Introduction
Soft tissue sarcomas (STS) are rare tumors that
account for <1% of cancers in adults. Leiomyosarcoma (LMS)
accounts for 10-20% of STS, is associated with a poor prognosis, a
high tendency toward distant recurrence, and decreased disease-free
survival rates (1). Systemic
chemotherapy is considered for advanced STS. Doxorubicin either
alone or combined with ifosfamide has served as first-line
chemotherapy. However, newer drugs such as trabectedin, pazopanib
and eribulin have proven effective against advanced STS including
LMS after the failure of first-line anthracycline-based
chemotherapy (2).
Trabectedin was tolerated well in clinical trials
(3). The most prevalent grade 3/4
adverse events were neutropenia (37%) and elevated serum aspartate
transaminase/alanine transaminase levels (13 and 26%, respectively)
followed by elevated creatine phosphokinase (5.3%) and
rhabdomyolysis (1.2%). The incidence of death caused by
drug-related adverse events was low (2.1%). Trabectedin
extravasation has been reported to cause serious soft tissue damage
(4-7).
A characteristic feature of trabectedin extravasation is the
paucity of associated symptoms such as pain, swelling and redness
during and immediately after extravasation. Therefore, patients and
medical staff often overlook the early stages of extravasation;
hence, detection and treatment may be delayed, which exacerbates
soft tissue damage. Delayed skin and soft tissue disorders can take
days to weeks to develop after trabectedin extravasation.
In the present case report, a patient who developed
skin and soft tissue disorders due to trabectedin extravasation is
presented. The Institutional Review Board at Mie University
Graduate School of Medicine waived the need for written informed
consent owing to the nature of the study. The patient provided
