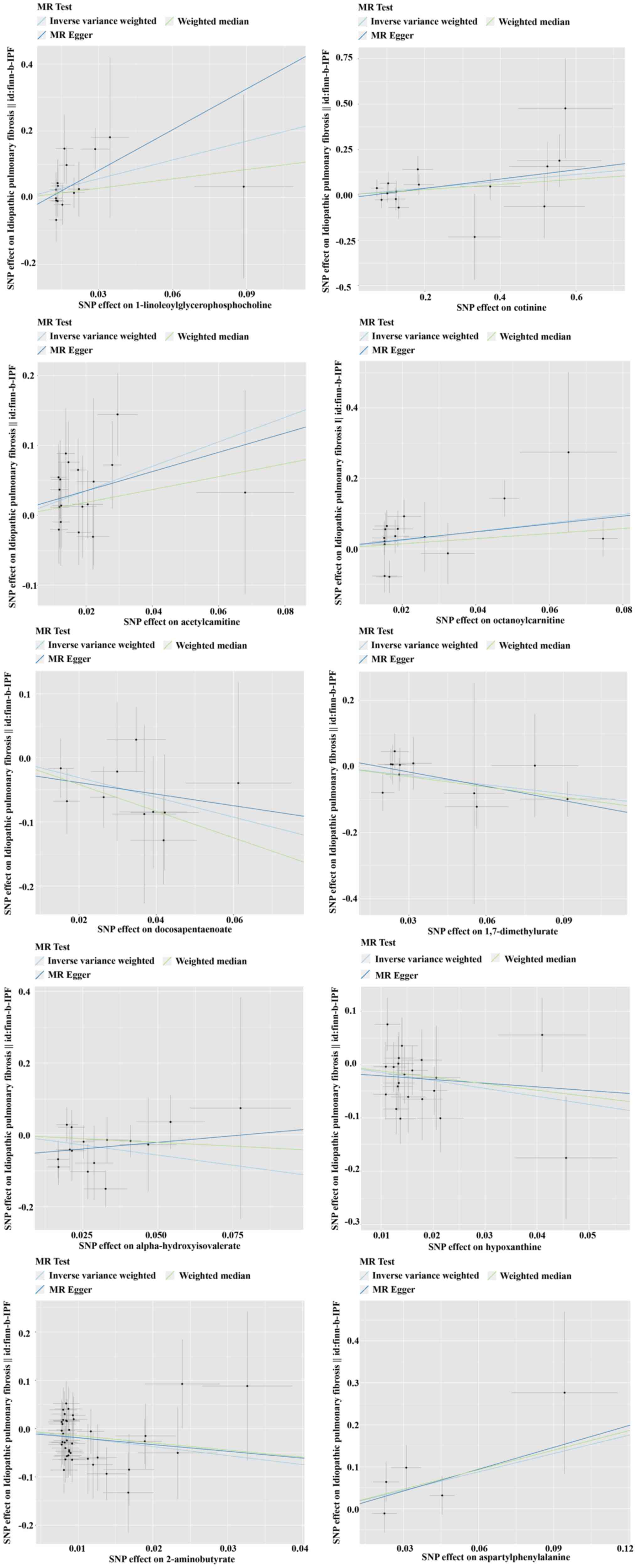
3). The scatter plot is displayed in Fig. 4.
 | Table ISensitivity analysis. |
Table I
Sensitivity analysis.
