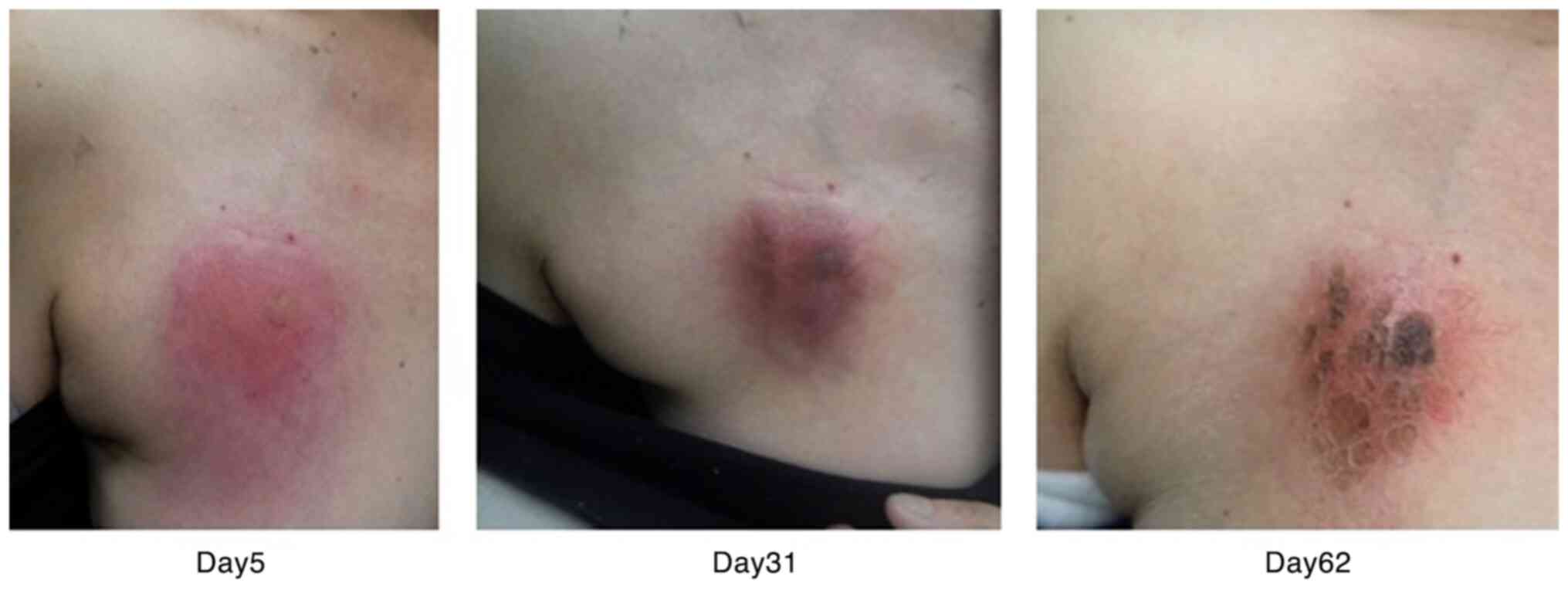
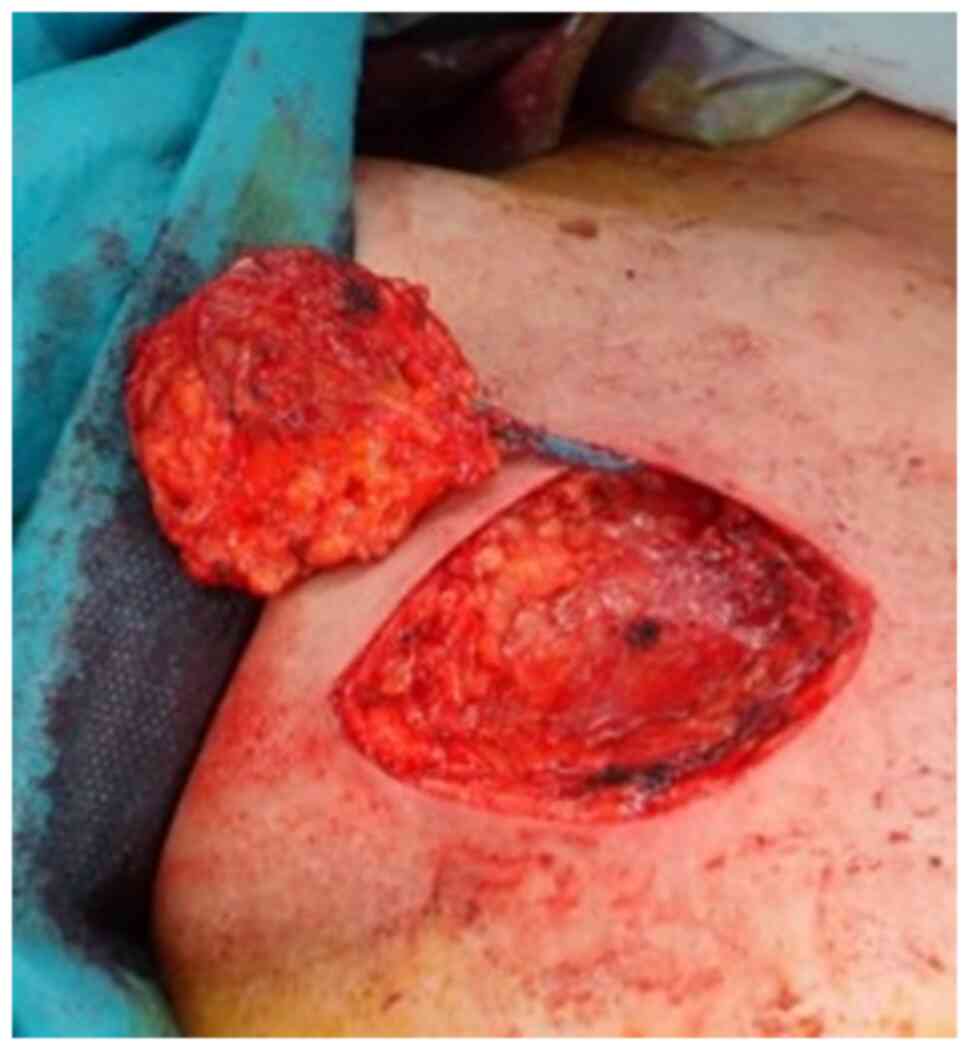
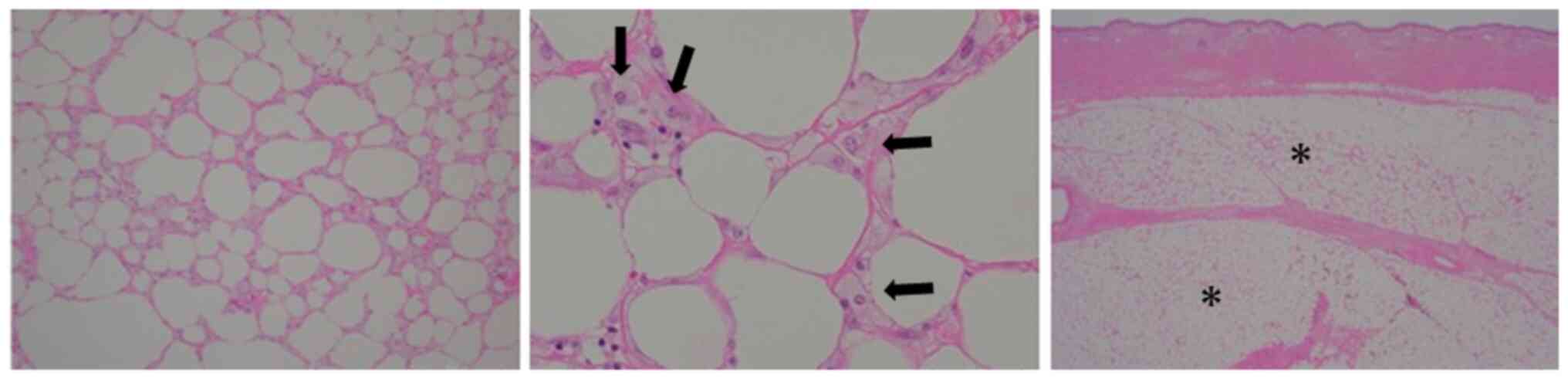
|
1
|
Delisle M, Alshamsan B, Nagaratnam K,
Smith D, Wang Y and Srikanthan A: Metastasectomy in leiomyosarcoma:
A systematic review and pooled survival analysis. Cancers (Basel).
14(3055)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nakamura T and Sudo A: The role of
trabectedin in soft tissue sarcoma. Front Pharmacol.