written informed consent to the collection of her data and
associated images for research purposes and the publication of the
present case report.
Case report
A 61-year-old woman was diagnosed with
retroperitoneal LMS that was resected at another hospital. Lung
metastasis developed one year later and she was referred to Mie
University Hospital for further treatment on January 2020. An
indwelling central venous (CV) port was deployed into the left
subclavian vein and the patient underwent seven courses of
chemotherapy using doxorubicin. Thereafter, eribulin was
administered as second-line chemotherapy. However, this failed and
was replaced with trabectedin. Trabectedin was continuously infused
intravenously (i.v.) into the CV port for 5 h. A total of 5 h after
trabectedin administration, the i.v. infusion device alarm
indicated a leak from the CV port at the right chest wall. Skin
symptoms other than swelling were not evident at that time;
however, trabectedin was immediately discontinued and a steroid
hydrocortisone sodium succinate (100 mg) was injected
subcutaneously (s.c.) around the CV port. Topical clobetasol
propionate was started on day 1 after the leak was recognized, as
erythema, induration and tenderness were observed. Although skin
symptoms were not exacerbated, the erythematous area expanded by
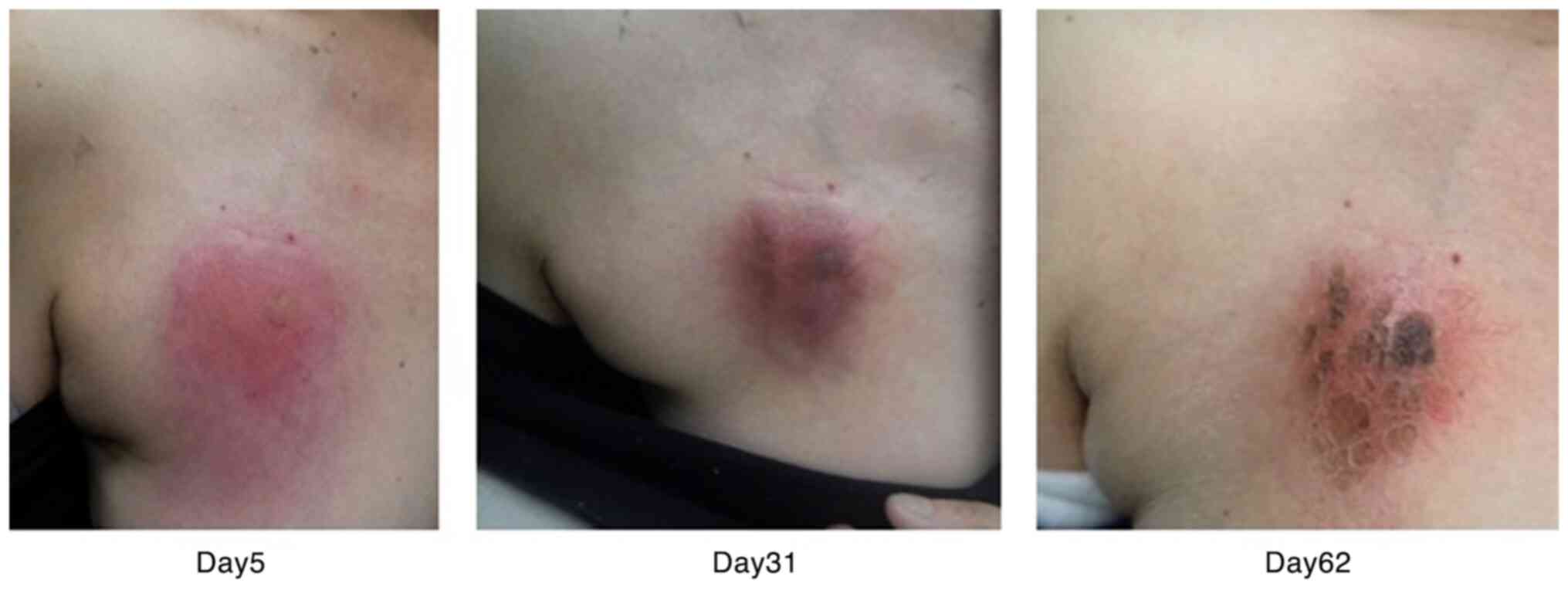
day 5 after the leak was determined. A total of 1 month after
extravasation, the central region of the erythematous area had
become crusted. As the skin condition had subsided, the CV port was
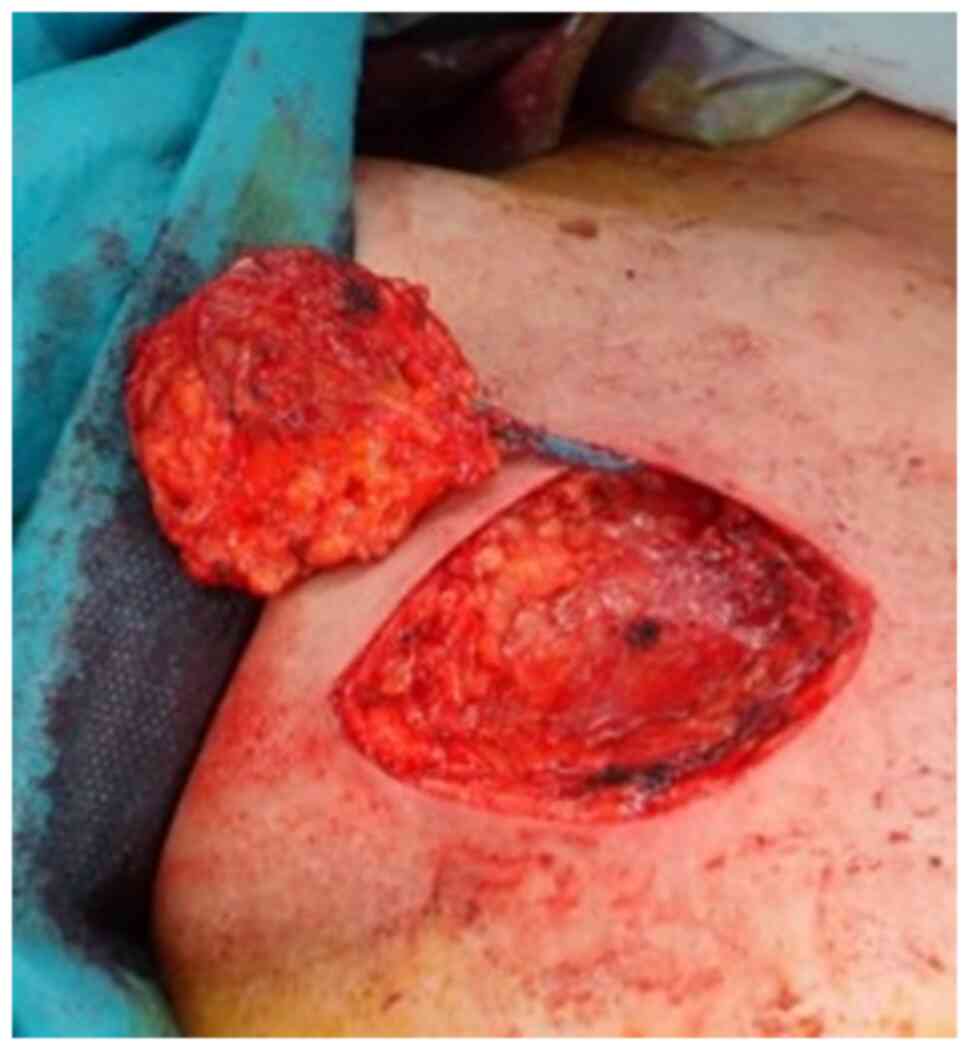
removed and necrotic soft tissue was debrided 62 days after
extravasation (Fig. 1; Table I). Soft tissue flap and skin graft
were not necessary. The CV port and catheter that were removed were
not damaged.