| Metabolites | Q_pval (IVW) |
MRegger_intercept |
MRegger_interpreter_pval |
|---|
| Cotinine | 0.634204819 | -0.016253799 | 0.594349888 |
| Hypoxanthine | 0.64539929 | -0.014255373 | 0.658951597 |
|
Aspartylphenylalanine | 0.507548038 | -0.0100362 | 0.8937339 |
| X-10395 | 0.421558055 | 0.005325463 | 0.697704685 |
| X-10810 | 0.942302112 | 0.016006743 | 0.482089942 |
|
Acetylcarnitine | 0.854090843 | 0.007536244 | 0.833125497 |
|
2-aminobutyrate | 0.94823233 | -0.004991164 | 0.84015343 |
| Docosapentaenoate
(n3 DPA; 22:5n3) | 0.857218151 | -0.020539902 | 0.729874938 |
| X-11261 | 0.752776285 | -0.029700565 | 0.386080027 |
| X-11795 | 0.812512936 | -0.032772067 | 0.537999752 |
| X-12189 | 0.19702192 | -0.02414237 | 0.332548791 |
| X-12428 | 0.49662327 | -0.009779736 | 0.805574022 |
| X-12443 | 0.748525852 | 0.004634389 | 0.921132003 |
|
Octanoylcarnitine | 0.198556909 | 0.003739538 | 0.894565868 |
|
Alpha-hydroxyisovalerate | 0.480656564 | -0.057310933 | 0.218243317 |
|
1,7-dimethylurate | 0.86047744 | 0.026635303 | 0.450327514 |
|
1-linoleoylglycerophosphocholine | 0.806629731 | -0.040877387 | 0.380697155 |
| X-12786 | 0.824668697 | 0.009070153 | 0.770926683 |
| X-13671 | 0.667042516 | 0.037205512 | 0.501665959 |
| X-14374 | 0.124767596 | -0.026570989 | 0.822873085 |
| X-14745 | 0.384835525 | -0.057805481 | 0.606030326 |
Discussion
The present study aimed to investigate the possible
causal effect of 486 blood metabolites on IPF progression.
Therefore, via combining two extensive GWAS datasets and
implementing a rigorous MR design, a total of 13 genetic
metabolites, namely cotinine, aspartyl phenylalanine,
acetyl-carnitine, octanoyl-carnitine,
1-linoleoylglycerophosphocholine, X-10395, X-10810, X-11261,
X-11795, X-12443, X-12786, X-13671 and X-14745, were identified,
which were associated with enhanced susceptibility to IPF. By
contrast, the analysis predicted eight genetic metabolites, namely
hypoxanthine, 2-aminobutyrate, docosapentaenoate,
alpha-hydroxy-isovalerate, 1,7-dimethylurate, X-12189, X-12428 and
X-14374, that could reduce the risk of IPF.
The rising morbidity and significant mortality of
IPF in recent years have imposed a substantial burden on
individuals globally, emphasizing the need for prompt screening and
prevention as a critical strategy. The emergence of metabolomics
technologies has sparked growing curiosity in investigating the
significance of metabolites associated with IPF. Due to the
chemical nature of exposures originating from both internal and
external sources, blood samples can serve as a means to identify
exposure groups (32). A previous
study supported the reliability of S100 calcium-binding protein A12
as a serum biomarker for assessing the severity and prognosis of
IPF (33). 5-methoxytryptophan
(5-MTP) has been recently identified as a tryptophan metabolite.
Therefore, a study indicated that 5-MTP could combat inflammation,
inhibit tumor growth, protect blood vessels and prevent fibrosis in
kidney disorders. Additionally, the aforementioned study showed
that 5-MTP could be also significantly involved in IPF and it was
therefore anticipated to serve as a treatment strategy for
pulmonary fibrosis (34).
Glutaminolysis, a crucial metabolic pathway in IPF, involves the
conversion of glutamine to glutamate by glutaminase and
subsequently to α-ketoglutarate, a circulating metabolite of the
tricarboxylic acid cycle (35).
Emerging evidence has suggested that the immune system also exerts
a significant role in the development of IPF. Therefore, it has
been reported that IL-1β, IL-6, IL-23 and IL-17A, involved in type
17 immunity, play a significant role in both pulmonary fibrosis and
acute exacerbation of pulmonary fibrosis. Targeting type 17
immunity could emerge as a novel therapeutic approach to hinder the
advancement or onset of pulmonary fibrosis (36). Although the existing literature has
provided conflicting results for particular metabolites,
significant differences in the expression of serum metabolites have
been identified between patients with IPF and healthy controls.
However, further studies are needed to establish a definitive
cause-and-effect association and gain a deeper comprehension of the
underlying mechanisms. In the present study, the c MR method was
used to elucidate the cause-and-effect association between blood
metabolites and IPF, along with the metabolic pathways implicated.