13(777872)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Demetri GD, von Mehren M, Jones RL,
Hensley ML, Schuetze SM, Staddon A, Milhem M, Elias A, Ganjoo K,
Tawbi H, et al: Efficacy and safety of trabectedin or dacarbazine
for metastatic liposarcoma or leiomyosarcoma after failure of
conventional chemotherapy: Results of a phase III randomized
multicenter clinical trial. J Clin Oncol. 34:786–793.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Theman TA, Hartzell TL, Sinha I, Polson K,
Morgan J, Demetri GD, Orgill DP and George S: Recognition of a new
chemotherapeutic vesicant: Trabectedin (ecteinascidin-743)
extravasation with skin and soft tissue damage. J Clin Oncol.
27:e198–e200. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Haslik W, Hacker S, Felberbauer FX,
Thallinger C, Bartsch R, Kornauth C, Deutschmann C and Mader RM:
Port-a-cath extravasation of vesicant cytotoxics: Surgical options
for a rare complication of cancer chemotherapy. Eur J Surg Oncol.
41:378–385. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pluschnig U, Haslik W, Bayer G, Soleiman
A, Bartsch R, Lamm W, Steger GG, Zielinski CC and Mader RM: Outcome
of chemotherapy extravasation in a large patient series using a
standardised management protocol. Support Care Cancer.
23:1741–1748. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Schöffski P, Cerbone L, Wolter P, De Wever
ID, Samson I, Dumez H, Clement P, Wildiers H and Stas M:
Administration of 24-h intravenous infusions of trabectedin in
ambulatory patients with mesenchymal tumors via disposable
elastomeric pumps: An effective and patient-friendly palliative
treatment option. Onkologie. 35:14–17. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pérez Fidalgo JA, García Fabregat L,
Cervantes A, Margulies A, Vidall C and Roila F: ESMO Guidelines
Working Group. Management of chemotherapy extravasation: ESMO-EONS
clinical practice guidelines. Ann Oncol. 23 (Suppl
7):vii167–vii173. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shiono M, Takahashi S, Takahashi M,
Yamaguchi T and Ishioka C: Current situation regarding central
venous port implantation procedures and complications: A
questionnaire-based survey of 11,693 implantations in Japan. Int J
Clin Oncol. 21:1172–1182. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yip D and Funaki B: Subcutaneous chest
ports via the internal jugular vein. A retrospective study of 117
oncology patients. Acta Radiol. 43:371–375. 2002.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tsuruta S, Goto Y, Miyake H, Nagai H,
Yoshioka Y, Yuasa N and Takamizawa J: Late complications associated
with totally implantable venous access port implantation via the
internal jugular vein. Support Care Cancer. 28:2761–2768.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nagasawa Y, Shimizu T, Sonoda H, Mekata E,
Wakabayashi M, Ohta H, Murata S, Mori T, Naka S and Tani T: A
comparison of outcomes and complications of totally implantable
access port through the internal jugular vein versus the subclavian
vein. Int Surg. 99:182–188. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Heitmann C, Durmus C and Ingianni G:
Surgical management after doxorubicin and epirubicin extravasation.
J Hand Surg Br. 23:666–668. 1998.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Seyfer AE and Solimando DA Jr: Toxic
lesions of the hand associated with chemotherapy. J Hand Surg Am.
8:39–42. 1983.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schummer W, Schummer C, Bayer O, Müller A,
Bredle D and Karzai W: Extravasation injury in the perioperative
setting. Anesth Analg. 100:722–727. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Aoyama R, Tanemura A, Takafuji M, Kotobuki
Y, Tanaka A, Katayama I and Takenaka S: A rare case of sever
muscular necrosis due to extravascular leakage of
trabectedin-severe tissue damage of trabectedin extravasation. J
Cosmet Dermatol Sci Appl. 8:6–9. 2018.
|
|
17
|
Verboom MC, Ouwerkerk J, Steeghs N,
Lutjeboer J, Martijn Kerst J, van der Graaf WTA, Reyners AKL,
Sleijfer S and Gelderblom H: Central venous access related adverse
events after trabectedin infusions in soft tissue sarcoma patients;
experience and management in a nationwide multi-center study. Clin
Sarcoma Res. 7(2)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yoshimi K, Wasa J, Otsuka M, Yoshikawa S,
Goto H, Omodaka T, Katagiri H, Murata H, Hosaka S and Kiyohara Y:
Differential diagnosis of trabectedin extravasation: A case report.
J Dermatol. 44:e200–e201. 2017.PubMed/NCBI View Article : Google Scholar
|