 | Table IThe course of events after
extravasation of TRB. |
Table I
The course of events after
extravasation of TRB.
| 1st day of TRB
administration | Patient report TRB
leakage after 5 h administration. → Discontinue administration
immediately. Local injection of steroids at the leakage area.
(SolcotephTM 100 mg + Lidocaine 5 ml) |
| Post leakage | |
| Day 1 | Topical steroids
applied to the leakage area. |
| Day 2 | Only mild redness at
the CV port area, no pain, discharged from hospital. |
| Day 5 | Expansion of the
erythematous area and tenderness appear. Thereafter, the disease
milder. (Painkillers and antimicrobials were not used.) |
| Day 62 | Skin symptoms have
settled and surgery is performed. CV port removed and leak area
debridement performed. A new CV port was constructed in the left
chest wall. |
| Day 66 | TRB administered from
new CV port. |
| Day 70 | Discharged without
problems in the post-operative course. |
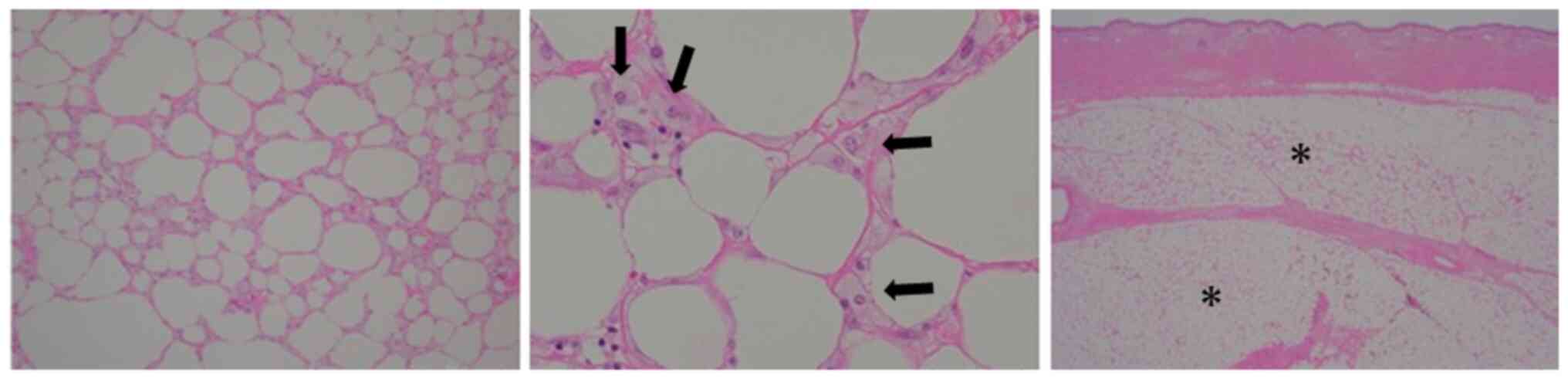
Necrotic changes of soft tissue were not grossly
visible in the subcutaneous tissue on the surface of the resected
area (Fig. 2), but microscopic
necrotic changes were evident in the subcutaneous tissue around the
insertion site of the port. These consisted of extensive
subcutaneous fat necrosis with some fibrosis and fibrin deposition.
The presence of a small amount of foam cells indicated mild
inflammatory cell infiltration (Fig.
3). Trabectedin was continued from a new indwelling CV port
positioned at left chest wall. The patient succumbed to sarcoma 21
months after trabectedin administration.
Discussion
The classification of cytotoxic with regard to their
subcutaneous toxicity was classified into three categories
(6): first, non-vesicant substances
which do not cause local irritations; second, irritant substances
which can cause local pain, swelling and local irritations but do
not result in necrosis; and third, vesicant substances including
trabectedin (8), which may induce
ulcerations and necrosis. Extravasations of vesicants may result in
scar formation and damage of skin and soft tissue requiring
surgical interventions. Severe skin and soft tissue disorders are
associated with trabectedin extravasation (4-7),
the initial characteristic of which is scant subjective symptoms.