The present study aimed to offer guidance for the screening and
management of IPF.
The study revealed that elevated levels of cotinine,
aspartyl phenylalanine, acetyl-carnitine, octanoyl-carnitine and
1-linoleoylglycerophosphocholine were associated with a heightened
susceptibility to IPF. A previous study demonstrated that cotinine,
which serves as a biomarker for smoking exposure, was associated
with an increased risk of kidney stones, coronary heart disease and
chronic obstructive pulmonary disease (37). In addition, high levels of serum
cotinine could also reduce muscle mass, hepatic steatosis and liver
fibrosis (38-40).
The present study verified that cotinine could be involved in
elevated risk of IPF, thus suggesting that the smoking cessation
management should be strengthened in clinical practice. Aspartyl
phenylalanine, which is formed when cholecystokinin-8 breaks down,
but not when angiotensin breaks down by angiotensin-converting
enzyme, has been associated with an increased risk of acute
coronary syndromes (41).
Additionally, another study revealed a cause-and-effect association
between aspartyl phenylalanine and hypertension and
hypertriglyceridemia (41). The
deficiency of alpha aspartyl phenylalanine hydrolase activity is
considered to be the reason for the possible adverse reactions of
aspartame intake (42,43). Therefore, reducing the intake of
aspartame could be beneficial for preventing IPF. However, further
research is needed to confirm this finding. Fatty acid metabolites,
such as acetyl-carnitine and octanoyl-carnitine, serve significant
roles in several cellular energy metabolic pathways and are
commonly utilized as nutritional supplements. The identification of
acyl-carnitines has become increasingly crucial for the study of
metabolism in various diseases, such as metabolic disorders, heart
disease, diabetes, depression, neurological disorders and
particular types of cancer (44).
Therefore, it was hypothesized that its effect on IPF could be
associated to its involvement in histone acetylation. However,
further studies are needed to verify this finding.
1-linoleoylglycerophosphocholine is a phospholipid, which is formed
from linoleic acid and glycerol. It is involved in several
processes in the human body, such as building and upkeeping cell
membranes and acting as a signaling molecule. It is also used as an
ingredient in nutritional supplements and medications. A previous
study suggested that 1-oleoylglycerophosphate choline could notably
reduce the risk of type 2 diabetes (45). In the present study, elevated
1-lipoloylglycophorophosphacholine levels were associated with
increased susceptibility to IPF. During the pathological process of
IPF, the cell membrane structure can be damaged, while the cellular
function can be disrupted. When the levels of
1-lipoloylglycophorophosphacholine are abnormally enhanced, it can
disrupt the lipid balance of the cell membrane, and affect membrane
protein function and the exchange and signal transmission of
substances inside and outside the cell. For example, it can
interfere with the binding of cell surface receptors and ligands,
thus affecting the response of cells to signaling molecules, such
as growth factors and cytokines, thereby promoting abnormal
activation and proliferation of fibroblasts, excessive deposition
of extracellular matrix, and advancing the process of pulmonary
fibrosis (46,47).
In the present study, the following metabolites with
protective effects on IPF were identified: Hypoxanthine,
2-aminobutyrate, docosapentaenoate, alpha-hydroxy-isovalerate and
1,7-dimethylurate. Therefore, the particular mechanism of their
protective effects on IPF requires further in-depth study.
Hypoxanthine is a purine base, which plays a significant role in
living organisms. It is a component of DNA and RNA, and is also
involved in the process of nucleotide synthesis. In addition,
hypoxanthine can be converted to uric acid via metabolic pathways,
thus affecting purine metabolism and acting as an indicator of
hypoxia. Purine plays a key role in regulating immune response
during wound healing and fibrosis and is a key signal hub for scar
tissue formation in the fibrotic region. The current study
strengthened the study on the improvement of IPF by hypoxanthine,
thus providing novel insights into the identification of potential
targets for IPF. 2-aminobutyrate, an amino acid, plays multiple
functions within the human body. Protein synthesis, metabolism, as
well as neurotransmitter synthesis are its areas of involvement. It
has been reported that 2-aminobutyrate can also have antioxidant
and anti-inflammatory effects, which could account for its
protective effects against IPF. Docosapentaenoic acid, a type of
omega-3 fatty acid, is specifically classified as a long-chain
polyunsaturated fatty acid. It is also known as DPA and is
particularly found in fish and marine oils. Docosapentaenoic acid
was found to exert similar health benefits with other omega-3 fatty
acids, such as reducing inflammation, supporting heart health and
potentially improving cognitive function (48). During the degradation of leucine,
alpha-hydroxy-isovalerate is produced as a metabolite.