Trabectedin should be immediately discontinued if extravasation is
suspected. Ward staff and patients should be informed to prevent
and detect extravasation as early as possible to minimize the
occurrence of skin and soft tissue disorders. Trabectedin is
administered via a central vein often with an indwelling CV
port. Most trabectedin leaks are caused by issues with the CV port
and puncture, or by the catheter. Although the cause of the leak in
our patient was not evident, septal deterioration may have been
involved. Issues with the puncture site of the CV port include a
puncture needle that is short, bent, anatomically unsuitable, or a
needle that floats owing to swelling after CV port deployment.
Puncture site fixation problems include dislodged needles due to
patient movement, including those while asleep. Catheter problems
include deterioration, a bent route, breakage (0.3-2.9%) and
occlusion due to thrombus such as a fibrin sheath (0.6-1.7%)
(1,9-12).
The clinical course after extravasation is difficult
to predict and is dependent on several factors such as the amount
of extravasated drug, the cytotoxic in the affected tissue and
vesicant potential of the drug (6).
Previous studies recommend early surgical intervention after
trabectedin extravasation; however, treatment tends to be
conservative (13,14). Trabectedin is a vesicant drug
(8) and it has been suggested that
leaks should be promptly dealt with owing to risk of delayed tissue
disorders (15). Trabectedin
extravasation caused tissue disorders in 4 (0.4%) of 950 patients
in a clinical trial using CV administration (16). Verboom et al (17) reported CV access-related adverse
events after trabectedin infusion in 127 patients with STS. The
most frequently adverse events at the venous access devices site
were erythema (30.7%), pain (28.3%), inflammation (11.8%) and
thrombosis (11.0%). Of 127 patients, extravasation developed in one
(0.8%). Therefore, trabectedin extravasation may be uncommon.
However, skin disorders have developed owing to trabectedin
extravasation after debridement and skin grafting (4). After extravasation, trabectedin was
immediately discontinued and topical and s.c. injected steroids
were started on the following day. It is presumed that early
discontinuation of trabectedin and additional treatment prevented
extensive skin disorders requiring a skin graft or flap. After the
skin symptoms at the extravasation site had subsided, the CV port
was removed and the skin was debrided. Pathological assessment
identified extensive necrosis at the site of the trabectedin
extravasation. Yoshimi et al (18) reported pathological findings of
necrosis without inflammatory cell infiltration, which was
consistent with our patient. Haslik et al (5) reported that a pathological
characteristic of trabectedin extravasation is necrosis with
various degrees of cellular infiltration similar to those of other
cytotoxic vesicants. It is difficult to determine whether a skin
lesion is due to infection around CV port or trabectedin
extravasation. Pathological findings of cell necrosis facilitate
differentiation between infection and extravasation. Nonetheless,
early biopsy of erythema far from the site of an implanted CV port
site is recommended as surgery and ulcers can induce cell
infiltration. In conclusion, a trabectedin leak was promptly
observed and treatment was promptly implemented to protect soft
tissue. However, our patient underwent additional treatment as
using the same CV port after extravasation would possibly impose
further extravasations and infection. Patients and medical staff
should be continuously educated about the risk of symptom-free
trabectedin extravasation to ensure a rapid response.
Acknowledgements
The authors express their appreciation to Dr Satoshi
Takenaka (Department of Musculoskeletal Oncology Service, Osaka
International Cancer Institute, Osaka, Japan) for his valuable
contributions to the study.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TN, TH and KA treated the patient and provided
follow-up care. YM and TN drafted the manuscript. YM, TN, HY and MH
prepared the figures and table, and confirm the authenticity of all
the raw data. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The Institutional Review Board at Mie University
Graduate School of Medicine waived the need for written informed
consent owing to the nature of the study.
Patient consent for publication
The patient provided written informed consent for
publication of her data and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Delisle M, Alshamsan B, Nagaratnam K,
Smith D, Wang Y and Srikanthan A: Metastasectomy in leiomyosarcoma:
A systematic review and pooled survival analysis. Cancers (Basel).
14(3055)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nakamura T and Sudo A: The role of
trabectedin in soft tissue sarcoma. Front Pharmacol.