Alpha-hydroxy-isovalerate is an organic compound involved in
several metabolic pathways in the body. Researchers are currently
examining its possible involvement in the identification and
treatment of different ailments, such as maple syrup urine disease
and other conditions associated with the metabolism of
branched-chain amino acids (49).
1,7-Dimethylurate is a metabolite of caffeine, also known as
dimethylxanthine. It is produced through the metabolism of caffeine
in the body and has antioxidant and anti-inflammatory properties
(50). For the unknown serum
metabolites found to be associated with IPF risk, further attention
should be paid in future studies to explore their possible
mechanisms underlying their effect on IPF, thus providing
assistance and insights for the clinical prevention and treatment
of IPF.
However, the present study has certain limitations.
Firstly, a limited number of SNPs, that could be used for
genome-wide level exposure, were detected. In order to tackle this
problem, slightly more lenient criteria were established for MR
analysis, a widely adopted approach. Nevertheless, the F-test
statistics for all chosen SNPs surpassed 10, thus suggesting that,
in the present study, the instrumental variable was adequately
strong. Furthermore, the research sample was presently restricted
to individuals of European descent. Therefore, the applicability to
other populations requires further investigation and confirmation.
Further exploration is required to uncover the precise mechanisms
underlying the effects of particular metabolites on the development
of IPF. Due to limited data sources, in the current study, only 486
metabolites were covered. Therefore, more studies on additional
blood metabolites associated with IPF should be performed. A purely
bioinformatic analysis of existing data from one source indeed
presents several limitations. Firstly, the data may not be
representative of the entire population. For example, if the source
database has a bias in terms of geographical location or ethnicity,
it could lead to inaccurate or incomplete conclusions about the
relationship between metabolites and the studied condition.
Secondly, the lack of experimental verification means that the
bioinformatic predictions may not accurately reflect the in
vivo or in vitro biological processes. There could be
confounding factors or interactions that are not accounted for in
the data. Thirdly, the static nature of the existing data may not
capture the dynamic changes in metabolite levels over time or in
response to different stimuli. To address these limitations, future
research could involve collecting data from multiple diverse
sources to improve representativeness. Experimental studies such as
cell culture and animal models could be conducted to validate the
bioinformatic findings and explore the underlying mechanisms.
Longitudinal studies could also be designed to monitor metabolite
fluctuations and their associations with disease progression.
In conclusion, the present magnetic resonance study
indicated that numerous blood metabolites could play a key role in
IPF, thus offering valuable perspectives on possible approaches for
the early detection, avoidance and management of IPF. By combining
genomics and metabolomics, this analysis could provide a point of
reference for investigating the cause and development of IPF.
Supplementary Material
List of the identification (ID) for
each of the 486 blood metabolites.
Analysis results of 486 metabolites
and idiopathic pulmonary causality.
Mendelian randomization analysis
results of 21 blood metabolites associated with idiopathic
pulmonary causality risk.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Shandong Taishan
Scholar Project (grant no. tsqn202306392).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
FW and XL designed the study and drafted the
manuscript. FW, JL, BL, WY, XZ and XL performed the data collection
and analysis. All authors read and approved the final version of
the manuscript. FW and XL confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Podolanczuk AJ, Thomson CC, Remy-Jardin M,
Richeldi L, Martinez FJ, Kolb M and Raghu G: Idiopathic pulmonary
fibrosis: State of the art for 2023. Eur Respir J.
61(2200957)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Moss BJ, Ryter SW and Rosas IO: Pathogenic
mechanisms underlying idiopathic pulmonary fibrosis. Annu Rev
Pathol. 17:515–546. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Thiam F, Phogat S, Abokor FA and Osei ET:
In vitro co-culture studies and the crucial role of
fibroblast-immune cell crosstalk in IPF pathogenesis. Respir Res.
24(298)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Platenburg MGJP, van Moorsel CHM, Wiertz
IA, van der Vis JJ, Vorselaars ADM, Veltkamp M and Grutters JC:
Improved survival of IPF patients treated with antifibrotic drugs
compared with untreated patients. Lung. 201:335–343.
2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tomassetti S, Ravaglia C, Piciucchi S, Ryu
J, Wells A, Donati L, Dubini A, Klersy C, Luzzi V, Gori L, et al:
Historical eye on IPF: A cohort study redefining the mortality
scenario. Front Med (Lausanne). 10(1151922)2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ogura T, Inoue Y, Azuma A, Homma S, Kondoh
Y, Tanaka K, Ochiai K, Sugiyama Y and Nukiwa T: Real-world safety
and tolerability of nintedanib in patients with idiopathic
pulmonary fibrosis: Interim report of a post-marketing surveillance
in Japan. Adv Ther. 40:1474–1493. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Spagnolo P and Lee JS: Recent advances in
the genetics of idiopathic pulmonary fibrosis. Curr Opin Pulm Med.
29:399–405. 2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Alonso-Gonzalez A, Tosco-Herrera E,
Molina-Molina M and Flores C: Idiopathic pulmonary fibrosis and the
role of genetics in the era of precision medicine. Front Med
(Lausanne). 10(1152211)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rajesh R, Atallah R and Bärnthaler T:
Dysregulation of metabolic pathways in pulmonary fibrosis.
Pharmacol Ther. 246(108436)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Roque W and Romero F: Cellular
metabolomics of pulmonary fibrosis, from amino acids to lipids. Am
J Physiol Cell Physiol. 320:C689–C695. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Johnson CH, Ivanisevic J and Siuzdak G:
Metabolomics: Beyond biomarkers and towards mechanisms. Nat Rev Mol
Cell Biol. 17:451–459. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Selvarajah B, Azuelos I, Anastasiou D and
Chambers RC: Fibrometabolism-An emerging therapeutic frontier in
pulmonary fibrosis. Sci Signal. 14(eaay1027)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhao YD, Yin L, Archer S, Lu C, Zhao G,
Yao Y, Wu L, Hsin M, Waddell TK, Keshavjee S, et al: Metabolic
heterogeneity of idiopathic pulmonary fibrosis: A metabolomic
study. BMJ Open Respir. Res. 4(e000183)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gaugg MT, Engler A, Bregy L,
Nussbaumer-Ochsner Y, Eiffert L, Bruderer T, Zenobi R, Sinues P and
Kohler M: Molecular breath analysis supports altered amino acid
metabolism in idiopathic pulmonary fibrosis. Respirology.
24:437–444. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mamazhakypov A, Schermuly RT, Schaefer L
and Wygrecka M: Lipids - two sides of the same coin in lung
fibrosis. Cell Signal. 60:65–80. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tedesco S, Scattolini V, Albiero M,
Bortolozzi M, Avogaro A, Cignarella A and Fadini GP: Mitochondrial
calcium uptake is instrumental to alternative macrophage
polarization and phagocytic activity. Int J Mol Sci.
20(4966)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kim HS, Moon SJ, Lee SE, Hwang GW, Yoo HJ
and Song JW: The arachidonic acid metabolite
11,12-epoxyeicosatrienoic acid alleviates pulmonary fibrosis. Exp
Mol Med. 53:864–874. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang Q, Xie Z, Wan N, Yang L, Jin Z, Jin
F, Huang Z, Chen M, Wang H and Feng J: Potential biomarkers for
diagnosis and disease evaluation of idiopathic pulmonary fibrosis.