13(777872)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Demetri GD, von Mehren M, Jones RL,
Hensley ML, Schuetze SM, Staddon A, Milhem M, Elias A, Ganjoo K,
Tawbi H, et al: Efficacy and safety of trabectedin or dacarbazine
for metastatic liposarcoma or leiomyosarcoma after failure of
conventional chemotherapy: Results of a phase III randomized
multicenter clinical trial. J Clin Oncol. 34:786–793.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Theman TA, Hartzell TL, Sinha I, Polson K,
Morgan J, Demetri GD, Orgill DP and George S: Recognition of a new
chemotherapeutic vesicant: Trabectedin (ecteinascidin-743)
extravasation with skin and soft tissue damage. J Clin Oncol.
27:e198–e200. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Haslik W, Hacker S, Felberbauer FX,
Thallinger C, Bartsch R, Kornauth C, Deutschmann C and Mader RM:
Port-a-cath extravasation of vesicant cytotoxics: Surgical options
for a rare complication of cancer chemotherapy. Eur J Surg Oncol.
41:378–385. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pluschnig U, Haslik W, Bayer G, Soleiman
A, Bartsch R, Lamm W, Steger GG, Zielinski CC and Mader RM: Outcome
of chemotherapy extravasation in a large patient series using a
standardised management protocol. Support Care Cancer.
23:1741–1748. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Schöffski P, Cerbone L, Wolter P, De Wever
ID, Samson I, Dumez H, Clement P, Wildiers H and Stas M:
Administration of 24-h intravenous infusions of trabectedin in
ambulatory patients with mesenchymal tumors via disposable
elastomeric pumps: An effective and patient-friendly palliative
treatment option. Onkologie. 35:14–17. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pérez Fidalgo JA, García Fabregat L,
Cervantes A, Margulies A, Vidall C and Roila F: ESMO Guidelines
Working Group. Management of chemotherapy extravasation: ESMO-EONS
clinical practice guidelines. Ann Oncol. 23 (Suppl
7):vii167–vii173. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shiono M, Takahashi S, Takahashi M,
Yamaguchi T and Ishioka C: Current situation regarding central
venous port implantation procedures and complications: A
questionnaire-based survey of 11,693 implantations in Japan. Int J
Clin Oncol. 21:1172–1182. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yip D and Funaki B: Subcutaneous chest
ports via the internal jugular vein. A retrospective study of 117
oncology patients. Acta Radiol. 43:371–375. 2002.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tsuruta S, Goto Y, Miyake H, Nagai H,
Yoshioka Y, Yuasa N and Takamizawa J: Late complications associated
with totally implantable venous access port implantation via the
internal jugular vein. Support Care Cancer. 28:2761–2768.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nagasawa Y, Shimizu T, Sonoda H, Mekata E,
Wakabayashi M, Ohta H, Murata S, Mori T, Naka S and Tani T: A
comparison of outcomes and complications of totally implantable
access port through the internal jugular vein versus the subclavian
vein. Int Surg. 99:182–188. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Heitmann C, Durmus C and Ingianni G:
Surgical management after doxorubicin and epirubicin extravasation.
J Hand Surg Br. 23:666–668. 1998.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Seyfer AE and Solimando DA Jr: Toxic
lesions of the hand associated with chemotherapy. J Hand Surg Am.
8:39–42. 1983.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schummer W, Schummer C, Bayer O, Müller A,
Bredle D and Karzai W: Extravasation injury in the perioperative
setting. Anesth Analg. 100:722–727. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Aoyama R, Tanemura A, Takafuji M, Kotobuki
Y, Tanaka A, Katayama I and Takenaka S: A rare case of sever
muscular necrosis due to extravascular leakage of
trabectedin-severe tissue damage of trabectedin extravasation. J
Cosmet Dermatol Sci Appl. 8:6–9. 2018.
|
|
17
|
Verboom MC, Ouwerkerk J, Steeghs N,
Lutjeboer J, Martijn Kerst J, van der Graaf WTA, Reyners AKL,
Sleijfer S and Gelderblom H: Central venous access related adverse
events after trabectedin infusions in soft tissue sarcoma patients;
experience and management in a nationwide multi-center study. Clin
Sarcoma Res. 7(2)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yoshimi K, Wasa J, Otsuka M, Yoshikawa S,
Goto H, Omodaka T, Katagiri H, Murata H, Hosaka S and Kiyohara Y:
Differential diagnosis of trabectedin extravasation: A case report.
J Dermatol. 44:e200–e201. 2017.PubMed/NCBI View Article : Google Scholar
|