Chin Med J (Engl). 136:1278–1290. 2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kanehisa M, Goto S, Sato Y, Furumichi M
and Tanabe M: KEGG for integration and interpretation of
large-scale molecular data sets. Nucleic Acids Res. 40(Database
issue):D109–D114. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dhindsa RS, Mattsson J, Nag A, Wang Q,
Wain LV, Allen R, Wigmore EM, Ibanez K, Vitsios D, Deevi SVV, et
al: Identification of a missense variant in SPDL1 associated with
idiopathic pulmonary fibrosis. Commun Biol. 4(392)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Skrivankova VW, Richmond RC, Woolf BAR,
Yarmolinsky J, Davies NM, Swanson SA, VanderWeele TJ, Higgins JPT,
Timpson NJ, Dimou N, et al: Strengthening the reporting of
observational studies in epidemiology using mendelian
randomization: The STROBE-MR statement. JAMA. 326:1614–1621.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Slob EAW and Burgess S: A comparison of
robust Mendelian randomization methods using summary data. Genet
Epidemiol. 44:313–329. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang X, Zhu Q, Zhang L, Pei Y, Xu X, Liu
X, Lu G, Pan J and Wang Y: Causal relationship between gut
microbiota and serum vitamin D: Evidence from genetic correlation
and Mendelian randomization study. Eur J Clin Nutr. 76:1017–1023.
2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Roze D: Causes and consequences of linkage
disequilibrium among transposable elements within eukaryotic
genomes. Genetics. 224(iyad058)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kamat MA, Blackshaw JA, Young R, Surendran
P, Burgess S, Danesh J, Butterworth AS and Staley JR: PhenoScanner
V2: An expanded tool for searching human genotype-phenotype
associations. Bioinformatics. 35:4851–4853. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sekula P, Del Greco MF, Pattaro C and
Köttgen A: Mendelian randomization as an approach to assess
causality using observational data. J Am Soc Nephrol. 27:3253–3265.
2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhao L, Wu R, Wu Z, Liu X, Li J, Zhang L
and Zhang S: Genetically predicted 486 blood metabolites concerning
risk of systemic lupus erythematosus: A Mendelian randomization
study. Sci Rep. 13(22543)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Burgess S and Thompson SG: Interpreting
findings from Mendelian randomization using the MR-Egger method.
Eur J Epidemiol. 32:377–389. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pierce BL, Ahsan H and VanderWeele TJ:
Power and instrument strength requirements for Mendelian
randomization studies using multiple genetic variants. Int J
Epidemiol. 40:740–752. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hemani G, Bowden J and Davey Smith G:
Evaluating the potential role of pleiotropy in mendelian
randomization studies. Hum Mol Genet. 27:R195–R208. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Burgess S, Bowden J, Fall T, Ingelsson E
and Thompson SG: Sensitivity analyses for robust causal inference
from Mendelian randomization analyses with multiple genetic
variants. Epidemiology. 28:30–42. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rappaport SM, Barupal DK, Wishart D,
Vineis P and Scalbert A: The blood exposome and its role in
discovering causes of disease. Environ Health Perspect.
122:769–774. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li Y, He Y, Chen S, Wang Q, Yang Y, Shen
D, Ma J, Wen Z, Ning S and Chen H: S100A12 as biomarker of disease
severity and prognosis in patients with idiopathic pulmonary
fibrosis. Front Immunol. 13(810338)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fang L, Chen H, Kong R and Que J:
Endogenous tryptophan metabolite 5-Methoxytryptophan inhibits
pulmonary fibrosis by downregulating the TGF-β/SMAD3 and PI3K/AKT
signaling pathway. Life Sci. 260(118399)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Bai L, Bernard K, Tang X, Hu M, Horowitz
JC, Thannickal VJ and Sanders YY: Glutaminolysis epigenetically
regulates antiapoptotic gene expression in idiopathic pulmonary
fibrosis fibroblasts. Am J Respir Cell Mol Biol. 60:49–57.
2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Senoo S, Higo H, Taniguchi A, Kiura K,
Maeda Y and Miyahara N: Pulmonary fibrosis and type-17 immunity.
Respir Investig. 61:553–562. 2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lei T, Li M, Zhu Z, Yang J, Hu Y and Hua
L: Comprehensive evaluation of serum cotinine on human health:
Novel evidence for the systemic toxicity of tobacco smoke in the US
general population. Sci Total Environ. 892(164443)2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Álvarez-Hernández J, Matía-Martín P,
Cáncer-Minchot E and Cuerda C: NUTRICOVID study group of SENDIMAD.
Long-term outcomes in critically ill patients who survived
COVID-19: The NUTRICOVID observational cohort study. Clin Nutr.
42:2029–2035. 2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen Z, Li H, Song C, Sun J and Liu W:
Association between serum cotinine and muscle mass: Results from
NHANES 2011-2018. BMC Public Health. 24(2093)2024.PubMed/NCBI View Article : Google Scholar
|
|
40
|
She D, Jiang S and Yuan S: Association
between serum cotinine and hepatic steatosis and liver fibrosis in
adolescent: A population-based study in the United States. Sci Rep.
14(11424)2024.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Qiu G, Lin Y, Ouyang Y, You M, Zhao X,
Wang H, Niu R, Li W, Xu X, Yan Q, et al: Nontargeted metabolomics
revealed novel association between serum metabolites and incident
acute coronary syndrome: A mendelian randomization study. J Am
Heart Assoc. 12(e028540)2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Stegink LD, Lindgren SD, Brummel MC,
Stumbo PJ and Wolraich ML: Erythrocyte L-aspartyl-L-phenylalanine
hydrolase activity and plasma phenylalanine and aspartate
concentrations in children consuming diets high in aspartame. Am J
Clin Nutr. 62:1206–1211. 1995.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Meng J, Liu W, Wu Y, Xiao Y, Tang H and
Gao S: Is it necessary to wear compression stockings and how long
should they be worn for preventing post thrombotic syndrome? A
meta-analysis of randomized controlled trials. Thromb Res.
225:79–86. 2023.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Dambrova M, Makrecka-Kuka M, Kuka J,
Vilskersts R, Nordberg D, Attwood MM, Smesny S, Sen ZD, Guo AC,
Oler E, et al: Acylcarnitines: Nomenclature, biomarkers,
therapeutic potential, drug targets, and clinical trials. Pharmacol
Rev. 74:506–551. 2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Vangipurapu J, Fernandes Silva L,
Kuulasmaa T, Smith U and Laakso M: Microbiota-related metabolites
and the risk of type 2 diabetes. Diabetes Care. 43:1319–1325.
2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zou X, Wang L, Wang S, Zhang Y, Ma J, Chen
L, Li Y, Yao TX, Zhou H, Wu L, et al: Promising therapeutic targets
for ischemic stroke identified from plasma and cerebrospinal fluid
proteomes: A multicenter Mendelian randomization study. Int J Surg.
110:766–776. 2024.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Meng J, Tang H, Xiao Y, Liu W, Wu Y, Xiong
Y and Gao S: Appropriate thromboprophylaxis strategy for COVID-19
patients on dosage, antiplatelet therapy, outpatient, and
postdischarge prophylaxis: A meta-analysis of randomized controlled
trials. Int J Surg. 110:3910–3922. 2024.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lu Y, Li D, Wang L, Zhang H, Jiang F,
Zhang R, Xu L, Yang N, Dai S, Xu X, et al: Comprehensive
investigation on associations between dietary intake and blood
levels of fatty acids and colorectal cancer risk. Nutrients.
15(730)2023.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kaplan RC, Williams-Nguyen JS, Huang Y,
Mossavar-Rahmani Y, Yu B, Boerwinkle E, Gellman MD, Daviglus M,
Chilcoat A, Van Horn L, et al: Identification of dietary
supplements associated with blood metabolites in the hispanic
community health study/study of latinos cohort study. J Nutr.
153:1483–1492. 2023.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Purpura M, Jäger R and Falk M: An
assessment of mutagenicity, genotoxicity, acute-, subacute and
subchronic oral toxicity of paraxanthine (1,7-dimethylxanthine).
Food Chem Toxicol. 158(112579)2021.PubMed/NCBI View Article : Google Scholar
|